Abstract
Background
Cardiac resynchronization therapy results in improved ejection fraction in patients with heart failure. We sought to determine whether these effects were mediated by changes in contractility, afterload, or volumes.
Methods and Results
In 610 patients with New York Heart Association class I/II heart failure from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study, we performed detailed quantitative echocardiography assessment prior to and following cardiac resynchronization therapy. We derived measures of contractility (the slope [end-systolic elastance] and the volume intercept of the end-systolic pressure–volume relationship, stroke work, and preload recruitable stroke work), measures of arterial load and ventricular–arterial coupling, and measures of chamber size (volume intercept, end-systolic and end-diastolic volumes). At 6 and 12 months, cardiac resynchronization therapy was associated with a reduction in the volume intercept and end-systolic and end-diastolic volumes (P<0.01). There were no consistent effects on end-systolic elastance, stroke work, preload recruitable stroke work, or ventricular–arterial coupling. In the active cardiac resynchronization therapy population, baseline measures of arterial load were associated with the clinical composite score (odds ratio 1.30, 95% CI 1.04 to 1.63, P=0.02). The volume intercept was associated with mortality (hazard ratio 1.90, 95% CI 1.01 to 3.59, P=0.047) and more modestly with the combined end point of mortality or heart failure hospitalization (hazard ratio 1.48, 95% CI 0.8 to 2.25, P=0.06). In contrast, end-systolic elastance, stroke work, preload recruitable stroke work, and ventricular–arterial coupling were not associated with any outcomes.
Conclusion
In patients with NYHA Class I/II heart failure, cardiac resynchronization therapy exerts favorable changes in left ventricular end-systolic and end-diastolic volumes and the volume intercept. The volume intercept may be useful to gain insight into prognosis in heart failure.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00271154.
Keywords: cardiac resynchronization, echocardiography, heart failure
Cardiac resynchronization therapy (CRT) has altered the management of chronic systolic heart failure (HF), significantly reducing cardiovascular morbidity and mortality. The effects of CRT on measures of cardiac function, such as left ventricular ejection fraction (LVEF), are widely established.1,2 Nonetheless, the question of how CRT affects the physiological and structural components of LVEF, such as chamber contractility, afterload, and size, particularly in New York Heart Association (NYHA) class I/II HF, remains incompletely understood.
Noninvasive estimations of these parameters can be derived to gain insight into the effects of CRT on cardiac mechanics. Left ventricular (LV) end-systolic elastance (Ees), the slope of the end-systolic pressure volume relationship (ESPVR), quantifies ventricular stiffening at end-systole and provides some information regarding contractile function (Figure1). The volume intercept (V0) of the ESPVR is a measure of the ventricular volume at a theoretical systolic pressure of 0 mm Hg and further defines the state of the left ventricle in the pressure-volume plane. Ees can be related to effective arterial elastance (Ea), an index of resistive arterial load, and the Ea/Ees ratio provides insight into ventricular–arterial coupling and cardiac efficiency.3,4 Additional load-dependent and independent measures indicative of contractility, such as stroke work (SW) and the slope of the relationship between LV stroke volume and end-diastolic volume, termed preload recruitable stroke work (PRSW), can also be derived to gain insight into LV contractility. How these measures of contractile function, afterload, and LV volumes change with CRT in persons with NYHA class I/II HF remains largely unknown.
Figure 1.
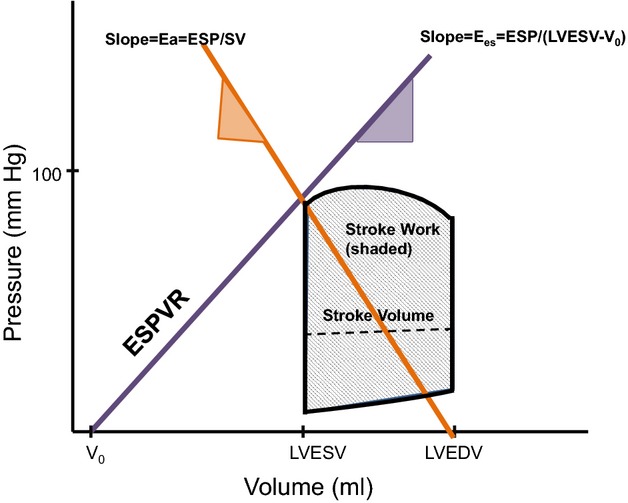
Schematic description of these measures of myocardial mechanics. The purple line denotes the end systolic pressure volume relationship (ESPVR), and the Ees represents the slope of the ESPVR. ESP denotes end-systolic pressure, and Eessb represents the noninvasively derived single-beat estimation of Ees. LVEDV is the end-diastolic volume, and LVESV is the end-systolic volume. V0 is the volume intercept of the ESPVR at an end-systolic pressure of 0 mm Hg. Ea represents the negative slope joining the end-systolic pressure-volume point to the point on the volume axis at end-diastole with this line denoted in orange. Stroke work is represented by the gray shaded region of the pressure-volume area. The dashed line within this region is the stroke volume (SV), or the difference between the LVEDV and LVESV. Ea indicates effective arterial elastance; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SV, stroke volume; V0, volume intercept.
We performed a comprehensive evaluation of echocardiography-derived measures of LV function (single beat–derived Ees [Eessb], V0, SW, PRSW), arterial load (Ea) and ventricular-arterial coupling (Ea/Eessb), and chamber size (V0, end-systolic and end-diastolic volumes) in a large randomized trial of CRT in patients with NYHA class I/II HF. A secondary objective of this study was to determine whether any of these measures could be used to identify patients who experienced improved clinical outcomes with CRT.
Methods
Study Design
The Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study was a prospective, double-blind, randomized controlled trial of CRT in patients with NYHA class I/II HF.2,5–7 All patients were in sinus rhythm with QRS duration ≥120 ms, LVEF ≤40%, and LV end-diastolic dimension ≥55 mm. Detailed patient inclusion and exclusion criteria have been published previously.2,5 All patients were receiving optimal medical HF therapy that included stable doses of an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker for at least 1 month and a β-adrenergic receptor blocker for at least 3 months. The ethics committee at each investigator site approved the protocol, and all patients gave written informed consent.
After baseline evaluation, patients underwent implantation of a CRT device with or without an implantable cardiac defibrillator and then were randomly assigned in a 2:1 model to the active (CRT ON) or control (CRT OFF) group. Device programming has been described previously in detail and was performed according to the patients’ randomization assignments. All CRT OFF patients were reprogrammed to have CRT turned on at 12 months in North America and at 24 months in Europe, and all patients were followed for a total of 5 years. Echocardiograms were regularly performed at standardized intervals throughout the study duration.
Assessment of LV Remodeling
For the purposes of this echocardiographic study, we focused on echocardiograms obtained at baseline and at 6 and 12 months to evaluate the impact of CRT on early changes in these parameters. Echocardiograms were analyzed in a core laboratory that was blinded to the randomization assignment. LV dimensions were recorded with 2-dimensional directed M-mode echocardiography at the tips of the mitral valve leaflets. Echocardiograms were digitized to obtain LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) by Simpson’s method of discs, as recommended by the American Society of Echocardiography, from which LVEF was calculated.8 LV mass was calculated at end diastole as: (5/6×LV short-axis myocardial area at the midlevel×LV length×1.055).8 Overall, <7% of echocardiograms were unanalyzable secondary to image quality, which is similar to or improved from what has been reported in the literature from our other cohort studies.9,10 Missing echocardiography data were not secondary to mortality or follow-up status but were secondary to lack of analyzability from image quality.
Assessment of LV Contractile Function and Ventricular–Arterial Interaction
End systolic pressure (ESP) was estimated as 0.90×systolic pressure, obtained by manual blood pressure cuff measurement.11 Blood pressure was recorded on the day of the echocardiogram at baseline and at 6 and 12 months. Stroke volume (SV) was measured from the difference between the LVEDV and LVESV. Effectiveness (Ea) was defined as the ratio of ESP/SV. Ees was determined using a modified single-beat algorithm described by Chen et al using arm-cuff pressures, echo-derived Doppler stroke volumes, and several timing intervals (isovolumic contraction time, total systolic period) and is denoted as Eessb.11 Ventricular–arterial coupling was estimated by the Ea/Eessb ratio. Additional indices of the ESPVR and LV size, V0, were also estimated from Eessb, end-systolic volume, and ESP.
Stroke work (SW) was derived as mean arterial blood pressure multiplied by SV12 and was also indexed to end-diastolic volume (SW/LVEDV). The slope of the PRSW relationship13 was derived according to the single-beat estimation method, assuming a constant (k) of 0.7, and incorporating volumes, SW, and LV mass.
Clinical Outcomes
The primary clinical end point of the REVERSE study was the HF clinical composite score (CCS), which was categorized as worsened, unchanged, or improved.2,5 Patients were judged to have worsened if they died or were hospitalized due to or associated with worsening HF, crossed over or permanently discontinued double-blind treatment due to worsening HF, or demonstrated worsening in NYHA class or moderately marked worsening of patient global assessment. Patients were judged to be improved if they had not worsened and demonstrated improvement in NYHA class and/or moderately marked improvement in patient global assessment. Patients who were not worsened or improved were classified as unchanged. For this study, we examined this primary end point as well as death and HF-related hospitalizations over a period from baseline to the end of randomization (12 months in North America and 24 months in Europe).
Statistical Methods
All randomized subjects with available echocardiographic values were included in the analyses. Differences in changes in echocardiographic parameters over 6 and 12 months were evaluated using 2-sample t tests. If paired values were reported, only patients with values at both time points for each echocardiography measure were included. An ordered (improved, unchanged, worsened) logistic regression model was used to assess the relationship between baseline measurements of echocardiographic parameters and the CCS at 12 months after randomization, with the effect modification of CRT tested by an interaction term of randomization (CRT ON or CRT OFF) and the variable of interest. Cox proportional hazard models were used to analyze the association between echocardiographic measures obtained at baseline versus time to death and time to first HF hospitalization or all-cause death during the randomization period (12 months in North America and 24 months in Europe), with the effect modification of CRT tested by an interaction term of randomization (CRT ON or CRT OFF) and the variable of interest. Cox proportional hazard models were also used to compare the change in echo parameters from baseline to 6 months versus time to death over the subsequent 4.5 years of follow-up. In these models, echocardiographic variables were examined individually with unadjusted associations reported. All analyses were performed using SAS version 9.2 (SAS Institute).
Results
Patient Characteristics
Baseline characteristics of the study population are detailed in Table1. In this NYHA class I/II HF cohort of 610 participants (419 CRT ON and 191 CRT OFF), patients were predominantly male and white, and the vast majority were treated with a standard regimen of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta blockers. The mean LVEF at baseline was 27.0±6.6%. Slightly more than half of the patient population had an ischemic etiology of HF. There were 368 patients with a left bundle-branch block.
Table 1.
Baseline Characteristics
| Clinical or Echocardiographic Parameter | CRT OFF (n=191) | CRT ON (n=419) | All Patients (n=610) | Normal Values* |
|---|---|---|---|---|
| Age, y | 61.8 (11.6) | 62.9 (10.6) | 62.5 (11.0) | |
| Male | 79.6% | 78.0% | 78.5% | |
| Race | ||||
| Black | 3.4% | 7.3% | 6.1% | |
| White | 93.2% | 89.9% | 90.9% | |
| Other | 3.4% | 2.8% | 3.0% | |
| NYHA | ||||
| Class I | 16.8% | 17.9% | 17.5% | |
| Class II | 83.3% | 82.1% | 82.5% | |
| Diabetes | 24.1% | 21.7% | 22.5% | |
| Hypertension | 51.3% | 52.0% | 51.8% | |
| Heart rate | 68.2 (10.9) | 66.9 (10.3) | 67.3 (10.5) | |
| Body mass index, kg/m2 | 29.0 (5.4) | 28.3 (5.2) | 28.5 (5.2) | |
| Ischemic etiology | 50.8% | 56.3% | 54.6% | |
| Systolic blood pressure, mm Hg | 123 (19) | 125 (19) | 125 (19) | |
| Diastolic blood pressure, mm Hg | 72 (12) | 72 (11) | 72 (11) | |
| ACE-I or ARB | 97.4% | 96.4% | 96.7% | |
| Beta blocker | 93.7% | 95.7% | 95.1% | |
| Eessb, mm Hg/mL | 0.83 (0.41) | 0.85 (0.39) | 0.84 (0.40) | 2.10 (0.67) |
| Stroke work, mL mm Hg | 6145 (2084) | 6351 (2282) | 6286 (2221) | 7560 (1770) |
| Stroke work/LVEDV, mm Hg | 23.6 (7.2) | 24.6 (7.2) | 24.3 (7.2) | 49 (9) |
| PRSW, g/cm2 | 38.7 (12.2) | 40.4 (11.2) | 39.8 (11.6) | 71 (14) |
| Ea, mm Hg/mL | 1.8 (0.6) | 1.8 (0.7) | 1.8 (0.7) | 1.39 (0.34) |
| Ea/Eessb | 2.4 (1.1) | 2.3 (0.8) | 2.3 (1.0) | 0.69 (0.15) |
| V0, mL | 39.4 (99.9) | 38.5 (73.6) | 38.8 (82.6) | −9 (17) |
| Stroke volume, mL | 68.9 (20.7) | 70.6 (23.2) | 70.0 (22.4) | 85 (17) |
| LVESV, mL | 203 (91) | 197 (74) | 199 (80) | 51 (19) |
| LVEDV, mL | 272 (102) | 268 (89) | 269 (93) | 136 (32) |
| LV mass, g/m2 | 137 (35) | 136 (33) | 137 (34) | 95 (19) |
| LVEF, % | 26.4 (6.7) | 27.2 (6.6) | 27.0 (6.6) | 64 (7) |
Continuous variables expressed as mean (SD); categorical variables as percentages. ACE-I indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CRT OFF, control group; CRT ON, active cardiac resynchronization therapy group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NYHA, New York Heart Association; PRSW, preload recruitable stroke work; V0, volume intercept.
Normal values previously reported.12
Baseline Levels and Interval Changes in Echocardiography-Derived Measures of Myocardial Mechanics With CRT
At baseline, all echocardiographic parameters (including Eessb, SW, PRSW, Ea, Ea/Eessb, V0, LVESV, LVEDV, and LVEF) were not significantly different between the CRT ON and CRT OFF groups (Table1). Many of these values were markedly abnormal compared with previously published reference normal values, which have defined Ea as 1.39±0.34 mm Hg/mL, SW as 7560±1770 mL mm Hg, PRSW as 71±14 g/cm2, Eessb as 2.10±0.67 mm Hg/mL, Ea/Eessb as 0.69±0.15, and V0 as −9±17 mL.12 Specifically, Ea, Ea/Eessb, and V0 were substantially greater in the HF population, whereas Eessb, SW, and PRSW were markedly lower.
At 6 months, there were no significant changes in Eessb, Ea, Ea/Eessb, or SW compared with baseline levels in either the CRT ON or CRT OFF groups (Table2). In the CRT ON group, the mean change in Ea was 0.03±0.70 mm Hg/mL and Eessb was 0.06±0.46 mm Hg/mL, which was not significantly different from the mean changes in the CRT OFF group (P=0.49 and P=0.15, respectively). There was also no change in Ea/Eessb during the first 6 months of CRT in either group (P=0.87). Modest changes in PRSW were observed, from 40.3 to 43.7 g/cm2. In contrast, V0, LVEDV, and LVESV all significantly changed over time (Figure2), on the order of −22 to −29 mL, which represented a 10% to 14% relative change in the CRT ON group compared with the CRT OFF group (P=0.007 for V0 and P<0.001 for LVESV and LVEDV).
Table 2.
Mean Changes in Echocardiographic Parameters From Baseline to 6 Months
| CRT OFF | CRT ON | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 6-Month | Change (SD) | n | Baseline | 6-Month | Change (SD) | ||
| Eessb, mm Hg/mL | 150 | 0.81 | 0.81 | 0.00 (0.43) | 325 | 0.85 | 0.91 | 0.06 (0.46) | 0.15 |
| Stroke work, mL mm Hg | 167 | 6177 | 6211 | 34 (2104) | 352 | 6379 | 6323 | −55 (2322) | 0.66 |
| Stroke work/LVEDV, mm Hg | 167 | 23.5 | 24.1 | 0.6 (7.0) | 352 | 24.6 | 28.1 | 3.4 (8.8) | 0.0001 |
| PRSW, g/cm2 | 104 | 38.1 | 38.2 | 0.0 (12.2) | 200 | 40.3 | 43.7 | 3.4 (13.4) | 0.03 |
| Ea, mm Hg/mL | 167 | 1.75 | 1.73 | −0.02 (0.70) | 352 | 1.78 | 1.80 | 0.03 (0.70) | 0.49 |
| Ea/Eessb | 150 | 2.47 | 2.42 | −0.05 (1.26) | 324 | 2.30 | 2.27 | −0.03 (1.32) | 0.87 |
| V0, mL | 150 | 34.3 | 36.0 | 2 (93) | 323 | 37.9 | 16.2 | −22 (84) | 0.007 |
| Stroke volume, mL | 167 | 69.6 | 69.7 | 0.1 (20) | 353 | 70.7 | 69.6 | −1 (24) | 0.52 |
| LVEDV, mL | 167 | 274 | 269 | −5 (49) | 353 | 268 | 239 | −29 (66) | <0.0001 |
| LVESV, mL | 167 | 204 | 199 | −5 (42) | 353 | 197 | 170 | −27 (55) | <0.0001 |
| LVEF, % | 167 | 26.4 | 27.2 | 0.8 (6.6) | 353 | 27.2 | 30.8 | 3.6 (8.3) | <0.0001 |
P value compares the mean changes (2-sample t test) between CRT OFF and CRT ON. CRT OFF indicates control group; CRT ON, active cardiac resynchronization therapy group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; PRSW, preload recruitable stroke work; V0, volume intercept.
Figure 2.
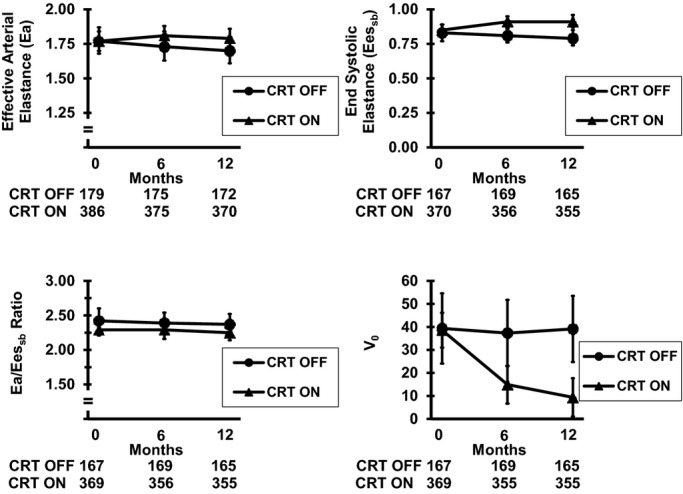
Changes in echocardiography parameters over 6 and 12 months. Mean values along with 95% CIs are presented according to CRT ON or CRT OFF for Ea, Eessb, Ea/Eessb, and V0. The numbers below each figure represent the number of participants in each subgroup (CRT OFF or CRT ON). The most pronounced changes were observed in V0. CRT indicates cardiac resynchronization therapy; CRT OFF, control group; CRT ON, active group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; V0, volume intercept.
Changes in echocardiographic parameters at 12 months mostly paralleled the 6-month findings (Table3, Figure2). The most robust and sustained changes were again observed in V0, LVEDV, and LVESV (all P<0.0001) and were on the order of a 13% to 76% relative change. There were also changes in Eessb of borderline significance, on the order of 5.8% (P=0.052). These data suggest that CRT results in the most substantive changes in measures indicative of LV size, primarily reflected by volumetric measures: V0, LVEDV, and LVESV. There were no significant changes in afterload, as assessed by Ea, or ventricular–arterial coupling, as assessed by Ea/Eessb.
Table 3.
Mean Changes in Echocardiographic Parameters From Baseline to 12 Months
| CRT OFF | CRT ON | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | 12-Month | Change (SD) | n | Baseline | 12-Month | Change (SD) | ||
| Eessb, mm Hg/mL | 146 | 0.82 | 0.79 | −0.03±0.44 | 321 | 0.86 | 0.91 | 0.05±0.45 | 0.052 |
| Stroke work, mL mm Hg | 163 | 6125 | 6100 | −26 (2232) | 343 | 6277 | 6147 | −123 (2289) | 0.63 |
| Stroke work/LVEDV, mm Hg | 163 | 23.4 | 23.9 | 0.5 (6.9) | 343 | 24.6 | 28.6 | 4.0 (9.6) | <0.0001 |
| PRSW, g/cm2 | 94 | 37.4 | 38.5 | 1.0 (13.6) | 172 | 40.4 | 43.6 | 3.2 (13.9) | 0.23 |
| Ea, mm Hg/mL | 163 | 1.77 | 1.70 | −0.06±0.60 | 343 | 1.78 | 1.80 | 0.01±0.65 | 0.20 |
| Ea/Eessb | 146 | 2.46 | 2.35 | −0.11±1.10 | 321 | 2.30 | 2.26 | −0.04±1.22 | 0.53 |
| V0, mL | 146 | 38.8 | 43.2 | 4±91 | 321 | 38.4 | 9.1 | −29±84 | 0.0001 |
| Stroke volume, mL | 165 | 69.2 | 69.5 | 0.3±21.8 | 344 | 69.8 | 68.7 | −1.1±21.9 | 0.50 |
| LVEDV, mL | 165 | 274 | 271 | −2±57 | 344 | 265 | 230 | −35±65 | <0.0001 |
| LVESV, mL | 165 | 204 | 202 | −2±48 | 344 | 195 | 161 | −34±57 | <0.0001 |
| LVEF, % | 165 | 26.3 | 27.1 | 0.8±6.6 | 344 | 27.3 | 31.8 | 4.6±9.3 | <0.0001 |
P value compares the mean changes (2-sample t test) between CRT OFF and CRT ON. CRT OFF indicates control group; CRT ON, active cardiac resynchronization therapy group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; PRSW, preload recruitable stroke work; V0, volume intercept.
Association Between Baseline Cardiovascular Structure and Function and Outcomes
Over a follow-up time of 12 months, 107 patients worsened their CCS. Only baseline Ea levels were significantly associated with the CCS, and those were in the CRT ON group only. For each SD increase in Ea, there were 30% increased odds of an improved clinical outcome (P=0.02) (Table4); however, there was no significant interaction between any of the baseline echocardiographic measures and CRT on clinical outcomes (all P>0.05). Parameters of LV function, remodeling, chamber stiffness, and ventricular–arterial coupling measured at baseline did not appear to influence the effect of CRT on overall HF clinical status, as defined by CCS.
Table 4.
The Association Between Baseline Echocardiographic Parameters and Clinical Composite Score at 12 Months
| Baseline Variable | Units* | CRT OFF | CRT ON | ||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Eessb, mm Hg/mL | 0.4 | 1.09 (0.82 to 1.44) | 0.56 | 1.02 (0.84 to 1.25) | 0.83 |
| Stroke work, mL mm Hg | 2221 | 0.96 (0.72 to 1.28) | 0.77 | 0.85 (0.70 to 1.02) | 0.07 |
| Stroke work/LVEDV, mm Hg | 7.2 | 0.97 (0.74 to 1.27) | 0.80 | 0.94 (0.78 to 1.14) | 0.54 |
| PRSW, g/cm2 | 11.6 | 1.08 (0.79 to 1.48) | 0.64 | 0.88 (0.69 to 1.12) | 0.30 |
| Ea, mm Hg/mL | 0.7 | 1.15 (0.85 to 1.56) | 0.37 | 1.30 (1.04 to 1.63) | 0.02 |
| Ea/Eessb | 1.0 | 1.00 (0.78 to 1.29) | 0.99 | 1.21 (0.95 to 1.54) | 0.12 |
| V0, mL | 82.6 | 0.93 (0.74 to 1.18) | 0.55 | 0.91 (0.73 to 1.14) | 0.42 |
| LVESV, mL | 80 | 0.87 (0.69 to 1.11) | 0.27 | 0.90 (0.73 to 1.10) | 0.31 |
| LVEF, % | 6.6 | 0.96 (0.73 to 1.26) | 0.79 | 0.92 (0.76 to 1.12) | 0.41 |
CRT OFF indicates control group; CRT ON, active cardiac resynchronization therapy group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; OR, odds ratio; PRSW, preload recruitable stroke work; V0, volume intercept.
Units based on 1 SD in echocardiographic parameter. The ORs presented represent the OR per unit change.
We further evaluated the relationship between baseline echocardiographic measures and clinical outcomes by examining both mortality alone and the combined outcome of mortality and HF hospitalizations across the entire study population. There were 19 deaths and 64 participants with HF hospitalization or death from the beginning to the end of randomization, which was a period of 12 months in North America and 24 months in Europe. Within the CRT ON group, of all parameters, V0 was modestly associated with mortality and with the combined end point of mortality or HF hospitalizations (hazard ratio [HR] 1.90, 95% CI 1.01 to 3.59, P=0.047, and HR 1.48, 95% CI 0.98 to 2.25, P=0.06, respectively, for each SD increase) (Table5 and Figure3). Interestingly, V0 was also associated with the combined end point of mortality or HF hospitalizations in the CRT OFF group (HR 1.49, 95% CI 1.06 to 2.08, P=0.02). The association between LVESV and mortality alone and with the combined end point of mortality or HF hospitalizations in the CRT ON population trended toward significance (HR 1.49, 95% CI 0.89 to 2.48, P=0.13, and HR 1.37, 95% 1.00 to 1.88, P=0.05, respectively). There was no interaction by CRT on the associations between echocardiographic parameters and clinical outcomes, suggesting that these measures did not differ between the CRT ON and CRT OFF groups (all P>0.05).
Table 5.
The Association Between Baseline Echocardiographic Parameters and Mortality or the Combined End Point of Mortality and Subsequent HF Hospitalization Over 12 to 24 Months
| Baseline Variable | Units* | CRT OFF | CRT ON | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Mortality (baseline to end of randomization) | |||||
| Eessb, mm Hg/mL | 0.4 | 1.18 (0.61 to 2.28) | 0.62 | 1.11 (0.63 to 1.95) | 0.73 |
| Stroke work, mL mm Hg | 2221 | 0.85 (0.36 to 2.04) | 0.72 | 0.87 (0.48 to 1.58) | 0.66 |
| Stroke work/LVEDV, mm Hg | 7.2 | 0.91 (0.38 to 2.17) | 0.83 | 0.69 (0.37 to 1.28) | 0.24 |
| PRSW, g/cm2 | 11.6 | 0.75 (0.17 to 3.31) | 0.70 | 0.98 (0.45 to 2.12) | 0.96 |
| Ea, mm Hg/mL | 0.7 | 0.79 (0.30 to 2.11) | 0.64 | 0.95 (0.51 to 1.77) | 0.86 |
| Ea/Eessb | 1.0 | 0.52 (0.18 to 1.57) | 0.25 | 0.88 (0.42 to 1.86) | 0.73 |
| V0, mL | 82.6 | 1.43 (0.68 to 3.00) | 0.34 | 1.90 (1.01 to 3.59) | 0.047 |
| LVESV, mL | 80 | 0.92 (0.35 to 2.44) | 0.87 | 1.49 (0.89 to 2.48) | 0.13 |
| LVEF, % | 6.6 | 0.96 (0.40 to 2.28) | 0.92 | 0.67 (0.36 to 1.23) | 0.19 |
| HF hospitalization or mortality (baseline to end of randomization) | |||||
| Ea, mm Hg/mL | 0.7 | 0.82 (0.52 to 1.30) | 0.41 | 0.82 (0.55 to 1.24) | 0.36 |
| Stroke work, mL mm Hg | 2221 | 1.07 (0.73 to 1.57) | 0.74 | 0.88 (0.61 to 1.26) | 0.48 |
| Stroke work/LVEDV, mm Hg | 7.2 | 0.75 (0.49 to 1.16) | 0.20 | 0.64 (0.43 to 0.94) | 0.02 |
| PRSW, g/cm2 | 11.6 | 0.69 (0.41 to 1.15) | 0.15 | 0.86 (0.55 to 1.35) | 0.52 |
| Eessb, mm Hg/mL | 0.4 | 0.87 (0.58 to 1.32) | 0.51 | 0.83 (0.55 to 1.24) | 0.35 |
| Ea/Eessb | 1.0 | 0.93 (0.62 to 1.42) | 0.75 | 0.99 (0.65 to 1.51) | 0.97 |
| V0, mL | 82.6 | 1.49 (1.06 to 2.08) | 0.02 | 1.48 (0.98 to 2.25) | 0.06 |
| LVESV, mL | 80 | 1.36 (1.06 to 1.74) | 0.02 | 1.37 (1.00 to 1.88) | 0.05 |
| LVEF, % | 6.6 | 0.70 (0.45 to 1.08) | 0.10 | 0.67 (0.46 to 0.98) | 0.04 |
CRT OFF indicates control group; CRT ON, active cardiac resynchronization therapy group; Ea, effective arterial elastance; Eessb, single beat–derived end-systolic elastance; HF, heart failure; HR, hazard ratio; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; PRSW, preload recruitable stroke work; V0, volume intercept.
Units based on 1 SD in echocardiographic parameter. Each row represents 3 univariate analyses using Cox proportional hazards. The HRs presented represent the HR per unit change.
Figure 3.
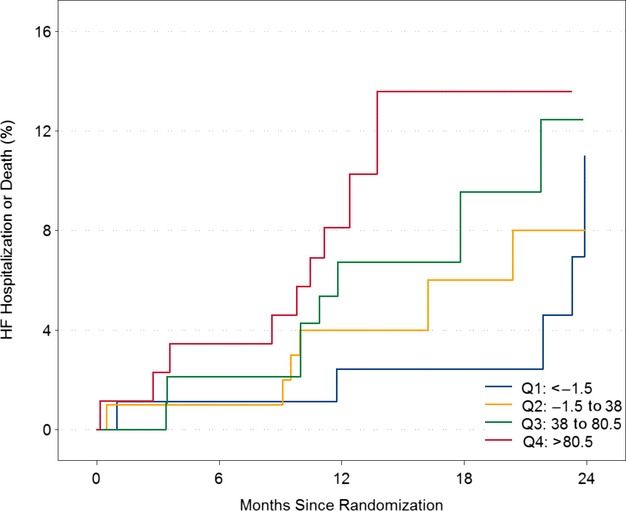
Relationship between baseline V0 and time to first HF hospitalization or death in CRT ON. Kaplan–Meier plots (inverted) describing the relationship between V0 and time to first heart failure hospitalization or death in CRT ON alone. V0 expressed as quartiles. Q1 represents V0<−1.5 mL, Q2 represents −1.5 to 38 ml, Q3 represents 38 to 80.5 mL, and Q4 represents >80.5 mL. CRT indicates cardiac resynchronization therapy; CRT ON, active group; HF, heart failure; Q, quartile; V0, volume intercept.
Discussion
In this population of 610 NYHA class I/II HF patients randomized to CRT ON versus CRT OFF, we derived detailed quantitative echocardiographic measures of contractile function, total arterial load, ventricular-arterial coupling, and LV remodeling. We determined that the primary and most significant changes were in volumetric measures: V0 and end-systolic and end-diastolic volumes. These findings were evident within 6 months of CRT implantation in the CRT ON group and were sustained at 12 months. In the CRT ON population, higher baseline values of V0, indicative of worse LV remodeling, were significantly associated with an increased risk of mortality, suggesting potential utility as a prognostic measure in HF.
As such, no substantive or consistent changes were observed in Ea or Ea/Eessb in the CRT ON population compared with CRT OFF over a 6- or 12-month period. There were modest changes in PRSW at 6 months (P=0.03) and Eessb at 12 months (P=0.052), which may be indicative of some effects of CRT on these measures of contractility; however, these parameters were also not uniformly associated with clinical outcomes. Only baseline Ea was associated with the primary study outcome measure, CCS. These findings support the hypothesis that the clinical benefits observed with CRT and the improvements in LVEF are likely less secondary to changes in load or ventricular–arterial coupling and more related to changes in V0, LVESV, and LVEDV. Our findings provide human data to support initial important observations in animals from the 1980s on the effects of LV pacing on measures such as V0.14,15
Previously published small human studies of 78 HF participants and a mean NYHA class of III reported significant differences in Ea, Eessb, and Ea/Eessb over 1 year with CRT therapy16; however, these patients had more advanced HF, as reflected by higher Ees, lower LVEF, and worse Ea/Eessb. In contrast, the REVERSE population consisted of patients with mild HF and less severe abnormalities in baseline levels of Ea/Eessb. Of note, a large proportion of patients were also treated with optimal medical therapy including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta blockers. We did not observe significant changes in Ea/Eessb with CRT or association with clinical outcomes, and this is potentially related to the lesser severity of disease. Another smaller study of 25 participants with NYHA class III and IV HF suggested that changes in Ea, Eessb and Ea/Eessb occurred early with CRT, on the order of 23±12 days.17 These participants tended to have dramatic alterations in LVEF, from 28% to 42%. We hypothesize that these discrepancies between prior published studies and our present results are secondary to differences in disease severity, heterogeneity of CRT response, duration of follow-up, and sample size. Indeed, larger studies evaluating additional noninvasive measures of contractile function, such as global longitudinal strain, corroborate the importance of functional changes in NYHA class I/II HF and CRT.18
The lack of association of Eessb with outcomes is not inconsistent with prior human studies in chronic HF.10 Although we cannot entirely exclude measurement error accounting for the lack of more substantive change in Eessb, we feel this is less likely, given that our calculated methods were derived using an algorithm that was verified using comparisons with invasive analyses.11 In addition, as revealed in patients with HF with preserved ejection fraction, normal Eessb can exist despite abnormal systolic and diastolic function. Moreover, and most important, Eessb alone is not a comprehensive measure of LV function in the pressure-volume plane. Fully describing the contractile state of the ventricle requires specification of both the slope (Ees) and the intercept (V0) of the ESPVR, and they have been noted in seminal papers as important descriptors of LV function.14,15 We detected an association between V0 and prognosis in patients with mild HF. Interestingly, V0 was only weakly correlated with LVESV (R=0.38 to 0.45) and LVEDV (R=0.21 to 0.31). Finally, it can be challenging to distinctly separate contractility from remodeling, particularly in the setting of HF with reduced ejection fraction, and a single measure alone to assess contractility is likely inadequate.
This study should be interpreted in light of certain limitations. These measures of chamber mechanics are derived, and not specifically in the CRT population, although they have been well validated across a broad variety of cardiac remodeling phenotypes.10,19 We were also unable to derive measures of end-systolic wall stress or strain, which would have provided additional insight into stress–strain relationships and cardiac function. Furthermore, we were unable to report long-term data on changes in specific echocardiographic measures, given lack of blood pressure recording after 1 year. Given trial design, we focused on short-term outcomes, in the time period of 12 to 24 months, and it may be that the significance of these findings is greater in the long term. Reflecting the mild disease state of our patients, our event rate was relatively low, which reduced our power to detect significant associations. We also note that data were missing for some of our echocardiography measures, primarily due to technical limitations in analyzability secondary to image quality.
In conclusion, in a population of NYHA class I/II HF patients, CRT primarily affected measures of LV volumes and less so measures of chamber or arterial stiffness or ventricular–arterial coupling. Volumetric measures were modestly associated with clinical outcomes. These findings suggest that with further study, noninvasive assessment of V0 could be used as an additional parameter to gain insight into the effects of CRT.
Sources of Funding
The REVERSE study was sponsored and funded by Medtronic, Inc. The study was designed and conducted in collaboration between physician experts and the Medtronic Clinical Research Department. Linde was supported by the Swedish Heart Lung Foundation (grants 20080498 and 20110406) and the Stockholm County Council (grants 20090376 and 20110610).
Disclosures
Dr Linde reports research grants, speaker honoraria, and consulting fees from Medtronic and Cardio3, and speaker honoraria and consulting fees from St Jude Medical. Dr Daubert reports speaker honoraria and consulting fees from Medtronic and St Jude Medical. Dr St John Sutton reports research grant support, speaker honoraria, and consulting fees from Medtronic as well as research support from Paracore. Dr Ghio reports consulting fees from Medtronic. Dr Gold reports consulting fees from Medtronic, St Jude Medical and Boston Scientific, lecture fees from Medtronic and Boston Scientific and research grants from Medtronic, Boston Scientific, and St Jude Medical. The remaining authors have nothing to disclose. Dr Chirinos is a consultant for OPKO HealthCare, Bristol Myers Squibb and Fukuda Denshi.
References
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study Group. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation. 2009;120:1858–1865. doi: 10.1161/CIRCULATIONAHA.108.818724. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Heart Fail Clin. 2009;5:217–228. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde C, Abraham WT, Gold MR, Daubert C REVERSE Study Group. Cardiac resynchronization therapy in asymptomatic or mildly symptomatic heart failure patients in relation to etiology: results from the REVERSE (REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction) study. J Am Coll Cardiol. 2010;56:1826–1831. doi: 10.1016/j.jacc.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Linde C, Gold M, Abraham WT, Daubert JC REVERSE Study Group. Rationale and design of a randomized controlled trial to assess the safety and efficacy of cardiac resynchronization therapy in patients with asymptomatic left ventricular dysfunction with previous symptoms or mild heart failure—the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Am Heart J. 2006;151:288–294. doi: 10.1016/j.ahj.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing G, American Society of Echocardiography’s Guidelines and Standards, Committee, European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St John Sutton M, Cappola TP. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:H744–H750. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
- Park RC, Little WC, O’Rourke RA. Effect of alteration of left ventricular activation sequence on the left ventricular end-systolic pressure-volume relation in closed-chest dogs. Circ Res. 1985;57:706–717. doi: 10.1161/01.res.57.5.706. [DOI] [PubMed] [Google Scholar]
- Little WC, O’Rourke RA. Effect of regional ischemia on the left ventricular end-systolic pressure-volume relation in chronically instrumented dogs. J Am Coll Cardiol. 1985;5:297–302. doi: 10.1016/s0735-1097(85)80050-4. [DOI] [PubMed] [Google Scholar]
- Zanon F, Aggio S, Baracca E, Pastore G, Corbucci G, Boaretto G, Braggion G, Piergentili C, Rigatelli G, Roncon L. Ventricular-arterial coupling in patients with heart failure treated with cardiac resynchronization therapy: may we predict the long-term clinical response? Eur J Echocardiogr. 2009;10:106–111. doi: 10.1093/ejechocard/jen184. [DOI] [PubMed] [Google Scholar]
- Zocalo Y, Bia D, Armentano RL, Gonzalez-Moreno J, Varela G, Calleriza F, Reyes-Caorsi W. Resynchronization improves heart-arterial coupling reducing arterial load determinants. Europace. 2013;15:554–565. doi: 10.1093/europace/eus285. [DOI] [PubMed] [Google Scholar]
- Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ, Bourgoun M, Foster E, Zareba W, Goldenberg I, McNitt S, Pfeffer MA, Moss AJ, Solomon SD Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy Investigators. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]


