Abstract
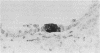
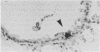
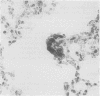
The nature and distribution of bronchopulmonary endocrine cells immunoreactive for calcitonin was studied in normal adult Wistar albino rats by immunoenzyme histochemistry with the peroxidase-antiperoxidase technique. A widespread distribution of both solitary endocrine cells and neuroepithelial bodies immunoreactive for calcitonin was found. Many, but not all, were also immunoreactive for neurone specific enolase. Although both classes of cells were present in airways and parenchyma, most of the solitary cells were found in alveolar ducts and alveoli whereas most of the neuroepithelial bodies were located in bronchi and bronchioles. Bronchopulmonary endocrine cells are generally regarded as being sparse in the adult rat. It is suggested that this may be a consequence of the use of inadequate methods in attempting to identify them. So far as is known, this is the first time that calcitonin has been demonstrated by immunohistochemical methods in the lungs of rats.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker K. L., Monaghan K. G., Silva O. L. Immunocytochemical localization of calcitonin in Kulchitsky cells of human lung. Arch Pathol Lab Med. 1980 Apr;104(4):196–198. [PubMed] [Google Scholar]
- Becker K. L., Nash D. R., Silva O. L., Snider R. H., Moore C. F. Urine calcitonin levels in patients with bronchogenic carcinoma. JAMA. 1980 Feb 15;243(7):670–672. [PubMed] [Google Scholar]
- Becker K. L., Nash D., Silva O. L., Snider R. H., Moore C. F. Increased serum and urinary calcitonin levels in patients with pulmonary disease. Chest. 1981 Feb;79(2):211–216. doi: 10.1378/chest.79.2.211. [DOI] [PubMed] [Google Scholar]
- Becker K. L., Silva O. L. Hypothesis: the bronchial Kulchitsky (K) cell as a source of humoral biologic activity. Med Hypotheses. 1981 Jul;7(7):943–949. doi: 10.1016/0306-9877(81)90049-9. [DOI] [PubMed] [Google Scholar]
- Cook R. D., King A. S. A neurite-receptor complex in the avian lung: electron microscopical observations. Experientia. 1969 Nov 15;25(11):1162–1164. doi: 10.1007/BF01900251. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Track N. S. Bombesin, calcitonin and leu-enkephalin immunoreactivity in endocrine cells of human lung. Experientia. 1981 Jul 15;37(7):765–767. doi: 10.1007/BF01967969. [DOI] [PubMed] [Google Scholar]
- Edmondson N. A., Lewis D. J. Distribution and ultrastructural characteristics of Feyrter cells in the rat and hamster airway epithelium. Thorax. 1980 May;35(5):371–374. doi: 10.1136/thx.35.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEYRTER F. Zur Pathologie des argyrophilen Helle-Zellen-Organes im Bronchialbaum des Menschen. Virchows Arch Pathol Anat Physiol Klin Med. 1954;325(6):723–732. doi: 10.1007/BF00955103. [DOI] [PubMed] [Google Scholar]
- Galan F. G., Hurtado J., Cano R. P., Peralta M. G. High plasma calcitonin concentrations in chronic bronchitis. Br Med J (Clin Res Ed) 1982 Sep 25;285(6345):850–851. doi: 10.1136/bmj.285.6345.850-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V. E. The endocrine lung. Lab Invest. 1983 May;48(5):507–509. [PubMed] [Google Scholar]
- Hage E. Electron microscopic identification of several types of endocrine cells in the bronchial epithelium of human foetuses. Z Zellforsch Mikrosk Anat. 1973 Aug 3;141(3):401–412. doi: 10.1007/BF00307413. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Klein E. F., Jr, Graves S. A. "Hot pot" tracheitis. Chest. 1974 Feb;65(2):225–226. doi: 10.1378/chest.65.2.225. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere J., Theunynck P. Serotonin producing neuroepithelial bodies in rabbit respiratory mucosa. Science. 1973 Apr 27;180(4084):410–413. doi: 10.1126/science.180.4084.410. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere M., Theunynck P. Neuro-epithelial bodies in the respiratory mucosa of various mammals. A light optical, histochemical and ultrastructural investigation. Z Zellforsch Mikrosk Anat. 1972;135(4):569–592. doi: 10.1007/BF00583438. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Liebens M. Microspectrography of formaldehyde and fluorescamine-induced fluorescence in rabbit pulmonary neuroepithelial bodies: demonstration of a new, probably polypeptide intracytoplasmic substance. Experientia. 1977 Nov 15;33(11):1510–1511. doi: 10.1007/BF01918840. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Peuskens J. C. Argyrophil (kinin and amine producing?) cells in human infant airway epithelium. Life Sci. 1969 Jun 1;8(11):577–585. doi: 10.1016/0024-3205(69)90019-8. [DOI] [PubMed] [Google Scholar]
- Moosavi H., Smith P., Heath D. The Feyrter cell in hypoxia. Thorax. 1973 Nov;28(6):729–741. doi: 10.1136/thx.28.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano J. R., Kleinerman J. APUD cells and neuroepithelial bodies in hamster lung: methods, quantitation, and response to injury. Thorax. 1980 May;35(5):363–370. doi: 10.1136/thx.35.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G., Takor T. Embryology of the diffuse neuroendocrine system and its relationship to the common peptides. Fed Proc. 1979 Aug;38(9):2288–2294. [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Sidhu G. S. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979 Jul;96(1):5–20. [PMC free article] [PubMed] [Google Scholar]
- Taylor W. Pulmonary argyrophil cells at high altitude. J Pathol. 1977 Jul;122(3):137–144. doi: 10.1002/path.1711220304. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y., Osamura R. Y., Watanabe K., Yanaihara N. Immunohistochemical studies on gastrin-releasing peptide- and adrenocorticotropic hormone-containing cells in the human lung. Lab Invest. 1983 May;48(5):623–632. [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Cole G. A., Marangos P. J., Pearse A. G. Neuron-specific enolase as an immunocytochemical marker for the diffuse neuroendocrine system in human fetal lung. J Histochem Cytochem. 1981 Dec;29(12):1359–1364. doi: 10.1177/29.12.7033363. [DOI] [PubMed] [Google Scholar]