Abstract
Autologous spermatogonial stem cell (SSC) transplantation is a potential therapeutic modality for patients with azoospermia following cancer treatment. For this promise to be realized, definitive membrane markers of prepubertal and adult human SSCs must be characterized in order to permit SSC isolation and subsequent expansion. This study further characterizes the markers of male gonocytes, prespermatogonia, and SSCs in humans. Human fetal, prepubertal, and adult testicular tissues were analyzed by confocal microscopy, fluorescence activated cell sorting (FACS), and qRT-PCR for expression of unique germ cell membrane markers. During male fetal development, THY1 and C-Kit are transient markers of gonocytes but not in prespermatogonia and post-natal SSCs. Although C-Kit expression is detected in gonocytes, THY1 expression is also detected in the somatic component of the fetal testes in addition to gonocytes. In the third trimester of gestation, THY1 expression shifts exclusively to the somatic cells of the testes where it continues to be detected only in the somatic cells postnatally. In contrast, SSEA-4 expression was only detected in the gonocytes, prespermatogonia, SSCs, and Sertoli cells of the fetal and prepubertal testes. After puberty, SSEA-4 expression can only be detected in primitive spermatogonia. Thus, although THY1 and C-Kit are transient markers of gonocytes, SSEA-4 is the only common membrane marker of gonocytes, prespermatogonia, and SSCs from fetal through adult human development. This finding is essential for the isolation of prepubertal and adult SSCs, which may someday permit fertility preservation and reversal of azoospermia following cancer treatment.
INTRODUCTION
The isolation, expansion, and successful reconstitution of spermatogenesis after spermatogonial stem cell (SSC) transplantation in the murine model have opened new doors for potential human therapeutic applications such as fertility treatments and preservation (Brinster & Avarbock 1994, Brinster & Zimmermann 1994, Kanatsu-Shinohara et al. 2003). Unfortunately, 20 years after the first reports of successful testicular germ cell transplantations in mice, progress in human SSC research has been limited mainly due to the scarcity of available human testicular tissues for research and the lack of an effective in vivo model fully capable of supporting human spermatogenesis (Brinster & Avarbock 1994, Brinster & Zimmermann 1994, Dym et al. 2009, Cheng & Mruk 2010).
Successful in vitro expansion of prepubertal and adult human SSCs, capable of engrafting in mouse seminiferous tubules in a human murine xenograft model, had been reported using testicular cells from digested seminiferous tubules (Sadri-Ardekani et al. 2009, Liu et al. 2011, Sadri-Ardekani et al. 2011, Mirzapour et al. 2012). Additionally, these in vitro derived human SSCs were shown to possess pluripotent properties similar to embryonic stem cells (Conrad et al. 2008, Golestaneh et al. 2009, Kossack et al. 2009, Mizrak et al. 2010). However, these in vitro expanded pluripotent human SSCs, capable of multi-lineage differentiation, have been questioned as recent studies demonstrated that these cells exhibited mesenchymal rather than germ cell properties highlighting the need for further characterization of the SSC population (Ko et al. 2011, Tapia et al. 2011, Chikhovskaya et al. 2012). Most prior human SSC studies used a mixed population of testicular cells (somatic and germ cells combined) from enzymatically digested seminiferous tubules for culture, expansion, and transplantation. Thus, the precise identity of the primitive, pluripotent germ cells within the testicular cell population capable of expansion and engraftment is uncertain.
In order to permit fertility preservation using cryopreservation and subsequent transplantation of autologous SSCs in prepubertal boys undergoing sterilizing oncologic treatments, it is vital to develop the ability to isolate SSCs capable of engraftment through the use of unique membrane markers, thus eliminating the risks of malignant cell contamination from the testicular cell population (Fujita et al. 2005, Fujita et al. 2006, Hermann et al. 2011, Dovey et al. 2013). While definitive markers of SSCs have been identified in mice, unique membrane markers of primate and human SSCs have yet to be fully characterized (Ebata et al. 2005, Gashaw et al. 2007, Seandel et al. 2007, Conrad et al. 2008, Muller et al. 2008, Dym et al. 2009, Grisanti et al. 2009, Maki et al. 2009, Wu et al. 2009, He et al. 2010, Izadyar et al. 2011, Eildermann et al. 2012, Dovey et al. 2013, Kossack et al. 2013). Although THY1, GFRα1R, GPR125, EPCAM, and SSEA-4 have been reported to be unique membrane markers of adult human SSCs, many of these markers (GFRα1R, GPR125, and EPCAM) are also expressed in adult testicular stromal cells, limiting their potential use as markers for SSC isolation (Conrad et al. 2008, Wu et al. 2009, He et al. 2010, Izadyar et al. 2011, Dovey et al. 2013). Although primary adult human testicular cells expressing SSEA-4 were shown to engraft in mouse seminiferous tubules, SSEA-4 expression was not detected in human SSCs by others (Izadyar et al. 2011, Dovey et al. 2013). Thus, there is a lack of consensus agreement on the unique membrane markers of human SSCs.
A logical approach to identify the unique membrane markers of human SSCs is to study human male germ cells during development (gonocytes, prespermatogonia), prior to the onset of spermatogenesis and follow them through puberty (spermatogonia) and adulthood. If a unique membrane marker of primitive germ cells were present through all stages of development it may serve as an important marker of SSCs. Recent studies report two distinct populations of primitive germ cells, gonocytes and prespermatogonia, within the male fetal testes during the first two trimesters of gestation (Pauls et al. 2006, Anderson et al. 2007, Gkountela et al. 2012, Jorgensen et al. 2012). OCT4A and C-Kit were expressed in gonocytes during the late first and early second trimesters; however, expression of VASA was not found in these gonocytes (Anderson et al. 2007, Gkountela et al. 2012). During the second trimester, prespermatogonia began to appear in the testes, presumably from differentiation of gonocytes (Gkountela et al. 2012). While the prespermatogonia expressed VASA, they no longer expressed OCT4A and C-Kit (Pauls et al. 2006, Anderson et al. 2007, Gkountela et al. 2012). Therefore, C-Kit was the only membrane marker in fetal gonocytes, but such an identifying marker unique to prespermatogonia and spermatogonia remains to be investigated.
Thus, we aim to characterize the expression of human primitive germ cell membrane markers from fetal development (gonocytes and prespermatogonia) through adulthood (spermatogonia and SSCs).
MATERIALS AND METHODS
Testicular Tissues
All tissues were obtained after informed consent in accordance with the study protocol approved by the University of California, San Francisco (UCSF) IRB. Human fetal testes (13–24 weeks of gestation) were collected following elective terminations of pregnancy excluding cases with fetal anomalies (n=33). Gestational age was determined by last menstrual cycle and confirmed with ultrasound and subsequent foot length measurement. Autopsied prepubertal testicular tissues were obtained from deceased subjects, whose death was not related to disorders of their reproductive system (n=3). Adult testicular biopsy samples were collected from patients (n=3) with normal spermatogenesis who underwent testicular spermatocelectomy, vasovasostomy, and testicular excisional sperm extraction due to anejaculation.
Confocal microscopy
Tissues were fixed in 4% paraformaldehyde, embedded in optimal cutting temperature compound (O.T.C) (Sakura Finetek, Torrance, CA), and cryosectioned at 5 μm. Sections were permeabilized with 0.1% Triton-X-100 PBS (Sigma-Aldrich, St. Louis, MO), blocked in 5% BSA-PBS, and incubated overnight at 4°C with the following antibodies: goat anti-VASA (R&D-AF2030 at 1:100 dilution, Minneapolis, MN), anti-THY1 (BD-559869 at 1:50, San Jose, CA, and R&D-AF206 at 1:40), rabbit anti-WT1 (Santa Cruz-SC-192 at 1:75, Dallas, TX), anti-OCT4A (Santa Cruz SC-9081 at 1:75, and SC-8628 at 1:75), mouse anti-SSEA-4 (BD-560308 at 1:50), anti-C-Kit (Santa Cruz SC-5535 at 1:50 and M14 at 1:50), mouse anti-SSEA-1(R&D- FAB2155A at 1:50), mouse anti-TRA-1-81 (BD-560885 at 1:50), mouse anti-TRA-1-60 (BD-560071 at 1:50), goat anti- GFRα-1R (R&D-AF714 at 1:50), rabbit anti-MAGEA4 (Abcam ab76177 at 1:50), rat anti-SSEA-3 (Abcam ab16286 at 1:100), and rabbit anti-GPR125 (Abcam-ab51705 at 1:50, Cambridge, MA). Primary species specific isotypes were used for controls. Donkey anti-goat Alexa 488 and 555, donkey anti-sheep Alexa 555, donkey anti-rabbit Alexa 555 and 594, donkey anti-mouse Alexa 488, 555 and 594 (BD) were applied accordingly the following day at 1:200–1:500 dilutions at room temperature for 1 hour. Images were captured using a Leica SP5 AOBS confocal microscope (Leica Microsystems Inc, Buffalo Grove, IL), and analyzed using ImageJ v1.6 (rsbweb.nih.gov).
Testicular Cell Isolation and Fluorescence Activated Cell Sorting (FACS)
Tissues were subjected to a two-step enzymatic digestion with collagenase IV (1mg/ml) (Sigma-Aldrich) in DMEM/F12 + Glutamax (Invitrogen, Grand Island, NY) for 20 min at 37°C, followed by trypsin EDTA 0.25% (UCSF Cell Culture Facility) and DNase I (50 μg/ml) (Sigma-Aldrich) for 20 min, and filtered through a 70 μm cell strainer. Cells were incubated with the following antibodies: anti-SSEA-4 FITC (BD-560308), anti-THY-1 APC (BD-559869), anti-c-KIT PE (R&D-FAB332P), anti-TRA-1-81 PE (BD-560885) and anti-SSEA-1 APC (R&D-FAB2155A), in 1% bovine serum albumin (BSA) for 30 min at 37°C. Cell sorting was performed on a BD FACS Aria Flow Cytometer and analyzed using FlowJo v9.6 (Ashland, OR). 50,000–200,000 events were acquired for analyses.
Molecular Analyses
Subpopulations of testicular cells were sorted directly into RNA lysis buffer. Total RNA was isolated using the RNeasy Micro Kit (QIAGEN, Valencia, CA) and cDNA was synthesized using qScript cDNA Super Mix (Quanta Biosciences, Gaithersburg, MD). Each qPCR amplification was performed in triplicate at 250 cells/reaction using FastStart Universal SYBR Green Master Mix with ROX (Roche, Pleasanton, CA) and Applied Biosystem 7500 Real-time PCR System (Carlsbad, CA). Please see supplemental table for list of primers and sequences. Gene expression was analyzed using the 2−(ΔC(t)) and 2−(ΔΔC(t)) methods. ANOVA were used for statistical analyses.
RESULTS
Male fetal testes contain two populations of germ cells defined by the relative expression of VASA
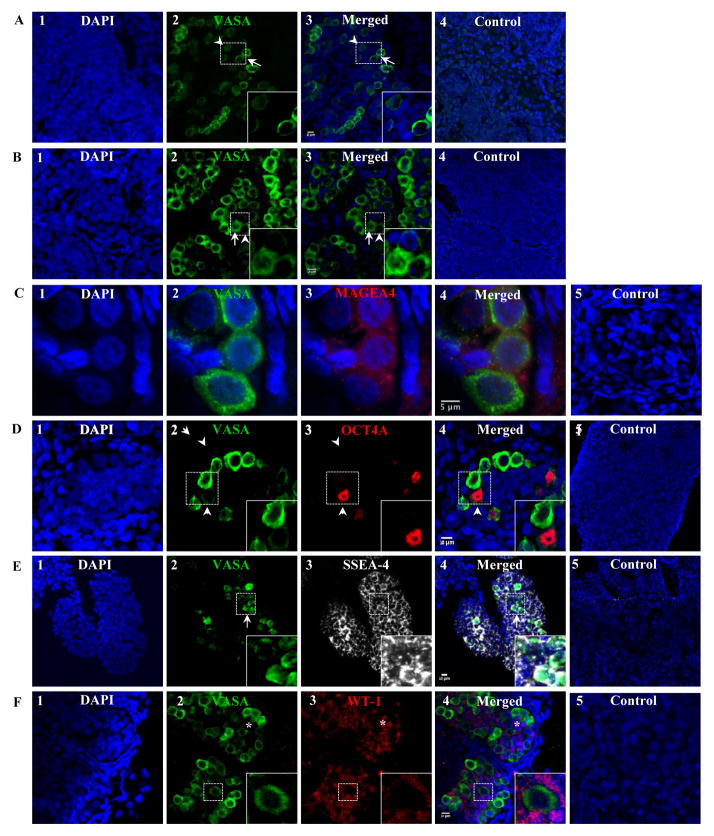
In addition to published membrane markers of human and mouse SSCs (GPR125, GFRα1R, SSEA-1, SSEA-4, C-Kit, and THY1), expression of embryonic stem cell membrane markers (TRA1-60 and TRA1-81) were also evaluated in both gonocytes and prespermatogonia. GPR125, GFRα1R, TRA1-60, TRA1-81, and SSEA-1 expression was not detected in either early or late second trimester testes by confocal microscopy and flow cytometry (data not shown). Germ cells expressing VASA were first detected at 13 weeks of gestation (the earliest time point examined) and continued to increase in number throughout gestation. At 13 weeks of gestation, two populations of germ cells were identified based on relative expression of VASA, VASA dim (VASAD) and VASA bright (VASAB), (Fig. 1A). Moreover, the number of VASAB cells increased with advancing gestation. At gestational week 13 and 24, the ratio of VASAD/VASAB germ cells decreased from 3/2 (Fig. 1A) to 1/3 when all VASA+ cells from 10 cords were counted (Fig. 1B), respectively, suggesting that the VASAB population represent the prespermatogonia population as the number of VASAB cells increased with gestation. In contrast, the VASAD population may represent the rare gonocyte population. Both VASAD and VASAB cells co-expressed MAGEA further confirming that they are indeed primitive germ cells (Fig. 1C). Given the prior finding of OCT4A expression in VASA negative gonocytes, we evaluated VASAD expression in these cells. Rare OCT4A positive cells were detected only in cells expressing low levels of VASA (Fig. 1D), demonstrating that VASAD cells are indeed gonocytes.
Figure 1.
The dynamic of VASA, OCT4A, and SSEA-4 expression in male testes at 13–24 weeks of gestation. (A) Two populations of germ cells were observed based on relative expression of VASA, VASA dim (VASAD) vs. VASA bright (VASAB), shown here at 13 weeks of gestation. Ratio of VASAD/VASAB germ cells ~3/2. (B) The ratio of VASAD/VASAB germ cells (~1/3) decreased with advancing gestation, shown here at 24 weeks of gestation. (C) All VASA+ cells co-expressed MAGEA4, shown at 20 weeks of gestation. (D) Only VASAD germ cells expressed OCT4A during the second trimester, shown here at 21 weeks of gestation. (E) Both VASAD and VASAB germ cells expressed similar levels of SSEA-4 shown at 20 weeks of gestation. (E–F) Fetal Sertoli cells also expressed SSEA-4. Arrow head, arrow, and asterisk indicate VASAd, VASAb, and Sertoli cells, respectively. Ratio of VASAD/VASAB cells was determined by counting all VASA+ cells from 10 cords. Donkey anti-goat Alexa 488 (VASA), anti-mouse Alexa 594 (MAGEA4), anti-rabbit Alexa 594 (OCT4A, WT-1), and anti-mouse Alexa 555 (SSEA-4) were used.
SSEA-4 is a common membrane marker for gonocytes, prespermatogonia, and Sertoli cells
All of the cells within the seminiferous cord, during the second and third trimesters, expressed SSEA-4 (Fig. 1E). Both VASAD and VASAB cells expressed SSEA-4 at similar levels indicating SSEA-4 to be a common marker for both gonocytes and prespermatogonia. However, SSEA-4 expression was also detected in the remaining VASA negative cells making up the cord. WT1 expression was then evaluated to determine whether these SSEA-4+/VASA− cells were Sertoli cells. WT1 was expressed exclusively in all SSEA-4+/VASA− cells (Fig. 1F) confirming that all the non-germ cells expressing SSEA-4+ in the seminiferous cords were Sertoli cells.
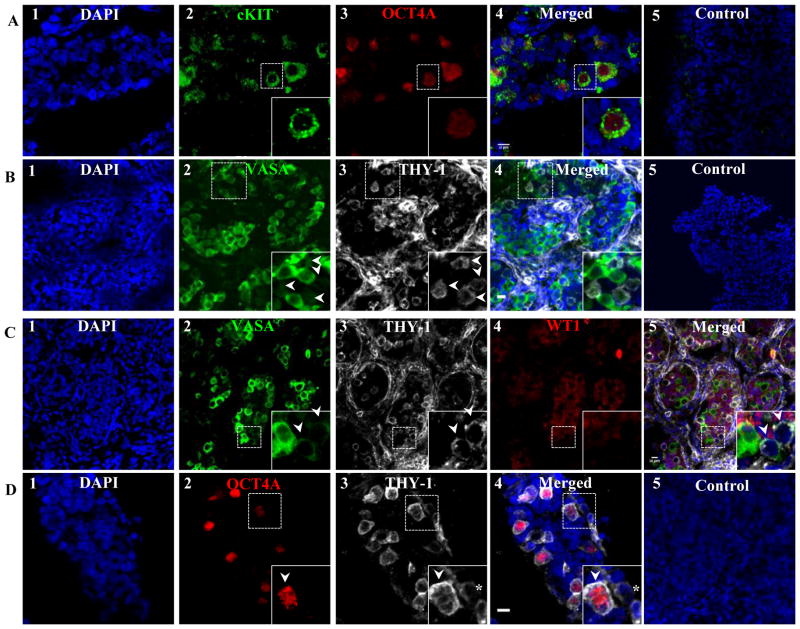
Gonocytes transiently express C-Kit and THY1
C-Kit and THY1 expression in gonocytes, prespermatogonia, and Sertoli cells was also evaluated. Gonocytes expressing OCT4A were found to co-express C-Kit, in addition to VASAD (Fig. 2A). In contrast to SSEA-4, the majority of THY1 expression was detected on cells outside of the seminiferous cords with the exception of a few cell clusters within the cords during the second trimester (Fig. 2B). Within the seminiferous cord, THY1+ cells were arranged in small clusters of 3–6 cells and expressed low levels of VASA (VASAD) (Fig. 2C). In contrast, VASAB cells never expressed THY1. Furthermore, WT1 expression was never detected in THY1+cells indicating that SSEA-4+/THY1+ cells are primitive gonocytes (Fig. 2C). Within the cords, THY1+ cells co-expressed OCT4A further confirming that SSEA-4, THY1, and C-Kit are membrane markers on gonocytes (Fig. 2D).
Figure 2.
Extracellular membrane markers of fetal male gonocytes. Gonocytes also co-expressed C-Kit (A) and THY1 (B), shown at 15 and 19 weeks of gestation, respectively. While SSEA-4+ is a general marker for all germ and Sertoli cells in the fetal testes, THY1 expression within the SSEA-4 population was restricted only to gonocytes that expressed low level of VASA (VASAD) (C) and OCT4A (D), shown at 21 and 18 weeks of gestation, respectively. Arrow heads indicate VASAD cells (gonocytes). Donkey anti-goat Alexa 488 (VASA), anti-mouse Alexa 488 (C-Kit), anti-rabbit Alexa 594 (OCT4A), and anti-mouse Alexa 555 (THY1) were used.
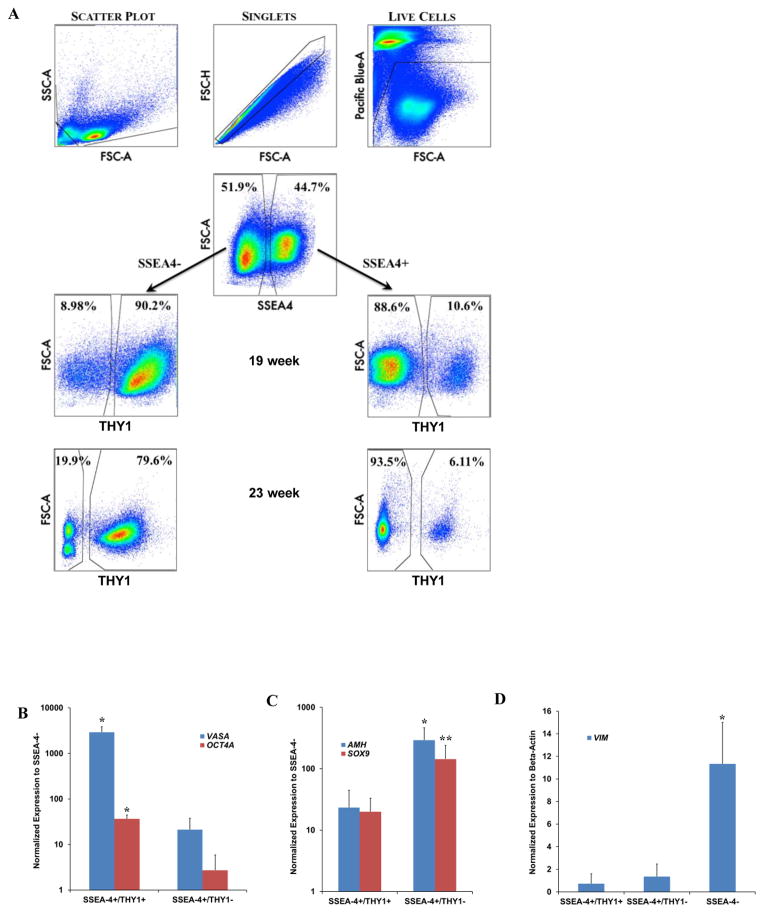
To confirm that SSEA-4+/THY1+ cells were in fact gonocytes, male fetal testes were digested, FACS sorted and individually analyzed for SSEA-4 expression. SSEA-4+ and SSEA-4− cells were individually assessed for THY1 expression (Fig. 3A). At 19 weeks of gestation, ~10% of the total SSEA-4+ cells were THY1+ (gonocytes) (Fig. 3A). The remaining ~90% of the SSEA-4+ cells were THY1 negative (prespermatogonia and Sertoli cells). In contrast, >90% of SSEA-4− cells expressed THY1. Similar to confocal microscopy observations that the number of VASAD cells decreased with advancing gestation (Fig. 1A–B), the number of gonocytes (SSEA-4+/THY1+) decreased to ~6% at 23 weeks of gestation (Fig. 3A).
Figure 3.
Molecular analyses of fetal gonocytes, prespermatogonia, and Sertoli cells. (A) SSEA-4 and THY1 can be used as markers for separating gonocytes from prespermatogonia/Sertoli cells by FACS. Cellular debris clumps, and dead cells were gated out prior to sorting. SSEA-4+ and SSEA-4− cells were evaluated individually for THY1 expression. Consistent with confocal microscopy findings, the ratio of fetal gonocytes to prespermatogonia (VASAD/VASAB) declined with advancing gestation as demonstrated here between 19 and 23 weeks of gestation. (B) SSEA-4+/THY1+ cells (gonocytes) expressed VASA and OCT4A at significantly higher levels than SSEA-4+/THY1− (prespermatogonia/Sertoli cells) cells. (C) SSEA-4+/THY1− cells expressed significantly higher levels of genes (AMH) specific to Sertoli cells. SOX9 expression was not evaluated statistically for significance because only 2 biological samples were analyzed in the SSEA-4+/THY1− population. (D) Very low level of stromal marker VIM was detected in the SSEA-4+ populations. All qPCR reactions were ran in triplicates with 3 biological samples per group at 19 weeks of gestation except for one condition in which only 2 biological samples were analyzed indicated as **. * indicates statistical significant with p<0.01.
These findings were confirmed at the molecular level by qPCR analysis. SSEA-4+/THY1+ (gonocytes) expressed high levels of VASA (139-fold) and OCT4A (13-fold) (Fig 3B). Although, VASA and OCT4A were also detected in SSEA-4+/THY1− (prespermatogonia and Sertoli cells) their levels were significantly lower than the pure gonocyte population, confirming that this population contains both prespermatogonia and Sertoli cells (Fig. 3B). Both AMH and SOX9 were more highly expressed in the SSEA-4+/THY1− population than the SSEA-4+/Thy1+ population providing further support for the presence of Sertoli cells (Fig. 3C). Lastly, VIM expression was significantly higher in the somatic SSEA-4− population in comparison to both SSEA-4+/THY1+ and SSEA-4+THY1− population (Fig. 3D).
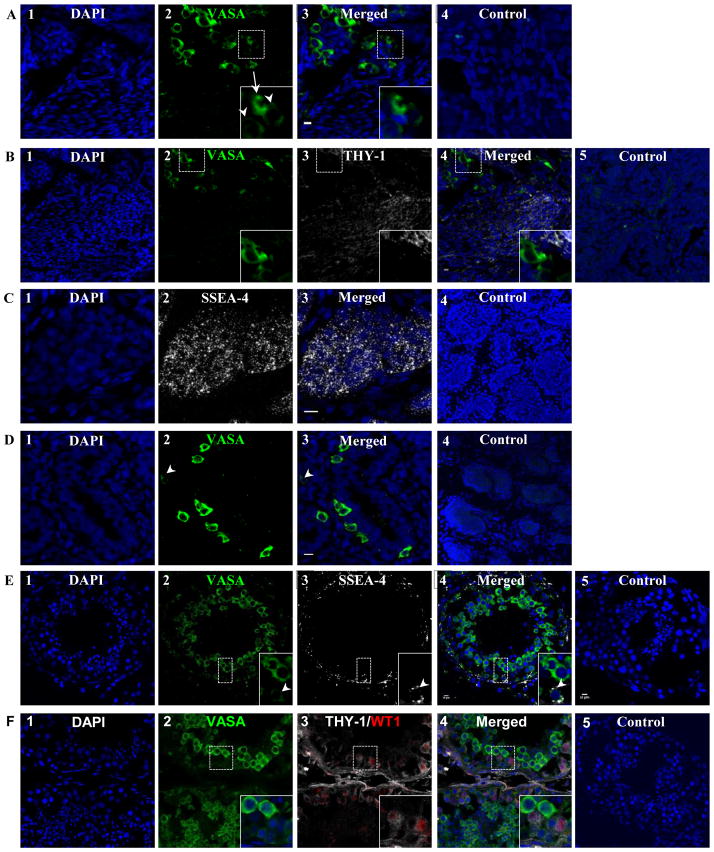
After 32 weeks of gestation, all cells (gonocytes, prespermatogonia, and Sertoli cells) within the seminiferous cord continued to express SSEA-4 (data not shown). The ratio of VASAD/VASAB cells decreased to less than 1/5 when all VASA+ cells from 10 cords were counted, consistent with the continuous differentiation of gonocytes to prespermatogonia during fetal development (Fig. 4A). After the second trimester, THY1 expression was detected exclusively in somatic cells outside of the seminiferous cords (Fig. 4B), while, C-Kit and OCT4A expression were no longer detected in either VASAD or VASAB cells (data not shown).
Figure 4.
Changes in germ cell marker expression in the fetal testes after 24 weeks of gestation and after birth. (A) The number of gonocytes (VASAD cells) per testicular cord continued to decrease with advancing gestation. The ratio of VASAD/VASAB cells was <20% at 32 weeks of gestation as shown here. (B) Although THY-1 continued to be expressed in the somatic cells outside of the seminiferous cords, all germ cells ceased to express THY-1, shown at 37 weeks of gestation. (C) In contrast, SSEA-4 continued to be expressed in all cells within the seminiferous cord postnatally, shown here at 4 years of age. (D) The ratio of VASAD/VASAB germ cells (<10%) continued to decline postnatally, shown here at 4 months of age. (E) Seminiferous tubules matured and formed lumen post pubertally as shown here from a normal adult sample. Primitive spermatogonia, located at the basement membrane, expressed low level of VASA (VASAD) whereas differentiating spermatocytes expressed high level of VASA (VASAB). While SSEA-4 continued to be expressed in VASAD spermatogonia, it was no longer expressed in Sertoli cells. (F) THY-1 continued to be the marker of somatic cells within the seminiferous tubules. Additionally, as Sertoli cells ceased to express SSEA-4 after puberty, they began to express THY-1 as shown with co-expression of WT1. Arrow heads and arrows indicate VASAD and VASAB cells, respectively. Donkey anti-goat Alexa 488 (VASA), anti-rabbit Alexa 594 (WT-1), and anti-mouse Alexa 555 (SSEA-4) were used.
SSEA-4 continues to be the membrane marker for spermatogonial stem cells (SSCs) postnatally
Both SSCs and Sertoli cells from 4-month and 4-year old boys continued to express SSEA-4 within the seminiferous cord, similar to the fetal testes in the third trimester (Fig. 4C). Neither THY1 nor C-Kit expression was detected within the seminiferous cord of these prepubertal testes (data not shown). However, in contrast to the fetal testes, <10% of spermatogonia were VASAD postnatally (Fig. 4D).
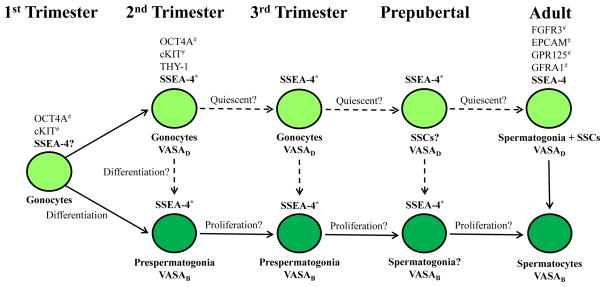
When adult seminiferous tubules were examined, two populations of germ cells, VASAD and VASAB, were also detected. VASAD germ cells were seen in the basement membrane, where primitive spermatogonia and SSCs are located (Fig. 4E). In contrast, VASAB germ cells were seen nearer toward the lumen consistent with mature spermatocytes. Interestingly, SSEA-4 expression was detected only in VASAD germ cells indicating that SSEA-4 remained to be the membrane marker of primitive spermatogonia (Fig. 4E). Although THY1 expression had been found in early fetal gonocytes, THY1 expression was restricted solely to somatic cells (Sertoli cells, peritubular interstitial cells, and cells making up the lamina propria) in adult men (Fig 4F). Figure 5 summarizes the membrane markers of gonocytes and primitive spermatogonia (SSCs) during fetal and postnatal development.
Figure 5.
Markers of human gonocytes, pre-spermatogonia, and spermatogonia during development.
*Indicates also a marker of Sertoli cells
#Previously reported as markers of gonocytes and adult human SSCs
VASAD = VASA dim; VASAB = VASA bright
DISCUSSION
We conducted a comprehensive in vitro characterization of germ cell membrane markers in human gonocytes, prespermatogonia, and spermatogonial stem cells from 13 weeks of gestation through adulthood. We report dynamic changes in the expression of known germ cell markers THY1, C-Kit, OCT4A, and VASA and identified SSEA-4 as a conserved extracellular membrane marker of male primitive germ cells during human male germ cell development.
In murine studies, VASA and OCT4 are co-expressed in primordial germ cells during their migration to the gonadal ridge (Fujiwara et al. 1994, Tanaka et al. 2000). Prior human studies reported that male germ cells do not express VASA until after 14 weeks of gestation (Anderson et al. 2007). Although OCT4A was described as the quintessential marker of human gonocytes during the first trimester, there was an uncoupling of OCT4A expressing gonocytes during the second trimester, as most gonocytes ceased to express OCT4A and differentiated into VASA expressing prespermatogonia (Pauls et al. 2006, Anderson et al. 2007). However, a recent study demonstrates that human gonocytes co-expressed OCT4A, C-Kit, and VASA during the first trimester (7–11 weeks of gestation) (Gkountela et al. 2012). Similarly, there was also an uncoupling of OCT4A+/C-Kit+/VASA+ gonocytes into OCT4A+/C-Kit+/VASA− gonocytes and OCT4A−/C-Kit−/VASA+ prespermatogonia during the second trimester (Anderson et al. 2007, Gkountela et al. 2012). Due to limited sample availability, we focused our studies on male testes at 13 weeks of gestation and beyond. In the present study we described VASAD and VASAB cells as two temporally and spatially distinct populations of germ cells that persisted through the second and third trimester. Our findings suggest a similar uncoupling of gonocytes and prespermatogonia to that previously reported in humans (Pauls et al. 2006, Anderson et al. 2007). While Gkountela and colleagues described two major distinct populations of male human germ cells (C-Kit+/VASA−, and C-Kit−/VASA+) in second trimester testes, we did not detect any C-Kit+/VASA− gonocytes at any time points in our studies. Since the same anti-VASA antibody was used, it is possible that differences in tissue processing and our use of confocal microscopy may account for the discrepancy in the relative detection of VASA expression between studies. Although none of the VASAB germ cells in our study expressed markers associated with gonocytes (OCT4A and C-Kit), all VASAD germ cells co-expressed both OCT4A and C-Kit suggesting that they were the same population of primitive gonocytes previously reported (Pauls et al. 2006, Gkountela et al. 2012). Similar to the decline in C-Kit+ and OCT4A+ gonocytes seen previous studies, the number of OCT4A+/C-Kit+/VASAD gonocytes also declined with advancing gestation in our studies (Pauls et al. 2006, Anderson et al. 2007, Gkountela et al. 2012).
Comprehensive screening of previously reported extracellular membrane markers of SSCs and embryonic stem cells revealed that C-Kit, THY1, and SSEA4 are markers of human gonocytes. Specifically, C-Kit was found to be a transient marker of gonocytes during the second trimester; thereafter, its expression was not detected within the primitive germ cell compartment thereafter, consistent with previous human studies (Pauls et al. 2006, Gkountela et al. 2012). While THY1 was shown to be a marker of mouse SSCs, its role as marker of primate and adult human SSCs is controversial (Kubota et al. 2003, Conrad et al. 2008, Ko et al. 2010, Ko et al. 2011, Tapia et al. 2011, Chikhovskaya et al. 2012, Eildermann et al. 2012). We recently demonstrated, using highly purified population of adult human testicular THY1+ cells for analyses, that THY1+ is a marker of adult testicular somatic cells, rather than SSCs which expressed SSEA-4 (Yango et al. 2013). The findings of transient THY1 expression within the gonocyte population during the second trimester of gestation confirm that THY1 is not a marker of human SSCs postnatally (Ko et al. 2011, Tapia et al. 2011, Chikhovskaya et al. 2012, Yango et al. 2013).
SSEA-4 is also a known marker of undifferentiated pluripotent human embryonic stem cells, cleavage to blastocyst stage embryos, and bone marrow derived mesenchymal stem cells (MSCs) (Henderson et al. 2002, Rosu-Myles et al. 2013). Although associated with undifferentiated cells, the function of SSEA-4 is currently unknown and remained to be investigated (Brimble et al. 2007). We demonstrated that SSEA-4 was the common marker of human gonocytes, prespermatogonia, and primitive spermatogonia starting at 13 weeks of gestation through post-puberty, in contrast to the transient expression of THY1 and C-Kit seen in gonocytes. Although restricted to the seminiferous cord, SSEA-4 expression was not exclusively expressed in the germ cell compartment within the fetal and prepubertal testes. In addition to gonocytes and prespermatogonia, SSEA-4 was also found to be a marker of human Sertoli cells prior to puberty as demonstrated by confocal microscopy and confirmed by molecular analyses of subpopulations of SSEA-4 expressing cells. However, there was a significant change in SSEA-4 expression within the seminiferous tubules after puberty. Whereas SSEA-4 expression continued to be restricted to the primitive spermatogonia in adult, Sertoli cells no longer expressed SSEA-4. Our findings are consistent with recent reports that SSEA-4 expression is restricted exclusively to primitive spermatogonia within the adult primate and human seminiferous tubules (Muller et al. 2008, Maki et al. 2009, Izadyar et al. 2011, Pacchiarotti et al. 2013). To assess the specificity of the SSEA-4 antibody, we also evaluated for SSEA-1 and SSEA-3 expression by FACS and confocal microscopy and found that neither SSEA-1 and SSEA-3 was expressed in human testicular tissues (data not shown), consistent with previous studies in primates (Muller et al. 2008).
SSEA-4 is a conserved extracellular marker of primitive male human germ cells through all stages of development as described here. Recent studies also report fibroblast growth factor receptor 3 (FGFR3) as a potential conserved membrane maker of human primitive spermatogonia (von Kopylow et al. 2010, von Kopylow et al. 2012, Kossack et al. 2013). However, additional studies are still needed to further characterize this population (von Kopylow et al. 2010, von Kopylow et al. 2012). In contrast to prior studies, we did not detect GPR125 and GFRα1R expression in the fetal testes (Wu et al. 2009, He et al. 2010, Dovey et al. 2013). Additionally, GPR125, GFRα1R, and EPCAM expression does not appear to be specific to germ cells (Wu et al. 2009, He et al. 2010, Dovey et al. 2013). We recognize that GFRα1R may be expressed in human fetal testes at a defined gestational window that we may not have evaluated, as mouse studies have demonstrated that Gfrα1 mRNA is detected in the testes up to dpc 14 and undetectable thereafter (Golden et al. 1999).
Recent studies demonstrated that highly purified sorted adult human testicular SSEA-4+ cells are germ cells that have not entered meiosis and can give rise to SSC colonies capable of expansion in vitro (Yango et al. 2013). Enriched adult testicular SSEA-4+ cells were able to colonize mouse seminiferous tubules after transplantation confirming that the SSEA-4+ population is highly enriched for SSCs (Izadyar et al. 2011). Thus, current evidence supports the use of SSEA-4 as a membrane marker to isolate human primitive spermatogonia postnatally for in vitro expansion and differentiation. Currently, only one study demonstrated the ability to expand human SSCs in vitro by culture of unpurified prepubertal testicular tissue, however the membrane markers of these pre-pubertal SSCs were not evaluated (Sadri-Ardekani et al. 2011).
In summary, we have described and characterized the dynamic changes in the expression of extracellular membrane markers of human male primitive germ cells from 13 weeks of gestation through adult. Specifically, SSEA-4 was shown to be a unique ontogenically conserved marker of human spermatogonia through all stages of development. This finding contributes to the knowledge gap of identifying primitive spermatogonia for future transplantation studies.
Supplementary Material
Acknowledgments
Funding: NDT is supported by ASRM new investigator award, UCSF Rap Grant, and Weston Haven Foundation.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S, Merrill AH, Jr, Robins AJ, Schulz TC. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25:54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philos Trans R Soc Lond B Biol Sci. 2010;365:1459–1463. doi: 10.1098/rstb.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhovskaya JV, Jonker MJ, Meissner A, Breit TM, Repping S, van Pelt AM. Human testis-derived embryonic stem cell-like cells are not pluripotent, but possess potential of mesenchymal progenitors. Hum Reprod. 2012;27:210–221. doi: 10.1093/humrep/der383. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J Clin Invest. 2013;123:1833–1843. doi: 10.1172/JCI65822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- Ebata KT, Zhang X, Nagano MC. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev. 2005;72:171–181. doi: 10.1002/mrd.20324. [DOI] [PubMed] [Google Scholar]
- Eildermann K, Gromoll J, Behr R. Misleading and reliable markers to differentiate between primate testis-derived multipotent stromal cells and spermatogonia in culture. Hum Reprod. 2012 doi: 10.1093/humrep/des091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, Matsumiya K, Wakayama T, Okuyama A. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. The Journal of Clinical Investigation. 2005;115:1855–1861. doi: 10.1172/JCI24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Tsujimura A, Miyagawa Y, Kiuchi H, Matsuoka Y, Takao T, Takada S, Nonomura N, Okuyama A. Isolation of Germ Cells from Leukemia and Lymphoma Cells in a Human In vitro Model: Potential Clinical Application for Restoring Human Fertility after Anticancer Therapy. Cancer Res. 2006;66:11166–11171. doi: 10.1158/0008-5472.CAN-06-2326. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proceedings of the National Academy of Sciences. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashaw I, Dushaj O, Behr R, Biermann K, Brehm R, Rubben H, Grobholz R, Schmid KW, Bergmann M, Winterhager E. Novel germ cell markers characterize testicular seminoma and fetal testis. Mol Hum Reprod. 2007;13:721–727. doi: 10.1093/molehr/gam059. [DOI] [PubMed] [Google Scholar]
- Gkountela S, Li Z, Vincent JJ, Zhang KX, Chen A, Pellegrini M, Clark AT. The ontogeny of cKIT(+) human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat Cell Biol. 2012;15:113–122. doi: 10.1038/ncb2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–1126. doi: 10.1089/scd.2008.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, Fuso A, Berno V, Boitani C, Stefanini M, Vicini E. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–3052. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Salati J, Sheng Y, Chu T, Orwig KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Human Reproduction. 2011;26:3222–3231. doi: 10.1093/humrep/der343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, Yuen C, Greilach S, Zhao HH, Chow M, Chow YC, Rao J, Barritt J, Bar-Chama N, Copperman A. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Nielsen JE, Jensen MB, Graem N, Rajpert-De Meyts E. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod. 2012;18:523–534. doi: 10.1093/molehr/gas030. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Ko K, Arauzo-Bravo MJ, Tapia N, Kim J, Lin Q, Bernemann C, Han DW, Gentile L, Reinhardt P, Greber B, Schneider RK, Kliesch S, Zenke M, Scholer HR. Human adult germline stem cells in question. Nature. 2010;465:E1. doi: 10.1038/nature09089. discussion E3. [DOI] [PubMed] [Google Scholar]
- Ko K, Reinhardt P, Tapia N, Schneider RK, Arauzo-Bravo MJ, Han DW, Greber B, Kim J, Kliesch S, Zenke M, Scholer HR. Brief report: evaluating the potential of putative pluripotent cells derived from human testis. Stem Cells. 2011;29:1304–1309. doi: 10.1002/stem.671. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack N, Terwort N, Wistuba J, Ehmcke J, Schlatt S, Scholer H, Kliesch S, Gromoll J. A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum Reprod. 2013 doi: 10.1093/humrep/det336. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki CB, Pacchiarotti J, Ramos T, Pascual M, Pham J, Kinjo J, Anorve S, Izadyar F. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum Reprod. 2009;24:1480–1491. doi: 10.1093/humrep/dep033. [DOI] [PubMed] [Google Scholar]
- Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44:41–55. doi: 10.1111/j.1439-0272.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM, de la Rosette JJ, Knegt AC, Belmonte JC, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158–167. doi: 10.1093/humrep/dep354. [DOI] [PubMed] [Google Scholar]
- Muller T, Eildermann K, Dhir R, Schlatt S, Behr R. Glycan stem-cell markers are specifically expressed by spermatogonia in the adult non-human primate testis. Hum Reprod. 2008;23:2292–2298. doi: 10.1093/humrep/den253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacchiarotti J, Ramos T, Howerton K, Greilach S, Zaragoza K, Olmstead M, Izadyar F. Developing a Clinical-Grade Cryopreservation Protocol for Human Testicular Tissue and Cells. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/930962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls K, Schorle H, Jeske W, Brehm R, Steger K, Wernert N, Buttner R, Zhou H. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 2006;21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- Rosu-Myles M, McCully J, Fair J, Mehic J, Menendez P, Rodriguez R, Westwood C. The globoseries glycosphingolipid SSEA-4 is a marker of bone marrow-derived clonal multipotent stromal cells in vitro and in vivo. Stem Cells Dev. 2013;22:1387–1397. doi: 10.1089/scd.2012.0547. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, Valenzuela DM, Hobbs RM, Pandolfi PP, Rafii S. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes & Development. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- Tapia N, Arauzo-Bravo MJ, Ko K, Scholer HR. Concise review: challenging the pluripotency of human testis-derived ESC-like cells. Stem Cells. 2011;29:1165–1169. doi: 10.1002/stem.669. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, Steinkraus V, Spiess AN. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Staege H, Schulze W, Will H, Kirchhoff C. Fibroblast growth factor receptor 3 is highly expressed in rarely dividing human type A spermatogonia. Histochem Cell Biol. 2012;138:759–772. doi: 10.1007/s00418-012-0991-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, Ginsberg JP, Brinster RL. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yango P, Smith JF, Altman E, Klatsky P, Tran N. Testicular Niche Required for Human Spermatogonial Stem Cell Expansion. American Society of Reproductive Medicine annual meeting. 2013 doi: 10.5966/sctm.2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.