Abstract
Background
Left atrial appendage (LAA) exclusion has been performed in patients with atrial fibrillation (AF) to prevent thrombus formation and subsequent cardioembolic events. Left atrial electrical remodeling is a recognized factor in the recurrence of AF. The effects of LAA exclusion on P-wave characteristics and left atrial electrical remodeling have not been well described. The purpose of this study was to evaluate the effect of LAA ligation on P-wave morphology in patients with AF.
Methods and Results
Fifteen patients who were in sinus rhythm during the LAA ligation procedure were included in the study. We evaluated the P-wave characteristics, including P-wave duration, P-wave amplitude, PQ interval, and P-wave dispersion, before and after ligation. Eleven patients had paroxysmal AF and 4 patients had persistent AF (12 male patients and 3 female patients). P-wave duration immediately after ligation was significantly shorter compared with baseline in all limb leads except lead aVR (P<0.05). P-wave amplitude immediately after ligation was significantly greater compared with baseline in inferior leads; however, P-wave amplitude after 1 to 3 months was significantly lower compared with immediately after ligation. PQ interval immediately after ligation was significantly shorter compared with baseline (P=0.01), and P-wave dispersion after 1 to 3 months was significantly shorter compared with baseline (P=0.02).
Conclusions
LAA exclusion produces consistent P-wave changes consistent with decreased atrial mass and decreased atrial dispersion that may represent reverse electrical atrial remodeling. This is a potential mechanism to explain the role of LAA ligation in maintaining sinus rhythm in patients with AF.
Keywords: atrial fibrillation, atrial remodeling, left atrial appendage, ligation, P wave
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and it may cause symptoms associated with heart failure and stroke. Pulmonary vein isolation (PVI) is a well-established treatment option for rhythm control in patients with paroxysmal AF. In a follow-up of 5 years, the multiple ablation success rate was ≈80% in patients with paroxysmal AF,1–3 while the multiple ablation success rate was ≈45% in patients with persistent AF.4 The role of left atrial (LA) appendage (LAA) has been recognized in the initiation and maintenance of AF.5,6 It has been reported that the LAA is responsible for recurrence of AF/AT in at least 27% of patients presenting for repeat procedures and that the electric isolation of the LAA can improve success regarding the recurrence of AF. P-wave duration (PWD) on the surface electrocardiogram (ECG) is a reflection of atrial depolarization time. The major factors that influence PWD are atrial size, LA pressure, and interatrial conduction time.7 PWD measured by 12-lead ECG has been shown to collate with atrial conduction abnormalities and predicts the recurrence of AF.8 Recently, percutaneous LAA ligation has been performed in patients with AF at risk for cardioembolic stroke who have contraindications or intolerance to oral anticoagulation therapy.9–11 However, it is unknown whether LAA ligation is associated with a change in P-wave characteristics or LA electrical remodeling. We described the effect of LAA ligation on P-wave characteristics such as PWD, P-wave amplitude (PWA), P-wave dispersion, and recurrence of AF.
Methods
Patient Population
Among 24 patients who underwent percutaneous LAA ligation for symptomatic AF between August 2011 and September 2014 in our medical center, 15 patients who were in sinus rhythm (SR) during the ligation procedure underwent an evaluation of P-wave characteristics, including PWD, PWA, PQ interval, and P-wave dispersion. Eleven patients had a history of paroxysmal AF and 4 patients had a history of persistent AF. Paroxysmal AF was defined as AF terminating spontaneously within 7 days, and persistent AF was defined as non–self-terminating AF lasting >7 days and requiring pharmacologic or electrical cardioversion to restore SR. We selected the patients who have all of the following criteria: (1) aged ≥18 years, (2) ≥1 risk factor of embolic stroke (CHADS2 score [estimates risk based on the presence of congestive heart failure, hypertension, aged ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack] ≥1), (3) ineligible for long-term anticoagulation therapy and/or an anticoagulation agent failure (ie, transient ischemic attack or stroke during anticoagulation therapy), and (4) nonvalvular AF. We excluded patients who have the following criteria: (1) history of PVI, (2) history of pericarditis, (3) history of cardiac surgery, (4) recent myocardial infarction within 3 months, (5) pectus excavatum, (6) prior embolic event within the past 30 days, (7) New York Heart Association functional class IV heart failure symptoms, (8) left ventricular function <35%, and (9) history of thoracic radiation. Patients meeting the criteria for the study enrollment underwent a screening contrast cardiac computed tomography (CT) scan. This study was approved by an institutional review committee. All patients provided written informed consent for the procedure.
P-Wave Measurement and Analysis
Standard 12-lead ECG was recorded before LAA ligation, immediately after conclusion of the ligation procedure, and 1 to 3 months after ligation in normal SR. Standard 12-lead ECG was done in supine position and was recorded at a 25 mm/s and 1 mV/cm standardization with a band-pass filter of frequencies between 0.05 and 40 Hz. P-wave characteristics were analyzed carefully by 2 independent investigators, and we magnified the ECG by 5× by using computer and manually measured P-wave characteristics. All parameters were measured 3×. PWD was defined as the time difference between the P-wave onset and offset from the equipotential reference line. PWA was defined as the difference between the highest and lowest peak of the P wave to the nadir crossing the isoelectric. P-wave dispersion was defined as the difference between the maximum and the minimum of the PWD among 12-lead ECGs.
LAA Ligation Procedure
All patients underwent transesophageal echocardiography (TEE) before the procedure to rule out a preexisting thrombus in the LA/LAA. LAA ligation using the LARIAT suture delivery system (Senterheart, Inc, Redwood City) has been reported previously.9 The LAA ligation is performed in 4 steps: (1) percutaneous epicardial and transseptal access, (2) placement of an endocardial magnet-tipped guidewire in the apex of the LAA with balloon identification of the LAA ostium, (3) connection of the epicardial and endocardial magnet-tipped guidewires for stabilization of the LAA, and (4) snare capture of the LAA with closure confirmation and release of the pretied suture for LAA ligation. Conformation of LAA closure was confirmed with the use of LA angiography and assessment of leaks by TEE. We directly measured LA pressure before and immediately after LAA ligation procedure in 3 patients.
Follow-up
Patients were evaluated every 1 to 3 months in an outpatient clinic after ligation. A follow-up ECG was obtained in the identical manner as at the time of LAA ligation and was used for analysis. A follow-up TEE and/or transthoracic echocardiography was performed within 1 to 3 months of ligation. A follow-up 24-hour Holter recording was performed within 3 months of ligation and at any time that the patient had any symptoms suggestive of AF, such as palpitations, dizziness, or syncope. Continuous event monitoring was performed 6 months after the procedure.
Statistical Analysis
Continuous variables are presented as the mean±SD and compared by using the Student t test. Differences among study groups were analyzed by using 1-way ANOVA, followed by Scheffé test and the nonparametric Mann–Whitney U test, where appropriate. The change in PWD and PWA were examined by using repeated-measurements ANOVA, with the time periods. Categorical variables were compared by using a χ2 or Fisher exact test as appropriate. P-values <0.05 were considered statistically significant.
Results
Population Characteristics
Baseline characteristics are shown in Table 1. We identified 15 patients with AF referred for the LAA ligation during SR. There were 12 men and 3 women, and their mean age was 64±11 years. Among these patients, 11 (73%) patients had a history of paroxysmal AF and 4 (27%) patients had a history of persistent AF. The mean CHADS2 score was 2.4±1.7 and the mean CHA2DS2-VASc (vascular disease and sex category) score was 3.1±1.9 in these patients. Five (33%) patients had a bleeding event before LAA ligation. The individual clinical characteristics of AF patients are shown in Table 2. All 4 patients with persistent AF underwent electrical or pharmacological cardioversion before LAA ligation. The average follow-up period was 389±133 days. All patients returned for 1 to 3 months post ligation and underwent ECG.
Table 1.
Baseline Characteristics of Patients With AF
| Characteristic | LAA Ligation Patients (n=15) |
|---|---|
| Age, y | 64±11 |
| Sex, male | 11 (73%) |
| EF, % | 63±8.3 |
| BMI, kg/m2 | 29±4.3 |
| Paroxysmal AF | 11 (73%) |
| Persistent AF | 4 (27%) |
| Bleeding event | 5 (33%) |
| CHADS2 score | 2.4±1.7 |
| History of CHF | 6 (40%) |
| Hypertension | 11 (73%) |
| Age (≧75 years) | 2 (16%) |
| Diabetes mellitus | 3 (20%) |
| History of stroke | 4 (26%) |
| CHA2DS2-VASc score | 3.1±1.8 |
| HAS-BLED score | 3.1±1.4 |
| Medical therapy, n (%) | |
| Class III | 6 (40%) |
| Class I | 5 (33%) |
| β-Blockers | 11 (73%) |
| ACEI/ARB | 3 (20%) |
| Anticoaguration | 10 (66%) |
AF indicates atrial fibrillation; LAA, left atrial appendage; EF, ejection fraction; BMI, body mass index; CHA2DS2-VASc, estimates risk based on the presence of congestive heart failure, hypertension, aged ≥75 y, diabetes mellitus, and prior stroke or transient ischemic attack plus vascular disease and sex category; CHF, congestive heart failure; HAS-BLED, risk stratification scheme is one of several that has been validated to estimate baseline risk of major hemorrhage (defined as hemorrhage involving a critical anatomic site, for example, intracranial, or a bleed requiring hospitalization, transfusion of ≥2 units of packed cells, or associated with a decrease in hemoglobin level of ≥2 g/L; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin type II blocker.
Table 2.
Clinical Characteristics of Patients With AF
| Patient No. | Age, y | Sex | Type | Ejection Fraction (%) | CHADS2 | Body Mass Index, kg/m2 | Bleeding | Recurrence | Cardioversion | Complication | Flow (LA/LAA) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | Paro | 60 | 2 | 34.2 | 0 | AF | N/A | None | (−) |
| 2 | 58 | M | Paro | 61 | 2 | 38.7 | 0 | SR | N/A | None | (−) |
| 3 | 56 | M | Paro | 70 | 1 | 29.8 | 0 | SR | N/A | None | (−) |
| 4 | 60 | M | Paro | 61 | 1 | 22.8 | 1 | AF | N/A | None | (−) |
| 5 | 57 | M | Paro | 58 | 1 | 31.2 | 1 | SR | N/A | Pericarditis | (−) |
| 6 | 82 | F | Paro | 72 | 3 | 29.5 | 0 | AF | N/A | None | (+) |
| 7 | 66 | M | Paro | 66 | 4 | 28.7 | 0 | SR | N/A | None | (−) |
| 8 | 55 | M | Paro | 45 | 3 | 27.2 | 0 | AF | N/A | None | (−) |
| 9 | 71 | M | Paro | 62 | 4 | 32.4 | 1 | AF | N/A | None | (−) |
| 10 | 50 | M | Per | 65 | 1 | 33.3 | 0 | SR | Electrical | None | (−) |
| 11 | 65 | M | Per | 55 | 1 | 33.3 | 0 | SR | Electrical | None | (−) |
| 12 | 82 | M | Per | 63 | 1 | 23.7 | 1 | SR | Pharma | None | (−) |
| 13 | 65 | M | Per | 68 | 2 | 28.1 | 1 | SR | Electrical | None | (−) |
| 14 | 72 | M | Paro | 70 | 3 | 29.2 | 0 | AF | N/A | None | (−) |
| 15 | 59 | F | Paro | 69 | 2 | 28.5 | 0 | SR | N/A | None | (−) |
AF indicates atrial fibrillation; CHADS2, estimates risk based on the presence of congestive heart failure, hypertension, aged ≥75 y, diabetes mellitus, and prior stroke or transient ischemic attack; LA, left atrial; LAA, left atrial appendage; Paro, paroxysmal AF; N/A, not applicable; SR, sinus rhythm; Per, persistent AF; Pharma, pharmacological cardioversion.
LAA Ligation
All patients had complete closure of the LAA. There were no procedural complications. Pericarditis was observed in 1 patient after ligation and required nonsteroidal anti-inflammatory drugs. At the follow-up TEE, 13 (86%) of 15 patients had complete closure of the LAA, and 2 patients had a mild leak (<2 mm) on color-flow Doppler (Table 2). There was no complication of stroke during the follow-up period. In 3 patients, LA pressure was measured during the LAA ligation procedure. Two patients showed an increase in LA pressure immediately after ligation, and 1 patient did not have an increase in LA pressure.
P-Wave Characteristics
Figure 1 shows the change in PWD at baseline, immediately after ligation, and after 1 to 3 months. PWDs in lead I and aVL were significantly shorter immediately after ligation compared with baseline (I: 110±24 ms versus 97±17 ms, P=0.01; aVL: 108±21 ms versus 92±15 ms, P=0.01). PWDs in inferior leads were significantly shorter immediately after ligation compared with baseline (II: 106±19 ms versus 95±18 ms, P=0.02; III: 100±20 ms versus 90±21 ms, P=0.02; aVF: 112±15 ms versus 101±18 ms, P=0.03). We also investigated the changes in PWA before, immediately after ligation, and 1 to 3 months later (Figure 2). PWAs in inferior leads were significantly higher immediately after ligation compared with baseline (II: 0.13±0.03 mV versus 0.17±0.04 mV, P=0.02; III: 0.14±0.04 mV versus 0.18±0.04 mV, P=0.01; aVF: 0.14±0.05 mV versus 0.17±0.04 mV, P=0.03). PWAs in inferior lead were significantly lower at 1 to 3 months later compared with immediately after ligation (II: 0.17±0.04 mV versus 0.14±0.03 mV, P=0.03; III: 0.15±0.04 mV versus 0.12±0.05 mV, P=0.01; aVF: 0.16±0.03 mV versus 0.12±0.04 mV, P=0.03). Table 3 shows the ΔPWD and PWA (immediately after ligation—before ligation) in patients with maintenance SR (n=9) compared with recurrence AF (n=6). Changes in PWD in leads I, aVL, V1, and V2 were significantly higher in patients with maintenance SR compared with those with recurrence AF. Changes in PWA in V1 and V2 were significantly higher in patients with maintenance SR compared with those with recurrence AF. Figure 3 shows the PQ interval and P-wave dispersion before, immediately after ligation, and 1 to 3 months later. Average PQ interval at immediately after ligation was significantly shorter compared with that before ligation (166±16 versus 158±17 ms; P=0.01). Average P-wave dispersion after 1 to 3 months was significantly shorter compared with baseline (38±21 versus 30±13 ms; P=0.02). Figure 4 shows an example of changes of P-wave morphology in a patient with maintenance of SR. PWDs after 2 months were shorter compared with baseline. PWAs immediately after ligation were higher compared with baseline in the inferior lead, while PWA after 2 months was lower compared with baseline.
Figure 1.
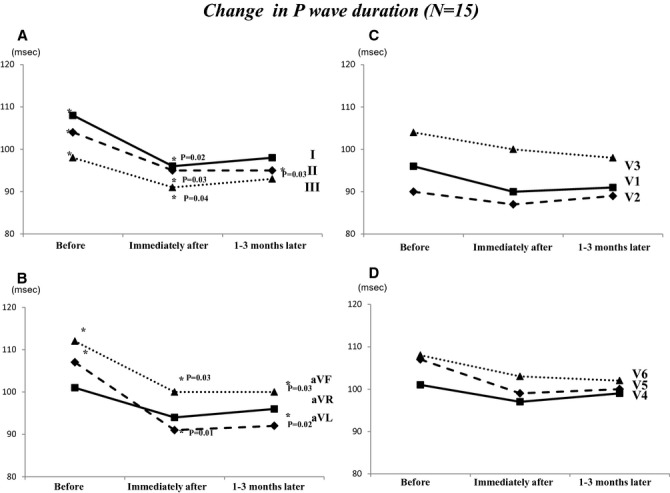
Changes in PWD before, immediately after LAA ligation, and after 3 months. A, This panel showed the changes in PWD in leads I, II, and III. B, This panel showed the changes in PWD in leads aVR, aVL, and aVF. C, This panel showed the changes in PWD in leads V1, V2, and V3. D, This panel showed the changes in PWD in leads V4, V5, and V6. LAA indicates left atrial appendage; PWD, P-wave duration.
Figure 2.
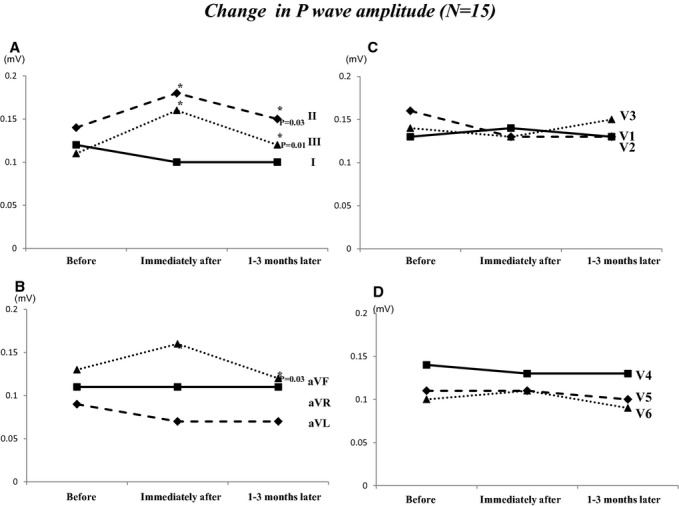
Changes in PWA before, immediately after LAA ligation, and after 3 months. A, This panel showed the changes in PWA in leads I, II, and III. B, This panel showed the changes in PWA in leads aVR, aVL, and aVF. C, This panel showed the changes in PWA in leads V1, V2, and V3. D, This panel showed the changes in PWA in leads V4, V5, and V6. LAA indicates left atrial appendage; PWA, P-wave amplitude.
Table 3.
Change in PWD and PWA Before PVI
| Lead | ΔPWD (Immediately After–Before) | ΔPWA (Immediately After–Before) | ||||
|---|---|---|---|---|---|---|
| SR (n=9) | AF (n=6) | P Value | SR (n=9) | AF (n=6) | P Value | |
| I | −25 | −5 | 0.01 | −0.02 | −0.01 | 0.78 |
| II | −13 | −9 | 0.51 | 0.06 | 0.03 | 0.31 |
| III | −7.2 | −6.2 | 0.71 | 0.04 | 0.02 | 0.71 |
| aVR | −6.2 | −6.8 | 0.87 | −0.02 | 0.02 | 0.12 |
| aVL | −25 | −12 | 0.04 | −0.03 | −0.01 | 0.61 |
| aVF | −13 | −12 | 0.81 | 0.07 | −0.03 | 0.09 |
| V1 | −8.2 | 3.1 | 0.03 | −0.03 | 0.07 | 0.04 |
| V2 | −10.5 | 2.1 | 0.04 | −0.05 | 0.05 | 0.03 |
| V3 | −3.4 | 1.2 | 0.42 | −0.03 | 0.01 | 0.29 |
| V4 | −4.6 | −4.1 | 0.91 | −0.02 | −0.02 | 0.61 |
| V5 | −6.9 | −9.1 | 0.7 | −0.02 | −0.01 | 0.83 |
| V6 | −7.2 | −5.6 | 0.71 | −0.03 | −0.02 | 0.48 |
AF indicates atrial fibrillation; PVI, pulmonary vein isolation; PWA, P-wave amplitude; PWD, P-wave duration; SR, sinus rhythm.
Figure 3.
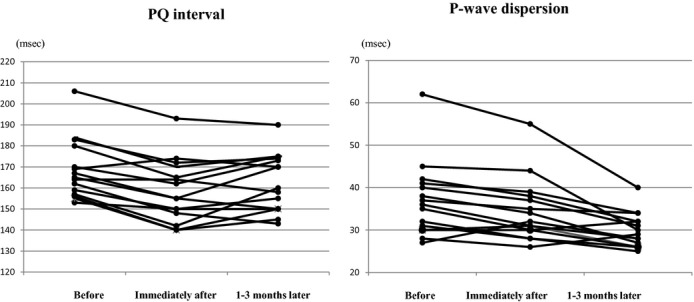
Spaghetti plots of PQ interval and P-wave dispersion with 12 patients before, immediately after left atrial appendage ligation, and after 1 to 3 months.
Figure 4.
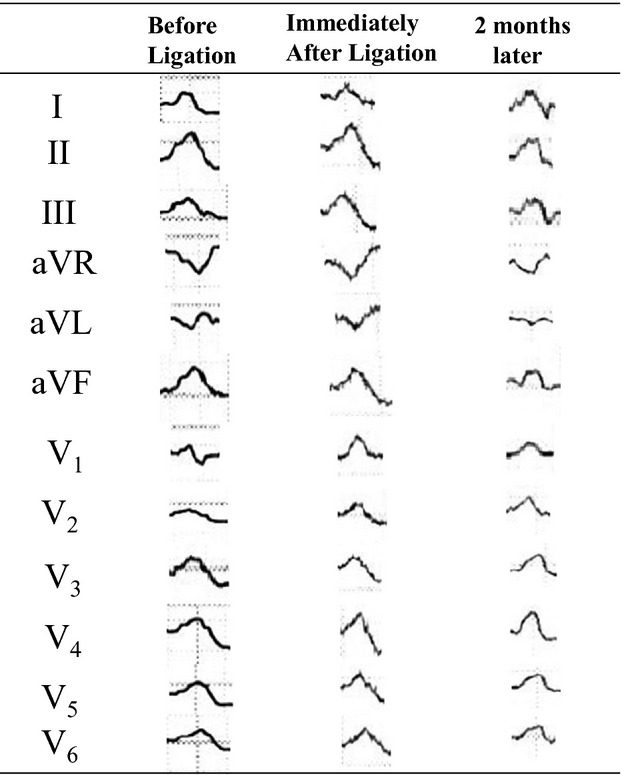
Example of changes in P-wave morphology in a patient who maintained sinus rhythm after left atrial appendage ligation.
Discussion
This is the first study describing P-wave characteristics on the surface ECG before and after LAA ligation by using the LARIAT suture delivery system. The findings of this study should not be extrapolated to other methods of LAA exclusion that do not lead to necrosis of LAA. The significant shortening of PWD and decrease in P-wave dispersion are consistent with LA debulking after LAA ligation. PWD is an electrophysiological parameter of entire atrial depolarization including intra-atrial conduction time. It has been shown that patients with AF have longer intra-atrial and interatrial conduction times.12,13 PVI alters LA electrophysiological substrate and leads to change in PWD on the surface ECG. A decrease in PWD after PVI had been associated with maintenance of SR.14,15 Therefore, we excluded patients who had undergone PVI. PWD after ligation was significantly shorter compared with baseline in our study. The P wave is recorded when the depolarization first begins in the right atrium close to the sinus node, and ejection of LAA, which is far from the sinus node, occurs after the propagation of impulse to LAA.16,17 Two prior studies described that the P-LAA interval was an effective predictor of recurrence of AF.18,19 Jiang et al showed that LAA activation delay affected the PQ interval on the surface ECG.20 Our study showed a decrease in both PWD and PQ interval after LAA ligation. This is a potential explanation for decrease in AF after LAA ligation. P-wave dispersion is a marker of anisotropic or inhomogeneous atrial conduction.21,22 It has been associated with LA remodeling and vulnerability to AF.23,24 Changes in PWD (immediately after ligation—before ligation) in leads I and V1 were significantly higher in patients with maintenance of SR and were significantly lower in patients with recurrence of AF. Changes in PWD in leads I and V1 might be a predictor of recurrence of AF.
PWA immediately after ligation was significantly higher compared with baseline in the inferior leads, while it is decreased after 1 to 3 months. These changes are more likely due to inflammation and tissue ischemia and to transient LA pressure changes. We directly measured LA pressure in 3 patients during LAA ligation procedure. Two patients showed an increase in LA pressure immediately after ligation, and these patients had increased PWA. However, PWA after 1 to 3 months was significantly lower compared with immediately after ligation. Later changes may represent reverse atrial remodeling. Higher positive PWA in lead V1 has been shown to be a predictor of postoperative AF.25 In our study, ΔPWA in V1 tended to be higher in patients with recurrence of AF compared with those in SR.
AT is known to originate from the LAA and may be responsible for recurrence of AF in at least 27% of persistent AF patients presenting for repeat PVI.5 Catheter-based electrical isolation of the LAA has been associated with 1.8% incidence of cardiac tamponade and increased incidence of LAA thrombus with subsequence embolic events.5 LAA exclusion has the benefit of preventing thrombus formation within the LAA and eliminates the risk of perforation within the LAA. LAA exclusion has been demonstrated to produce LAA electrical isolation and elimination of atrial arrhythmias.26–28 Additionally, LAA is trabeculated due to the extensive pectinate muscle network that produces heterogeneous fiber orientation and favors areas of slow conduction, conduction block, and initiation of reentrant circuits. As demonstrated in this report, LAA exclusion decreases P-wave dispersion and may contribute to maintenance of SR when combined with PVI. Recent histological examination of patients undergoing LAA ligation demonstrates atrophy of the LAA with transmural scarring involving both the LA and LAA adjacent to the suture ligation.29 The ligament of Marshall is usually in direct connection with the coronary sinus myocardial sleeves and extends in the region between the LAA and the left superior pulmonary vein. Bachmann bundle is generally located near the right pulmonary veins and has several connections with muscle bundles that diverge at the base of the LAA.30–32 These muscle sleeves have been recognized in the initiation and maintenance of AF.33 It is conceivable that LAA ligation includes ligation of these muscle bundles that have been shown to act as a trigger in patients with persistent AF. A future prospective randomized study comparing PVI alone to LAA ligation and PVI is required in patients with persistent AF to test the hypothesis that LAA ligation leads to better efficacy.
Study Limitations
This study has 3 limitations. First, the present study used a standard 12-lead ECG recorded at 4-week follow-up clinic visits and 24-hour monitoring and event monitor performed at 3 to 6 months. We did not use implantable loop monitor that could have detected asymptomatic episodes of AF. However, this study was not designed to assess the incidence of AF before and after LAA ligation procedure. In addition, this study included only symptomatic episodes of AF. Second, this study had a small number of patients. These results have to be interpreted with caution. We believe that the statistical methodology was rigorous, and P-wave characteristics and AF recurrence were well validated, which substantiates the main conclusions. However, further prospective and randomized studies using the implantable loop monitor will be required to be certain of P-wave characteristics with AF recurrence. Third, antiarrhythmic drugs were administered in these patients before and after LAA ligation, which may have an impact on the P-wave parameters. However, all patients continue to take the same drugs during follow-up.
Conclusion
This is the first study to show P-wave characteristics before and after LAA ligation. Decrease in PWD, PWA, and P-wave dispersion might represent reverse electrical atrial remodeling. This is a potential mechanism explaining the role of LAA ligation in maintaining SR in patients with AF.
Disclosures
Dr Lee is an equity holder and consultant for SentreHEART, Inc. The other authors have no conflicts to declare.
References
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- Elayi CS, Verma A, Di Biase L, Ching CK, Patel D, Barrett C, Martin D, Rong B, Fahmy TS, Khaykin Y, Hongo R, Hao S, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Potenza D, Fanelli R, Massaro R, Arruda M, Schweikert RA, Natale A. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658–1664. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Cheema A, Dong J, Dalal D, Marine JE, Henrikson CA, Spragg D, Cheng A, Nazarian S, Bilchick KC, Almasry I, Sinha S, Scherr D, Halperin H, Berger R, Calkins H. Circumferential ablation with pulmonary vein isolation in permanent atrial fibrillation. Am J Cardiol. 2007;99:1425–1428. doi: 10.1016/j.amjcard.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F. Catheter ablation of long-standing persistent atrial fibrillation; 5-years outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–1929. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM, Zagrodzky JD, Santangeli P, Hao S, Hongo R, Beheiry S, Themistoclakis S, Bonso A, Rossillo A, Corrado A, Raviele A, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Lewis WR, Natale A. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- Hocini M, Shah AJ, Nault I, Sanders P, Wright M, Narayan SM, Takahashi Y, Jaïs P, Matsuo S, Knecht S, Sacher F, Lim KT, Clémenty J, Haïssaguerre M. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1853–1861. doi: 10.1016/j.hrthm.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiano P, D'Aloia A, Zanelli E, Gualeni A, Musatti P, Giordano A. Contribution of left atrial pressure and dimension to signal-averaged P-wave duration in patients with chronic congestive heart failure. Am J Cardiol. 1997;79:219–222. doi: 10.1016/s0002-9149(96)00720-5. [DOI] [PubMed] [Google Scholar]
- Gonna H, Gallagher MM, Guo XH, Yap YG, Hnatkova K, Camm AJ. P-wave abnormality predicts recurrence of atrial fibrillation after electrical cardioversion: a prospective study. Ann Noninvasive Electrocardiol. 2014;19:57–62. doi: 10.1111/anec.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, Han F, Bednarek J, Myc J, Kapelak B, Sadowski J, Lelakowski J, Bartus S, Yakubov SJ, Lee RJ. Percutaneous left atrial appendage suture ligation using the LARIAT in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Massumi A, Chelu MG, Nazeri A, May SA, Afshar-Kharaghan H, Saeed M, Razavi M, Rasekh A. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol. 2013;111:869–873. doi: 10.1016/j.amjcard.2012.11.061. [DOI] [PubMed] [Google Scholar]
- Koneru JN, Badhwar N, Ellenbogen KA, Lee RJ. LAA ligation using the LARIAT suture delivery device: tips and tricks for a successful procedure. Heart Rhythm. 2014;11:911–921. doi: 10.1016/j.hrthm.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Kuhne M, Ho SY, Morady F, Chugh A. Elimination of left atrial appendage potentials during radiofrequency ablation near the right superior pulmonary vein. Heart Rhythm. 2008;5:475–478. doi: 10.1016/j.hrthm.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Akimitsu S, Kawahira K, Kawanami F, Yamanouchi Y, Hiroki T, Arakawa K. Electrophysiological properties in chronic lone atrial fibrillation. Circulation. 1991;84:1662–1668. doi: 10.1161/01.cir.84.4.1662. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Kumagai K, Vakulenko V, Yasuda T, Siegerman C, Garfinkel A, Chen PS, Saku K. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:931–938. doi: 10.1111/j.1540-8167.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Van Beeumen K, Houben R, Tavernier R, Ketels S, Duytschaever M. Changes in P-wave area and P-wave duration after circumferential pulmonary vein isolation. Europace. 2010;12:798–804. doi: 10.1093/europace/eup410. [DOI] [PubMed] [Google Scholar]
- De Ponti P, Ho SY, Salerno-Uriarte JA, Tritto M, Spadacini G. Electroanatomic analysis of sinus impulse propagation in normal human atria. J Cardiovasc Electrophysiol. 2002;13:1–10. doi: 10.1046/j.1540-8167.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- Lemery R, Birnie D, Tang AS, Green M, Gollob M, Hendry M, Lau E. Normal atrial activation and voltage during sinus rhythm in the human heart: an endocardial and epicardial mapping study in patients with a history of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:402–408. doi: 10.1111/j.1540-8167.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- Kinay O, Nazli C, Ergene O, Dogan A, Gedikli O, Hoscan Y, Acar G, Altinbas A. Time interval from the initiation of the electrocardiographic P wave to the start of left atrial appendage ejection flow: a novel method for predicting atrial fibrillation recurrence. J Am Soc Echocardiogr. 2002;15:1479–1484. doi: 10.1067/mje.2002.127515. [DOI] [PubMed] [Google Scholar]
- Karaca M, Kinay O, Nazli C, Biceroglu S, Vatansever F, Ergene AO. The time interval from the initiation of the P-Wave to the start of left atrial appendage ejection flow: does it reflect interatrial conduction time? Echocardiography. 2007;24:810–815. doi: 10.1111/j.1540-8175.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- Jiang CX, Sang CH, Dong JZ, Liu XP, Long DY, Yu RH, Tang RB, Wu JH, Ning M, Liu C, Ma CS. Significant left atrial appendage activation delay complicating aggressive septal ablation during catheter ablation of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:652–660. doi: 10.1111/j.1540-8159.2010.02753.x. [DOI] [PubMed] [Google Scholar]
- Dilaveris P, Batchvarov V, Gialafos J, Malik M. Comparison of different methods for manual P wave duration measurement in 12-lead electrocardiograms. Pacing Clin Electrophysiol. 1999;22:1532–1538. doi: 10.1111/j.1540-8159.1999.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M, Gialafos JE, Toutouzas PK. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–738. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- Guntekin U, Gunes Y, Tuncer M, Gunes A, Sahin M, Simsek H. Long-term follow-up of P-wave duration and dispersion in patients with mitral stenosis. Pacing Clin Electrophysiol. 2008;31:1620–1624. doi: 10.1111/j.1540-8159.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- Fogari R, Derosa G, Ferrari I, Corradi L, Zoppi A, Lazzari P, Santoro T, Preti P, Mugellini A. Effect of valsartan and ramipril on atrial fibrillation recurrence and P-wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation. Am J Hypertens. 2008;21:1034–1039. doi: 10.1038/ajh.2008.217. [DOI] [PubMed] [Google Scholar]
- Rader F, Costantini O, Jarrett C, Gorodeski EZ, Lauer MS, Blackstone EH. Quantitative electrocardiography for predicting postoperative atrial fibrillation after cardiac surgery. J Electrocardiol. 2011;44:761–767. doi: 10.1016/j.jelectrocard.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang J, Ma J, Jia Y, Zheng Z, Wang H, Su X, Zhang S. Management of focal atrial tachycardias originating from the atrial appendage with the combination of radiofrequency catheter ablation and minimally invasive atrial appendectomy. Heart Rhythm. 2014;11:17–25. doi: 10.1016/j.hrthm.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Starcka CT, Steffelb J, Emmerta MY, Plassa A, Mahapatrac S, Falka V, Salzberga SP. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012;15:416–419. doi: 10.1093/icvts/ivs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han FT, Bartus K, Lakkireddy D, Rojas F, Bednarek J, Kapelak B, Bartus M, Sadowski J, Badhwar N, Earnest M, Valderrabano M, Lee RJ. The effects of LAA ligation on LAA electrical activity. Heart Rhythm. 2014;11:864–870. doi: 10.1016/j.hrthm.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, Morelli RL, Szczepanski W, Kapelak B, Sadowski J, Lee RJ. Anatomic analysis of the LAA following closure with the LARIAT device. Circ Arrhythm Electrophysiol. 2014;7:764–767. doi: 10.1161/CIRCEP.113.001084. [DOI] [PubMed] [Google Scholar]
- Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen P-S, Fishbein MC. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–1327. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Jr, Foster JR, Gettes LS. Atrial excitability and conduction in patients with interatrial conduction defects. Am J Cardiol. 1982;50:1331–1337. doi: 10.1016/0002-9149(82)90471-4. [DOI] [PubMed] [Google Scholar]
- Chik WW, Chan JK, Ross DL, Wagstaff J, Kizana E, Thiagalingam A, Kovoor P, Thomas SP. Atrial tachycardias utilizing the ligament of Marshall region following single ring pulmonary vein isolation for atrial fibrillation. Pacing Clin Electrophysiol. 2014;37:1149–1158. doi: 10.1111/pace.12423. [DOI] [PubMed] [Google Scholar]


