Abstract
Accumulating evidence suggests that CNS α7 nicotinic acetylcholine receptors (nAChRs) are important targets for the development of therapeutic approaches for Parkinson’s disease. This progressive neurodegenerative disorder is characterized by debilitating motor deficits, as well as autonomic problems, cognitive declines, changes in affect and sleep disturbances. Currently L-dopa is the gold standard treatment for Parkinson’s disease motor problems, particularly in the early disease stages. However, it does not improve the other symptoms, nor does it reduce the inevitable disease progression. Novel therapeutic strategies for Parkinson’s disease are therefore critical. Extensive pre-clinical work using a wide variety of experimental models shows that nicotine and nAChR agonists protect against damage to nigrostriatal and other neuronal cells. This observation suggests that nicotine and/or nAChR agonists may be useful as disease modifying agents. Additionally, studies in several parkinsonian animal models including nonhuman primates show that nicotine reduces L-dopa-induced dyskinesias, a side effect of L-dopa therapy that may be as incapacitating as Parkinson’s disease itself. Work with subtype selective nAChR agonists indicate that α7 nAChRs are involved in mediating both the neuroprotective and antidyskinetic effects, thus offering a targeted strategy with optimal beneficial effects and minimal adverse responses. Here, we review studies demonstrating a role for α7 nAChRs in protection against neurodegenerative effects and for the reduction of L-dopa-induced dyskinesias. Altogether, this work suggests that α7 nAChRs may be useful targets for reducing Parkinson’s disease progression and for the management of the dyskinesias that arise with L-dopa therapy.
Keywords: Alpha7, L-dopa-induced dyskinesias, Neuroprotection, Nicotinic receptors, Parkinson’s disease
Graphical Abstract
1. Introduction
The idea that nicotine may be useful as a therapy for Parkinson’s disease initially stemmed from the results of epidemiological studies [1–6]. An extensive literature demonstrated a reduced risk of Parkinson’s disease among current and former smokers, which correlated with smoking duration, intensity, and recentness [7, 8]. This decline in Parkinson’s disease was also observed with other forms of tobacco and with environmental smoke exposure [9, 10]. Moreover, the incidence of Parkinson’s disease within twin pairs was less in the twin that smoked compared to the nonsmoker [11]. A recent report suggested that the apparent neuroprotective effect may be due to the propensity of Parkinson’s disease patients to quit smoking early rather than to neuroprotection [12]. However, the greater majority of studies are consistent with the idea that the reduced frequency of Parkinson’s disease is due to a true biological effect of tobacco use.
The results of the above studies, coupled with the finding that nicotine, a key component in tobacco products, stimulates dopamine release [13–16] led to the premise that nicotine may underlie the beneficial effect of smoking in Parkinson’s disease. Extensive studies now substantiate this hypothesis. Experimental evidence shows that nicotine partially protects against nigrostriatal and other forms of CNS damage. Moreover, nicotine administration reduces the abnormal involuntary movements or dyskinesias that arise as a side effect of L-dopa treatment for Parkinson’s disease motor symptoms. Multiple nAChR populations exist in the brain, with several subtypes implicated in these beneficial effects of nicotine. This review will focus on the role of α7 nAChRs and discuss the potential utility of α7 nAChR agonists as a novel treatment strategy for Parkinson’s disease to reduce disease progression and alleviate L-dopa-induced dyskinesias (LIDs).
2. α7 nAChRs
Neuronal nAChRs are pentameric ligand-gated ion channels composed of diverse combinations of α and β transmembrane subunits [17–19]. There is a mandatory requirement for an α subunit, which binds the naturally occurring neurotransmitter acetylcholine and also exogenous agonists such as nicotine. In addition, β subunits may be present in the receptor complex; these do not express a neurotransmitter recognition site although they contribute significantly to receptor properties. The main nAChR subtypes in the brain expressing both α and β subunits are the α4β2*, α6β2* and α3β4* populations, with the asterisk indicating the possible presence of other nAChR subunits in the receptor complex. Another primary nAChR subtype in the brain is one composed only of α7 subunits (Fig. 1). Not only are α7 nAChRs structurally different from the other subtypes, they are also phylogenetically and functionally distinct [18, 20]. They display the lowest nicotine sensitivity and exhibit the fastest desensitization kinetics of all nAChRs, a property which initially made their functional characterization very difficult. They have a very high calcium permeability which allows them to regulate numerous calcium-dependent cellular mechanisms important for optimal CNS function [21, 22].
Fig. 1.
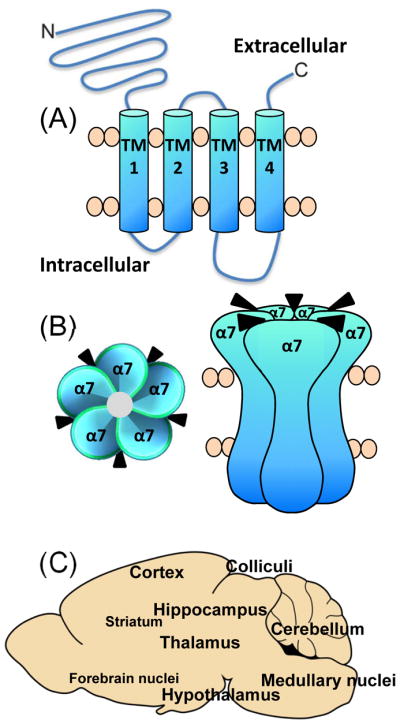
Schematic representation of α7 nAChR structure and localization. The α7 nAChR subunit consists of a large extracellular N-terminal region, four transmembrane (TM) domains and an extracellular carboxy-terminal (A). The α7 receptor is membrane bound and composed of 5 identical α subunits, with five agonist binding sites, with (B) depicting a top view and a side view of the receptor in the cell membrane. α7 nAChRs are very widely distributed in numerous brain regions (C). The smaller font size represents a lower density of α7 nAChRs in the various brain areas.
α7 nAChRs are uniquely localized throughout the brain, although there is substantial overlap in their distribution with other nAChR subtypes. α7 nAChRs are very widespread, with a dense localization in regions such as the hypothalamus, geniculate nuclei, colliculi, hippocampus, medial habenula, thalamus, cortex, amygdala, and sparse expression in striatum, forebrain, medulla and various brain nuclei (Fig. 1) [23–29]. Consistent with their extensive CNS localization, α7 nAChRs are implicated in numerous functions including development, maintenance, survival, synaptic plasticity, neurotransmitter release and/or immune responsiveness. Their acute and long term effects on these cellular processes may modulate behaviors such as anxiety, attention, learning, memory, movement and sensory gating, with consequent implications for Alzheimer’s disease, Parkinson’s disease, schizophrenia, traumatic brain injury, autism, addiction, pain and immune/inflammatory disorders [30–36].
3. α7 nAChRs and neuroprotection
3.1. Trophic role and protection against toxic insults
A trophic role for α7 nAChRs was originally suggested several decades ago [37, 38]. Studies using cultured cells showed that drugs acting at α7 nAChRs modulated neuritic outgrowth via α7 nAChR-mediated alterations in intracellular calcium [39–41]. Further support for a trophic action stemmed from experiments in intact animals, which demonstrated that α7 nAChR expression was modified with neuronal development, growth, maintenance and survival [42–45].
The idea that α7 nAChRs had a role in neuroprotection initially arose from results showing that α7 nAChR antagonists prevented nicotine, choline or acetylcholinesterase inhibitor-mediated protection against toxic insults in neuronal cell cultures (Table 1). α7 nAChR antagonists such as methyllycaconitine (MLA) or α-bungarotoxin (α-BTX) attenuated nAChR-mediated protection against amyloid-β, glutamate and NMDA toxicity, as well as growth factor and oxygen-glucose deprivation in cell lines and primary neuronal cultures [46–58].
Table 1.
α7 nAChR-mediated protection against induced toxicity in culture and in vivo.
| Toxic insult | Model | Cholinergic drug | Neuroprotection prevented by | References |
|---|---|---|---|---|
| Amyloid-β | Rat cortical cultures | Nicotine | α-BTX, MLA | [47, 48, 51, 52] |
| Rat cortical cultures | DMXB | --- | [47] | |
| SH-SY5Y cell line | Galantamine | α-BTX, MLA | [59, 60, 148] | |
| PC12 cell line | TC-1698 | --- | [62] | |
| Glutamate | Rat cortical cultures | Nicotine | α-BTX, MLA | [48, 49, 51, 55] |
| Rat spinal cultures | Nicotine | α-BTX | [53] | |
| Rat cortical cultures | Galantamine | --- | [48] | |
| Rat cortical cultures | Donepezil | MLA | [46, 57] | |
| NMDA | Rat hippocampal cultures | Nicotine | α-BTX, MLA | [56] |
| Mouse cortical cultures | Nicotine | Dominant negative α7 knockin | [70] | |
| Growth factor deprivation | PC12 cell line | Nicotine | MLA | [54] |
| PC12 cell line | DMXB | MLA | [64] | |
| PC12 cell line | Pyrrolidinecholine | MLA | [58] | |
| Ethanol | Rat hippocampal cultures | DMXB | MLA | [65, 66] |
| Mouse cortical cultures | --- | α7 knockout | [72] | |
| Oxygen-glucose deprivation | Mouse hippocampal slices | Nicotine | α7 knockout | [69] [68] |
| Okadaic acid | SH-SY5Y cell line | PNU-282987 | PNU-120596 | [63] |
| Oxidative stress | SH-SY5Y cell line | PNU-282987 | MLA | [61] |
| Amyloid-β | Mouse in vivo | --- | α7 knockout | [73] |
| Ischemia | Mouse in vivo | Neostigmine + anisodamine | α7 knockout | [71] |
| Septohippocampal axotomy | Mouse in vivo | 4OH-GTS-21 | --- | [130] |
Not done (---)
Conversely, α7 nAChR agonists afforded neuroprotection. For example, the α7 nAChR allosteric modulator galantamine decreased amyloid-β and glutamate-induced toxicity in cell lines or cortical cultures [48, 59, 60]. Additionally, the α7 nAChR agonist TC-1698 reduced amyloid-β toxicity in pheochromocytoma (PC12) cells, while PNU-282987 protected SH-SY5Y cells against oxidative stress and okadaic acid-induced toxicity [61–63]. Another agonist 3-[2,4-dimethoxybenzylidene]anabaseine (DMXB) attenuated amyloid-β and ethanol-induced toxicity, as well as growth factor deprivation, in both cell lines and primary cultures [47, 64–66]. Furthermore, DMXB protected cholinergic neurons against septohippocampal axotomy in mice, demonstrating effectiveness in vivo [67].
The use of α7 knockout mice also proved valuable in demonstrating a protective role for α7 nAChRs. Nicotine failed to protect against oxygen-glucose deprivation in hippocampal slices from α7 knockout mice and against NMDA toxicity in cortical cultures from transgenic mice expressing dominant-negative α7 nAChRs [68–70]. In addition, while combined neostigmine/anisodamine treatment was protective in a mouse model of ischemia, no effect was observed in α7 nAChR knockout mice [71]. Deletion of α7 nAChRs also worsened ethanol-induced toxicity in primary cortical cultures and exacerbated early-stage cognitive declines in a mouse model of Alzheimer’s disease [72, 73].
Overall, results using several different approaches including the use of receptor targeted drugs and genetically modified mice implicate α7 nAChRs in neuroprotection against varying toxic insults in multiple neuronal systems.
3.2. Protection against nigrostriatal damage in parkinsonian animal models
A role for α7 nAChRs in protection against dopaminergic degeneration initially stemmed from studies using the general nAChR agonist nicotine [74–76]. The first of these by Janson and coworkers showed that nicotine administered before or at the time of lesioning significantly improved both striatal and nigral dopaminergic markers in rats with a hemi-transection of the medial forebrain bundle [77–79]. Since then the neuroprotective effect of nicotine has been demonstrated in numerous rodent models of dopaminergic nigrostriatal damage. This includes protection against 6-hydroxydopamine (6-OHDA)-induced nigrostriatal damage in rats [80–83] and against MPTP-induced nigrostriatal degeneration in mice. However, neuroprotection in this latter animal model did not consistently occur raising questions about nicotine’s protective potential [76, 84–88]. Subsequent work in parkinsonian nonhuman primates again showed that nicotine improved various striatal dopaminergic measures, including tyrosine hydroxylase, the dopamine transporter, the vesicular monoamine transporter and dopamine levels [89, 90]. These latter findings in a model exhibiting symptoms closely resembling those of the human disease, coupled with the rodent work, provide strong evidence for nicotine’s neuroprotective potential.
With respect to the nAChR subtypes involved in neuroprotection against nigrostriatal damage, pharmacological studies showed that the α4β2* nAChR agonist ABT-089 protected against 6-OHDA-induced nigrostriatal damage in rats (Table 2) [91]. By contrast, nicotine-mediated protection against nigrostriatal damage was not observed in α4 nAChR knockout mice, which lack α4β2* nAChRs (Table 2) [81]. Thus, drug and genetic studies support the idea that β2* nAChRs are important. Nicotine may exert its neuroprotective effect by chaperoning β2* nAChRs to the surface of the cell. This may induce changes in the structure and function of the endoplasmic reticulum, the Golgi apparatus and secretory vesicles of cells thereby reducing ER stress and enhancing cell survival [92, 93].
Table 2.
α7 and β2* nAChR-mediated protection against nigrostriatal toxicity in vivo
| nAChR subtype | Model system | Neuroprotection induced by nAChR agonist | Neuroprotection prevented by nAChR antagonist | References |
|---|---|---|---|---|
| β2* | 6-OHDA lesioned rat | ABT-089 | --- | [91] |
| Methamphetamine-treated α4 knockout mice | Nicotine | --- | [81] | |
|
| ||||
| α7 | MPTP-treated mouse | Nicotine | Methylycaconitine | [99, 136] |
| MPTP-treated mouse | PNU-282987 | --- | [98] | |
| 6-OHDA lesioned rat | DMXB | Methylycaconitine | [95, 96] | |
| 6-OHDA lesioned rat | ABT-107 | --- | [95] | |
The use of subtype selective α7 nAChR agonists and antagonists also supports a protective role for α7 nAChRs (Table 2) [74, 75, 94]. Drugs with varying agonist properties, such as the α7 nAChR allosteric modulator galantamine, and the α7 agonists DMXB and ABT-107 all protected against 6-OHDA-induced nigrostriatal damage in rats [95–97]. Additionally, the α7 agonist PNU-282987 protected against nigrostriatal damage in the MPTP-lesioned mouse [98], while α7 nAChR antagonists such as methylycaconitine blocked the neuroprotective effect of nicotine [99]. These studies with α7 nAChR agonists and antagonists provide compelling evidence for a role for these receptors in protection against nigrostriatal damage.
In summary, both α7 and β2* nAChR agonists reduce nigrostriatal damage in several parkinsonian animal models, suggesting that drugs targeting these receptors may be useful for attenuating progression of the motor symptoms that arise in Parkinson’s disease. As mentioned earlier, nicotine and nAChR agonists also reduce numerous other forms of neuronal toxicity. nAChR drugs may therefore also ameliorate non-motor symptoms that arise with Parkinson’s disease by reducing/halting other neurodegenerative processes in the brain.
4. α7 nAChR agonists reduce L-dopa-induced dyskinesias (LIDs)
In addition to a putative neuroprotective role, nicotine treatment may also be useful in the management of LIDs. These are debilitating abnormal involuntary movements that occur with L-dopa administration, the gold-standard therapy for Parkinson’s disease [100–103]. Although L-dopa very successfully treats Parkinson’s disease motor symptoms, LIDs develop in the majority of patients by 10 years on L-dopa treatment. The only approved pharmacological therapy is with amantadine; however, the beneficial effects of this drug are variable across patients and are generally lost with sustained treatment [103–105]. Although new drugs are under study, effective treatments for LIDs remain elusive.
Recent preclinical work in rodents and nonhuman primates indicates that drugs interacting with nAChRs may be of benefit in reducing LIDs (Table 3) [34]. Initial studies with the general nAChR agonist nicotine showed a 60% reduction in LIDs in parkinsonian monkeys and rodents, indicating effectiveness across species [106–111]. Importantly, nicotine’s effects persisted with long term treatment, with one study in nonhuman primates carried out for over a year [108]. Nicotine reduced LIDs whether given orally via the drinking water, by systemic injection or by minipump, showing the effect is independent of treatment mode. Varenicline, another general nAChR agonist [112–114] also reduced LIDs by ~50% (Table 3) [115]. At least a month was required for the antidyskinetic effect of nicotine to maximally develop [108]. Notably, such a time period was also required for the nicotine-mediated antidyskinetic effect to dissipate after drug discontinuation [115]. These combined observations suggest that long term molecular changes are most likely involved in the nicotine-mediated reduction in LIDs.
Table 3.
α7 and β2* and nAChR drugs decrease LIDs in parkinsonian nonhuman primates
| nAChR subtype | nAChR drug | Monkey model | Mode of treatment | % decline in LIDs | References |
|---|---|---|---|---|---|
| Multiple | Nicotine | Saimiri sciureus | Drinking water | ≤ 60% | [106–108] |
| Varenicline | Saimiri sciureus | Oral | ≤ 50% | [115] | |
|
| |||||
| α7 | ABT-107 | Saimiri sciureus | Oral | ≤ 60% | [149] |
| ABT-126 | Saimiri sciureus | Oral | ≤ 60% | [117] | |
| AQW051 | Macaca fascicularis | Oral | ≤ 60% | [118] | |
|
| |||||
| β2* | TC-8831 | Saimiri sciureus | Oral | ≤ 50% | [115] |
| TC-8831 | Macaca fascicularis | Oral | ≤ 50% | [119] | |
| ABT-089 | Saimiri sciureus | Oral | ≤ 50% | [122] | |
| ABT-894 | Saimiri sciureus | Oral | ≤ 60% | [122] | |
| AZD1446 | Macaca fascicularis | Oral | 30% | [121] | |
These data with nicotine prompted work to investigate the nAChR subtypes that contributed to the antidyskinetic effect. Two approaches that proved valuable included the use of genetically modified mice and drugs targetting the different nAChR subtypes. Genetic deletion of α7 nAChRs increased LIDs in L-dopa-treated parkinsonian mice compared to wildtype littermates suggesting that α7 nAChRs exert an inhibitory influence on expression of these abnormal movements [116]. However, nicotine still reduced LIDs in α7 nAChR null mutant mice, indicating that α7 nAChRs are not essential for nicotine’s antidyskinetic action. Similar studies with mice lacking the β2, α4 and α6 nAChR subunits yielded somewhat different results. Nicotine exerted no antidyskinetic effect in any of these knockout mice, suggesting that the α4β2* and α6β2* nAChRs are essential. In addition, mice lacking the β2 or α6 nAChR subunits expressed fewer LIDs compared to their wildtype littermates. Overall, the results indicate that α7 and β2* nAChRs regulate the expression of LIDs but in somewhat unique manners.
Pharmacological studies also support the idea that drugs interacting at α7 or β2* nAChR subtypes influence the occurrence of LIDs. Drugs that selectively act at α7 nAChRs decreased LIDs, with no change in parkinsonism when the animals were administered L-dopa (on L-dopa) or in the absence of the drug (off L-dopa) (Table 3). This includes work in Saimiri sciureus in which the α7 agonist ABT-107 reduced LIDs ~60% (Table 3, Fig. 2). The decrease persisted with several months of treatment [115]. Notably, the ABT-107-induced antidyskinetic effect persisted for about a month after its discontinuation suggesting that long term molecular changes were involved. A study with another α7 agonist ABT-126 yielded similar results (Table 3) [117]. Administration of the α7 nAChR agonist AQW051 to Macaca fascicularis also resulted in ~60% decrease in LIDs, with no worsening of parkinsonism [118], demonstrating the effectiveness of α7 nAChR agonists in another nonhuman primate.
Fig. 2.
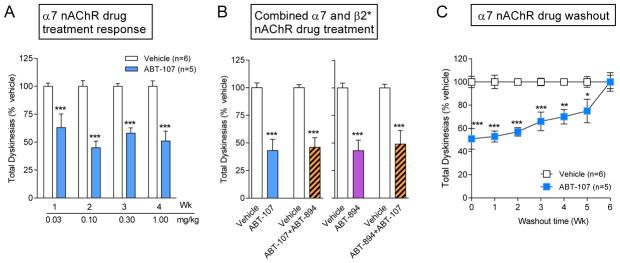
The α7 nAChR agonist ABT-107 reduces LIDs in parkinsonian monkeys. MPTP-lesioned monkeys were gavaged with L-dopa plus carbidopa twice daily 5 days per week, with the α7 nAChR agonist ABT-107 given orally 30 min before L-dopa. The left panel (A) shows that ABT-107 significantly reduced LIDs by 60%. The middle panel (B) shows that the reduction in LIDs with combined ABT-107 plus ABT-894 treatment was similar to that with ABT-107 or ABT-894 alone. The right panel (C) shows ABT-107 discontinuation leads to a return of LIDs to vehicle-treated levels by 6 wk. Values are the mean ± SEM of 5–6 monkeys. Significance of difference from vehicle, *p < 0.05, **p < 0.01, ***P < 0.001 using two-way ANOVA. Taken in modified form from [149].
The antidyskinetic effect was not unique to α7 nAChRs drugs, as β2* nAChR agonists also improved LIDs (Table 3). This includes TC-8831, which reduced LIDs by 50% in macaques and squirrel monkeys without affecting parkinsonism, although a drawback of this drug was induction of emesis [119, 120]. Another β2* nAChR agonist AZD1446 also attenuated LIDs in macaques, although it was not as effective with only a 30% decline possibly due to a lower nAChR affinity [121]. In addition, the β2* nAChR agonists ABT-089 and ABT-894 decreased LIDs, with the most pronounced effect with ABT-894 which resulted in a 60% decline in LIDs in squirrel monkeys (Table 3) [122]. Neither ABT-089 nor ABT-894 was associated with emesis. Like ABT-107, the decline in LIDs was dose dependent, did not diminish with time and required about a month to washout [122].
Notably, the reduction in LIDs observed with either an α7 or β2* nAChR drug on its own was not increased by administration of both nAChR subtype drugs together. Combined administration of ABT-894 with the α7 agonist ABT-107 resulted in a reduction in LIDs similar to that observed with either drug alone (Fig. 2). These findings suggest that drugs acting at either α7 or β2* nAChRs are sufficient to achieve the antidyskinetic effect observed with general agonists such as nicotine. These data also indicate that α7 and β2* nAChR agonists reduce LIDs via some final common mechanism.
In summary, both α7 and β2* nAChR drugs reduce LIDs up to 60% with no detrimental effects on parkinsonism. Because α7 nAChR agonist appear to be associated with few side effects and are linked to positive effects on cognition (which may be deficient in Parkinson’s disease), they may represent ideal candidate drugs to improve LIDs in Parkinson’s disease.
5. Mechanisms underlying α7 nAChR-mediated effects in the brain
A question that arises is the molecular and cellular mechanisms whereby an interaction at α7 nAChRs modulates behaviors. One of the first steps in α7 nAChR-mediated transduction involves changes in calcium signaling (Fig. 3). α7 nAChRs exhibit a greater relative calcium permeability than other nicotinic receptors and readily flux calcium [40, 45, 123, 124]. Resultant changes in intracellular calcium may enhance cholinergic transmission in the short term, with consequent long term changes in neuronal plasticity.
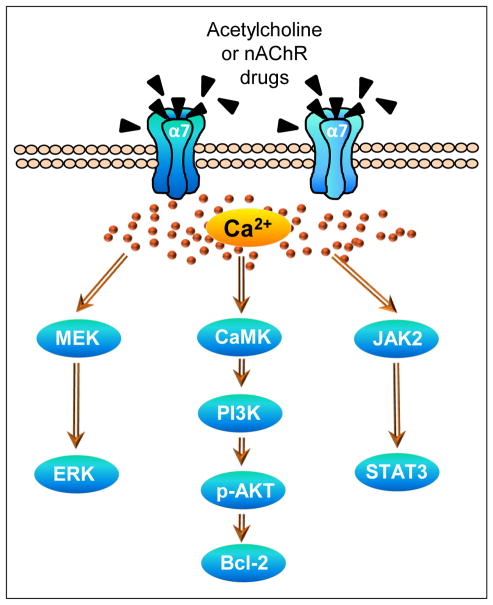
Fig. 3.
Various intracellular signaling pathways have been linked to α7 nAChR activation as described in the text. These lead to secondary events within the same cell or others, with consequent alterations in synaptic plasticity, development, maintenance, survival, apoptosis, neurotransmitter release, immune responsiveness or others. These subsequently modulate movement, addiction, anxiety, attention, learning, memory, sensory gating, inflammation, and neuroprotection. Bcl, B-cell lymphoma; CaMK, Ca2+/calmodulin-dependent protein kinase; ERK, extracellular signal-regulated kinases; MEK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; p-AKT, phosphorylated AKT.
Several intracellular mechanisms have been shown to mediate protective effect of α7 nAChR activation against toxic insults (Fig. 3). For instance, nicotine reduces Aβ-induced toxicity by enhancing phosphatidylinositol 3-kinase to lead to increased levels of phosphorylated AKT, and also Src, B-cell lymphoma (Bcl) 2 and Bcl-x with a consequent protection [51, 125]. The mitogen-activated protein kinase/extracellular signal-regulated kinases pathway and the JAK2/STAT3 pathway have also been implicated in α7 nAChR-mediated neuroprotection against a variety of toxic insults in PC12 cells, spinal cultures and keratinocytes [126–129]. Other downstream mechanisms associated with α7 nAChR-mediated protection include increases in phospholipase C [126], nerve growth factor [130], hemeoxygenase [61] and proinflammatry cytokines (tumor necrosis factor-α and interleukin-1β) [131], while nitric oxide [49], caspases, reactive oxygen species [61] are linked to toxicity.
With respect to α7 nAChR-mediated protection against nigrostriatal damage, the first step appears to be an increase in calcium influx via the α7 nAChR and also voltage-gated calcium channels [132]. This may then result in activation of a survival pathway involving the calcium effector protein calmodulin, phosphatidylinositol 3-kinase and phosphorylated AKT with subsequent upregulation of Bcl-2 [132, 133]. In addition, α7 nAChRs on astrocytes may contribute to nicotine’s protective effect against nigrostriatal damage. α7 nAChRs have been identified on astrocytes and shown to mediate changes in intracellular calcium mobilization [134, 135]. α7 nicotinic cholinergic signaling may subsequently alter expression of a variety of astrocytic transduction mechanisms including extracellular signal-regulated kinase1/2, p38, tumor necrosis factor-α, glial derived neurotrophic factor, glial fibrillary acidic protein, CD68, and caspase 9 [96, 99, 136] to modulate nigrostriatal plasticity.
The mechanisms linked to the α7 nAChR-mediated improvement in LIDs are less studied but probably include long and short term molecular and cellular changes. One of these may consist of alterations in striatal dopamine release. It is well established that LIDs are associated with an aberrant dopamine release from striatal terminals [137]. Nicotine decreases dopamine release via β2 nAChR-mediated nAChR desensitization and down regulation [138]. Striatal α7 nAChRs on glutamatergic afferents from the cortex may be similarly involved. Stimulation of these α7 nAChRs increases glutamate release, which in turn acts at glutamate receptors on dopamine terminals to modulate dopamine release/turnover [139]. Additionally, α7 nAChRs in the substantia nigra may influence the release of striatal dopamine [140].
Long term mechanisms most likely also play a role in the nAChR-mediated antidyskinetic effect. This possibility is based on findings that one to two months of treatment with nicotine or nAChR drugs are required for an optimal antidyskinetic effect and also for its decline with drug discontinuation [107, 108]. LIDs have been associated with structural changes in spine morphology in the striatum. This includes the appearance of aberrant spines, the development of new spines or a loss of existing spines in neurons of the corticostriatal, direct and/or indirect pathways [141–144]. Nicotine may restore aberrant signaling that contributes to LIDs by modulating spine formation/morphology as it is well known to modulate neuronal morphology via an interaction at α7 nAChRs [39, 41, 145].
6. Summary
Emerging studies suggest that α7 nAChR drugs may be useful in the management of Parkinson’s disease. Of particular importance is the finding that α7 nAChR agonists have disease modifying potential as they protect against nigrostriatal deficits in parkinsonian nonhuman primate and rodent models. In addition, α7 nAChRs mediate neuroprotective effects against numerous other toxic insults and thus be of benefit against some of the other CNS deficits associated with Parkinson’s disease. Preclinical studies in nonhuman primates also show that α7 nAChR agonists reduce LIDs, a serious side effect of L-dopa therapy. Altogether, these data suggest that α7 nAChR agonists, which may exhibit fewer side effects than other subtype selective nAChR agonists [146, 147], may be useful for reducing Parkinson’s disease progression and also decreasing LIDs.
Acknowledgments
This work was supported by NIH grants NS59910.
Abbreviations
- Bcl
B-cell lymphoma
- α-BTX
α-bungarotoxin
- DMXB
3-[2,4-Dimethoxybenzylidene]anabaseine
- LIDs
L-dopa-induced dyskinesias
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- PC
pheochromocytoma
- *
indicates the possible presence of other subunits in the receptor complex
Footnotes
Conflict of interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52:115–9. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 2.Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson’s disease: systematic review of prospective studies. Mov Disord. 2004;19:614–21. doi: 10.1002/mds.20029. [DOI] [PubMed] [Google Scholar]
- 3.Elbaz A, Moisan F. Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol. 2008;21:454–60. doi: 10.1097/WCO.0b013e3283050461. [DOI] [PubMed] [Google Scholar]
- 4.Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Annals of neurology. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25 (Suppl 1):S58–62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- 6.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26 (Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 7.Thacker EL, O’Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–8. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly EJ, McCullough ML, Chao A, Henley SJ, Calle EE, Thun MJ, et al. Smokeless tobacco use and the risk of Parkinson’s disease mortality. Mov Disord. 2005;20:1383–4. doi: 10.1002/mds.20587. [DOI] [PubMed] [Google Scholar]
- 9.Searles Nielsen S, Gallagher LG, Lundin JI, Longstreth WT, Jr, Smith-Weller T, Franklin GM, et al. Environmental tobacco smoke and Parkinson’s disease. Mov Disord. 2012;27:293–6. doi: 10.1002/mds.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–7. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 11.Tanner CM, Goldman SM, Aston DA, Ottman R, Ellenberg J, Mayeux R, et al. Smoking and Parkinson’s disease in twins. Neurology. 2002;58:581–8. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- 12.Ritz B, Lee PC, Lassen CF, Arah OA. Parkinson disease and smoking revisited: Ease of quitting is an early sign of the disease. Neurology. 2014 doi: 10.1212/WNL.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990;54:937–45. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- 14.Lichtensteiger W, Hefti F, Felix D, Huwyler T, Melamed E, Schlumpf M. Stimulation of nigrostriatal dopamine neurones by nicotine. Neuropharmacology. 1982;21:963–8. doi: 10.1016/0028-3908(82)90107-1. [DOI] [PubMed] [Google Scholar]
- 15.Westfall TC, Besson MJ, Giorguieff MF, Glowinski J. The role of presynaptic receptors in the release and synthesis of 3H-dopamine by slices of rat striatum. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:279–87. doi: 10.1007/BF00517390. [DOI] [PubMed] [Google Scholar]
- 16.Giorguieff MF, Le Floc’h ML, Westfall TC, Glowinski J, Besson MJ. Nicotinic effect of acetylcholine on the release of newly synthesized (3H)dopamine in rat striatal slices and cat caudate nucleus. Brain research. 1976;106:117–31. doi: 10.1016/0006-8993(76)90077-9. [DOI] [PubMed] [Google Scholar]
- 17.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 18.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson’s Disease. Pharmacol Rev. 2011;63:938–66. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–59. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–80. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–23. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- 23.Pauly JR, Stitzel JA, Marks MJ, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain. Brain Res Bull. 1989;22:453–9. doi: 10.1016/0361-9230(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 24.Marks MJ, Pauly JR, Grun EU, Collins AC. ST/b and DBA/2 mice differ in brain alpha-bungarotoxin binding and alpha 7 nicotinic receptor subunit mRNA levels: a quantitative autoradiographic analysis. Brain Res Mol Brain Res. 1996;39:207–22. doi: 10.1016/0169-328x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- 25.Hunt S, Schmidt J. Some observations on the binding patterns of alpha-bungarotoxin in the central nervous system of the rat. Brain research. 1978;157:213–32. doi: 10.1016/0006-8993(78)90025-2. [DOI] [PubMed] [Google Scholar]
- 26.Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, et al. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–98. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- 28.Quik M, Polonskaya Y, Gillespie A, Jakowec M, Lloyd GK, Langston JW. Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol. 2000;425:58–69. doi: 10.1002/1096-9861(20000911)425:1<58::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–15. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunological reviews. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bencherif M, Narla ST, Stachowiak MS. Alpha7 neuronal nicotinic receptor: a pluripotent target for diseases of the central nervous system. CNS Neurol Disord Drug Targets. 2014;13:836–45. doi: 10.2174/1871527313666140711094525. [DOI] [PubMed] [Google Scholar]
- 32.Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–61. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- 33.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144:50–9. doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yohn NL, Turner JR, Blendy JA. Activation of alpha4beta2*/alpha 6beta2* Nicotinic Receptors Alleviates Anxiety During Nicotine Withdrawal Without Upregulating Nicotinic Receptors. J Pharmacol Exp Ther. 2014;349:348–54. doi: 10.1124/jpet.113.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelso ML, Oestreich JH. Traumatic brain injury: central and peripheral role of alpha7 nicotinic acetylcholine receptors. Curr Drug Targets. 2012;13:631–6. doi: 10.2174/138945012800398964. [DOI] [PubMed] [Google Scholar]
- 37.Quik M, Geertsen S. Neuronal nicotinic alpha-bungarotoxin sites. Canadian journal of physiology and pharmacology. 1988;66:971–9. doi: 10.1139/y88-160. [DOI] [PubMed] [Google Scholar]
- 38.Halvorsen SW, Berg DK. Specific down-regulation of the alpha-bungarotoxin binding component on chick autonomic neurons by ciliary neuronotrophic factor. J Neurosci. 1989;9:3673–80. doi: 10.1523/JNEUROSCI.09-10-03673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coronas V, Durand M, Chabot JG, Jourdan F, Quirion R. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience. 2000;98:213–9. doi: 10.1016/s0306-4522(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 40.Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–62. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- 41.Chan J, Quik M. A role for the nicotinic alpha-bungarotoxin receptor in neurite outgrowth in PC12 cells. Neuroscience. 1993;56:441–51. doi: 10.1016/0306-4522(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 42.Broide RS, Robertson RT, Leslie FM. Regulation of alpha7 nicotinic acetylcholine receptors in the developing rat somatosensory cortex by thalamocortical afferents. J Neurosci. 1996;16:2956–71. doi: 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko WM, Britto LR, Lindstrom JM, Karten HJ. Distribution of the alpha7 nicotinic acetylcholine receptor subunit in the developing chick cerebellum. Brain research Developmental brain research. 1998;105:141–5. [PubMed] [Google Scholar]
- 45.Broide RS, Leslie FM. The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol. 1999;20:1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- 46.Takada Y, Yonezawa A, Kume T, Katsuki H, Kaneko S, Sugimoto H, et al. Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. J Pharmacol Exp Ther. 2003;306:772–7. doi: 10.1124/jpet.103.050104. [DOI] [PubMed] [Google Scholar]
- 47.Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, et al. Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Annals of neurology. 1997;42:159–63. doi: 10.1002/ana.410420205. [DOI] [PubMed] [Google Scholar]
- 48.Kihara T, Sawada H, Nakamizo T, Kanki R, Yamashita H, Maelicke A, et al. Galantamine modulates nicotinic receptor and blocks Abeta-enhanced glutamate toxicity. Biochem Biophys Res Commun. 2004;325:976–82. doi: 10.1016/j.bbrc.2004.10.132. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko S, Maeda T, Kume T, Kochiyama H, Akaike A, Shimohama S, et al. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain research. 1997;765:135–40. doi: 10.1016/s0006-8993(97)00556-8. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, King MA, Grimes J, Smith N, de Fiebre CM, Meyer EM. Alpha7 nicotinic receptor mediated protection against ethanol-induced cytotoxicity in PC12 cells. Brain research. 1999;816:225–8. doi: 10.1016/s0006-8993(98)01153-6. [DOI] [PubMed] [Google Scholar]
- 51.Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, et al. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–6. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 52.Yu W, Mechawar N, Krantic S, Quirion R. alpha7 Nicotinic receptor activation reduces beta-amyloid-induced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem. 2011;119:848–58. doi: 10.1111/j.1471-4159.2011.07466.x. [DOI] [PubMed] [Google Scholar]
- 53.Nakamizo T, Kawamata J, Yamashita H, Kanki R, Kihara T, Sawada H, et al. Stimulation of nicotinic acetylcholine receptors protects motor neurons. Biochem Biophys Res Commun. 2005;330:1285–9. doi: 10.1016/j.bbrc.2005.03.115. [DOI] [PubMed] [Google Scholar]
- 54.Jonnala RR, Terry AV, Jr, Buccafusco JJ. Nicotine increases the expression of high affinity nerve growth factor receptors in both in vitro and in vivo. Life Sci. 2002;70:1543–54. doi: 10.1016/s0024-3205(01)01529-6. [DOI] [PubMed] [Google Scholar]
- 55.Shimohama S, Akaike A, Kimura J. Nicotine-induced protection against glutamate cytotoxicity. Nicotinic cholinergic receptor-mediated inhibition of nitric oxide formation. Ann N Y Acad Sci. 1996;777:356–61. doi: 10.1111/j.1749-6632.1996.tb34445.x. [DOI] [PubMed] [Google Scholar]
- 56.Dajas-Bailador FA, Lima PA, Wonnacott S. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39:2799–807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 57.Shen H, Kihara T, Hongo H, Wu X, Kem WR, Shimohama S, et al. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. Br J Pharmacol. 2010;161:127–39. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonnala RR, Graham JH, 3rd, Terry AV, Jr, Beach JW, Young JA, Buccafusco JJ. Relative levels of cytoprotection produced by analogs of choline and the role of alpha7-nicotinic acetylcholine receptors. Synapse (New York, NY. 2003;47:262–9. doi: 10.1002/syn.10176. [DOI] [PubMed] [Google Scholar]
- 59.Arias E, Ales E, Gabilan NH, Cano-Abad MF, Villarroya M, Garcia AG, et al. Galantamine prevents apoptosis induced by beta-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology. 2004;46:103–14. doi: 10.1016/s0028-3908(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 60.Li Q, Wu D, Zhang L, Zhang Y. Effects of galantamine on beta-amyloid release and beta-site cleaving enzyme 1 expression in differentiated human neuroblastoma SH-SY5Y cells. Exp Gerontol. 2010;45:842–7. doi: 10.1016/j.exger.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Parada E, Egea J, Romero A, del Barrio L, Garcia AG, Lopez MG. Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via alpha7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med. 2010;49:1815–21. doi: 10.1016/j.freeradbiomed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- 63.Del Barrio L, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, et al. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by alpha7 and beta2* nicotinic stimulation. Toxicol Sci. 2011;123:193–205. doi: 10.1093/toxsci/kfr163. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Papke RL, He YJ, Millard WJ, Meyer EM. Characterization of the neuroprotective and toxic effects of alpha7 nicotinic receptor activation in PC12 cells. Brain research. 1999;830:218–25. doi: 10.1016/s0006-8993(99)01372-4. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Meyer EM, Walker DW, Millard WJ, He YJ, King MA. Alpha7 nicotinic receptor activation inhibits ethanol-induced mitochondrial dysfunction, cytochrome c release and neurotoxicity in primary rat hippocampal neuronal cultures. J Neurochem. 2002;81:853–8. doi: 10.1046/j.1471-4159.2002.00891.x. [DOI] [PubMed] [Google Scholar]
- 66.de Fiebre NC, de Fiebre CM. Alpha 7 nicotinic acetylcholine receptor-mediated protection against ethanol-induced neurotoxicity. Alcohol. 2003;31:149–53. doi: 10.1016/j.alcohol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Ren XQ, Cheng SB, Treuil MW, Mukherjee J, Rao J, Braunewell KH, et al. Structural determinants of alpha4beta2 nicotinic acetylcholine receptor trafficking. J Neurosci. 2005;25:6676–86. doi: 10.1523/JNEUROSCI.1079-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosa AO, Egea J, Gandia L, Lopez MG, Garcia AG. Neuroprotection by nicotine in hippocampal slices subjected to oxygen-glucose deprivation: involvement of the alpha7 nAChR subtype. J Mol Neurosci. 2006;30:61–2. doi: 10.1385/JMN:30:1:61. [DOI] [PubMed] [Google Scholar]
- 69.Egea J, Rosa AO, Sobrado M, Gandia L, Lopez MG, Garcia AG. Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience. 2007;145:866–72. doi: 10.1016/j.neuroscience.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 70.Gahring LC, Meyer EL, Rogers SW. Nicotine-induced neuroprotection against N-methyl-D-aspartic acid or beta-amyloid peptide occur through independent mechanisms distinguished by pro-inflammatory cytokines. J Neurochem. 2003;87:1125–36. doi: 10.1046/j.1471-4159.2003.02074.x. [DOI] [PubMed] [Google Scholar]
- 71.Qian J, Zhang JM, Lin LL, Dong WZ, Cheng YQ, Su DF, et al. A combination of neostigmine and anisodamine protects against ischemic stroke by activating alpha7nAChR. Int J Stroke. 2015 doi: 10.1111/ijs.12458. [DOI] [PubMed] [Google Scholar]
- 72.de Fiebre NC, de Fiebre CM. alpha7 Nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not beta-amyloid-induced neurotoxicity. Neurosci Lett. 2005;373:42–7. doi: 10.1016/j.neulet.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 73.Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30:2442–53. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Target CNS Neurol Disord. 2002;1:399–411. doi: 10.2174/1568007023339166. [DOI] [PubMed] [Google Scholar]
- 75.Kawamata J, Suzuki S, Shimohama S. alpha7 nicotinic acetylcholine receptor mediated neuroprotection in Parkinson’s disease. Curr Drug Targets. 2012;13:623–30. doi: 10.2174/138945012800399026. [DOI] [PubMed] [Google Scholar]
- 76.Quik M, O’Neill M, Perez XA. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007;28:229–35. doi: 10.1016/j.tips.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Janson AM, Fuxe K, Agnati LF, Kitayama I, Harfstrand A, Andersson K, et al. Chronic nicotine treatment counteracts the disappearance of tyrosine-hydroxylase-immunoreactive nerve cell bodies, dendrites and terminals in the mesostriatal dopamine system of the male rat after partial hemitransection. Brain research. 1988;455:332–45. doi: 10.1016/0006-8993(88)90092-3. [DOI] [PubMed] [Google Scholar]
- 78.Janson AM, Hedlund PB, Hillefors M, von Euler G. Chronic nicotine treatment decreases dopamine D2 agonist binding in the rat basal ganglia. Neuroreport. 1992;3:1117–20. doi: 10.1097/00001756-199212000-00021. [DOI] [PubMed] [Google Scholar]
- 79.Janson AM, Meana JJ, Goiny M, Herrera-Marschitz M. Chronic nicotine treatment counteracts the decrease in extracellular neostriatal dopamine induced by a unilateral transection at the mesodiencephalic junction in rats: a microdialysis study. Neurosci Lett. 1991;134:88–92. doi: 10.1016/0304-3940(91)90515-u. [DOI] [PubMed] [Google Scholar]
- 80.Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain research. 2001;888:336–42. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- 81.Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–6. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Visanji NP, Fox SH, Johnston T, Reyes G, Millan MJ, Brotchie JM. Dopamine D(3) receptor stimulation underlies the development of L-DOPA-induced dyskinesia in animal models of Parkinson’s disease. Neurobiol Dis. 2008;35:184–92. doi: 10.1016/j.nbd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Lopez-Real AM, Labandeira-Garcia JL. Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson’s disease. Biochem Pharmacol. 2002;64:125–35. doi: 10.1016/s0006-2952(02)01070-5. [DOI] [PubMed] [Google Scholar]
- 84.Parain K, Marchand V, Dumery B, Hirsch E. Nicotine, but not cotinine, partially protects dopaminergic neurons against MPTP-induced degeneration in mice. Brain research. 2001;890:347–50. doi: 10.1016/s0006-8993(00)03198-x. [DOI] [PubMed] [Google Scholar]
- 85.Janson AM, Fuxe K, Agnati L, Sundstrom E, Goldstein M. The effect of chronic nicotine treatment on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degneration of nigrostriatal dopamine neurons in the black mouse. Advances in Pharmacological Sciences. 1991;1:323–9. [Google Scholar]
- 86.Behmand RA, Harik SI. Nicotine enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. J Neurochem. 1992;58:776–9. doi: 10.1111/j.1471-4159.1992.tb09786.x. [DOI] [PubMed] [Google Scholar]
- 87.Sershen H, Hashim A, Wiener HL, Lajtha A. Effect of chronic oral nicotine on dopaminergic function in the MPTP-treated mouse. Neurosci Lett. 1988;93:270–4. doi: 10.1016/0304-3940(88)90094-8. [DOI] [PubMed] [Google Scholar]
- 88.Hadjiconstantinou M, Hubble JP, Wemlinger TA, Neff NH. Enhanced MPTP neurotoxicity after treatment with isoflurophate or cholinergic agonists. J Pharmacol Exp Ther. 1994;270:639–44. [PubMed] [Google Scholar]
- 89.Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26:4681–9. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, et al. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98:1866–75. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- 91.Bordia T, Decker MW, Quik M. Unpublished observation [Google Scholar]
- 92.Srinivasan R, Richards CI, Xiao C, Rhee D, Pantoja R, Dougherty DA, et al. Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response. Mol Pharmacol. 2012;81:759–69. doi: 10.1124/mol.112.077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, et al. Nicotine up-regulates {alpha}4{beta}2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimohama S. Nicotinic receptor-mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull. 2009;32:332–6. doi: 10.1248/bpb.32.332. [DOI] [PubMed] [Google Scholar]
- 95.Bordia T, McGregor M, Papke RL, Decker MW, McIntosh JM, Quik M. The alpha7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp Neurol. 2015;263:277–84. doi: 10.1016/j.expneurol.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suzuki S, Kawamata J, Matsushita T, Matsumura A, Hisahara S, Takata K, et al. 3-[(2,4-Dimethoxy)benzylidene]-anabaseine dihydrochloride protects against 6-hydroxydopamine-induced parkinsonian neurodegeneration through alpha7 nicotinic acetylcholine receptor stimulation in rats. J Neurosci Res. 2013;91:462–71. doi: 10.1002/jnr.23160. [DOI] [PubMed] [Google Scholar]
- 97.Yanagida T, Takeuchi H, Kitamura Y, Takata K, Minamino H, Shibaike T, et al. Synergistic effect of galantamine on nicotine-induced neuroprotection in hemiparkinsonian rat model. Neurosci Res. 2008;62:254–61. doi: 10.1016/j.neures.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Stuckenholz V, Bacher M, Balzer-Geldsetzer M, Alvarez-Fischer D, Oertel WH, Dodel RC, et al. The alpha7 nAChR Agonist PNU-282987 Reduces Inflammation and MPTP-Induced Nigral Dopaminergic Cell Loss in Mice. J Parkinsons Dis. 2013;3:161–72. doi: 10.3233/JPD-120157. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Hu J, Wu J, Zhu C, Hui Y, Han Y, et al. alpha7 nicotinic acetylcholine receptor-mediated neuroprotection against dopaminergic neuron loss in an MPTP mouse model via inhibition of astrocyte activation. J Neuroinflammation. 2012;9:98. doi: 10.1186/1742-2094-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pahwa R, Lyons KE. Treatment of early Parkinson’s disease. Curr Opin Neurol. 2014;27:442–9. doi: 10.1097/WCO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 101.Vijverman AC, Fox SH. New treatments for the motor symptoms of Parkinson’s disease. Expert Rev Clin Pharmacol. 2014;7:761–77. doi: 10.1586/17512433.2014.966812. [DOI] [PubMed] [Google Scholar]
- 102.Heumann R, Moratalla R, Herrero MT, Chakrabarty K, Drucker-Colin R, Garcia-Montes JR, et al. Dyskinesia in Parkinson’s disease: mechanisms and current non-pharmacological interventions. J Neurochem. 2014;130:472–89. doi: 10.1111/jnc.12751. [DOI] [PubMed] [Google Scholar]
- 103.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311:1670–83. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 104.Pahwa R, Tanner CM, Hauser RA, Sethi K, Isaacson S, Truong D, et al. Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study) Mov Disord. 2015;30:788–95. doi: 10.1002/mds.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaeffer E, Pilotto A, Berg D. Pharmacological Strategies for the Management of Levodopa-Induced Dyskinesia in Patients with Parkinson’s Disease. CNS Drugs. 2014;28:1155–84. doi: 10.1007/s40263-014-0205-z. [DOI] [PubMed] [Google Scholar]
- 106.Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62:588–96. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 107.Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in l-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013;50:30–41. doi: 10.1016/j.nbd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quik M, Mallela A, Ly J, Zhang D. Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2013;28:1398–406. doi: 10.1002/mds.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–38. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–47. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 111.Huang L, Grady SR, Quik M. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–41. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 113.Bordia T, Carroll FI, Quik M. Varenicline Markedly Decreases Antipsychotic-induced Tardive Dyskinesia in a Rodent Model. Soc Neurosci Abstr. 2013;33:32.07. [Google Scholar]
- 114.Ween H, Thorin-Hagene K, Andersen E, Gronlien JH, Lee CH, Gopalakrishnan M, et al. alpha3* and alpha7 nAChR-mediated Ca(2+) transient generation in IMR-32 neuroblastoma cells. Neurochem Int. 2010;57:269–77. doi: 10.1016/j.neuint.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Zhang D, Mallela A, Sohn D, Carroll FI, Bencherif M, Letchworth S, et al. Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;347:225–34. doi: 10.1124/jpet.113.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quik M, Campos C, Grady SR. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013;86:1153–62. doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang D, McGregor M, Bordia T, Perez X, Decker MW, Quik M. The alpha7 nicotinic receptor agonist ABT-126 decreases L-Dopa-induced dyskinesias in MPTP-lesioned monkeys. Soc Neurosci Abstr. 2015 [Google Scholar]
- 118.Di Paolo T, Gregoire L, Feuerbach D, Elbast W, Weiss M, Gomez-Mancilla B. AQW051, a novel and selective nicotinic acetylcholine receptor alpha7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys. Parkinsonism Relat Disord. 2014;20:1119–23. doi: 10.1016/j.parkreldis.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Johnston TH, Huot P, Fox SH, Koprich JB, Szeliga KT, James JW, et al. TC-8831, a nicotinic acetylcholine receptor agonist, reduces L-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology. 2013;73:337–47. doi: 10.1016/j.neuropharm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Zhang D, Mallela A, Carroll FI, Quik M. Nicotinic receptor agonists reduce L-dopa-induced dyskinesias in a monkey model of Parkinson’s disease. 2013 doi: 10.1124/jpet.113.207639. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mather J, Burdette, Cebers, Posener, Alexander, Leventer, et al. Potential of AZD1446, a novel nicotinic agonist, for the treatment of L-DOPA-induced dyskinesia in Parkinson’s disease. Society for Neuroscience Abstr. 2014;43 137.11/L1. [Google Scholar]
- 122.Zhang D, Bordia T, McGregor M, McIntosh JM, Decker MW, Quik M. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2014;29:508–17. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–6. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 124.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shimohama S, Kihara T. Nicotinic receptor-mediated protection against beta-amyloid neurotoxicity. Biol Psychiatry. 2001;49:233–9. doi: 10.1016/s0006-3223(00)01100-8. [DOI] [PubMed] [Google Scholar]
- 126.Ren K, Puig V, Papke RL, Itoh Y, Hughes JA, Meyer EM. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94:926–33. doi: 10.1111/j.1471-4159.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- 127.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 128.Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem. 2002;277:44920–4. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- 129.Toborek M, Son KW, Pudelko A, King-Pospisil K, Wylegala E, Malecki A. ERK 1/2 signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. J Cell Biochem. 2007;100:279–92. doi: 10.1002/jcb.21013. [DOI] [PubMed] [Google Scholar]
- 130.Ren K, King MA, Liu J, Siemann J, Altman M, Meyers C, et al. The alpha7 nicotinic receptor agonist 4OH-GTS-21 protects axotomized septohippocampal cholinergic neurons in wild type but not amyloid-overexpressing transgenic mice. Neuroscience. 2007;148:230–7. doi: 10.1016/j.neuroscience.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 131.Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochemistry international. 2010;56:135–42. doi: 10.1016/j.neuint.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 132.Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+ FASEB J. 2011;25:2563–73. doi: 10.1096/fj.11-182824. [DOI] [PubMed] [Google Scholar]
- 133.Takeuchi H, Yanagida T, Inden M, Takata K, Kitamura Y, Yamakawa K, et al. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson’s disease models. J Neurosci Res. 2009;87:576–85. doi: 10.1002/jnr.21869. [DOI] [PubMed] [Google Scholar]
- 134.Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4148–53. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen JX, Yakel JL. Functional alpha7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J Mol Neurosci. 2012;48:14–21. doi: 10.1007/s12031-012-9719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, et al. Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology. 2015;91:87–96. doi: 10.1016/j.neuropharm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 137.Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–43. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 138.Bordia T, McIntosh JM, Quik M. The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem. 2013;125:291–302. doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–8. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 140.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fieblinger T, Cenci MA. Zooming in on the small: The plasticity of striatal dendritic spines in l-DOPA-Induced dyskinesia. Mov Disord. 2015;30:484–93. doi: 10.1002/mds.26139. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci. 2013;33:11655–67. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MG, et al. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 2014;75:711–22. doi: 10.1016/j.biopsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Nishijima H, Suzuki S, Kon T, Funamizu Y, Ueno T, Haga R, et al. Morphologic changes of dendritic spines of striatal neurons in the levodopa-induced dyskinesia model. Mov Disord. 2014;29:336–43. doi: 10.1002/mds.25826. [DOI] [PubMed] [Google Scholar]
- 145.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14:889–96. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Othman AA, Lenz RA, Zhang J, Li J, Awni WM, Dutta S. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol. 2011;51:512–26. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- 147.Gault L, Ritchie CW, Robieson WZ, Pritchett Y, Othman AA, Lenz RA. A phase 2 randomized, controlled trial of the 7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia. Alzheimer’s & Dementia: Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2015 doi: 10.1016/j.trci.2015.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arias E, Gallego-Sandin S, Villarroya M, Garcia AG, Lopez MG. Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic receptors. J Pharmacol Exp Ther. 2005;315:1346–53. doi: 10.1124/jpet.105.090365. [DOI] [PubMed] [Google Scholar]
- 149.Zhang D, McGregor M, Decker MW, Quik M. The alpha7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys. J Pharmacol Exp Ther. 2014;351:25–32. doi: 10.1124/jpet.114.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]