Abstract
Transthyretin (TTR, or prealbumin) is a tetrameric protein found in plasma and cerebrospinal fluid. Its major role is to transport thyroid hormones (thyroxin-T4) and retinol (through association with retinol-binding protein). TTR has been studied extensively, due to the great number of point mutations that result in sequence heterogeneity. Many of these variants are associated with pathological conditions that result in extracellular deposition of amyloid fibers in tissues. In this work, we have developed a rapid mass spectrometric immunoassay for determination and quantification of TTR and its variants from human serum and plasma samples. The assay was fully characterized in terms of its precision, linearity and recovery characteristics. The new assay was also compared with a conventional TTR ELISA. Furthermore, we have applied the optimized method to analyze transthyretin and its modifications in 44 human plasma samples, and in the process, optimized a method for TTR proteolytic digestion and identification of point mutations.
Keywords: transthyretin, immunoassay, mass spectrometry, plasma, quantification, trypsin
1. Introduction
Human transthyretin (TTR) is a plasma protein tetramer consisting of four identical 127 amino acid subunits, each with a molecular weight of approximately 14,000 Da [1]. It is a polymorphic protein that transports holo-retinol-binding protein and thyroxine [2, 3]. TTR has been associated with several pathological conditions such as senile systemic amyloidoses (SSA), and familial amyloidotic polyneuropathy (FAP), which are mainly characterized by extracellular deposition of insoluble amyloid fibrils, constituted primarily of TTR or its variants [4–6]. Many of these pathological conditions occur as a result of more than 80 different TTR mutations that have been reported so far [7]. Most of them are amyloidogenic mutations and have been a subject of intensive studies [8–10]. Others have been reported as non-pathogenic mutations and can be found in normal samples [7]. However, identification and characterization of TTR mutation both in health and disease is of great importance for revealing the unknown mechanisms underlying TTR amyloidosis and associated pathological conditions.
Developing novel methods for high-throughput analysis of proteins and their isoforms is considered an important step toward understanding the intrinsic protein characteristics. Most techniques for protein analysis provide information about the total protein concentration and are not geared to fully quantify the protein isoforms. The utility of mass spectrometry for protein analysis lies in the ability to provide highly accurate molecular weight information both on intact proteins and variants containing protein modifications. When coupled with the specificity of immunoaffinity separation, the resulting mass spectrometry immunoassay is a novel technique that can be used for both qualitative and quantitative analysis of proteins directly from biological specimens [11]. Additional post-capture protein mapping, involving site-specific enzymatic degradation and mass spectrometric analysis of the resulting peptides, provides insight into the intrinsic protein characteristics and assists in the identification and verification of specific protein modifications [12]. Several mass spectrometry immunoassay methods have been developed primarily for qualitative determination of protein variants [13]. In this work, we are going a step further and develop a fully quantitative method for determination of TTR and its variants in human serum and plasma samples. Developing the method for transthyretin is challenging due to the heterogeneity in its structure and the presence of numerous protein modifications. The fact that there are more then 80 reported point mutation in the TTR structure, some of which are associated with pathological conditions, necessitates a thorough design and method execution in order to delineate and quantify these modifications. Presented here is the development of rapid and accurate mass spectrometric immunoassay for quantitative analysis of TTR and its variants in plasma and serum samples. In addition, we have introduced a streamlined TTR mass mapping protocol using in-situ proteolytic degradation in order to identify and verify TTR point mutations present in specific samples.
2. Materials and Methods
2.1. Reagents
Polyclonal rabbit anti-human antibody to transthyretin was obtained from DAKO (Carpinteria, CA, USA, Catalog No. A0002, 3.9 mg/mL). Rabbit anti-human polyclonal antibody to beta-lactoglobulin (BL) was obtained from GeneTex (Irvine, CA, Cat. No. GTX77272, 1 mg/mL). Beta-lactoglobulin from bovine milk (Cat. No. L8005), 1,1′ Carbonyldiimidazole (115533), TWEEN 20 ( P7949), TRIS (T-6128), Dithiothreitol (DTT, 43815) and α-cyano-4-hydroxycinnamic acid (476870) were obtained from Sigma-Aldrich (St. Lous, MO). Affinity pipettes fitted with porous microcolumns were obtained from Intrinsic Bioprobes (Tempe, AZ, Cat No. IBI-CMD-R96). Phosphate buffered saline was obtained from Thermo Scientific (Rockford, IL, Cat. No. 28374). Filtered, degassed HBS-N buffer (0.01 M HEPES pH 7.4; 0.15 M NaCl) was obtained form GE Healthcare Bio-Sciences AB (Piscataway, NJ)). Sterile water (Cat. No. AB02120), acetone (AB00636), MES (AB01235), acetonitrile (AB00120) and trifluoracetic acid (AB02010) were purchased from American Bionalytical (Natick, MA). 1-Methyl 2-pyrrolidone was obtained from EMD Chemicals (Gibbstown, NJ, Cat. No. MX1932-5). N-octylglucoside was obtained from Roche Applied Science (Indianapolis, IN, Cat. No. 10634425001). TTR ELISA kit was obtained from ALPCO Immunoassays (Salem, NH). Sequencing grade modified trypsin was obtained from Promega (Madison, WI, Cat. No. V511). Sequencing grade endoproteinase Arg-C was obtained from Roche Applied Science (Penzberg, Germany, Cat. No. 11370529001).
2.2. Instrumentation
Method development analyses were performed using an 8-channel Finnpipette Novus multichannel pipetter (Thermo Fisher Scientific, Waltham, MA). Derivatization of the affinity pipettes with antibodies and high-throughput mass spectrometric immunoassays were performed on a Multimek 96 automated 96-channel pipettor (Beckman Coulter, Brea, CA). Mass spectrometry was performed on an Autoflex II MALDI-TOF (linear spectra) and Autoflex II TOF/TOF MALDI-TOF (reflectron spectra) mass spectrometers (Bruker, Billerica, MA). ELISA readouts were obtained on Cary 50 spectrophotometer equiped with a microplate reader accessory (Varian Instruments, Walnut Creek, CA).
2.3. Human serum and plasma samples
Forty-four Na-heparin human plasma samples were used for the high-throughput analysis, whereas twenty human serum samples were used for the method development. All samples were obtained from ProMedDX (Norton, MA, USA), and were designated as normal based on their non-reactivity for common blood infectious agents and the donor information itself. To ensure proper privacy protection, the samples were labeled only with a barcode and supplied with an accompanying specification sheet containing information about the gender and age. Samples were aliquoted and stored at −80°C until used.
2.4. Preparation of affinity pipettes
Prior to use, the affinity pipettes need to be derivatized with antibodies. Therefore, ninety-six affinity pipettes were mounted on the head of the Multimek 96 pipettor and initially rinsed with 200 mM HCl (20 cycles, each cycle consisting of an aspiration and dispense of a 150 μL volume), followed by a water rinse (5 cycles) and acetone rinse (5 cycles). The surface of the microcolumns was activated by immersing the pipettes into a tray containing 100 mg/mL 1,1′ Carbonyldiimidazole (in methyl-1 pyrrolidone-2), and 500 cycles of 100 μL aspirations and dispenses through each affinity pipette were performed. Two rinses with 1-methyl 2-pyrrolidone (10 cycles each, 150 μL volumes) and a final rinse with acetone (10 cycles, 150 μL) followed. The affinity pipettes were then immediately immersed into a microwell plate containing the antibodies solutions (0.39 mg/mL transthyretin antibody, and 0.01 mg/mL beta-lactoglobulin antibody, in 10 mM MES) and 800 cycles of aspirations and dispenses of 50 μL volumes were performed to bind the antibodies to the activated microcolumns surfaces. Two rinses with 60 mM HCl followed (30 cycles each, 100 μL), ending with two final rinses with assay buffer (PBS w/0.1% TWEEN, 30 cycles each, 100 μL). The antibody-derivatized pipettes were stored at 4°C until used.
2.5. Preparation of standards and analytical samples
For the standard curve generation, reference serum was used as a primary standard. This approach was used in order to avoid the TTR signal intensity discrepancy that occurs when a monomeric purified TTR standard is used to generate a standard curve for the analysis of native tetrameric TTR. The dissociation of the TTR tetramer in the analytical plasma and serum samples effectively increases the TTR concentration 4-fold, and the resulting signal in the mass spectra is 4-times stronger than that obtained by the analysis of the monomeric purified TTR standard. The use of reference serum is not uncommon in quantitative assays [14–16]. Using ELISA, the serum total TTR concentration was rigorously determined and this „stock“solution was used to generate the serial dilutions for the standard curve. Six-point standard curve was made for the assay, by serially diluting the reference serum sample (Standard #1, with 2.5 mg/L TTR). Serial dilutions were done using assay buffer, preparing standards with 1.25 mg/L (Standard #2), 0.625 mg/L (Standard #3), 0.3125mg/L (Standard 4), 0.1563 mg/L (Standard #5) and 0.0781 mg/L (Standard #6). For the standard curve, ten microliters of each standard were added to microwells containing 130 μL assay buffer and 10 μL 10 mg/L beta-lactoglobulin (BL was co-isolated with TTR and used as an IRS during the analysis; the obtained signal of BL is used to normalize the TTR signals in the mass spectra). For the analytical samples, the standard solutions were substituted with human plasma or serum sample (diluted 300-fold into assay buffer).
2.6. Mass spectrometric immunoassay
The antibody-derivatized affinity pipettes were mounted onto the head of the Multimek pipettor and initially rinsed with assay buffer (10 aspirations and dispense cycles, 100 μL volumes each). Next, the pipettes were immersed into a microplate containing the samples and 100 aspirations and dispense cycles were performed (100 μL volumes each) allowing for affinity capture of both transthyretin and beta lactoglobulin from the samples. The pipettes were then rinsed with assay buffer (100 cycles), and twice with water (10 cycles each). In preparation of elution, the affinity pipettes containing the retrieved protein were rinsed with 1 mM N-octylglucoside (single cycle with a 150 μL aliquot) in order to homogenize the subsequent MALDI matrix draw and elution by completely wetting the porous microcolumns inside the pipettes. For elution of the captured proteins, 6 μL aliquots of MALDI matrix (25 g/L α-cyano-4-hydroxycinnamic acid in aqueous solution containing 33% (v/v) acetonitrile, and 0.4 % (v/v) trifluoroacetic acid) were aspired into the affinity pipettes, and after a 10 second delay (to allow for the dissociation of the protein from the capturing antibody), the eluates from all 96 affinity pipettes containing the targeted proteins were dispensed directly onto a 96-well formatted MALDI target. Following drying, linear mass spectra were acquired with a delayed extraction mode using a 1.7 kV draw out pulse, 200 ns delay, and a full accelerating potential of 20 kV. Five mass spectra were acquired from each sample spot, each consisting of three-hundred laser shots. The mass spectra were internally calibrated with the singly and doubly charged beta-lactoglobulin signals, and further processed (baseline subtracted and smoothed) with the Flex Analysis software (Bruker Daltonics). The peak heights for the TTR signals and beta-lactoglobulin were measured and entered into an Excel spreadsheet. The standard curve was obtained by dividing the peak heights of the TTR signals by that of the BL signal, summing the individual TTR/BL ratios, and plotting the average (from five mass spectra) of the summed TTR/BL ratios against the six standard TTR concentrations. The data was fitted with a trendline using Sigma Plot (Systat Software, San Jose, CA). This standard curve was then utilized to determine the absolute concentration of transthyretin and its variants in the analytical samples.
2.7. TTR variants identification
Enzyme digestion with trypsin and endoproteinase Arg-C, was performed to identify and verify the TTR variants. Tryptic digestion was performed in situ, directly on the transthyretin eluates deposited onto the MALDI target after the mass spectrometric immunoassay of the human serum samples. Tips only with TTR antibody (lacking BL) were used for the analysis. Also, sample preparation and elution steps were performed using HBS-N buffer, instead of PBS w/0.1%Tween. Following drying of the TTR eluates, 10 μL aliquot of 25 mM TRIS, pH 9.1, containing 5 × 10−4 mg/mL trypsin was added onto each sample spot and the entire MALDI plate was placed into a humidified enclave, at 40°C. To keep the samples solvated, one 10 μL aliquot of water was added to each spot at ~10 min into the digestion. Digestion was terminated after 20 min by air-drying the plate. The sample spots were re-hydrated with 5 μL aliquots of 0.8% TFA, and allowed to dry again. Following matrix re-crystallization, reflectron mass spectra were acquired with a delayed extraction mode using a 2.1 kV draw out pulse, 1100 ns delay, an ion mirror voltage of 20 kV, and a full accelerating potential of 19 kV.
In order to further confirm the identified TTR variants in the samples, additional proteolytic digestion was done using endoproteinase Arg-C. The digestion was also performed in situ, on the MALDI target, using 0.01 mg/mL Arg-C (in 25 mM TRIS, pH 9.1). The same digestion protocol was followed as for the trypsin digestion. Following digestion, hydratation and matrix re-crystalization, reflectron mass spectra were acquired.
3. Results
A typical mass spectrum resulting from the transthyretin mass spectrometric immunoassay of a human plasma sample is shown in Fig. 1. Present in the spectrum are signals for BL - the IRS, and TTR - the targeted protein, which shows as multiple signals. The predominant form is cysteinylated TTR (MW=13,880.4), followed by the native TTR (MW=13,761.4). Also noted in the mass spectra are signals resulting from oxidation (MW=13,793.4), sulfonation (MW 13,841.4), Cysteine-Glycine transformation (MW=13,715.4) and cysteineglycine (MW=13,937.4). All of these TTR variants occur as a result of the modifications of a single highly reactive cystein residue (Cys10) [17]. Analyzing these forms is of great importance due to the crucial role of the free Cys10 residue and a possible involvement of physiological factors that affect Cys 10 reactivity in the cases of TTR amyloidogenesis [18–20].
Fig. 1.
Typical mass spectrum resulting from analysis of transthyretin from a human plasma sample.
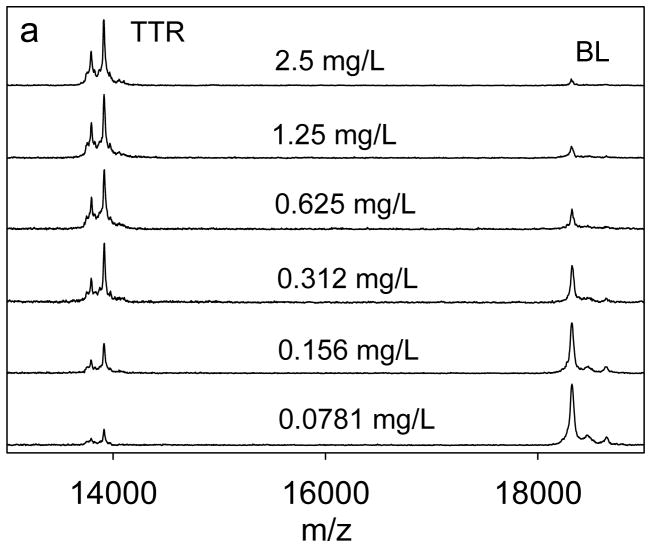
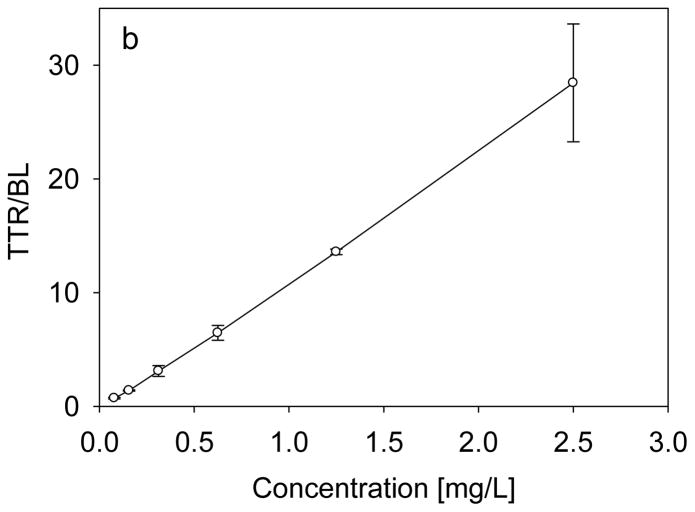
For the assay, a 6-point standard curve was selected, spanning the range from 0.0781 mg/L to 2.5mg/L. An example of the standard curve, along with representative mass spectra, is shown in Fig. 2. The trendline across the entire range, has a coefficient of determination (R2=0.999) and standard error of estimate (SEE=0.267). It was found that the range of the standard curve was sufficient to determine the concentration of transthyretin in all of the examined human plasma and serum samples. The limit of quantification for the assay was determined to be 0.08 mg/L, and the limit of detection was 0.04 mg/L. To determine the individual isoform concentrations, the ratio between each individual isoform signal and the IRS (BL) signal in the mass spectrum was first determined. Then, all the ratios were summed up, and using the previously generated standard equation, the total TTR concentration was calculated. The concentration of each form was then expressed as percentage from the total concentration. This method enabled the determination of the concentration of some of the TTR isoforms that are less abundant and that have peak heights ratios which fall bellow the standard curve range.
Fig. 2.
(a) Representative transthyretin standards mass spectra, and (b) Standard curve generated with the transthyretin (TTR) mass spectrometric immunoassay by using beta-lactoglobulin (BL) as an internal reference standard.
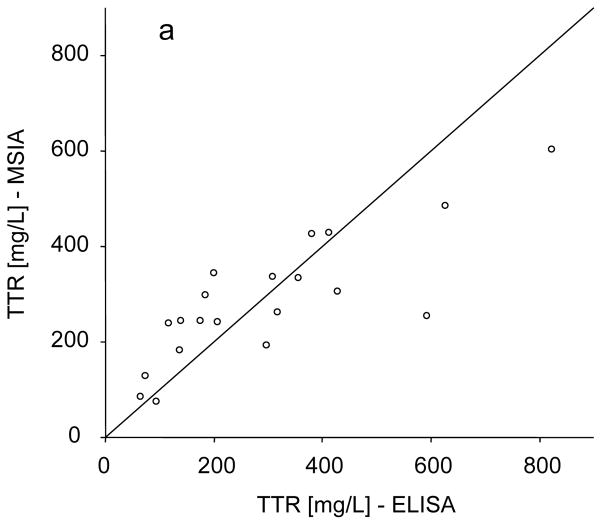
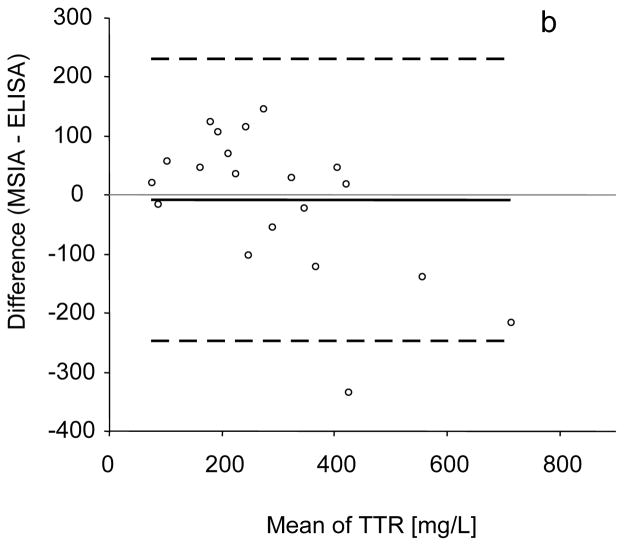
The intra-assay precision (within-run) was determined by analyzing three serum samples, in triplicates, each with a single standard curve. The inter-assay precision (run-to-run) was determined by analyzing one serum sample three times, on different days, with separate standard curves each time. The results are shown in Table 1, and indicate CVs of less than 10%. Linearity of the assay and spiking recovery were also performed, using serum samples with known transthyretin concentration. For the linearity determination, samples were serially diluted and analyzed for TTR concentrations. The results were then compared to those expected (Table 2). Spiking recovery experiments were performed by spiking serum samples with different amounts of recombinant human TTR, followed by analysis with the assay to determine the total transthyretin concentration, and comparison of the results with those expected (Table 3). In a final test, the developed assay was compared to conventional TTR ELISA. Twenty serum samples were analyzed both by the mass spectrometric immunoassay and a commercially available ELISA, and the TTR concentrations were determined. The graph shown in Fig. 3a indicates good correlation between the two set of numbers (Passing & Bablok fit of 96.5+0.67×, and Cusum linearity p-value of >0.1) [21], validating the results obtained with the new transthyretin assay. The Altman Bland test was also applied yielding a slight negative bias of 9% (Fig. 3b)
Table 1.
Determination of the intra-and inter-assay precision.
| Intra-assay CVs | |||
|---|---|---|---|
| Sample | 1 | 2 | 3 |
| STDV: | 13.1 | 6.13 | 13.2 |
| MEAN [mg/L]: | 298 | 297 | 339 |
| CV: | 4.41 | 2.07 | 3.89 |
| Inter-assay CV | |
|---|---|
| STDV | 19.6 |
| MEAN (mg/L) | 311 |
| CV | 6.29 |
Table 2.
Determination of the assay linearity.
| Linearity
| ||||
|---|---|---|---|---|
| Sample | Dilution | Observed [mg/L] | Expected [mg/L] | Recovery O/E % |
| 1 | 328 | |||
| 2× | 169 | 164 | 103 | |
| 4× | 78.4 | 82.0 | 95.6 | |
| 8× | 36.5 | 41.0 | 89.0 | |
|
| ||||
| 2 | 242 | |||
| 2× | 123 | 121 | 102 | |
| 4× | 53.8 | 60.6 | 88.8 | |
| 8× | 26.4 | 30.3 | 87.1 | |
Table 3.
Determination of the spiking and recovery.
| Spiking recovery
| |||
|---|---|---|---|
| Sample | Observed [mg/L] | Expected [mg/L] | Recovery O/E% |
| 1 | 490 | ||
| 1,480 | 1,490 | 99.3 | |
| 2,180 | 2,490 | 87.6 | |
| 4,770 | 4,490 | 106 | |
|
| |||
| 2 | 175 | ||
| 716 | 676 | 106 | |
| 1,200 | 1,180 | 102 | |
| 2,340 | 2,180 | 107 | |
Fig. 3.
(a) Transthyretin mass spectrometric immunoassay and standard ELISA method comparison, yielding a Passing & Bablok fit of 96.5+0.67×, and Cusum linearity p-value of >0.1. (b) Bland-Altman analysis showing slight negative bias of 9% (solid black line). Black dashed lines represent 95%limit of agreement.
The optimized mass spectrometric immunoassay was used to perform a complete quantitative analysis of TTR and TTR modifications in 44 human plasma samples. The standard curve samples were placed in column 1 of the microplate, along with a control sample with a known TTR concentration. Since TTR is an abundant plasma protein, all samples were diluted 300-fold in assay buffer prior to analysis. Eighty-eight analytical samples were prepared (each sample was prepared in duplicate) and placed in columns 2–12 of a 96-well microplate. The assays were executed using the Multimek 96 channel pipettor. Following mass spectrometry analysis and spectra processing, a standard curve was constructed from the data in column 1, and the performance of the assay verified with the control sample. The concentrations of transthyretin and its variants were then individually determined, averaged (from the two analyses for each sample) and are presented in Table 4. A total of six TTR isoforms were quantified in each of the 44 samples. In all samples, the predominant signal corresponds to the cysteinylated TTR form, followed by the native TTR peak. The other four Cys10 reactive forms also occur in all 44 samples, with relatively constant concentrations, with the exception of sulfonated Cys10 form which was present in higher quantities in samples obtained from individuals from Texas. This can be of potential importance when analyzing geographic distribution of different protein isoforms in future population proteomics studies, or can simply be a result of different sample handling procedures between the two sample collection sites.
Table 4.
Concentration of transthyretin and its variants in 44 human plasma samples. Samples labeled with an “*” contain a +30Da point mutation.
| Sample | native TTR [mg/L] | Cysteinilation (Cys10) [mg/L] | Cys10Gly [mg/L] | Oxidation (Cys10) [mg/L] | Sulfonation (Cys10) [mg/L] | CysGly [mg/L] | Total TTR [mg/L] | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31.2 | 57.4 | 13.8 | 14.6 | 15.4 | 11.7 | 144.1 | ||
| 2 | 24.7 | 39.5 | 10.1 | 14.4 | 53.2 | 8.49 | 150.4 | ||
| 3 | 20.5 | 34.6 | 7.66 | 10.2 | 10.0 | 7.52 | 90.5 | ||
| 4 | 18.5 | 37.9 | 8.19 | 8.57 | 9.43 | 7.67 | 90.3 | ||
| 5 | 31.1 | 66.6 | 12.0 | 18.7 | 55.0 | 15.2 | 198.6 | ||
| 6 | 18.6 | 31.0 | 7.36 | 9.00 | 10.1 | 7.17 | 83.2 | ||
| 7 | 28.8 | 58.7 | 13.8 | 15.4 | 41.1 | 11.7 | 169.5 | ||
| 8 | 42.1 | 84.1 | 17.3 | 21.4 | 57.4 | 18.0 | 240.3 | ||
| 9 | 33.8 | 55.8 | 17.7 | 16.4 | 18.4 | 13.7 | 155.8 | ||
| 10 | 19.9 | 47.5 | 9.44 | 11.7 | 25.7 | 9.68 | 123.9 | ||
| 11 | 51.0 | 95.5 | 21.9 | 24.3 | 25.5 | 24.4 | 242.6 | ||
| 12 | 28.0 | 57.8 | 14.2 | 15.9 | 40.0 | 13.2 | 169.1 | ||
| 13 | 59.8 | 106.7 | 26.5 | 29.8 | 34.2 | 26.2 | 283.2 | ||
| 14 | 24.3 | 37.9 | 8.62 | 10.3 | 10.8 | 9.08 | 101.0 | ||
| 15 | 29.3 | 74.5 | 14.4 | 13.5 | 16.7 | 12.3 | 160.7 | ||
| 16 | 24.9 | 55.0 | 12.0 | 13.1 | 16.1 | 10.6 | 131.7 | ||
| 17 | 36.5 | 63.1 | 16.4 | 26.6 | 55.8 | 14.3 | 212.7 | ||
| 18 | 35.0 | 56.5 | 17.6 | 19.0 | 32.4 | 15.1 | 175.6 | ||
| 19 | 26.5 | 52.7 | 12.3 | 14.6 | 42.3 | 11.3 | 159.7 | ||
| 20 | 34.6 | 51.3 | 14.8 | 22.1 | 65.8 | 11.0 | 199.6 | ||
| 21 | 37.5 | 73.5 | 16.2 | 20.8 | 54.2 | 17.1 | 219.3 | ||
| 22 | 26.9 | 42.7 | 12.9 | 14.5 | 40.8 | 10.9 | 148.7 | ||
| 23 | 32.7 | 78.2 | 14.2 | 19.0 | 51.7 | 15.9 | 211.7 | ||
| 24 | 12.7 | 21.4 | 6.84 | 6.22 | 6.61 | 5.70 | 59.5 | ||
| 25 | 23.5 | 48.5 | 11.5 | 11.0 | 12.8 | 8.72 | 116.0 | ||
| 26 | 32.9 | 66.5 | 12.6 | 18.7 | 62.5 | 12.4 | 205.6 | ||
| 27 | 18.8 | 50.7 | 8.10 | 8.46 | 11.8 | 9.25 | 107.1 | ||
| 28 | 45.8 | 87.3 | 17.1 | 20.0 | 23.1 | 21.9 | 215.2 | ||
| 29 | 33.4 | 73.3 | 17.2 | 17.1 | 37.7 | 15.3 | 194.0 | ||
| 30 | 22.9 | 49.8 | 9.32 | 9.95 | 12.4 | 9.37 | 113.7 | ||
| 31 | 47.0 | 100 | 22.3 | 25.4 | 62.1 | 22.1 | 278.9 | ||
| 32 | 34.5 | 68.9 | 15.2 | 18.5 | 46.2 | 13.5 | 196.8 | ||
| 33 | 33.0 | 59.5 | 12.5 | 15.7 | 38.9 | 11.7 | 171.3 | ||
| 34 | 33.5 | 66.9 | 13.3 | 13.2 | 17.6 | 12.7 | 157.2 | ||
| 35 | 32.9 | 65.3 | 13.4 | 15.9 | 41.7 | 14.9 | 184.1 | ||
| 36 | 30.0 | 48.4 | 13.2 | 14.8 | 43.0 | 9.99 | 159.4 | ||
| 37 | 15.8 | 26.7 | 6.04 | 6.51 | 7.15 | 5.92 | 68.1 | ||
| 38 | 47.9 | 55.8 | 17.6 | 23.8 | 49.6 | 16.2 | 210.9 | ||
| 39 | 10.4 | 17.9 | 5.08 | 4.73 | 5.01 | 4.68 | 47.8 | ||
| 40 | 30.3 | 69.5 | 12.4 | 14.7 | 36.1 | 12.9 | 175.9 | ||
| 41 | 26.7 | 83.7 | 10.7 | 11.8 | 19.3 | 13.1 | 165.3 | ||
| Δ(+30Da) | Δ (+30Da) | ||||||||
| * 42 | 20.6 | 20.6 | 41.4 | 32.7 | 7.85 | 24.0 | 6.89 | - | 154.0 |
| * 43 | 19.7 | 20.5 | 42.1 | 32.4 | 8.32 | 26.6 | 8.69 | - | 158.3 |
| * 44 | 29.2 | 30.0 | 41.1 | 36.7 | 6.02 | 42.8 | 9.84 | - | 195.7 |
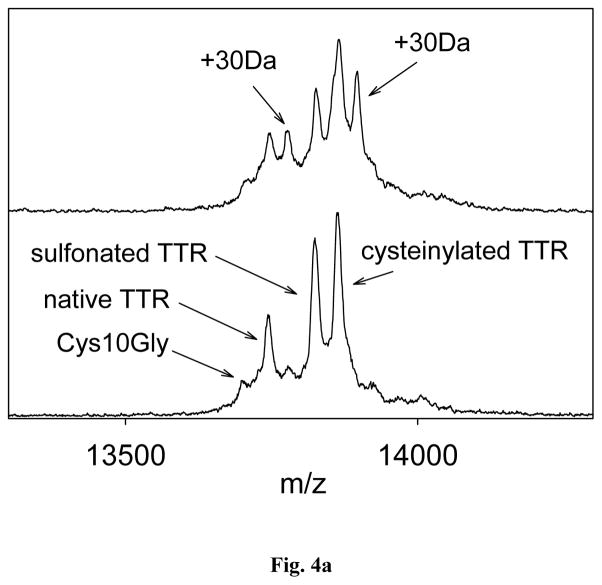
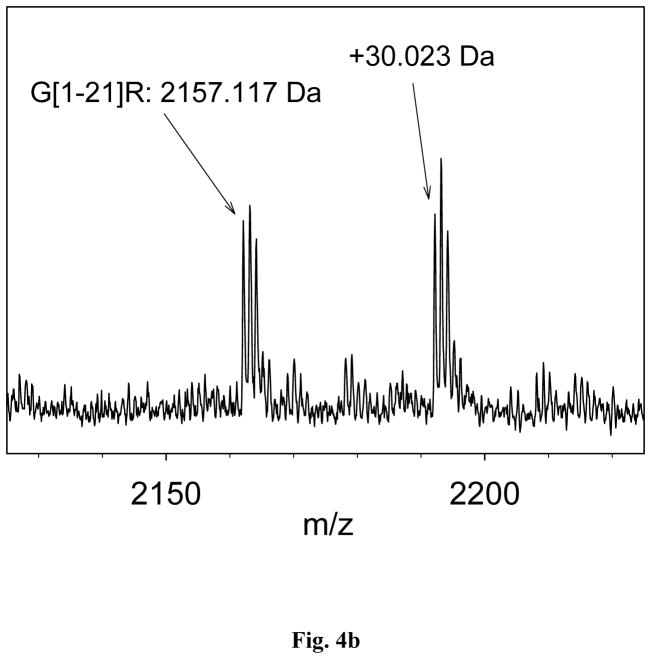
More than 80 point mutations have been reported for TTR [7]. Using the optimized mass spectrometry immunoassay method, a single point mutation was detected in 3 of the 44 plasma samples. The presence of the mutation was indicated by a peak shift of approximately +30Da noted for the native and cysteinylated TTR signals (Fig. 4a). There are several point mutations that can result in a +30 Da shifts [22–27]. In order to identify the point mutation, trypsin and endoproteinase Arg-C in situ proteolytic digests were performed on the samples containing the point mutations (identified with an “*” in Table 4), as well as on few normal samples (serving as controls). The trypsin proteolytic cleavage produced peptides that covered 87.4% of the TTR sequence, from residues 16 to 127. A mass shift of +30 Da was not observed in any of these proteolytic fragments, suggesting that the point mutation was located in residues 1–16. To further pinpoint the location, additional site-specific proteolytic digestion was performed using endoproteinase Arg-C. This enzyme cleaves peptide bonds at the C-terminal side of Arg residues. In the TTR sequence, there are three arginines; hence, endoproteinase Arg-C would produce three peptides that correspond to residues G[1-21]R, G[22-34]R and R[104-127]E. The first peptide covers the sequence that contains the mutation. After performing MALDI-TOF analysis (in reflectron mode), signals at m/z 2157.117 and, m/z 2187.140 (+30.023 Da) were noted in the endoproteinase Arg-C digest spectra (Fig. 4b.). Therefore, the mutations in all three samples were identified as Gly6Ser (+ 29,817 Da). This mutation is the most abundant non-pathogenic TTR mutation in the general population, with a frequency of 6–7% [28], which is in line with the results in the current study (7%). Other mutations were not detected, most likely as a result of the small cohort used (44 samples).
Fig. 4.
(a) Transthyretin mass spectra obtained from a sample with Gly6Ser mutation; (b) Mutation identification with endoproteinase Arg-C digestion.
4. Discussion
The most important clinical application of the TTR measurements is in detection of point mutations and presence (or absence) of variants that correspond to the onset of different types of amyloidosis and amyloidiotic polyneuropathies [10]. Early characterization of these variants was performed with the use of specific antibodies in various immunoassay formats [29, 30]. Other methods, such as IEF and DNA sequencing have also been used to detect TTR mutations [31, 32]. However, the additional detection with mass spectrometry introduces the technical advantage and ability to simultaneously quantify all these forms. Ever since mass spectrometry had been introduced for TTR analyses, several methods have been developed for detection of transthyretin variants. Some of them combine chromatography (usually LC) with mass spectrometry [33–36], while others utilize immuno-capture in combination with MALDI-TOF-MS [37–39]. Although these techniques provide excellent results in identifying TTR variants and mutations, most of them are time consuming, require great expertise in handling the samples, and none has the specificity to fully quantify these forms.
The method presented here is a step forward in quantifying individual TTR variants. Using the optimized mass spectrometry immunoassay, we were able to characterize and to fully quantify the different TTR forms present in human serum and plasma. The data presented result from a simple two-step approach in analyzing TTR. The immunoaffinity capture provides the specificity of conventional ELISA, utilizing a polyclonal antibody that recognizes most of the TTR variants. The subsequent mass spectrometry detection has the ability to differentiate between the different variants; especially those resulting from point mutations in the TTR sequence that usually result in changes in the molecular weight of TTR. What is more, the complete analysis is convenient, fast, and high-throughput (96 parallel assays were executed in less than 3 hours).
Identifying and quantifying TTR variants and mutations is of great importance due to their clinical significance. The fact that there are a great number of point mutations associated with formation of amyloid deposits, makes the TTR mass spectrometric immunoassay an attractive method for rapid screening and detection of their presence. Using the method presented here, we were able to identify 3 samples with point mutations among the 44 normal plasma samples. The Gly6Ser mutation results in a mass shift of ~30Da, which is easily observed in the linear MALDI TOF mass spectra. However, there are a small number of mutations (~10 out the ~100 known thus far [7]) that result in mass shifts of less than 10 Daltons – changes that are not easily detectable in linear MALDI MS. Hence, the initial linear MS is followed by enzymatic digestion and detection of peptide fragments via reflectron MS, which are then used to pinpoint the location of the mutation. Typically, protein digestion in situ is a process that is time consuming (over 12 hours of incubation, usually overnight). However, the on-the-spot digestion described in this work was executed in minutes, facilitated by the localization of the protein sample on the flat MALDI target surface, the high-enzyme concentration, and the denaturation effects of the MALDI matrix (which nonetheless does not interfere with the enzymatic activity). All of these factors contribute to fast identification of the point mutations.
The concept of mass spectrometry immunoassay, introduces a new, high-throughput, and simplified approach to analyzing TTR variants and mutations. The same method can be applied to other proteins as well, using antibodies specific toward those targets. The results from the application of these methods to protein analysis will provide valuable information about the intrinsic characteristics of the protein variants and their association in health and disease.
Acknowledgments
The project described was supported by Grant Number 1 R43 RR025701 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
The authors are employed by the affiliated institution and were paid to perform the research presented.
Abbreviations
- BL
beta-lactoglobulin
- IRS
internal reference standard
- MALDI-TOF
matrix assisted laser desorption/ionization time-of-flight
- TTR
transthyretin
References
- 1.Schreiber G, Richardson SJ. The evolution of gene expression, structure and function of transthyretin. Comp Biochem Physiol B Biochem Mol Biol. 1997;116:137–160. doi: 10.1016/s0305-0491(96)00212-x. [DOI] [PubMed] [Google Scholar]
- 2.Buxbaum JN, Reixach N. Transthyretin: the servant of many masters. Cell Mol Life Sci. 2009;66:3095–3101. doi: 10.1007/s00018-009-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming CE, Nunes AF, Sousa MM. Transthyretin: more than meets the eye. Prog Neurobiol. 2009;89:266–276. doi: 10.1016/j.pneurobio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Hou X, Aguilar MI, Small DH. Transthyretin and familial amyloidotic polyneuropathy. Recent progress in understanding the molecular mechanism of neurodegeneration. FEBS J. 2007;274:1637–1650. doi: 10.1111/j.1742-4658.2007.05712.x. [DOI] [PubMed] [Google Scholar]
- 5.Joao Saraiva M, Mendes Sousa M, Cardoso I, Fernandes R. Familial amyloidotic polyneuropathy: protein aggregation in the peripheral nervous system. J Mol Neurosci. 2004;23:35–40. doi: 10.1385/jmn:23:1-2:035. [DOI] [PubMed] [Google Scholar]
- 6.Saraiva MJ. Transthyretin amyloidosis: a tale of weak interactions. FEBS Lett. 2001;498:201–203. doi: 10.1016/s0014-5793(01)02480-2. [DOI] [PubMed] [Google Scholar]
- 7.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 8.Damas AM, Saraiva MJ. Review: TTR amyloidosis-structural features leading to protein aggregation and their implications on therapeutic strategies. J Struct Biol. 2000;130:290–299. doi: 10.1006/jsbi.2000.4273. [DOI] [PubMed] [Google Scholar]
- 9.Quintas A, Saraiva MJ, Brito RM. The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. FEBS Lett. 1997;418:297–300. doi: 10.1016/s0014-5793(97)01398-7. [DOI] [PubMed] [Google Scholar]
- 10.Saraiva MJ. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat. 2001;17:493–503. doi: 10.1002/humu.1132. [DOI] [PubMed] [Google Scholar]
- 11.Nedelkov D. Mass spectrometry-based immunoassays for the next phase of clinical applications. Expert Rev Proteomics. 2006;3:631–640. doi: 10.1586/14789450.3.6.631. [DOI] [PubMed] [Google Scholar]
- 12.Nedelkov D, Tubbs KA, Niederkofler EE, Kiernan UA, Nelson RW. High-Throughput Comprehensive Analysis of Human Plasma Proteins: A Step toward Population Proteomics. Anal Chem. 2004;76:1733–1737. doi: 10.1021/ac035105+. [DOI] [PubMed] [Google Scholar]
- 13.Nedelkov D. Population proteomics: investigation of protein diversity in human populations. Proteomics. 2008;8:779–786. doi: 10.1002/pmic.200700501. [DOI] [PubMed] [Google Scholar]
- 14.Agger SA, Marney LC, Hoofnagle AN. Simultaneous quantification of apolipoprotein A-I and apolipoprotein B by liquid-chromatography-multiple-reaction-monitoring mass spectrometry. Clin Chem. 56:1804–1813. doi: 10.1373/clinchem.2010.152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frajola WJ, Maurukas J. A stable liquid human reference serum. Health Lab Sci. 1976;13:25–33. [PubMed] [Google Scholar]
- 16.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88:511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 17.Kishikawa M, Sass JO, Sakura N, Nakanishi T, et al. The peak height ratio of S-sulfonated transthyretin and other oxidized isoforms as a marker for molybdenum cofactor deficiency, measured by electrospray ionization mass spectrometry. Biochim Biophys Acta. 2002;1588:135–138. doi: 10.1016/s0925-4439(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 18.Gales L, Saraiva MJ, Damas AM. Structural basis for the protective role of sulfite against transthyretin amyloid formation. Biochim Biophys Acta. 2007;1774:59–64. doi: 10.1016/j.bbapap.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Kingsbury JS, Laue TM, Klimtchuk ES, Theberge R, et al. The modulation of transthyretin tetramer stability by cysteine 10 adducts and the drug diflunisal. Direct analysis by fluorescence-detected analytical ultracentrifugation. J Biol Chem. 2008;283:11887–11896. doi: 10.1074/jbc.M709638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaoka Y, Ohta M, Miyakawa K, Nakamura O, et al. Cysteine 10 is a key residue in amyloidogenesis of human transthyretin Val30Met. Am J Pathol. 2004;164:337–345. doi: 10.1016/s0002-9440(10)63123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passing H. Bablok, A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 22.Fitch NJ, Akbari MT, Ramsden DB. An inherited non-amyloidogenic transthyretin variant, [Ser6]-TTR, with increased thyroxine-binding affinity, characterized by DNA sequencing. J Endocrinol. 1991;129:309–313. doi: 10.1677/joe.0.1290309. [DOI] [PubMed] [Google Scholar]
- 23.Harrison HH, Gordon ED, Nichols WC, Benson MD. Biochemical and clinical characterization of prealbuminCHICAGO: an apparently benign variant of serum prealbumin (transthyretin) discovered with high-resolution two-dimensional electrophoresis. Am J Med Genet. 1991;39:442–452. doi: 10.1002/ajmg.1320390415. [DOI] [PubMed] [Google Scholar]
- 24.Kishikawa M, Nakanishi T, Miyazaki A, Hatanaka M, et al. A new nonamyloid transthyretin variant, G101S, detected by electrospray ionization/mass spectrometry. Mutations in brief no. 201. Online. Hum Mutat. 1998;12:363. [PubMed] [Google Scholar]
- 25.Moses AC, Rosen HN, Moller DE, Tsuzaki S, et al. A point mutation in transthyretin increases affinity for thyroxine and produces euthyroid hyperthyroxinemia. J Clin Invest. 1990;86:2025–2033. doi: 10.1172/JCI114938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraiva MJ, do Almeida MR, Sherman W, Gawinowicz M, et al. A new transthyretin mutation associated with amyloid cardiomyopathy. Am J Hum Genet. 1992;50:1027–1030. [PMC free article] [PubMed] [Google Scholar]
- 27.Sekijima Y, Hammarstrom P, Matsumura M, Shimizu Y, et al. Energetic characteristics of the new transthyretin variant A25T may explain its atypical central nervous system pathology. Lab Invest. 2003;83:409–417. doi: 10.1097/01.lab.0000059937.11023.1f. [DOI] [PubMed] [Google Scholar]
- 28.Nedelkov D, Phillips DA, Tubbs KA, Nelson RW. Investigation of human protein variants and their frequency in the general population. Mol Cell Proteomics. 2007;6:1183–1187. doi: 10.1074/mcp.M700023-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Nakazato M, Kurihara T, Matsukura S, Kangawa K, Matsuo H. Diagnostic radioimmunoassay for familial amyloidotic polyneuropathy before clinical onset. J Clin Invest. 1986;77:1699–1703. doi: 10.1172/JCI112489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Hirai S, Matsubara E, Okamoto K, et al. Familial amyloidotic polyneuropathy without familial occurrence: carrier detection by the radioimmunoassay of variant transthyretin. J Neurol Neurosurg Psychiatry. 1988;51:576–578. doi: 10.1136/jnnp.51.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altland K, Benson MD, Costello CE, Ferlini A, et al. Genetic microheterogeneity of human transthyretin detected by IEF. Electrophoresis. 2007;28:2053–2064. doi: 10.1002/elps.200600840. [DOI] [PubMed] [Google Scholar]
- 32.Benson MD, Yazaki M, Magy N. Laboratory assessment of transthyretin amyloidosis. Clin Chem Lab Med. 2002;40:1262–1265. doi: 10.1515/CCLM.2002.218. [DOI] [PubMed] [Google Scholar]
- 33.Bergen HR, 3rd, Zeldenrust SR, Butz ML, Snow DS, et al. Identification of transthyretin variants by sequential proteomic and genomic analysis. Clin Chem. 2004;50:1544–1552. doi: 10.1373/clinchem.2004.033266. [DOI] [PubMed] [Google Scholar]
- 34.Lim A, Prokaeva T, McComb ME, O’Connor PB, et al. Characterization of transthyretin variants in familial transthyretin amyloidosis by mass spectrometric peptide mapping and DNA sequence analysis. Anal Chem. 2002;74:741–751. doi: 10.1021/ac010780+. [DOI] [PubMed] [Google Scholar]
- 35.Nepomuceno AI, Mason CJ, Muddiman DC, Bergen HR, 3rd, Zeldenrust SR. Detection of genetic variants of transthyretin by liquid chromatography-dual electrospray ionization fourier-transform ion-cyclotron-resonance mass spectrometry. Clin Chem. 2004;50:1535–1543. doi: 10.1373/clinchem.2004.033274. [DOI] [PubMed] [Google Scholar]
- 36.Theberge R, Connors L, Skinner M, Skare J, Costello CE. Characterization of transthyretin mutants from serum using immunoprecipitation, HPLC/electrospray ionization and matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1999;71:452–459. doi: 10.1021/ac980531u. [DOI] [PubMed] [Google Scholar]
- 37.Schweigert FJ, Wirth K, Raila J. Characterization of the microheterogeneity of transthyretin in plasma and urine using SELDI-TOF-MS immunoassay. Proteome Sci. 2004;2:5. doi: 10.1186/1477-5956-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theberge R, Connors LH, Skinner M, Costello CE. Detection of transthyretin variants using immunoprecipitation and matrix-assisted laser desorption/ionization bioreactive probes: a clinical application of mass spectrometry. J Am Soc Mass Spectrom. 2000;11:172–175. doi: 10.1016/S1044-0305(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 39.Ueda M, Misumi Y, Mizuguchi M, Nakamura M, et al. SELDI-TOF mass spectrometry evaluation of variant transthyretins for diagnosis and pathogenesis of familial amyloidotic polyneuropathy. Clin Chem. 2009;55:1223–1227. doi: 10.1373/clinchem.2008.118505. [DOI] [PubMed] [Google Scholar]