Abstract
The central mesencephalic reticular formation is physiologically implicated in oculomotor function and anatomically interwoven with many parts of the oculomotor system’s premotor circuitry. This study in Macaca fascicularis monkeys investigates the pattern of central mesencephalic reticular formation projections to the area in and around the extraocular motor nuclei, with special emphasis on the supraoculomotor area. It also examines the location of the cells responsible for this projection. Injections of biotinylated dextran amine were stereotaxically placed within the central mesencephalic reticular formation to anterogradely label axons and terminals. These revealed bilateral terminal fields in the supraoculomotor area. In addition, dense terminations were found in both the preganglionic Edinger-Westphal nuclei. The dense terminations just dorsal to the oculomotor nucleus overlap with the location of the C-group medial rectus motoneurons projecting to multiply innervated muscle fibers suggesting they may be targeted. Minor terminal fields were observed bilaterally within the borders of the oculomotor and abducens nuclei. Injections including the supraoculomotor area and oculomotor nucleus retrogradely labeled a tight band of neurons crossing the central third of the central mesencephalic reticular formation at all rostrocaudal levels, indicating a subregion of the nucleus provides this projection. Thus, these experiments reveal that a subregion of the central mesencephalic reticular formation may directly project to motoneurons in the oculomotor and abducens nuclei, as well as to preganglionic neurons controlling the tone of intraocular muscles. This pattern of projections suggests an as yet undetermined role in regulating the near triad.
Keywords: Oculomotor, Midbrain, Saccades, Vergence, Eye Movements, Edinger-Westphal
INTRODUCTION
The oculomotor system circuits controlling saccades are among the best understood motor systems in the brain. However, not all components of this system are equally well described. For example, the central mesencephalic reticular formation (cMRF) is connected to virtually all the saccade-related structures within the brainstem, suggesting it is an integral component of the oculomotor system (Edwards 1975; Edwards and de Olmos 1976). Yet its connections have received only limited study in primates. The first evidence that the midbrain reticular formation (MRF) was involved in oculomotor control came from lesion studies (Bender and Shanzer 1964; Komatsuzaki et al. 1972). These indicated effects on horizontal gaze and the optokinetic reflex for eye movements into the field across from the lesion. More specific evidence was provided by electrical stimulation studies (Bender and Shanzer 1964; Cohen et al. 1985; Cohen et al. 1986). MRF stimulation produced contraversive, horizontal saccades. Modification of most stimulation parameters did not notably affect saccade metrics, as in the superior colliculus, where target vectors are topographically coded. In fact, dorsal stimulation in the MRF resulted in smaller amplitude saccades than ventral stimulation. Using electrical stimulation, Cohen and colleagues (1985) defined a box-shaped region in the middle of the MRF as the zone producing saccadic eye movements, and termed this region the cMRF.
The results from electrical stimulation were reinforced by single cell recordings in awake behaving monkeys. These provided further evidence of the cMRF’s role in the saccadic circuitry (Cromer and Waitzman 2006; Cromer and Waitzman 2007; Waitzman et al. 1996). Recordings in head-fixed monkeys indicated that the cMRF contains a variety of cell types based on their saccade-related activity. They further indicated that the best movement directions tended to be in the horizontal plane towards targets in the hemifield opposite the side being recorded. However, others reported that vertical components are also encoded in the firing pattern of these cells (Handel and Glimcher 1997). It was later suggested that this discrepancy might be due to differences in the portion of the MRF where the units were recorded. Specifically, inactivation experiments indicated the rostral portion of the MRF, termed the peri-interstitial nucleus of Cajal region of the MRF (piMRF), was more related to vertical saccades, while the cMRF was more related to horizontal saccades (Waitzman et al. 2000a; Waitzman et al. 2000b). The inactivation also showed changes in head position. In view of this, the activity of cMRF neurons was investigated in monkeys whose heads were unrestrained (Pathmanathan et al. 2006a; Pathmanathan et al. 2006b). These studies indicated the cMRF contains both eye- and head-related neurons. The latter had appeared as cells whose peak firing followed saccades in head fixed animals.
Considering the multiple types of gaze-related neurons in the cMRF and its connections with the superior colliculus and pontine reticular formation, Waitzman and colleagues (1996) proposed that the cMRF might serve dual roles in upstream and downstream control of saccadic circuits. The best described and largest input to the cMRF is from the ipsilateral superior colliculus (Cohen et al. 1986; Harting 1977). Intracellular staining of tecto-bulbo-spinal axons in cats and squirrel monkeys indicates that this input comes from collaterals of predorsal bundle axons that originate from intermediate and deep gray layer neurons of the tectum (Grantyn and Grantyn 1982; Moschovakis et al. 1988a; Moschovakis et al. 1988b). MRF outputs, which were initially defined in the cat (Edwards 1975; Edwards and de Olmos 1976), bilaterally target the same structures as the superior colliculus outputs, including the paramedian pontine reticular formation (PPRF), raphe nuclei, the medullary reticular formation, and cervical spinal cord. In addition, the MRF projects heavily and bilaterally back to the intermediate and deep layers of the superior colliculus. This feedback projection was confirmed with intracellular staining of squirrel monkey cMRF neurons (Moschovakis et al. 1988b) and presence of this feedback circuit was used to anatomically define the cMRF portion of the MRF in monkeys (Chen and May 2000). Further examination of the feedback pathway revealed that the ipsilateral reticulotectal projection was GABAergic, and the contralateral projection contained both GABAergic and non-GABAergic elements (Wang et al. 2010). The role of the cMRF in controlling head movements also was supported by anatomical studies in cats and monkeys (Perkins et al. 2009; Warren et al. 2008; Zhou et al. 2008). Specifically, cMRF projections to the cervical cord and to reticulospinal neurons in the medullary reticular formation were demonstrated. Finally, a projection to the omnipause cells in nucleus raphe interpositus has been delineated (Wang et al. 2013). This projection derives primarily from GABAergic cMRF cells that receive collicular input, so it may help release paramedian pontine reticular formation (PPRF) burst neuron activity.
Taken together, these anatomical and physiological studies reveal that the cMRF is intimately involved in modulating the gaze circuits through projections onto premotor neurons and their inputs. However, trans-synaptic retrograde labeling of neurons following injection of rabies virus into extraocular muscles revealed labeling of cMRF neurons in monkeys and guinea pigs at the same survival times as known premotor populations (Graf et al., 2002; Ugolini et al. 2006). So the cMRF may also contain premotor neurons that directly modulate motoneuronal activity. In contrast, Edwards (1975) did not observe terminals in the oculomotor (III) or abducens (VI) nuclei following injections of tritiated amino acids into the cat MRF. We further examine this question in the present study. Specifically, we investigated whether the cMRF projects to the extraocular motor nuclei in Macaca fascicularis monkeys by injecting this region with anterograde tracers. To specify the location of the putative premotor neurons, we injected retrograde tracers into III.
METHODS & MATERIALS
Experiments were performed using 8 adult or young adult, male, macaque monkeys (Macaca fascicularis). Injections of one of the following tracers, 10% biotinylated dextran amine (10,000 MW)(BDA; n = 5; Invitrogen) or 2% Phaseolus vulgaris leucoagglutinin (PhaL; n = 1; Vector Labs) were stereotaxically placed into the central mesencephalic reticular formation (cMRF) to anterogradely label axons and boutons. In other animals, 10 % BDA (n=1) or 2 % wheatgerm agglutinin conjugated to horseradish peroxidase (WGA-HRP; n=1) was injected into the supraoculomotor area (SOA) and oculomotor nucleus (III) to retrogradely label cMRF cells. All applicable international, national and institutional guidelines for the care and use of animals were followed. Specifically, all procedures presented in this study are in accordance with NIH guidelines for animal care and use, and were approved by the University of Mississippi Medical Center’s IACUC.
Surgical Procedures
All surgeries were performed under sterile conditions. Prior to surgery, each animal was sedated with ketamine hydrochloride (10 mg/kg, IM), administered along with atropine sulfate (0.05 mg/kg, IM) to reduce airway secretions. Animals were anesthetized with and maintained on Isoflurane (1–3%). An intravenous line delivered fluid support. The animals received an injection of dexamethasone (2.5 mg/kg, IV) in order to prevent brain and tracheal edema. The animal’s vital signs were monitored throughout the procedure. With the head in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA), a unilateral craniotomy was made over the brainstem, and the dura was incised and reflected. A small portion of the medial parietal cortex was aspirated to reveal the anterior edge of the tentorium cerebelli. The aspirated region was then extended rostrally to visualize the superior colliculus and pulvinar. Injection coordinates for the cMRF and III were determined with the help of a stereotaxic atlas (Szabo and Cowan 1984) and previously reported work (Wang et al. 2010). In order to avoid the superior colliculus, the syringe needle was inserted through the pulvinar to reach the cMRF. A 1 μL Hamilton microsyringe held in a micromanipulator was angled 10°, tip medial in the coronal plane and rotated 10–11° clockwise when observed from above, and was lowered to ~7 mm below the surface measurement of the superior colliculus. Two 0.1 μL injections of 10% BDA were placed along a single track, 1 mm apart. In some cases, a second pair of injections was made at a different mediolateral position. PhaL injections were made using the same general approach. However, the PhaL was dissolved in pH 8.0, 0.1 M phosphate buffer (PB) and was ejected from a glass micropipette with a 15–20 μm tip by iontophoresis under the control of an iontophoretic current supply module (Model CS 3; Transkinetics; Canton, MA, USA), using 7 μA, 7 second, square wave pulses for 10 min at each injection site (see Wang et al., 2013 for details). In the case of the retrograde experiments, the initial approach was similar, however, the aspiration was extended to reveal the posterior commissure. The injection needle was then inserted through the third ventricle rostral to the commissure, entering the midbrain at the base of the entrance to the cerebral aqueduct. The syringe was angled 2° tip medial in the frontal plane, and 0–10° tip rostral in the sagittal plane. The injection needle was insulated by coating it with polyurethane so that it could be used to stimulate III and elicit eye movements, providing for more accurate injections. Eye movements were evoked using an A-M System isolated pulse stimulator (Model 2100) and WPI stimulus isolation unit (Model A320). The stimulus train had a burst width of 2.5 to 5 msec, with an interpulse period was 50–150 μsec. Pulse duration was a square wave of 25 – 75 μsec. Effective stimulus current ranged from 1–4 mA. BDA or WGA-HRP was then pressure injected into locations that elicited maximal eye movements. At the end of the surgery, incised tissues were rejoined and stabilized with sutures. Sensorcaine (0.5–1.0 ml) was then administered locally at the incision site, and buprenex (0.01 mg/kg, IM) was given as a postoperative analgesic.
BDA and PhaL were allowed 14–21 days for transport, but WGA-HRP only required 2 days. Animals were then heavily sedated with sodium pentobarbital (50 mg/kg, IM), and transcardially perfused with a 0.1 M, pH 7.2 phosphate buffered saline (PBS) pre-wash, followed by a 3 L of a mixture containing 1% paraformaldehyde and 1.25–1.5% glutaraldehyde in 0.1M, pH 7.2 PB. The brain was blocked in-situ, then tissue blocks containing the brainstem were post-fixed for an additional hour before being transferred to PB and stored at 4° C until histological analysis.
Histology & Analysis
Tissue was cut in the frontal plane using a vibratome (Leica). BDA was visualized using diaminobenzidine (DAB) as a chromagen in 100 μm sections, as previously described (Adams 1977; Chen and May 2000; Wang et al. 2013). Briefly, sections were rinsed with fresh 0.1 M, pH 7.2 PB then 0.05% Triton X-100 in the same PB. Next, sections were immersed in 1:500 Avidin D-HRP (Vector labs) diluted in 0.05% Triton X-100 in 0.1M, pH 7.2 PB for 24 h at 4° C. Sections were then rinsed in 0.1 M, pH 7.2 PB before being placed in a solution containing 0.5% DAB, 0.01% cobalt chloride, 0.01% nickel ammonium sulfate in PB. Addition of hydrogen peroxide to produce a 0.005 % solution catalyzed the reaction, resulting in a black reaction product where BDA was present in the tissue. PhaL was visualized in 50 μm sections by localizing the lectin with a biotinylated primary anti-PhaL antibody (raised in goat; Cat. No. BA-0224; Vector Labs), diluted 1:200 in 0.1 M, pH 7.2 PBS containing 0.1% of normal goat serum. Sections were then incubated in avidin-biotin-horseradish peroxidase complex (ABC Vectastain kit; Vector Labs as specified by ABC kit instructions) in 0.1 M, pH 7.2 PBS. Peroxidase activity was revealed by reacting tissue with a solution containing 0.5% DAB with 0.01% cobalt chloride and 0.01% nickel ammonium sulfate in 0.1 M, pH 7.2 PB (Gerfen and Sawchenko 1984; Zhou et al. 2008). After 10–30 minutes of preincubation, hydrogen peroxide was added to produce a 0.005 % solution. WGA-HRP was revealed by utilizing tetramethylbenzidine as a chromagen (Olucha et al. 1985; Warren et al. 2008). Following these reactions, sections were rinsed in PB, mounted, counterstained with cresyl violet, dehydrated, cleared and cover slipped.
Sections were drawn using a Leica Wild-M8 dissecting microscope. Distributions of anterogradely labeled terminals and retrogradely labeled cell bodies were charted at 10x magnification, and labeled cells and terminal arbors were drawn at 40x or 100x magnification by use of drawing tubes mounted on an Olympus BH-2 or Nikon Eclipse 80i microscope. Digital photographs were taken using NIS-Elements AR and a Nikon Eclipse E600 light microscope equipped with a 1.5 megapixel, Nikon DS-Ri1 high resolution camera. When necessary, images were adjusted for brightness, contrast and color using Adobe Photoshop CS5 to replicate the image as it appeared when visualized under the microscope.
RESULTS
The Distribution of cMRF Terminals in SOA
Injections of tracer into the cMRF resulted in a consistent pattern of labeled terminals across all observed anterograde labelling cases. These included terminals within the periaqueductal gray (PAG), supraoculomotor area (SOA) and within portions of the oculomotor nucleus (III). Within the PAG, terminal fields were much more dense ipsilateral to the injected side. Dense terminal fields could be observed bilaterally within the SOA and within the preganglionic Edinger-Westphal nucleus (EWpg). More scattered terminal arbors were observed within and between the oculomotor nuclei. The presence of terminals in III motivated us to also examine the other extraocular motor nuclei, where we noted terminal fields dorsal to the trochlear nucleus (IV) and within and dorsal to the abducens nucleus (VI).
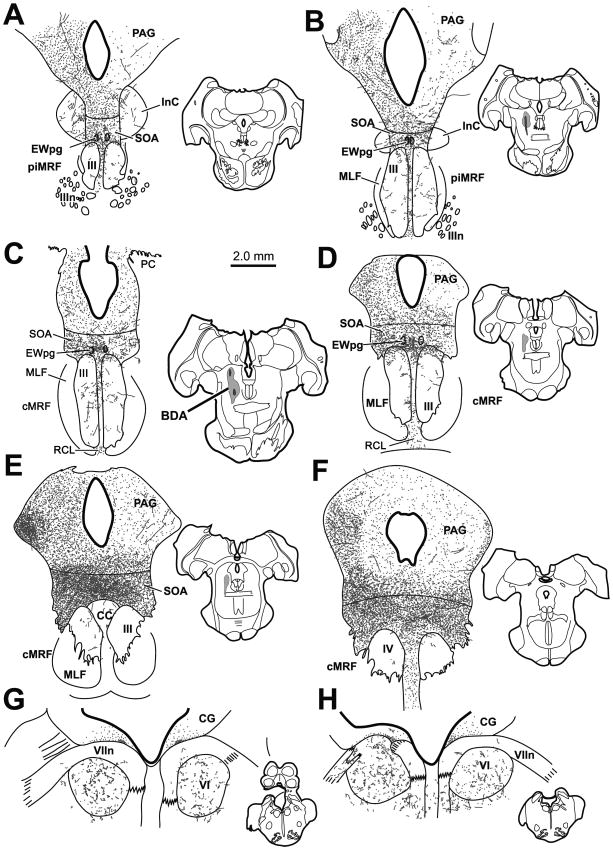
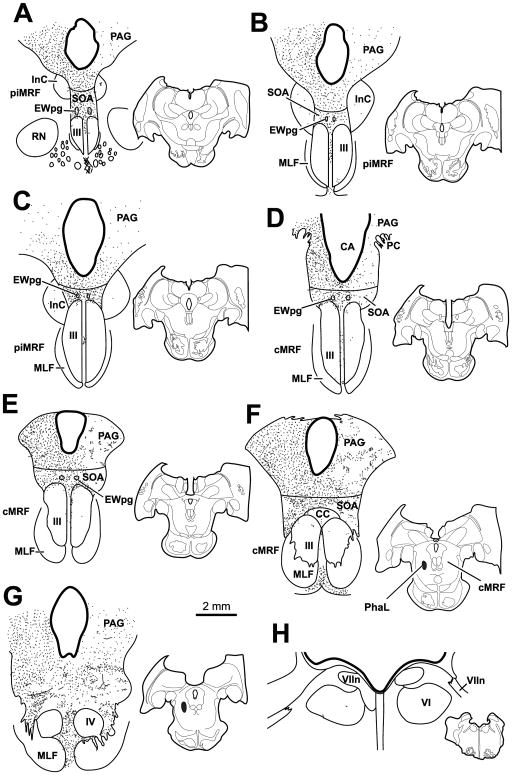
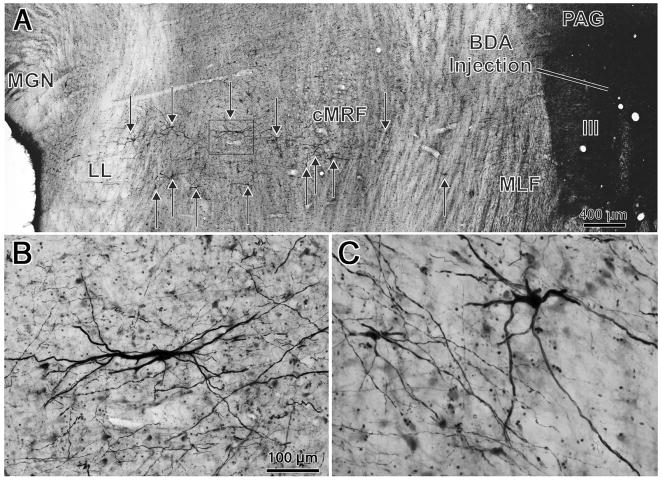
Figure 1 shows a case with a large BDA injection that extended along the rostral-caudal axis of the cMRF (Fig. 1B–E insets). The injection was largely constrained to the cMRF with minor spread rostrally into the piMRF. It occupied the medial half of the nucleus. This injection produced the densest labelling of all the cases. In this case, labeled terminals were found bilaterally in the periaqueductal gray, with a considerable ipsilateral predominance (Fig. 1A–F). PAG terminals were denser laterally and caudally. A few labeled axons were seen within the interstitial nucleus of Cajal (InC) (Fig. 1A&B). Of particular note, labeled terminals were observed bilaterally in the SOA, with a slight ipsilateral predominance. The terminal field stretched above III from its rostral to caudal pole (Fig. 1A–E), and continued above IV (Fig. 1F). The SOA terminal field was denser caudally, dorsal to the caudal central subdivision (CC) (Fig. 1E), and rostrally along the dorsal edge of III (Fig. 1C&D). Dense terminations were also present in and around the histologically defined EWpg (Fig. 1A–D). Sparse labeled axonal arbors were also observed within III and CC. These terminals were densest near the midline and in the area between the two oculomotor nuclei. Beneath III labeled terminals were found in the caudal linear nucleus of the raphe (RCL) (Fig. 1C&D). Labeled terminals were not observed within the trochlear nucleus (Fig. 1F). However, a notable number of BDA labeled terminal arbors were observed bilaterally within the abducens nucleus (VI) (Fig. 1G and H) and this sparse terminal field extended into and dorsal to the facial nerve (VIIn). This dorsal region receives input from other premotor gaze areas and projects to the cerebellum (Büttner-Ennever et al., 1989; May et al., 2013)
Figure 1.
A large BDA injection into the central mesencephalic reticular formation (cMRF) (insets B–E) produced dense terminal labeling (stipple) in the periaqueductal gray (PAG), supraoculomotor area (SOA) (A–E) and preganglionic Edinger-Westphal nucleus (EWpg) (A–D) and more limited terminal labeling in the oculomotor nucleus (III) (A–E) and its caudal central subdivision (CC) (E). Scattered terminals were also present in the trochlear (IV) (F) and abducens (VI) (G&H) nuclei. Terminals were also present dorsal to IV, as well as in and above the adjacent facial nerve (VIIn), and in the caudal linear raphe nucleus (RCL). All chartings arranged in rostral to caudal order, with insets to show level in this and other chartings. Injection sites are shown in the whole-section insets
A much smaller injection of BDA placed into the cMRF is shown in figure 2. (Fig. 2C–D). It was centered just rostral to the level of the posterior commissure (PC). Though less dense in comparison to the first case, the second case showed remarkable similarity in the resultant pattern of terminal boutons. Terminals were scattered bilaterally within the PAG, with an ipsilateral predominance (Fig. 2A–E) and a few labeled arbors were present in InC (Fig. 1A&B). The terminal fields were most dense within the SOA, particularly along the dorsal edge of III (Fig. 2D), and in the vicinity of EWpg (Fig. 2A–E). In this case, the increase in density caudally was not noted. A few labeled terminal arbors were present, within and between the oculomotor nuclei (Fig. 2A–D). Terminals were also observed bilaterally within VI and the adjacent facial nerve in this case (Fig. 2F&G), but these were fewer in number compared to the large injection case (Fig. 1G&H).
Figure 2.
A smaller BDA injection into the cMRF (insets C&D) produced considerable terminal labeling (stipple) in the periaqueductal gray (PAG), supraoculomotor area (SOA) and preganglionic Edinger-Westphal nucleus (EWpg) (A–E), and more limited terminal labeling in the oculomotor nucleus (III) (A–E). Terminals were also present in trochlear (IV) (E) and abducens (VI) (F&G) nuclei, as well as in and above the adjacent facial nerve (VIIn).
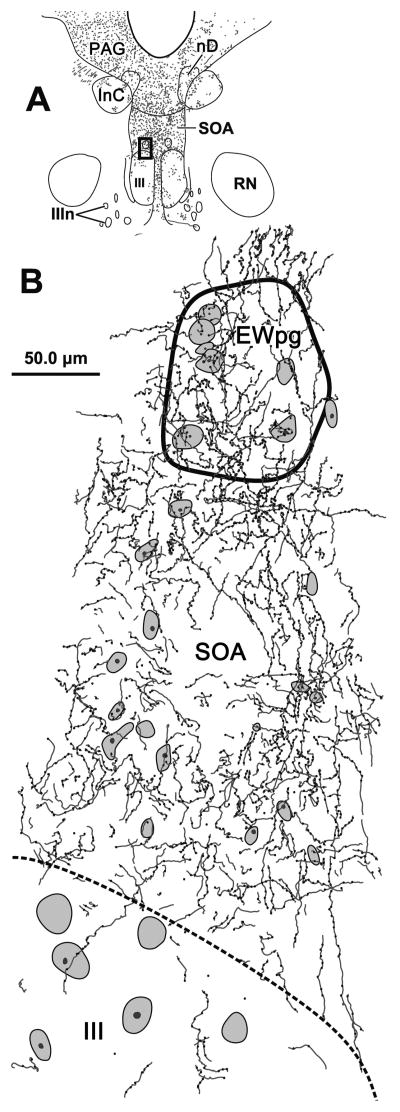
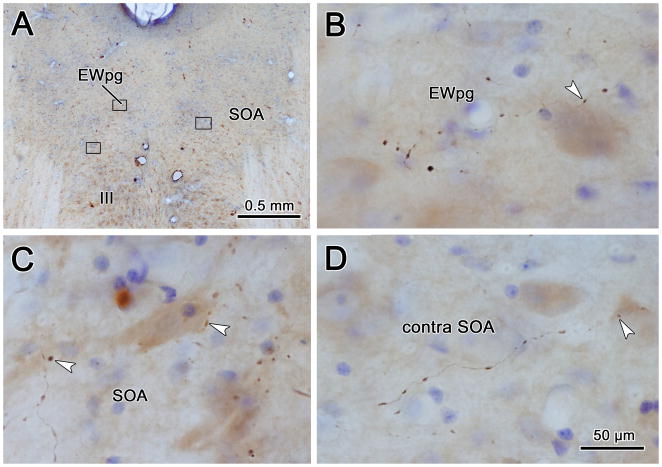
A better understanding of the morphology of the labeled axons can be obtained from the higher magnification illustration in figure 3. A section at the rostral end of III is illustrated (Fig 3A). The terminal field within SOA was denser at the dorsal edge of III, and this dense terminal field extended into EWpg. The axons primarily display en passant boutons and rarely observed to branch (Fig. 3B). Individual axons do not respect to the border between SOA and EWpg, but decreased dramatically ventral to the border of III.
Figure 3.
The pattern of axonal labeling from a BDA injection of the cMRF in a section located near the rostral pole of the oculomotor nucleus (III) (A). The box indicates the region shown in B, which includes the preganglionic Edinger-Westphal nucleus (EWpg) and the portion of the supraoculomotor area (SOA) between EWpg and III. The axons show few branches, display primarily en passant boutonal enlargements, and densely populate this region. They cross the EWpg border freely, but tail off when crossing the border of III. Cresyl violet stained cells are indicated by shading.
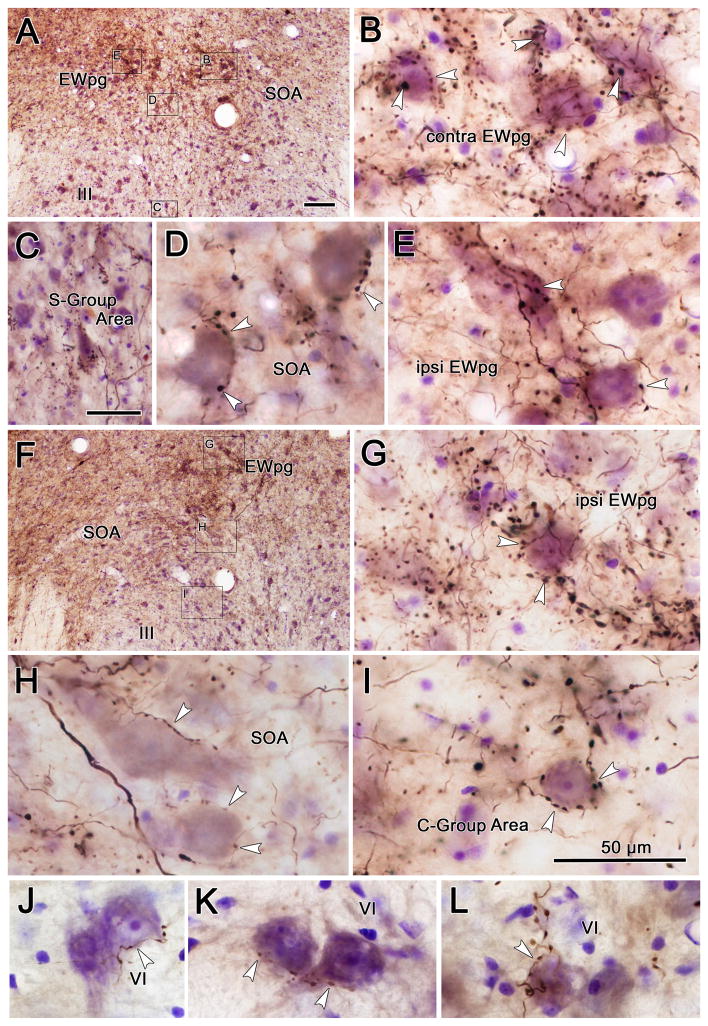
Examples of BDA labeled terminal arbors from the smaller illustrated injection into the cMRF (Fig. 2) are shown in figure 4. Plates A–E and F–I represent the pattern of label at more rostral and caudal levels, respectively. The axons show few branches or terminal boutons, but numerous en passant boutons. They nevertheless form a dense network within the tissue, which likely indicates some level of axonal arborization. Numerous close associations (arrowheads) between BDA labeled cMRF terminals and Nissl stained neurons were observed. Targeted cells were found in the SOA (Fig. 4D,H&I) and the ipsilateral and contralateral EWpg (Fig. 4B,E&G). A portion of the SOA terminals lay right along the dorsal border of III in the region that contains the C-group motoneurons (Fig. 4I). A smaller number of BDA labeled cMRF axons and terminals were present in the region between the two oculomotor nuclei where the S-group motoneurons are found (Fig. 4C). Lastly, close associations were also observed between cMRF axons and counterstained neurons within the abducens nucleus (Fig. 4J–L).
Figure 4.
Photographic plates showing the pattern of BDA labeling after a cMRF injection illustrated in figure 2. A&F show low magnification views from rostral (A) and caudal (F) sections through the oculomotor nucleus (III) to define the location of the high magnification views (B–E and G–I, respectively). Close associations (arrowhead) between labeled boutons and counterstained cell bodies were observed in the supraoculomotor area (SOA) (D&H), and preganglionic Edinger-Westphal nucleus (EWpg) (B,E&G). Associations were also present in the regions containing the C-group (I) and S-group (C) motoneurons adjacent to the oculomotor nucleus (III). Fewer close associations were seen in the abducens nucleus (J–L). All scale bars = 50 μm. Scale in A=F, Scale in I=B,D,E,G,H&J–L. Z-planes combined: B=5, C=7, D=1, E=3, G=5, H=3, I=1, J–K=1.
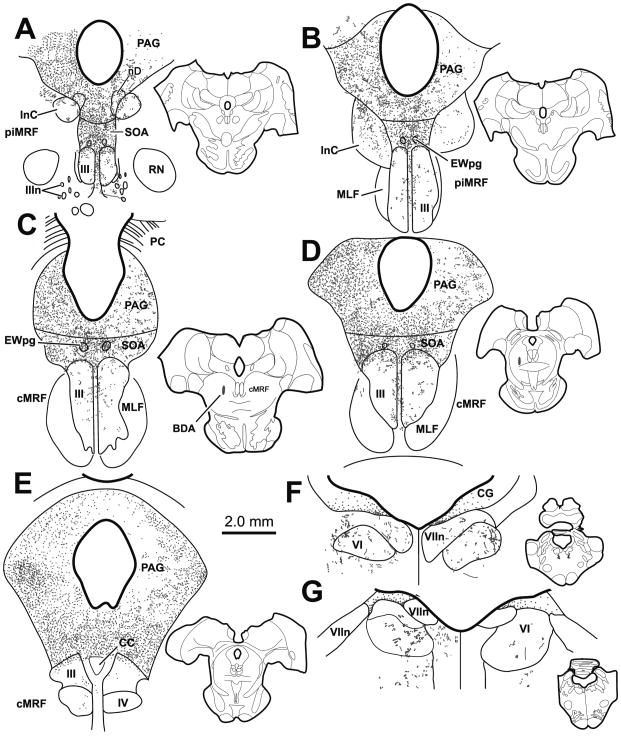
The axons of collicular neurons cross the medial cMRF before decussating and forming the predorsal bundle (Grantyn and Grantyn 1982; Harting 1977; Moschovakis et al. 1988a; Moschovakis et al. 1988b). These axons also terminate in the SOA (Edwards and Henkel 1978; Grantyn and Grantyn 1982; Harting 1977). Due to the possibility that the terminals observed after BDA injections were caused by fiber-of-passage uptake, we made injections of BDA into the lateral aspect of the cMRF to avoid the tectal axons. These injections resulted in very little axonal labeling in the SOA. As an alternative approach, we used PhaL, which is rarely taken up by passing fibers. When this tracer was injected into the cMRF, as shown in figure 5, the resultant injection site was at the cMRF’s caudal pole, near the level of the trochlear nucleus (IV) (Fig. 5F&G, insets). The distribution of labeled terminals observed after the PhaL injection was quite similar to that observed in the BDA cases. However, far fewer labeled terminal arbors were observed. Specifically, PhaL labeled terminals were found bilaterally in PAG with an ipsilateral predominance (Fig. 5A–G), but almost no label was seen in InC (Fig. 5A–C). Labeled axons were also found bilaterally in SOA and EWpg (Fig. 5A–F), but no obvious concentration differences were found within this field. Little or no label was observed within the borders of III, IV or VI (Fig. 5A–F, G and H, respectively), in contrast to the pattern observed with BDA injections. However, scattered terminals were present in CC (Fig. 5F).
Figure 5.
A small PhaL injection into the cMRF (insets F&G) produced considerable terminal labeling (stipple) in the periaqueductal gray (PAG), supraoculomotor area (SOA) and preganglionic Edinger-Westphal nucleus (EWpg) (A–E), and much more limited terminal labeling in the oculomotor nucleus (III) (A–F) and caudal central subdivision (CC) (F). Almost no terminals were present in trochlear IV (G) and abducens (VI) (H) nuclei.
Figure 6 shows photomicrographs of a representative section from the presented PhaL case. Similar to the BDA labeled axons, the PhaL labeled axons rarely branched and displayed numerous en passant enlargements. Close associations (arrowheads) were observed between PhaL labeled cMRF terminal boutons and counterstained neurons within the SOA on both the ipsilateral (Fig. 6C) and contralateral (Fig. 6D) side of the brainstem. A portion of these were located along the dorsal edge of the oculomotor nucleus (Fig. 6C). In addition, PhaL labeled terminals were observed making close associations with cells within the borders of the EWpg (Fig. 6B).
Figure 6.
Photographic plates showing the pattern of PhaL labeling after the cMRF injection illustrated in figure 5. A. shows a low magnification view to define the location of the high magnification views (B–D). Close associations (arrowheads) between labeled boutons and counterstained cell bodies were observed in the high magnification views of ipsilateral (C) and contralateral (D) supraoculomotor area (SOA), and in the ipsilateral preganglionic Edinger-Westphal nucleus (EWpg) (B). Scale in D=B&C. Z axis planes: B=3, C=4, D=1.
Cells of Origin of the cMRF-SOA Projection
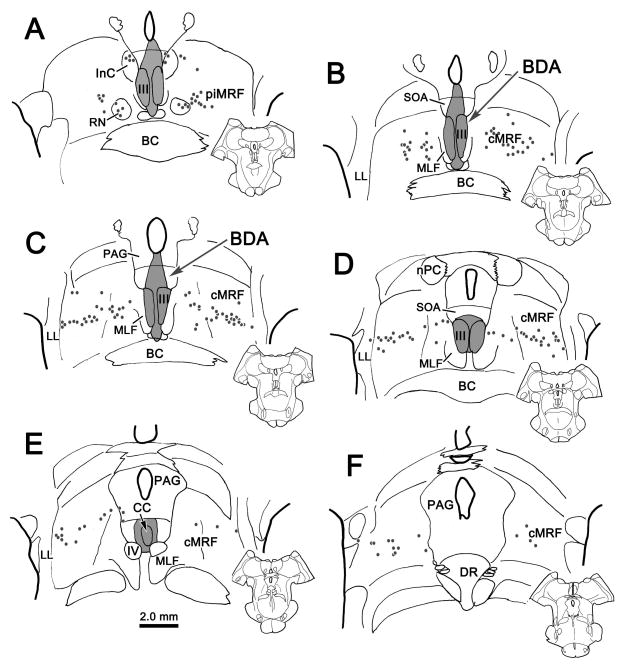
In order to identify the cells of origin of this cMRF projection, BDA or WGA-HRP was injected into the brainstem to encompass III, EWpg and SOA. As the findings were similar, the BDA injection is illustrated in figure 7. This injection completely involved III on both sides, and extended through the overlying SOA and EWpg (Fig. 7A–E). There was minor spread laterally into the MLF, dorsally into the PAG immediately beneath the cerebral aqueduct, and ventrally through RCL to the edge of the brachium conjunctivum (BC). This injection resulted in the retrograde labeling of a distinct population of neurons within the cMRF (Fig. 7B–F). Labeled neurons (dots) were also present within the piMRF (Fig. 7A), where they formed a tight cluster adjacent to labeled cells within the red nucleus (RN). The latter may have been labeled because their crossing axons were caught by the ventral edge of the injection site. Within the cMRF, the labeled cells formed a band running through the rostrocaudal extent of the nucleus, ending slightly caudal to the level of IV (Fig. 7B–F). This band of labeled cMRF cells stretched across the mediolateral extent of the nucleus, from the lateral lemniscus (LL) to the medial longitudinal fasciculus (MLF) or the edge of the PAG, where it meets the dorsolateral corner of III. However, it had a relatively compressed dorsoventral height, covering only the middle third of the cMRF. Rostrally (Fig. 7B), the population of cells was more dispersed and expanded to half the height of the cMRF. We compared the pattern of retrograde labeling following injection of III to the sites of 4 of the injections within the cMRF (Fig. 8). We found that the laterally placed injections (injections 2&3) involved only a small segment of the cells projecting to the oculomotor nucleus. These injections produced few labeled axons in the SOA. In contrast, those injections that involved substantial portions of the labeled population (1&4) produced numerous labeled terminals in the SOA, although involvement of medially running axons from laterally placed cells may have contributed to this difference.
Figure 7.
The pattern of retrograde labeling (dots) observed in the peri interstitial nucleus of Cajal portion of the mesencephalic reticular formation (piMRF) (A) and the central mesencephalic reticular formation (cMRF) (B–F) after an injection of BDA into the oculomotor nucleus (III) and supraoculomotor area (SOA) (A–E). In the cMRF, the cells are arranged as a band stretching across the mediolateral breadth of the nucleus.
Figure 8.
Relationship of the pattern of retrograde label (dots) following an oculomotor nucleus (III) injection (see Fig. 7) to the locations of four (1–4) BDA injection sites.
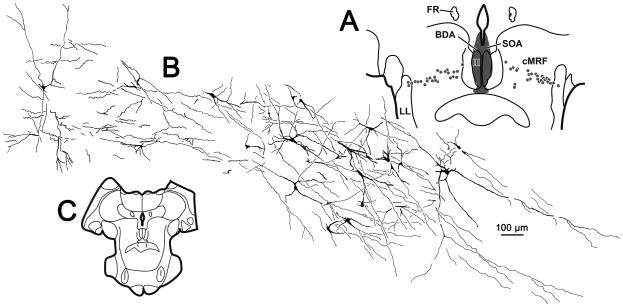
The BDA produced homogeneous labeling of the dendrites of these cMRF neurons. These cells were multipolar, with long, sparsely branching dendrites (Fig. 9B&C). The average soma area was 187.7 ± 4.3 μm2 (Mean ± SEM) and ranged from 50 to 763 μm2. Spines were not evident. This filling allowed examination of the organization of dendritic fields of these cMRF neurons (Fig. 10A&C). The vast majority of the dendrites of these neurons were constrained within a band formed by the labeled cells bodies. Furthermore, they were, for the most part, mediolaterally oriented (Fig. 10A&C).
Figure 9.
Photomicrographs showing the injection of BDA into the oculomotor nucleus (III) and supraoculomotor area (A), and the distribution of labeled cells (arrows) in the central mesencephalic reticular formation (cMRF) (A) that resulted from this injection. B&C show the morphology of the homogeneously labeled neurons within the cMRF. Note the poorly branched, tapering dendrites and range of cell sizes. Box in A shows location of B. Scale in B=C.
Figure 10.
Illustration of the dendritic fields of central mesencephalic reticular formation (cMRF) cells projecting to the oculomotor nucleus (III) within a single section (C). Following the injection shown in A, retrogradely labeled cells (dots) extend across the cMRF. The dendritic fields of the labeled cells on the right side of this section are illustrated in B. Note the morphology of the homogeneously labeled neurons within the cMRF and the fact their dendrites are largely constrained within the mediolaterally oriented band of labeled cells.
DISCUSSION
This is the first detailed study of cMRF projections to perioculomotor targets. Extensive projections by the cMRF to the SOA and to the EWpg were revealed. Minor projections to III, the caudal central subdivision (CC) and VI were also found. A striking aspect of all these projections is their bilaterality. The cMRF’s projection to the SOA might be related to a number of cell populations that will be discussed below in detail. The noteworthy density of terminals along the dorsal edge of III and IV, as well as the sparse terminations within the extraocular motor nuclei, point to the possibility of motoneuron targets. Dense terminal fields were also found in the Edinger-Westphal nucleus, which contains preganglionic motoneurons subserving pupillary constriction and lens accommodation. Injections of tracer into SOA and III resulted in a tightly clustered, band of neurons running through the middle third of the cMRF. The limited distribution of these cells suggests that there is a heretofore unrecognized, subdivision within this nucleus.
Technical Considerations
The reported results should be considered with acknowledgement of a few technical constraints. First, BDA is apt to be taken up by fibers of passage. This may be problematic in the case of cMRF injections, due to the fact the axons of the tecto-bulbo-spinal tract run through this nucleus and they are known to have collaterals that terminate in SOA (Grantyn and Grantyn 1982; Harting 1977; Moschovakis et al. 1988a; Moschovakis et al. 1988b). Thus, we can not exclude the possibility that some of the labeled terminals are tectal in origin. In fact, these injections retrogradely labeled cells in the superior colliculus. However, cMRF injections of PhaL, a tracer with little or no fibers of passage uptake, resulted in no retrograde labeling of collicular cells, but the same basic terminal field pattern as was observed using BDA, albeit a less dense one.
Further, injection of tracer into the SOA and III revealed the location of the cMRF projecting neurons, bidirectionally confirming that there are neurons located within the cMRF that project to the SOA and possibly to III. Another constraint is tracer spread. Tracer extended along the needle tract from the cMRF injection site dorsally into the pretectum (not illustrated). The olivary pretectal nucleus (OPt) subserves the pupillary light reflex and has direct projections to the EWpg motoneurons controlling pupilloconstriction. Thus, a portion of the terminals observed in EWpg following cMRF injections might be due to involvement of this luminance pathway. However, labeled terminals were found throughout EWpg, and only a small portion of the preganglionic population is believed to subserve pupillary control (Erichsen and May 2002; May et al. 2008; Warwick 1954). A constraint on interpreting the results of the retrograde experiment is the possibility of tracer spread beyond the confines of the SOA and III. The injection site spread ventrally below III (Fig. 7A–D), near where cMRF axons decussate en-route to lower brainstem and spinal levels (Edwards 1975; Edwards and de Olmos 1976). However, as will be discussed in the next section, the distribution of the retrogradely labeled neurons described after III injections is unlike that of previously reported populations of cMRF reticuloreticular or reticulospinal cells, and therefore it is unlikely that it resulted from fiber-of-passage uptake and retrograde labeling.
cMRF subdivisions
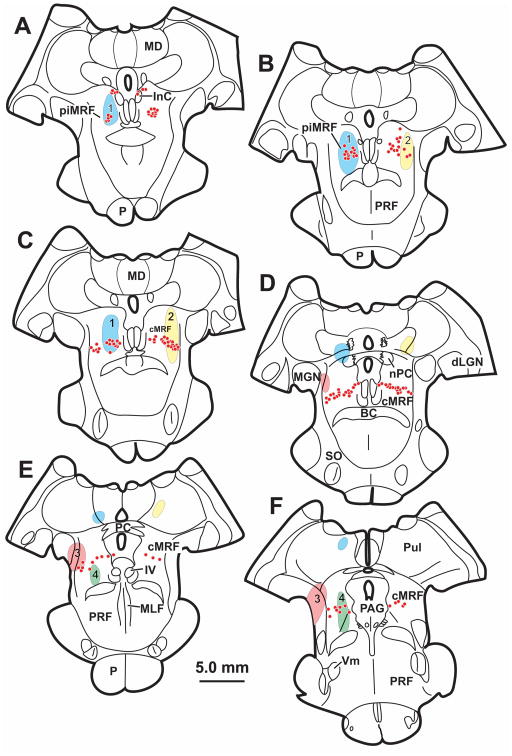
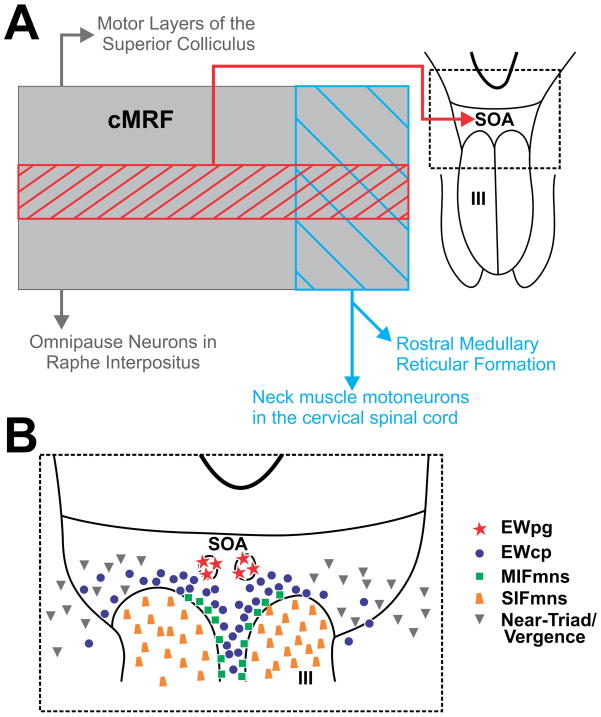
The cMRF can be divided based upon the location of cell populations using anatomical approaches. Figure 11 shows a schematic of the cMRF and the locations of known cell populations and their projection locations. The largest known and arguably best studied population of neurons within the cMRF are the reticulotectal neurons which are reciprocally connected with the superior colliculus [primate: (Chen and May 2000; Cohen and Büttner-Ennever 1984; Harting 1977; Wang et al. 2010; Zhou et al. 2008); cat: (Edwards and de Olmos 1976; Edwards et al. 1979; Graham 1977)]. The general distribution of these neurons is shown in gray in figure 11, reticulotectal cMRF neurons are evenly distributed from the MLF to the lateral lemniscus (LL), and extend through much of the dorsoventral extent of the midbrain reticular formation. The cMRF reticuloraphe neurons projecting to omnipause neurons in the nucleus raphe interpositus have a similar distribution, but are fewer in number (Fig. 11A) (Wang et al. 2013). Reticulospinal neurons projecting to cervical spinal cord and medullary reticuloreticular cMRF neurons projecting to the rostral medullary reticular formation occupy just the medial portion of the cMRF (Fig. 11A) [primate: (Castiglioni et al. 1978; Perkins et al. 2014; Robinson et al. 1994; Warren et al. 2008); cat: (Perkins et al. 2014; Satoda et al. 2002)]. As we have shown here, the presence of a narrow band of cells labeled from oculomotor injections, reveals an unreported subdivision with direct projections to the SOA and III (Fig. 11A).
Figure 11.
Schematic showing the subdivisions (A) and possible perioculomotor targets (B) of the central mesencephalic reticular formation (cMRF). A. location of the cells that are the source of the cMRF projection to the supraoculomotor area (SOA) (hatching) in comparison to the sources of other cMRF projections to the superior colliculus, pons, the spinal cord and medullary reticular formation (blue hatch). B. Potential target populations within the SOA and oculomotor nucleus (III).
In contrast to these anatomically defined subdivisions, there is less evidence for physiological divisions. A delineation has been made between the rostral and caudal MRF. It has been suggested that the rostral, piMRF is related to vertical movements, while the more caudal, cMRF is related to horizontal eye movements. Unfortunately, it is not clear whether these represent separate regions or are instead, two ends of a continuum of eye movement tuning (Handel and Glimcher 1997; Waitzman et al. 2000b). A continuum was reported by Cohen and colleagues (1985 & 1986), who showed that electrical stimulation at progressively more ventral sites through the cMRF increased the magnitude of the horizontal, contraversive saccade. This might be taken to suggest that the cMRF population projecting to the SOA represents a discrete set of eye movement amplitudes. However, no one has reported this topographic mapping of saccade amplitude in neurophysiological recordings. Furthermore, these stimulation results run counter to the anatomical findings that suggest head movements, which might be presumed to encode larger gaze shifts, are found medially. In fact, Pathmanathan and colleagues (2006a; 2006b) observed no topography in the location of cMRF cells coding for head movements.
Possible cMRF targets within the supraoculomotor area
The SOA was first described by Edwards and Henkel (1978) in cats. Based on the presence of tectal terminals in this area, they suggested that the ventral portion of the periaqueductal gray that rests just dorsal to III, represents a separate region whose function is tied to the underlying oculomotor nucleus. In fact, terminal fields have been observed in SOA that originate from a number of oculomotor-related structures, including the superior colliculus [primates: (Harting 1977)], the caudal fastigial nuclei [primate: (May et al. 1992)], lateral accessory optic nucleus [marmoset: (Blanks et al. 1995)], pretectum [primate: (Büttner-Ennever et al. 1996; Sun and May 2014; Wasicky et al. 2004)] and the frontal and supplemental eye fields [primate: (Shook et al. 1990; Stanton et al. 1988)]. The present findings add another oculomotor-related structure to the list of SOA inputs, strengthening the supposition about its function made by Edwards and Henkel (1978). However, as detailed below and schematized in figure 11B, the SOA contains a number of different cell populations, making interpretation of the actual cellular targets of these inputs challenging.
Medial rectus motoneurons
The SOA contains motoneurons that lie outside the borders of III. The extraocular muscles receive dual innervation (Spencer and Porter 2006). The innervation of one class of muscle fiber appears much like that of conventional muscle fibers observed elsewhere in the body. These muscle fibers each receive a single, plate-shaped (en plaque) neuromuscular junction. Consequently, they are termed singly-innervated fibers (SIFs). Their motoneurons are found within the borders of the extraocular motor nuclei (trapezoid, Fig. 11B) (Büttner-Ennever and Akert 1981; Porter et al. 1983). A second type of muscle fiber receives multiple, grape-shaped (en grappe) terminal boutons. These are distributed from the muscle fiber’s proximal origin to its distal insertion. These muscle fibers are termed multiply-innervated fibers (MIFs). Their motoneurons sit peripheral to the borders of the nuclei (boxes, Fig. 11B) (Büttner-Ennever and Akert 1981; Büttner-Ennever et al. 2001a; Eberhorn et al. 2005; Eberhorn et al. 2006; Porter et al. 1983; Tang et al., 2015). The MIF motoneurons that innervate the medial and inferior rectus muscles (termed the C-group neurons) sit immediately dorsal to the oculomotor nucleus, appearing to cap the nucleus. Superior rectus and inferior oblique MIF motoneurons (termed the S-group neurons) also sit outside the confines of the nucleus, on the midline, sandwiched between the two oculomotor nuclei. Differences between the SIF and MIF motoneurons suggest they have different functions. For example, MIF neurons have smaller cell bodies (Büttner-Ennever et al. 2001b), which results in a higher membrane resistance and which correlates with their having slower conducting axons (Nelson et al. 1986). MIF and SIF motoneurons also display histochemical differences, for example, SIF motoneurons contain nonphosphorylatead neurofilaments, parvalbumin and are surrounded by perineuronal nets, while MIF motoneurons show no immunoreactivity for these markers (Eberhorn et al. 2005; Eberhorn et al. 2006). The functional implications of these differences are not currently known. Inspection at the ultrastructural level revealed that there were significantly fewer terminals on C-group MIF motoneuron somata and proximal dendrites compared to SIF motoneurons (Erichsen et al. 2014). Close examination of the morphology of the medial rectus C-group motoneurons revealed that their dendrites extend dorsally into the SOA and into EWpg (Erichsen et al. 2014), where we observed dense cMRF terminations in the present study.
Taken together this evidence suggests it is possible that the cMRF makes a direct projection to MIF motoneurons, as the projection to the region containing C-group motoneurons is particularly intense. This would confirm the suggestion from transneuronal rabies studies that the cMRF contains premotor neurons (Ugolini et al. 2006). In fact, when Ugolini and colleagues injected rabies virus into the insertion of the lateral rectus, only MIF motoneurons were retrogradely labeled in VI. Second order neurons supplying these MIF motoneurons were trans-synaptically labeled within the cMRF, suggesting that the cMRF contains premotor neurons supplying abducens MIF motoneurons. These results agree with the present findings. In addition, the C-group also contains inferior rectus motoneurons and terminals were also observed in the area of the S-group, which contains superior rectus and inferior oblique motoneurons, so MIF populations other than the medial rectus may be targeted. The presence of such inputs could provide clues to the action of MIFs in eye movements.
Preganglionic motoneurons
Lens accommodation and pupillary constriction are controlled by a two neuron arc in which the postganglionic, parasympathetic motoneurons sit in the ciliary ganglion. The preganglionic, parasympathetic motoneurons in monkeys sit as a rostrocaudally running, columnar cluster of neurons lying dorsal to III (stars, Fig. 11B). The column extends beyond the rostral pole of III, where it penetrates the anteromedian nucleus (AM) (Akert et al. 1980; Burde and Loewy 1980). This population has traditionally been termed the Edinger-Westphal nucleus (EW). Stimulation of the primate EW results in accommodation of the lens and constriction of the pupil (Crawford et al. 1989) and the firing rates of these motoneurons have been directly correlated to lens accommodation (Gamlin et al. 1994). The density of cMRF terminals within the borders of this nucleus suggests that this portion of the reticular formation modulates the tone of the ciliary and pupillary sphincter muscles.
Peptidergic neurons
Not all neurons within the SOA have a function related to eye movements. A population of peptidergic neurons (blue circles, Fig. 11B) is found adjacent to III that has also been described as lying within EW (Maciewicz et al. 1983). The most widespread of these peptides is urocortin-1, a neuropeptide first described in rodents (Vaughan et al. 1995). It is part of a corticotropin-releasing factor family, made of four known peptides, corticotropin-releasing factor (CRF), urocortin-1, urocortin-2 and urocortin-3 (Bale and Vale 2004; Gysling et al. 2004; Ryabinin et al. 2005). The major peptide expressed within EW from this group is urocortin-1 (Kozicz et al. 2011). Terminals containing urocortin-1 are found in most areas of the brain, however, the cells from which most of these terminals originate can only be found in select areas (Bachtell et al. 2002; Kozicz et al. 1998; Yamamoto et al. 1998). In rodents, urocortin-1 in EW appears to play a role in drinking behavior and appetite (Ryabinin et al. 2012; Weitemier and Ryabinin 2006), and it has been associated with stress and reward (Gaszner et al. 2004; Kozicz et al. 2001). In addition to urocortin-1, other peptides found within EW in cats, rodents and frogs include neuronal populations immunoreactive for CCK, substance P, cocaine-and-amphetamine-regulated transcript (CART) and neuropeptide B [cat: (Maciewicz et al. 1983; Phipps et al. 1983); rat: (Dun et al. 2005; Hokfelt et al. 2002; Innis and Aghajanian 1986; Kozicz 2003; Kozicz et al. 1998); mouse: (Tanaka et al. 2003)]. Cells in this group also display catalytic enzymes for the production of nitric oxide in monkeys and cats (Erichsen and May 2012).
Initially, there was confusion between this peptidergic population and the cholinergic population of preganglionic motoneurons. This was due, in part, to the presence of species differences in their location and organization. However, direct comparison of the populations has led to a new terminology to describe them: the peptideric neurons have projections throughout the brain, and so are defined as Edinger-Westphal centrally projecting (EWcp) neurons, and the cholinergic motoneurons that supply the ciliary ganglion are defined as Edinger-Westphal preganglionic (EWpg) motoneurons (Horn et al. 2008; Kozicz et al. 2011; May et al. 2008a; May et al. 2008b). In monkeys, EWpg can be identified cytoarchitecturally as a nucleus lying dorsal to III (see figs 4A+F & 6A+E), but EWcp is more diffuse. These peptidergic cells are found between III in the same region as the S-group, extend between the C-group and EWpg, and then fan out within SOA. EWcp neurons clearly lie within the cMRF terminal field and so may be targeted by this projection. Since these cells are not apparently related to eye movements, we must temper our assumption that the cMRF projection is necessarily entirely related to eye movements.
Near response cells
Outside of the olivary pretectal nucleus inputs subserving the pupillary light reflex, little is known about the anatomy of inputs to the EW preganglionic motoneurons. Near response inputs would presumably be found, bilaterally, on all the components driving the near triad, including: preganglionic motoneurons for lens accommodation and pupillary constriction, as well as medial rectus motoneurons for convergence of the eyes. In fact, a set of near-response neurons with activity patterns that are linearly related to either vergence angle (eye position within the orbit) or vergence velocity (the dynamics of eye movement) have been recorded near III (triangles, Fig. 11B) (Das 2012; Judge and Cumming 1986; Mays 1984; Mays and Porter 1984; Mays et al. 1986). Near response neurons can also display activity that correlates with lens accommodation (Morley et al. 1992; Zhang et al. 1992). They can be antidromically driven from stimulating electrodes placed in the medial rectus subdivision of ipsilateral III (Zhang et al. 1992). Most near response cells were activated for convergence, while a smaller population fired for divergence. These studies suggested that midbrain near response neurons lie dorsolateral to III, and so might be found in the SOA (May et al. 1992). If these cells are indeed located within SOA, then they also represent a putative target of the cMRF projection. This may be clinically important, as strabismus affects these near response circuits (Das 2011; Das 2012).
Functional implications
A cMRF role in horizontal, conjugate gaze
Cohen and colleagues (1985 & 1986) showed that electrical stimulation of the cMRF produced contraversive, horizontal saccades. Single-unit neurophysiological recordings from this same region of the midbrain tegmentum revealed that the neurons were constitutively active when the eyes were held fixed in the center of the orbit, increased firing before and during visually guided contraversive saccades, and decreased firing or were silent during ipsiversive saccades. Recordings revealed that the movement fields of the cMRF neurons are preferentially distributed along the horizontal meridian, but also included varying degrees of vertical signal, as well (Cohen et al. 1986; Cromer and Waitzman 2006; Handel and Glimcher 1997; Waitzman et al. 1996). The bilateral cMRF projections observed in this study could fulfill the necessary projections for conjugate, saccadic movements, if the cMRF terminals target medial rectus motoneurons. An excitatory input to ipsilateral medial rectus motoneurons and an inhibitory input to contralateral medial rectus motoneurons would provide an appropriate input to induce conjugate horizontal saccadic eye movements. Furthermore, one might expect the opposite pattern of inhibitory and excitatory projection to the abducens motoneurons. In fact, we detected a bilateral projection to the region of the SOA that contains medial rectus and lateral rectus MIF motoneurons in this study. If these terminals actually target motoneurons, this could provide an anatomical substrate that would explain the oculomotor behaviors observed following stimulation of the cMRF. There is evidence from transneuronal retrograde tracing studies that the cMRF does indeed contain premotor neurons (Graf et al. 2002; Ugolini et al. 2006). There is also evidence that the cMRF produces both GABAergic, presumably inhibitory, and non-GABAergic, presumably excitatory projections to other targets (Appell and Behan 1990; Wang et al. 2013; Wang et al. 2010). Arguing against this interpretation is the fact that the sparse cMRF terminal field observed within the borders of III was not specifically localized to the medial rectus SIF motoneuron pools.
A cMRF role in the near-triad
If the bilateral projection by the cMRF contacts medial rectus motoneurons, they could also supply an anatomical substrate for vergence eye movements. A bilateral excitatory input from the cMRF would be appropriate for convergence or a bilateral inhibitory input could subserve divergence. Vergence, along with lens accommodation and pupillary constriction, are part of the near triad that adapts the eyes with respect to target distance. Lens accommodation and pupillary constriction are controlled by motoneurons in EWpg. This study has documented a dense, bilateral projection to this nucleus, as well as to the part of the SOA that is inhabited by medial rectus MIF motoneurons. A recent study of the latter population has suggested that they may play a special role in near triad functions (Erichsen et al. 2014). Indeed, multiply innervated fibers show graded responses, not fast twitch responses (Hess and Pilar, 1963). Furthermore, symmetric vergence movements are slower than saccades, and saccades made between targets at different distances from the viewer are slowed (Collewijn et al. 1995; Erkelens et al. 1989; van Leeuwen et al. 1998). Thus, the possibility that the cMRF input to the SOA underlies near triad function seems to fit our findings. Arguing against this interpretation is the fact that the cMRF has not been associated with a role in near triad control, only contraversive, horizontal saccades. However, it is possible that electrical stimulation produces these saccades through the cMRF’s descending projections to the pons (Edwards 1975) and that the vergence effects were swamped by these saccade effects. Furthermore, the majority of studies employing neurophysiological recording paired with eye movements were limited by the fact that these were only reporting on conjugate, saccadic eye movements and only measured the movements of one eye. In fact, vergence velocity neurons have been described as lying lateral to III (Mays et al. 1986). This location would place the recorded cells within the medial third of the population of cMRF cells described in the present study (Figs. 9&10).
A cMRF role in disconjugate saccades
A single report from Waitzman et al. (2008) describes neurons within the cMRF that do not encode conjugate information during saccades. In this study, animals were trained to make disjunctive saccades in which one eye moved, while the other did not. Single-unit recording from neurons within the cMRF revealed that some neurons can encode conjugate movements of both eyes, but others fired preferentially for movements of a single eye, i.e. they were eye-specific (Waitzman et al. 2008). Furthermore, stimulation at some sites resulted in horizontal, contraversive saccades, while stimulation at other sites elicited a disconjugate, movement. The direct projections from the cMRF to the SOA may be an integral component for disjunctive saccadic eye movements, by allowing the nervous system to have independent control of each eye.
However, at present, the projections of the eye-specific cMRF neurons are unknown. The area of the cMRF terminations also includes the other populations of MIF motoneurons: the inferior rectus in the C-group and the superior rectus and inferior oblique in the S-group. If the cMRF provides substantial input to any of these populations, it would argue against this pathway playing a role in either conjugate or convergent horizontal gaze. Thus, it is clear that to understand the role the cMRF plays, we will need to identify the specific targets of its terminal projections to the SOA by examining the relationship of these axon terminals with the various populations of neurons sitting within the cMRF terminal field: motoneurons of the oculomotor nucleus, preganglionic motoneurons in EWpg controlling the intrinsic eye muscles, or even the diffusely projecting peptidergic neurons in EWcp that are unrelated to movements of the eyes.
Acknowledgments
This work was supported by a National Institutes of Health, National Eye Institute grant EY014263 to Paul J. May, PhD and Susan Warren, PhD. We are grateful to Jinrong Wei for her technical support in the histological processing of the tissue.
ABBREVIATIONS
- III
Oculomotor Nucleus
- IIIn
Oculomotor Nerve
- IV
Trochlear Nucleus
- AM
Anteromedian Nucleus
- BC
Brachium Conjunctivum
- BDA
Biotinylated Dextran Amine
- CC
Caudal Central subdivision
- CG
Central Gray
- cMRF
Central Mesencephalic Reticular Formation
- DR
Dorsal Raphe
- EWcp
Edinger-Westphal, Centrally Projecting Nucleus
- EWpg
Edinger-Westphal, Preganglionic Nucleus
- FR
Fasciculus Retroflexus
- InC
Interstitial nucleus of Cajal
- LL
Lateral Lemniscus
- MD
Mediodorsal Thalamic Nucleus
- MGN
Medial Geniculate Nucleus
- MIF
Multiply Innervated Fibers
- MLF
Medial Longitudinal Fasciculus
- MRF
Midbrain Reticular Formation
- nD
Nucleus of Darkschewitsch
- nPC
Nucleus of the Posterior Commissure
- OPt
Olivary Pretectal Nucleus
- P
Pyramidal Tract
- PAG
Periaqueductal Gray
- PC
Posterior Commissure
- PhaL
Phaseolus vulgaris Leucoagglutinin
- piMRF
Peri-Interstitial Nucleus of Cajal region of the MRF
- PPRF
Paramedian Pontine Reticular Formation
- PRF
Pontine Reticular Formation
- Pul
Pulvinar
- RCL
Caudal Linear Nucleus of the Raphe
- RN
Red Nucleus
- SIF
Singly Innervated Fibers
- SO
Superior Olivary Nucleus
- SOA
Supraoculomotor Area
- VI
Abducens Nucleus
- VIIn
Facial Nerve
- Vm
Trigeminal Motor Nucleus
- WGA-HRP
Wheatgerm Agglutinin Conjugated Horseradish Peroxidase
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest pursuant to the publication of this research.
Reference List
- Adams JC. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980;184(2):491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302(1):143–158. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302(2):516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bender MB, Shanzer S. Oculomotor pathways defined by electrical stimulation and lesions in the brainstem of monkey. In: Bender MB, editor. The Oculomotor System. Hoeber Medical Division, Harper & Row; New York, USA: 1964. pp. 81–140. [Google Scholar]
- Blanks RH, Clarke RJ, Lui F, Giolli RA, Van PS, Torigoe Y. Projections of the lateral terminal accessory optic nucleus of the common marmoset (Callithrix jacchus) J Comp Neurol. 1995;354(4):511–532. doi: 10.1002/cne.903540404. [DOI] [PubMed] [Google Scholar]
- Burde RM, Loewy AD. Central origin of oculomotor parasympathetic neurons in the monkey. Brain Res. 1980;198(2):434–439. doi: 10.1016/0006-8993(80)90757-X. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Akert K. Medial rectus subgroups of the oculomotor nucleus and their abducens internuclear input in the monkey. J Comp Neurol. 1981;197(1):17–27. doi: 10.1002/cne.901970103. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366(2):348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AK, Scherberger H, D’Ascanio P. Motoneurons of twitch and nontwitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438(3):318–335. doi: 10.1002/cne.1318. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AK, Schmidtke K. Cell groups of the medial longitudinal fasciculus and paramedian tracts. Rev Neurol (Paris) 1989;145(8–9):533–539. [PubMed] [Google Scholar]
- Castiglioni AJ, Gallaway MC, Coulter JD. Spinal projections from the midbrain in monkey. J Comp Neurol. 1978;178(2):329–346. doi: 10.1002/cne.901780208. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. The feedback circuit connecting the superior colliculus and central mesencephalic reticular formation: a direct morphological demonstration. Exp Brain Res. 2000;131(1):10–21. doi: 10.1007/s002219900280. [DOI] [PubMed] [Google Scholar]
- Cohen B, Büttner-Ennever JA. Projections from the superior colliculus to a region of the central mesencephalic reticular formation (cMRF) associated with horizontal saccadic eye movements. Exp Brain Res. 1984;57(1):167–176. doi: 10.1007/BF00231143. [DOI] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Fradin J, Raphan T. Horizontal saccades induced by stimulation of the central mesencephalic reticular formation. Exp Brain Res. 1985;57(3):605–616. doi: 10.1007/BF00237847. [DOI] [PubMed] [Google Scholar]
- Cohen B, Waitzman DM, Büttner-Ennever JA, Matsuo V. Horizontal saccades and the central mesencephalic reticular formation. Prog Brain Res. 1986;64:243–256. doi: 10.1016/S0079-6123(08)63419-6. [DOI] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Voluntary binocular gaze-shifts in the plane of regard: dynamics of version and vergence. Vision Res. 1995;35(23–24):3335–3358. doi: 10.1016/0042-6989(95)00082-P. [DOI] [PubMed] [Google Scholar]
- Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503(2):265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Neurones associated with saccade metrics in the monkey central mesencephalic reticular formation. J Physiol. 2006;570(3):507–523. doi: 10.1113/jphysiol.2005.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Comparison of saccade-associated neuronal activity in the primate central mesencephalic and paramedian pontine reticular formations. J Neurophysiol. 2007;98(2):835–850. doi: 10.1152/jn.00308.2007. [DOI] [PubMed] [Google Scholar]
- Das VE. Cells in the supraoculomotor area in monkeys with strabismus show activity related to the strabismus angle. Ann N Y Acad Sci. 2011;1233:85–90. doi: 10.1111/j.1749-6632.2011.06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das VE. Responses of cells in the midbrain near-response area in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2012;53(7):3858–3864. doi: 10.1167/iovs.11-9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Mizuo K, Yang J, Chang JK, Dun NJ. Neuropeptide B immunoreactivity in the central nervous system of the rat. Brain Res. 2005;1045(1–2):157–163. doi: 10.1016/j.brainres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Eberhorn AC, Ardeleanu P, Büttner-Ennever JA, Horn AK. Histochemical differences between motoneurons supplying multiply and singly innervated extraocular muscle fibers. J Comp Neurol. 2005;491(4):352–366. doi: 10.1002/cne.20715. [DOI] [PubMed] [Google Scholar]
- Eberhorn AC, Büttner-Ennever JA, Horn AK. Identification of motoneurons supplying multiply- or singly-innervated extraocular muscle fibers in the rat. Neuroscience. 2006;137(3):891–903. doi: 10.1016/j.neuroscience.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Edwards SB. Autoradiographic studies of the projections of the midbrain reticular formation: descending projections of nucleus cuneiformis. J Comp Neurol. 1975;161(3):341–358. doi: 10.1002/cne.901610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB, de Olmos JS. Autoradiographic studies of the projections of the midbrain reticular formation: ascending projections of nucleus cuneiformis. J Comp Neurol. 1976;165(4):417–431. doi: 10.1002/cne.901650403. [DOI] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184(2):309–329. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- Edwards SB, Henkel CK. Superior colliculus connections with the extraocular motor nuclei in the cat. J Comp Neurol. 1978;179(2):451–467. doi: 10.1002/cne.901790212. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, May PJ. The pupillary and ciliary components of the cat Edinger-Westphal nucleus: a transsynaptic transport investigation. Vis Neurosci. 2002;19(1):15–29. doi: 10.1017/S0952523801191029. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, May PJ. A perioculomotor nitridergic population in the macaque and cat. Invest Ophthalmol Vis Sci. 2012;53(9):5751–5761. doi: 10.1167/iovs.12-10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen JT, Wright NF, May PJ. Morphology and ultrastructure of medial rectus subgroup motoneurons in the macaque monkey. J Comp Neurol. 2014;522(3):626–641. doi: 10.1002/cne.23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkelens CJ, Steinman RM, Collewijn H. Ocular vergence under natural conditions. II. Gaze shifts between real targets differing in distance and direction. Proc R Soc Lond B Biol Sci. 1989;236(1285):41–465. doi: 10.1098/rspb.1989.0030. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Zhang Y, Clendaniel RA, Mays LE. Behavior of identified Edinger-Westphal neurons during ocular accommodation. J Neurophysiol. 1994;72(5):2368–2382. doi: 10.1152/jn.1994.72.5.2368. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480(2):170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290(2):219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf W, Gerrits N, Yatim-Dhiba N, Ugolini G. Mapping the oculomotor system: the power of transneuronal labelling with rabies virus. Eur J Neurosci. 2002;15(9):1557–1562. doi: 10.1046/j.1460-9568.2002.01994.x. [DOI] [PubMed] [Google Scholar]
- Graham J. An autoradiographic study of the efferent connections of the superior colliculus in the cat. J Comp Neurol. 1977;173(4):629–654. doi: 10.1002/cne.901730403. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46(2):243–256. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Gysling K, Forray MI, Haeger P, Daza C, Rojas R. Corticotropin-releasing hormone and urocortin: redundant or distinctive functions? Brain Res Rev. 2004;47(1–3):116–125. doi: 10.1016/j.brainresrev.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Response properties of saccade-related burst neurons in the central mesencephalic reticular formation. J Neurophysiol. 1997;78(4):2164–2175. doi: 10.1152/jn.1997.78.4.2164. [DOI] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior collicullus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173(3):583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Hess A, Pilar G. Slow fibers in the extraocular muscles of the cat. J Physiol. 1963;169(4):780–798. doi: 10.1113/jphysiol.1963.sp007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Blacker D, Broberger C, Herrera-Marschitz M, Snyder G, Fisone G, Cortes R, Morino P, You ZB, Ogren SO. Some aspects on the anatomy and function of central cholecystokinin systems. Pharmacol Toxicol. 2002;91(6):382–386. doi: 10.1034/j.1600-0773.2002.910617.x. [DOI] [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Hartig W, Ardeleanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507(3):1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Cholecystokinin-containing and nociceptive neurons in rat Edinger-Westphal nucleus. Brain Res. 1986;363(2):230–238. doi: 10.1016/0006-8993(86)91008-5. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55(5):915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki A, Alpert J, Harris HE, Cohen B. Effects of mesencephalic reticular formation lesions on optokinetic nystagmus. Exp Neurol. 1972;34(3):522–534. doi: 10.1016/0014-4886(72)90047-7. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116(2):315–320. doi: 10.1016/S0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J Comp Neurol. 2011;519(8):1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Li M, Arimura A. The activation of urocortin immunoreactive neurons in the Einger-Westphal nucleus following stress in rats. Stress. 2001;4(2):85–90. doi: 10.3109/10253890109115724. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391(1):1–10. doi: 10.1002/(SICI)1096-9861(19980202)391:1<1::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Maciewicz R, Phipps BS, Foote WE, Aronin N, DiFiglia M. The distribution of substance P-containing neurons in the cat Edinger-Westphal nucleus: relationship to efferent projection systems. Brain Res. 1983;270(2):217–230. doi: 10.1016/0006-8993(83)90595-4. [DOI] [PubMed] [Google Scholar]
- May PJ, Bohlen MO, Warren S. Morphology and distribution of two inputs to the oculomotor nucleus in the macaque. Soc Neurosci Abst. 2013;39:363.03. [Google Scholar]
- May PJ, Porter JD, Gamlin PD. Interconnections between the primate cerebellum and midbrain near-response regions. J Comp Neurol. 1992;315(1):98–116. doi: 10.1002/cne.903150108. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008a;507(3):1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Sun W, Erichsen JT. Defining the pupillary component of the perioculomotor preganglionic population within a unitary primate Edinger-Westphal nucleus. Prog Brain Res. 2008b;171:97–106. doi: 10.1016/S0079-6123(08)00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984;51(5):1091–1108. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD. Neural control of vergence eye movements: activity of abducens and oculomotor neurons. J Neurophysiol. 1984;52(4):743–761. doi: 10.1152/jn.1984.52.4.743. [DOI] [PubMed] [Google Scholar]
- Mays LE, Porter JD, Gamlin PD, Tello CA. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986;56(4):1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- Morley JW, Judge SJ, Lindsey JW. Role of monkey midbrain near-response neurons in phoria adaptation. J Neurophysiol. 1992;67(6):1475–1492. doi: 10.1152/jn.1992.67.6.1475. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I Morphological classification of efferent neurons. J Neurophysiol. 1988a;60(1):232–262. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II Morphological identity of presaccadic neurons. J Neurophysiol. 1988b;60(1):263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- Nelson JS, Goldberg SJ, McClung JR. Motoneuron electrophysiological and muscle contractile properties of superior oblique motor units in cat. J Neurophysiol. 1986;55(4):715–726. doi: 10.1152/jn.1986.55.4.715. [DOI] [PubMed] [Google Scholar]
- Olucha F, Martinez-Garcia F, Lopez-Garcia C. A new stabilizing agent for the tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP) J Neurosci Methods. 1985;13(2):131–138. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Cromer JA, Cullen KE, Waitzman DM. Temporal characteristics of neurons in the central mesencephalic reticular formation of head unrestrained monkeys. Exp Brain Res. 2006a;168(4):471–492. doi: 10.1007/s00221-005-0105-z. [DOI] [PubMed] [Google Scholar]
- Pathmanathan JS, Presnell R, Cromer JA, Cullen KE, Waitzman DM. Spatial characteristics of neurons in the central mesencephalic reticular formation (cMRF) of head-unrestrained monkeys. Exp Brain Res. 2006b;168(4):455–470. doi: 10.1007/s00221-005-0104-0. [DOI] [PubMed] [Google Scholar]
- Perkins E, May PJ, Warren S. Feed-forward and feedback projections of midbrain reticular formation neurons in the cat. Front Neuroanat. 2014;7:55. doi: 10.3389/fnana.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E, Warren S, May PJ. The mesencephalic reticular formation as a conduit for primate collicular gaze control: tectal inputs to neurons targeting the spinal cord and medulla. Anat Rec (Hoboken) 2009;292(8):1162–1181. doi: 10.1002/ar.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps BS, Maciewicz R, Sandrew BB, Poletti CE, Foote WE. Edinger-Westphal neurons that project to spinal cord contain substance P. Neurosci Lett. 1983;36(2):125–131. doi: 10.1016/0304-3940(83)90253-7. [DOI] [PubMed] [Google Scholar]
- Porter JD, Guthrie BL, Sparks DL. Innervation of monkey extraocular muscles: localization of sensory and motor neurons by retrograde transport of horseradish peroxidase. J Comp Neurol. 1983;218(2):208–219. doi: 10.1002/cne.902180208. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346(1):43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;134(4):1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsoory MM, Kozicz T, Thiele TE, Neufeld-Cohen A, Chen A, Lowery-Gionta EG, Giardino WJ, Kaur S. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46(4):349–357. doi: 10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoda T, Matsumoto H, Zhou L, Rose PK, Richmond FJ. Mesencephalic projections to the first cervical segment in the cat. Exp Brain Res. 2002;144(3):397–413. doi: 10.1007/s00221-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol. 1990;301(4):618–642. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271(4):493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Sun W, May PJ. Central pupillary light reflex circuits in the cat: I. The olivary pretectal nucleus. J Comp Neurol. 2014;522:3960–3977. doi: 10.1002/cne.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis) J Comp Neurol. 1984;222(2):265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yoshida T, Miyamoto N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, Garry MG, Lerner MR, King DS, O’Dowd BF, Sakurai T, Yanagisawa M. Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100(10):6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Doldan DM, Dubayle D, Brandi AM, Büttner-Ennever JA, Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol. 2006;498(6):762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- van Leeuwen AF, Collewijn H, Erkelens CJ. Dynamics of horizontal vergence movements: interaction with horizontal and vertical saccades and relation with monocular preferences. Vision Res. 1998;38(24):3943–3954. doi: 10.1016/S0042-6989(98)00092-3. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378(6554):287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, Cohen B. Central mesencephalic reticular formation (cMRF) neurons discharging before and during eye movements. J Neurophysiol. 1996;75(4):1546–1572. doi: 10.1152/jn.1996.75.4.1546. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. I Hypermetric goal-directed saccades. J Neurophysiol. 2000a;83(4):2260–2284. doi: 10.1152/jn.2000.83.4.2260. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. II Hypometric vertical saccades. J Neurophysiol. 2000b;83(4):2285–2299. doi: 10.1152/jn.2000.83.4.2285. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Van Horn MR, Cullen KE. Neuronal evidence for individual eye control in the primate cMRF. Prog Brain Res. 2008;171:143–150. doi: 10.1016/S0079-6123(08)00619-5. [DOI] [PubMed] [Google Scholar]
- Wang N, Perkins E, Zhou L, Warren S, May PJ. Anatomical evidence that the superior colliculus controls saccades through central mesencephalic reticular formation gating of omnipause neuron activity. J Neurosci. 2013;33(41):16285–16296. doi: 10.1523/JNEUROSCI.2726-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Warren S, May PJ. The macaque midbrain reticular formation sends side-specific feedback to the superior colliculus. Exp Brain Res. 2010;201(4):701–717. doi: 10.1007/s00221-009-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Waitzman DM, May PJ. Anatomical evidence for interconnections between the central mesencephalic reticular formation and cervical spinal cord in the cat and macaque. Anat Rec (Hoboken) 2008;291(2):141–160. doi: 10.1002/ar.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat. 1954;88(1):71–93. [PMC free article] [PubMed] [Google Scholar]
- Wasicky R, Horn AK, Büttner-Ennever JA. Twitch and nontwitch motoneuron subgroups in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479(2):117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137(4):1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Maeda T, Fujimura M, Fujimiya M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger-Westphal nucleus of rat. Neurosci Lett. 1998;243(1–3):21–24. doi: 10.1016/S0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mays LE, Gamlin PD. Characteristics of near response cells projecting to the oculomotor nucleus. J Neurophysiol. 1992;67(4):944–960. doi: 10.1152/jn.1992.67.4.944. [DOI] [PubMed] [Google Scholar]
- Zhou L, Warren S, May PJ. The feedback circuit connecting the central mesencephalic reticular formation and the superior colliculus in the macaque monkey: tectal connections. Exp Brain Res. 2008;189(4):485–496. doi: 10.1007/s00221-008-1444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]