Abstract
(Pinacolato)boryl ortho-silyl(hetero)aryl triflates are presented as a new class of building blocks for arylation. They demonstrate unique versatility by delivering boronate or (hetero)aryne reactivity chemoselectively in a broad range of transformations. This approach enables the unprecedented postfunctionalization of fluoride-activated (hetero)aryne precursors, for example, as substrates in transition-metal catalysis, and offers valuable new possibilities for aryl boronate postfunctionalization without the use of specialized protecting groups.
Keywords: arynes, boron, C–H activation, chemoselectivity, synthetic methods
The development of versatile (hetero)arylation strategies is a key pursuit in organic synthetic methodology.[1] Dramatic advances on this front are exemplified by the use of aryl boronate reagents[2] and aryne intermediates.[3] Both participate in a seemingly inordinate range of reactions, including C–C, C–N, carbon–chalcogen and carbon–halogen bond formation. Aryl boronates have also attracted attention as organocatalysts,[4] as well as for medicinal[5] and materials[6] applications. Meanwhile, (hetero)arynes enable simultaneous, regioselective functionalization at two adjacent carbon atoms[7] and may be generated under mild conditions from ortho-silyl (hetero)aryl triflates using fluoride.[8] Such advantages have fueled their increasing popularity in the synthesis of natural products,[9] functional materials,[10] and organometallic compounds.[11]
The versatility of aryl boronates and ortho-silyl aryl triflates renders the prospect of their selective postfunctionalization exceptionally attractive. It promises greatly to broaden the range of possible derivatives, obviate tedious/inefficient de novo syntheses, and enable iterative approaches to their elaboration.[12] However, the same mild and versatile reactivity that imparts aryl boronates and ortho-silyl aryl triflates with such broad appeal makes their postfunctionalization commensurately more challenging.
For aryl boronates, responses to this problem have largely relied on the use of protecting groups to mitigate the reactivity of the boron center with respect to that of (pseudo)halide,[13] stannane,[14] or orthogonally protected boronate[15] groups present on the same arene. However, this approach has been exploited almost exclusively in C–C bond coupling; methods to introduce other valuable functionality remain scarce.[16]
Remarkably, the postfunctionalization of ortho-silyl aryl triflates has never been reported, despite their growing popularity and the passing of three decades since the seminal report by Kobayashi and co-workers on their use as aryne precursors.[8] Variants requiring even ostensibly simple substitution patterns often need inconvenient, multistep preparation.
We envisaged that the unique reactivities of aryl boronates and arynes could be harnessed for their mutual, chemoselective postfunctionalization (Figure 1). This approach would address both problems from common intermediates and offer versatile building blocks for arylation. Elegant recent studies by Akai and co-workers on the unusual influence of 3-boryl substituents over the regioselectivity of benzyne capture stands as the only previous juxtaposition of aryne and boronate reactivities.[17] However, the requirement that the boronate be placed at the benzyne C3 position in these studies necessitated multistep preparation and revealed an incompatibility with fluoride in the absence of extremely robust protecting groups.[17c] It also seemingly precluded use of the boronate prior to capture of the aryne and therefore the prospect of postfunctionalizing the aryne precursor. We reasoned that the remarkable functional-group tolerance of iridium-catalyzed C–H borylation[18] and its preference for the least sterically hindered position of simple arenes[19] would provide a direct way to introduce a B(pin) substituent selectively into precursors 1, thereby preserving various R groups at C3, where they are known to influence aryne reactivity most profoundly.[20]
Figure 1.
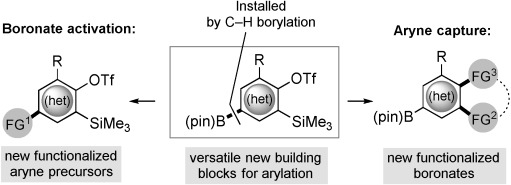
Underlying concept of this study. B(pin)=(pinacolato)boryl, Tf=trifluoromethanesulfonyl.
At the outset of our study, we found that the treatment of benzyne precursor 1 a (R=H) with [{Ir(μ-OMe)cod}2], 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy), and B2(pin)2 under the conditions shown in Scheme 1 gave 2 a quantitatively as a 3:1 mixture of regioisomers. The scope of the reaction was extended to the synthesis of 2 b–g with complete regioselectivity and generally excellent yields on scales up to 2 g. The reaction of 4,5-indolyne precursor 1 h[21] under the same conditions gave inseparable mixtures of C2- and C7-borylated products; the use of more B2(pin)2 led exclusively to diboronate 2 h.[22]
Scheme 1.
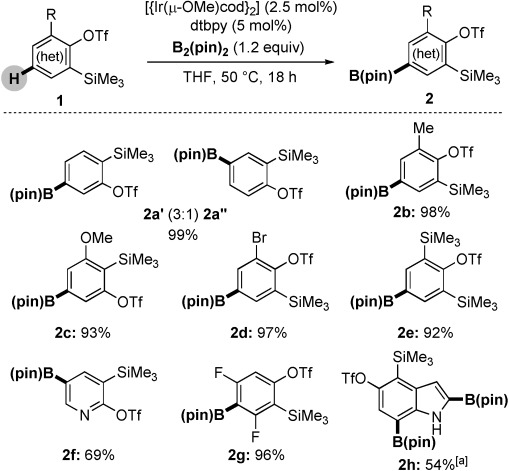
Iridium-catalyzed C–H borylation of aryne precursors 1. [a] The reaction was carried out using 2 equivalents of B2(pin)2 in a reaction time of 42 h. cod=1,5-cyclooctadiene.
We next explored chemoselective aryne generation from 2. We found that CsF (4 equiv) and 18-crown-6 (1 equiv) in MeCN enabled a broad range of functionalization reactions (Scheme 2), including attack by N- (products 4 a–c), O- (products 4 e–g), and S-based nucleophiles (product 4 i), as well as [4+2] cycloaddition (product 4 j), cyclization with an aryl azide[23] (product 4 k) or nitrone (product 4 l,m), and insertion into a cyclic urea[24] (product 4 n). An exception was substrate 2 g, which invariably succumbed to rapid deborylation (to give 4 d),[25] as is common for polyfluoroaryl boronates.[26] Substrate 3 a (R=H) underwent preferential attack by heteroatoms at the carbon atom meta to the B(pin) group (products 4 a,l). Benzynes are usually biased by electronegative substituents to favor attack by nucleophiles at the distal carbon atom of the triple bond;[27] we attribute the opposite selectivity in the formation of 4 a and 4 l to the electropositive character of boron.[17a]–[d] Otherwise, the regioselectivity of aryne capture was governed by the R group. For example, the OMe group of 2 c directed nucleophilic addition exclusively to C1 of the corresponding aryne 3 c (products 4 b,f,i), whereas 2 e gave mixtures of regioisomers (products 4 c and 4 g) arising from the competing steric and electronic influences of the SiMe3 substituent.[28] The activation of 2 d in the presence of pyridine N-oxide gave exclusively the 3-substituted pyridine 4 h through a rearrangement.[29]
Scheme 2.
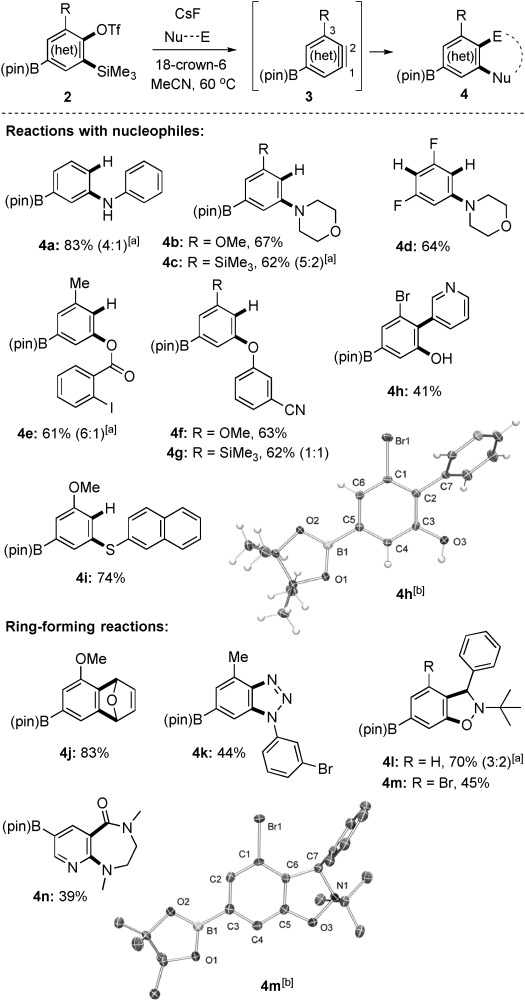
Elaboration of boronates 2 through (hetero)aryne trapping. [a] A mixture of regioisomers was obtained; the major regioisomer is shown. [b] Crystal structure. Ellipsoids are drawn at 50 % probability.
In most cases, the trapping reagent for boryl (hetero)arynes 3 was chosen to highlight the difficulty in accessing the anticipated products by established borylation methods in the final step. Thus, the halogen substituents in 4 e, 4 h, 4 k, and 4 m would make problematic their synthesis by borylation relying on lithiation or Pd catalysis.[15c, 30] Similarly, the presence of several sterically accessible Ar–H bonds in 4 e–i and 4 k–m precludes their selective preparation by iridium-catalyzed C–H borylation.[19, 31] The presented method is therefore a valuable and systematic new approach to aryl boronate postfunctionalization with a wide variety of functional groups. Furthermore, no examples have been reported previously in which the convenience of fluoride-induced aryne generation was possible without concomitant loss of the boronate or the use of specialized protecting groups.
We next addressed the chemoselective manipulation of the boronate group of 2 to effect further ortho-silyl (hetero)aryl triflate postfunctionalization. Suzuki–Miyaura coupling (SMC) proceeded very well with various (hetero)aryl bromides without compromising the ortho-silyl triflate moiety (Scheme 3). We anticipate that this method will provide a flexible route to novel substituted polyaromatic systems, for which arynes are increasingly exploited as building blocks.[10] Competitive activation of the bromide and/or ortho-silyl triflate unit of 2 d was circumvented by a complementary strategy with diaryl iodonium reagents (see below).
Scheme 3.
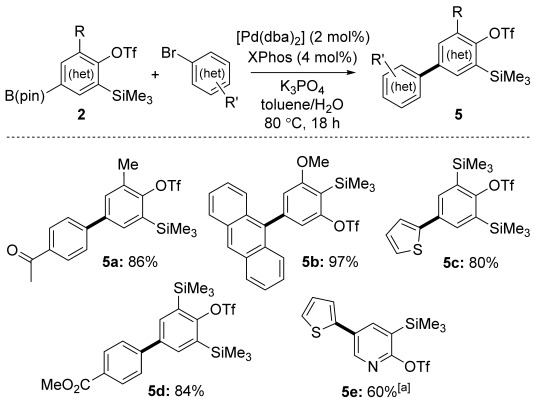
Arylation of precursors 2 by SMC. [a] Reaction conditions in this case: [Pd(dba)2] (4 mol %), XPhos (8 mol %), K2CO3 (3 equiv), toluene/H2O, 80 °C, 20 h. dba=dibenzylideneacetone, XPhos=2-dicyclohexylphosphanyl-2′,4′,6′-triisopropylbiphenyl.
To extend the utility of the boryl group for aryne-precursor postfunctionalization, compounds 2 were deprotected by treatment with diethanolamine (DEA) and acid hydrolysis.[32] This mild, expedient method furnished boronic acids 6 (Scheme 4), which proved indefinitely stable at room temperature in air.[25]
Scheme 4.
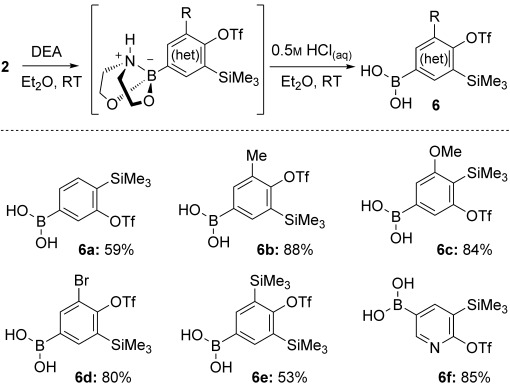
Deprotection of 2 to give the parent boronic acids. In each case the corresponding boroxine was formed as a minor by-product; yields are given with respect to boron.
Boronic acids 6 participated in a broad selection of high-yielding transformations with complete preservation of the ortho-silyl triflate (Scheme 5). The conversion of 6 c through palladium-catalyzed allylation into 7 a or trifluoroborate[33] 7 b is notable for its use of KF (up to 4 equiv), which is also commonly exploited to activate ortho-silyl aryl triflates. It demonstrates the following practicable order of functional-group reactivity with respect to fluoride: B(OH)2>ortho-silyl triflate>B(pin), and that this reactivity order can serve selective arene functionalization. Rhodium-catalyzed 1,4-conjugate addition[34] to chalcone afforded 7 c,d in excellent yields. The palladium-catalyzed oxidative coupling of 6 b and 6 d with diaryl iodonium reagents[35] also proved efficient. Thus, bromide substituents on either coupling partner could be preserved to give 7 e,f, obviating problems with their activation in SMC (Scheme 3) and leaving scope for further derivatization. Acid 6 f underwent copper-catalyzed iodination with N-iodosuccinimide to give 7 g in 93 % yield. This transformation is notable given the paucity of general methods reported for the iodination of electron-poor heteroaryl boronic acids. Copper-catalyzed Chan–Lam amination[36] (products 7 h,i) further highlights the unique versatility of the boryl (hetero)aryne building blocks: the same nucleophile may be introduced by using either the electrophilic (aryne) or nucleophilic (boronate) functionality under mild conditions with meta selectivity with respect to R (compare 4 b,c and 7 h,i).
Scheme 5.
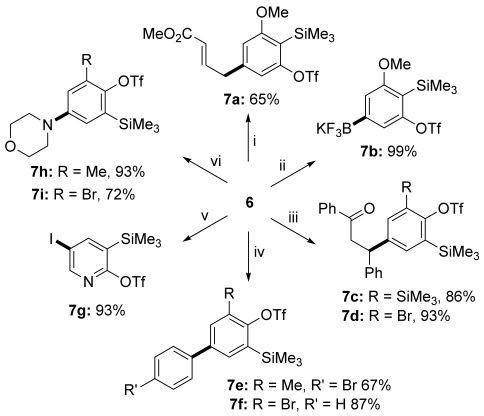
Transformations of aryl boronic acids 6: i) palladium-catalyzed allylation; ii) boronate conversion; iii) rhodium-catalyzed 1,4-conjugate addition; iv) palladium-catalyzed arylation with [Ar2I]X; v) copper-catalyzed iodination; vi) Chan–Lam amination.
To the best of our knowledge, the transformations in Schemes 1, 3, and 5 (reactions i, iii–vi) are the first to use ortho-silyl (hetero)aryl triflates as substrates in catalysis not involving activation of the aryne or mediation of its reactivity by a transition metal.[11, 37] This points to broad new possibilities for incorporating aryne precursors into more complex chemical environments.
In summary, we have developed a class of arylation reagents able to deliver orthogonally boronate or aryne reactivity for a wide variety of transformations. They enable the postfunctionalization of ortho-silyl (hetero)aryl triflate aryne precursors and significantly expand the options available for decorating aryl boronates selectively. The generality inherent in this approach offers a flexible new route to diversely functionalized (hetero)aromatic compounds.
Acknowledgments
We thank the Swedish Research Council (Vetenskapsrådet) and Carl Tryggers Stiftelse for funding. We are grateful to Prof. Adolf Gogoll for help with NMR spectroscopy and Prof. Lars Engman, Dr. Eszter Borbas, Dr. Johanna Larsson, and Carina Sollert for manuscript proof-reading.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Ackermann L. Modern Arylation Methods. Weinheim: Wiley-VCH; 2009. [Google Scholar]
- 2.Hall DG. Boronic Acids. Weinheim: Wiley-VCH; 2011. [Google Scholar]
- 3a.Wenk HH, Winkler M, Sander W. Angew. Chem. Int. Ed. 2003;42:502–528. doi: 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2003;115:518–546. [Google Scholar]
- 3b.Bhunia A, Yetra SR, Biju AT. Chem. Soc. Rev. 2012;41:3140–3152. doi: 10.1039/c2cs15310f. [DOI] [PubMed] [Google Scholar]
- 4.Georgiou I, Ilyashenko G, Whiting A. Acc. Chem. Res. 2009;42:756–768. doi: 10.1021/ar800262v. [DOI] [PubMed] [Google Scholar]
- 5.Trippier PC, McGuigan C. MedChemComm. 2010;1:183–198. [Google Scholar]
- 6.Severin K. Dalton Trans. 2009:5254–5264. doi: 10.1039/b902849h. [DOI] [PubMed] [Google Scholar]
- 7a.Peña D, Pérez D, Guitián E. Angew. Chem. Int. Ed. 2006;45:3579–3581. doi: 10.1002/anie.200600291. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2006;118:3659–3661. [Google Scholar]
- 7b.Sapountzis I, Lin W, Fischer M, Knochel P. Angew. Chem. Int. Ed. 2004;43:4364–4366. doi: 10.1002/anie.200460417. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2004;116:4464–4466. [Google Scholar]
- 7c.García-López J-A, Çetin M, Greaney MF. Angew. Chem. Int. Ed. 2015;54:2156–2159. doi: 10.1002/anie.201410751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2015;127:2184–2187. [Google Scholar]
- 8.Himeshima Y, Sonoda T, Kobayashi H. Chem. Lett. 1983:1211–1214. [Google Scholar]
- 9a.Gampe CM, Carreira EM. Angew. Chem. Int. Ed. 2012;51:3766–3778. doi: 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:3829–3842. [Google Scholar]
- 9b.Tadross PM, Stoltz BM. Chem. Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]
- 10a.Pérez D, Peña D, Guitián E. Eur. J. Org. Chem. 2013:5981–6013. [Google Scholar]
- 10b.Alonso JM, Diaz-Alvarez AE, Criado A, Perez D, Pena D, Guitian E. Angew. Chem. Int. Ed. 2012;51:173–177. doi: 10.1002/anie.201104935. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:177–181. [Google Scholar]
- 10c.Schuler B, Collazos S, Gross L, Meyer G, Pérez D, Guitián E, Peña D. Angew. Chem. Int. Ed. 2014;53:9004–9006. doi: 10.1002/anie.201403707. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2014;126:9150–9152. [Google Scholar]
- 11a.Oliva-Madrid M-J, Saura-Llamas I, Bautista D, Vicente J. Chem. Commun. 2013;49:7997–7999. doi: 10.1039/c3cc43049a. [DOI] [PubMed] [Google Scholar]
- 11b.Oliva-Madrid M-J, García-López J-A, Saura-Llamas I, Bautista D, Vicente J. Organometallics. 2014;33:6420–6430. doi: 10.1021/acs.organomet.0c00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c.Dyke AM, Hester AJ, Lloyd-Jones GC. Synthesis. 2006:4093–4112. [Google Scholar]
- 12.Wang C, Glorius F. Angew. Chem. Int. Ed. 2009;48:5240–5244. doi: 10.1002/anie.200901680. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2009;121:5342–5346. [Google Scholar]
- 13a.Noguchi H, Hojo K, Suginome M. J. Am. Chem. Soc. 2007;129:758–759. doi: 10.1021/ja067975p. [DOI] [PubMed] [Google Scholar]
- 13b.Gillis EP, Burke MD. J. Am. Chem. Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]
- 13c.Ito M, Itani I, Toyoda Y, Morimoto K, Dohi T, Kita Y. Angew. Chem. Int. Ed. 2012;51:12555–12558. doi: 10.1002/anie.201206917. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:12723–12726. [Google Scholar]
- 14a.Heinrich ACJ, Thiedemann B, Gates PJ, Staubitz A. Org. Lett. 2013;15:4666–4669. doi: 10.1021/ol401923j. [DOI] [PubMed] [Google Scholar]
- 14b.Linshoeft J, Heinrich ACJ, Segler SAW, Gates PJ, Staubitz A. Org. Lett. 2012;14:5644–5647. doi: 10.1021/ol302571t. [DOI] [PubMed] [Google Scholar]
- 15a.Noguchi H, Shioda T, Chou C-M, Suginome M. Org. Lett. 2008;10:377–380. doi: 10.1021/ol702420x. [DOI] [PubMed] [Google Scholar]
- 15b.Xu L, Ding S, Li P. Angew. Chem. Int. Ed. 2014;53:1822–1826. doi: 10.1002/anie.201309546. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2014;126:1853–1857. [Google Scholar]
- 15c.Xu L, Li P. Chem. Commun. 2015;51:5656–5659. doi: 10.1039/c5cc00231a. [DOI] [PubMed] [Google Scholar]
- 15d.Fyfe JWB, Seath CP, Watson AJB. Angew. Chem. Int. Ed. 2014;53:12077–12080. doi: 10.1002/anie.201406714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. 2014;126:12273–12276. [Google Scholar]
- 16a.Ihara H, Suginome M. J. Am. Chem. Soc. 2009;131:7502–7503. doi: 10.1021/ja902314v. [DOI] [PubMed] [Google Scholar]
- 16b.Ihara H, Koyanagi M, Suginome M. Org. Lett. 2011;13:2662–2665. doi: 10.1021/ol200764g. [DOI] [PubMed] [Google Scholar]
- 16c.Al-Zoubi RM, Hall DG. Org. Lett. 2010;12:2480–2483. doi: 10.1021/ol100537x. [DOI] [PubMed] [Google Scholar]
- 16d.Qiu D, Mo F, Zheng Z, Zhang Y, Wang J. Org. Lett. 2010;12:5474–5477. doi: 10.1021/ol102350v. [DOI] [PubMed] [Google Scholar]
- 16e.Lee C-Y, Ahn S-J, Cheon C-H. J. Org. Chem. 2013;78:12154–12160. doi: 10.1021/jo402174v. [DOI] [PubMed] [Google Scholar]
- 17a.Ikawa T, Takagi A, Kurita Y, Saito K, Azechi K, Egi M, Kakiguchi K, Kita Y, Akai S. Angew. Chem. Int. Ed. 2010;49:5563–5566. doi: 10.1002/anie.201002191. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2010;122:5695–5698. [Google Scholar]
- 17b.Takagi A, Ikawa T, Kurita Y, Saito K, Azechi K, Egi M, Itoh Y, Tokiwa H, Kita Y, Akai S. Tetrahedron. 2013;69:4338–4352. [Google Scholar]
- 17c.Takagi A, Ikawa T, Saito K, Masuda S, Ito T, Akai S. Org. Biomol. Chem. 2013;11:8145–8150. doi: 10.1039/c3ob41787e. [DOI] [PubMed] [Google Scholar]
- 17d.Ikawa T, Takagi A, Goto M, Aoyama Y, Ishikawa Y, Itoh Y, Fujii S, Tokiwa H, Akai S. J. Org. Chem. 2013;78:2965–2983. doi: 10.1021/jo302802b. [DOI] [PubMed] [Google Scholar]
- 17e.García-López J-A, Greaney MF. Org. Lett. 2014;16:2338–2341. doi: 10.1021/ol5006246. for a recent report on the generation of arynes from 2-(pseudo)halo phenylboronic esters, see. [DOI] [PubMed] [Google Scholar]
- 18a.Preshlock SM, Ghaffari B, Maligres PE, Krska SW, Maleczka RE, Smith MR. J. Am. Chem. Soc. 2013;135:7572–7582. doi: 10.1021/ja400295v. [DOI] [PubMed] [Google Scholar]
- 18b.Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- 19a.Hartwig JF. Chem. Soc. Rev. 2011;40:1992–2002. doi: 10.1039/c0cs00156b. [DOI] [PubMed] [Google Scholar]
- 19b.Larsen MA, Hartwig JF. J. Am. Chem. Soc. 2014;136:4287–4299. doi: 10.1021/ja412563e. [DOI] [PubMed] [Google Scholar]
- 19c.Hurst TE, Macklin TK, Becker M, Hartmann E, Kügel W, Parisienne-La Salle J-C, Batsanov AS, Marder TB, Snieckus V. Chem. Eur. J. 2010;16:8155–8161. doi: 10.1002/chem.201000401. [DOI] [PubMed] [Google Scholar]
- 20.Medina JM, Mackey JL, Garg NK, Houk KN. J. Am. Chem. Soc. 2014;136:15798–15805. doi: 10.1021/ja5099935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010. doi: 10.1021/ol802958a. [DOI] [PubMed] [Google Scholar]
- 21b.Cheong PHY, Paton RS, Bronner SM, Im GYJ, Garg NK, Houk KN. J. Am. Chem. Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21c.Im GYJ, Bronner SM, Goetz AE, Paton RS, Cheong PHY, Houk KN, Garg NK. J. Am. Chem. Soc. 2010;132:17933–17944. doi: 10.1021/ja1086485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21d.Bronner SM, Goetz AE, Garg NK. Synlett. 2011:2599–2604. [Google Scholar]
- 22. Owing to the instability of 2 h to chromatography and problems with selective activation of its boronate groups, we omitted this compound from further study.
- 23a.Shi F, Waldo JP, Chen Y, Larock RC. Org. Lett. 2008;10:2409–2412. doi: 10.1021/ol800675u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23b.Ikawa T, Kaneko H, Masuda S, Ishitsubo E, Tokiwa H, Akai S. Org. Biomol. Chem. 2015;13:520–526. doi: 10.1039/c4ob01627k. [DOI] [PubMed] [Google Scholar]
- 24a.Saito N, Nakamura K, Shibano S, Ide S, Minami M, Sato Y. Org. Lett. 2013;15:386–389. doi: 10.1021/ol303352q. [DOI] [PubMed] [Google Scholar]
- 24b.Yoshida H, Shirakawa E, Honda Y, Hiyama T. Angew. Chem. Int. Ed. 2002;41:3247–3249. doi: 10.1002/1521-3773(20020902)41:17<3247::AID-ANIE3247>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2002;114:3381–3383. [Google Scholar]
- 25. 1H and 19F NMR spectroscopy showed no decomposition of 6 after 3 months at room temperature and a further 2 months at −20 °C.
- 26.Lozada J, Liu Z, Perrin DM. J. Org. Chem. 2014;79:5365–5368. doi: 10.1021/jo500734z. [DOI] [PubMed] [Google Scholar]
- 27a.Yoshida H, Fukushima H, Ohshita J, Kunai A. J. Am. Chem. Soc. 2006;128:11040–11041. doi: 10.1021/ja064157o. [DOI] [PubMed] [Google Scholar]
- 27b.Tadross PM, Gilmore CD, Bugga P, Virgil SC, Stoltz BM. Org. Lett. 2010;12:1224–1227. doi: 10.1021/ol1000796. [DOI] [PubMed] [Google Scholar]
- 28a.Ikawa T, Nishiyama T, Shigeta T, Mohri S, Morita S, Takayanagi S, Terauchi Y, Morikawa Y, Takagi A, Ishikawa Y, Fujii S, Kita Y, Akai S. Angew. Chem. Int. Ed. 2011;50:5674–5677. doi: 10.1002/anie.201100360. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123:5792–5795. [Google Scholar]
- 28b.Bronner SM, Mackey JL, Houk KN, Garg NK. J. Am. Chem. Soc. 2012;134:13966–13969. doi: 10.1021/ja306723r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raminelli C, Liu Z, Larock RC. J. Org. Chem. 2006;71:4689–4691. doi: 10.1021/jo060523a. [DOI] [PubMed] [Google Scholar]
- 30.Pilarski LT, Szabó KJ. Angew. Chem. Int. Ed. 2011;50:8230–8232. doi: 10.1002/anie.201102384. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2011;123:8380–8382. [Google Scholar]
- 31a.Vanchura IIBA, Preshlock SM, Roosen PC, Kallepalli VA, Staples RJ, Maleczka JRE, Singleton DA, Smith MR., III Chem. Commun. 2010;46:7724–7726. doi: 10.1039/c0cc02041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31b.Tajuddin H, Harrisson P, Bitterlich B, Collings JC, Sim N, Batsanov AS, Cheung MS, Kawamorita S, Maxwell AC, Shukla L, Morris J, Lin Z, Marder TB, Steel PG. Chem. Sci. 2012;3:3505–3515. [Google Scholar]
- 31c.Sadler SA, Hones AC, Roberts B, Blakemore D, Marder TB, Steel PG. J. Org. Chem. 2015;80:5308–5314. doi: 10.1021/acs.joc.5b00452. [DOI] [PubMed] [Google Scholar]
- 31d.Sadler SA, Tajuddin H, Mkhalid IAI, Batsanov AS, Albesa-Jove D, Cheung MS, Maxwell AC, Shukla L, Roberts B, Blakemore DC, Lin Z, Marder TB, Steel PG. Org. Biomol. Chem. 2014;12:7318–7327. doi: 10.1039/c4ob01565g. [DOI] [PubMed] [Google Scholar]
- 31e.Mkhalid IAI, Coventry DN, Albesa-Jove D, Batsanov AS, Howard JAK, Perutz RN, Marder TB. Angew. Chem. Int. Ed. 2006;45:489–491. doi: 10.1002/anie.200503047. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2006;118:503–505. [Google Scholar]
- 32.Sun J, Perfetti MT, Santos WL. J. Org. Chem. 2011;76:3571–3575. doi: 10.1021/jo200250y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Lennox AJJ, Lloyd-Jones GC. Angew. Chem. Int. Ed. 2012;51:9385–9388. doi: 10.1002/anie.201203930. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2012;124:9519–9522. [Google Scholar]
- 33b.Darses S, Genet J-P. Chem. Rev. 2008;108:288–325. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]
- 34a.Edwards HJ, Hargrave JD, Penrose SD, Frost CG. Chem. Soc. Rev. 2010;39:2093–2105. doi: 10.1039/b919762c. [DOI] [PubMed] [Google Scholar]
- 34b.Tajuddin H, Shukla L, Maxwell AC, Marder TB, Steel PG. Org. Lett. 2010;12:5700–5703. doi: 10.1021/ol102518v. [DOI] [PubMed] [Google Scholar]
- 35.Kang S-K, Lee H-W, Jang S-B, Ho P-S. J. Org. Chem. 1996;61:4720–4724. doi: 10.1021/jo960195m. [DOI] [PubMed] [Google Scholar]
- 36.Quach TD, Batey RA. Org. Lett. 2003;5:4397–4400. doi: 10.1021/ol035681s. [DOI] [PubMed] [Google Scholar]
- 37a.Yoshida H, Tanino K, Ohshita J, Kunai A. Angew. Chem. Int. Ed. 2004;43:5052–5055. doi: 10.1002/anie.200460189. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. 2004;116:5162–5165. [Google Scholar]
- 37b.Bhuvaneswari S, Jeganmohan M, Cheng C-H. Org. Lett. 2006;8:5581–5584. doi: 10.1021/ol0622918. [DOI] [PubMed] [Google Scholar]
- 37c.Peng X, Wang W, Jiang C, Sun D, Xu Z, Tung C-H. Org. Lett. 2014;16:5354–5357. doi: 10.1021/ol5025426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


