Abstract
The alpha-7 subtype of the nicotinic acetylcholine receptor (α7-nAChR) is fundamental to physiology; it mediates various brain functions and represents an important target for drug discovery. Exploration of the brain nicotinic acetylcholine receptors (nAChRs) using positron-emission tomography (PET) will make it possible to better understand the important role of this receptor and to study its involvement in schizophrenia, bipolar disorder, Alzheimer's and Parkinson's diseases, drug dependence, inflammation and many other disorders and simplify the development of nicotinic drugs for treatment of these disorders.
Until recently, PET imaging of α7-nAChRs has been impeded by the absence of good radiotracers. This review describes various endeavors to develop α7-nAChR PET tracers by several research groups including the author’s group. Most initial PET tracers for imaging α7-nAChRs did not exhibit suitable imaging properties due to their low specific binding. Recently discovered [18F]ASEM is the first highly specific α7-nAChR radioligand and it was recently translated to human PET imaging.
Keywords: positron emission tomography, PET, α7-nAChR, alpha 7, nicotinic receptor, [18F]ASEM

1. Introduction
Nicotinic cholinergic receptors (nAChRs) are neurotransmitter-gated cationic channels that are present in the central nervous system (CNS), autonomic and sensory ganglia, and various non-neuronal cells. Two nAChR subtypes, α4β2- and α7-nAChR, are the most abundant nAChRs in the CNS[1].
The α7-nAChR subtype is highly expressed in the human brain and this subtype has been implicated in the pathophysiology of a variety of brain disorders and conditions including schizophrenia, Alzheimer's disease, bipolar disorder, traumatic brain injury, anxiety, depression, multiple sclerosis, inflammation, and drug addiction[1-10].
As was demonstrated in post-mortem studies, the density of α7-nAChRs in human brain tissue is significantly altered in many disorders:
Schizophrenia
In autoradiography and immunochemistry studies, Freedman et al[11] and others [12-17] have demonstrated a significant post-mortem reduction (25-54%) of α7-nAChR binding or expression in the hippocampus and cortex of subjects with schizophrenia vs. controls.
Alzheimer’s disease
A characteristic of Alzheimer’s disease is degeneration of cholinergic neurons[18]. A number of reports have described a significant loss of α7-nAChRs in the cortex and hippocampus of patients with Alzheimer’s disease [15, 19] (see also review[20]).
Bipolar disorder
An autoradiography study using an α7-nAChR radiotracer demonstrated an increased binding in the hippocampus and perirhinal cortex in the brain slices of the subjects suffering from bipolar disorder[21].
Traumatic brain injury
Traumatic brain injury is a significant public health problem with almost 2 million documented cases per year in the USA, with a mortality of 20%[22, 23]. Several reports found a significant reduction (30-70%, ex vivo or in vitro) of α7-nAChRs in animal models of traumatic brain injury [8, 24-26], suggesting that alteration of the α7-nAChR is a crucial component of the biochemical perturbation caused by traumatic brain injury.
The difference in the denstity of α7-nAChRs in the brain between healthy subjects and patients suffering from various disorders was quantified in post-mortem studies, but it never was observed in the living human brain. Non-invasive quantification of α7-nAChRs in humans would provide a better understanding of their role in various CNS disorders and could also simplify the development of nicotinic drugs for treatment of these disorders [27-32].
PET provides the best opportunity for quantification of receptors in the human brain – better than any other clinical imaging modality [33, 34]. However, since the invention of the PET technique in 1975 fewer than 40 of the existing receptors in the human brain have been imaged due to the lack of available PET radiotracers (see http://www.nimh.nih.gov/research-priorities/therapeutics/cns-radiotracer-table.shtml). Until recently, one of the major cerebral receptors lacking an appropriate PET radioligand for human imaging was α7-nAChR. The recently developed PET radioligand [18F]ASEM has opened new avenues in noninvasive imaging of this receptor system in human subjects.
2. Initial PET radioligands for α7-nAChRs
In principle, a quality α7-nAChR radioligand for PET should exhibit the same set of characteristics as PET tracers for most other brain receptors: 1) a high specific and low non-specific binding in vivo; 2) high selectivity versus non-target binding sites; 3) reversible brain kinetics with good blood-brain barrier permeability; 4) radiochemistry that is suitable for short-lived isotopes; and 5) low radiation burden and toxicity. These general requirements for PET radiotracers have been summarized in many reviews [35-37].
While all general requirements must be met, the high specific binding is the most demanding property in the development of α7-nAChR radiotracers. Specific PET tracers for brain receptors are expected to obey the Eckelman’s criterion that Bmax/KD ≥ 10 (Bmax = binding site density; KD = binding affinity constant of the radiotracer)[38, 39]. The concentration of the α7-nAChR binding sites in the primate brain is low (Bmax = 5 – 15 fmol/mg protein or 1.5 – 12 fmol/mg tissue)[12, 40, 41]. Consequently, the expected binding affinity for a quality α7-nAChR PET radioligand must be in a sub-nanomolar range. This binding affinity requirement challenged the development of suitable α7-nAChR radioligands (see reviews[35, 42, 43]).
Investigators have been attempting to develop α7-nAChR radioligands for in vivo imaging since the pioneering work of the Dolle[44] (Orsay, France) and Pomper[45] (Baltimore, US) in 2001-2005. Both groups radiolabeled quinuclidine derivatives (Fig. 1) that were previously reported by AstraZeneca as potential α7-nAChR drugs. Unfortunately, these radiotracers did not exhibit a sufficient signal-to-noise ratio in lab animals and were not translated to humans.
Fig. 1.
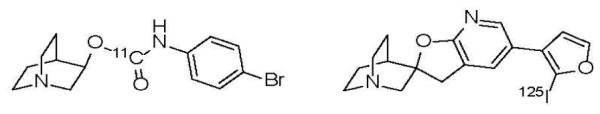
First radioligands for emission tomography imaging of α7-nAChR [44, 45].
In 2005-2010 many researchers, including our own group, worked on the development of a clinically viable α7-nAChR PET radioligand, and about two dozen α7-nAChR compounds were radiolabeled with [18F] or [11C]. As summarized in the recent reviews[35, 46-48], those efforts did not lead to an α7-nAChR PET radioligand with sufficient in vivo specificity.
[11C]CHIBA-1001 was the only α7-nAChR radioligand translated to human PET in the past, but it showed low target-to-non-target ratios in the brain (<1.3) in a single human PET scan[49] (Fig. 2). Further blocking experiments using PET in humans demonstrated some specificity of the [11C]CHIBA-1001 binding that, however, was not sufficiently high for reliable receptor quantification. This result led [11C]CHIBA-1001 inventors to a conclusion that a better PET radioligand is necessary[50]. The low specific binding of [11C]CHIBA-1001 in the human brain is in agreement with its relatively low in vitro binding affinity ([3H]CHIBA-1001, rat or human KD = 120 - 193 nM) and its inadequate α7-nAChR regional distribution in the rodent brain[51, 52].
Fig. 2.
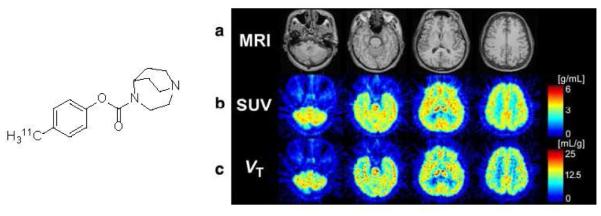
Left: Structure of [11C]CHIBA-1001. Right: PET images of human brain with [11C]CHIBA-1001. Panel a Magnetic resonance images (MRI) of the corresponding slices. Panel b Static images acquired from 0 to 90 min after injection of [11C]CHIBA-1001 expressed as SUV. Panel c A parametric image for the total distribution volume of [11C]CHIBA-1001 generated using Logan graphical analysis. The data from 30 to 90 min were applied to the Logan plot analysis. Reprinted from[49] with permission of Copyright Clearance Center's RightsLink service.
The most recent α7-nAChR PET radioligands, [18F]AZ11637326[53, 54], [11C]NS14492[55] and [18F]NS10743[56, 57] (Fig. 3), exhibited some specific binding in the brains of lab animals, but their specificities were also insufficient for human PET. The main reason for the deficient PET properties of most of the initial radioligands was due to the low binding affinity of these compounds (see for review[48]). Another recent PET tracer [11C]A752274 (Fig. 3) that was developed by collaboration of Abbott Laboratories and Johns Hopkins University exhibited very high binding affinity (Ki = 0.092 nM). Unfortunately, [11C]A752274 is a polar compound with low lipophilicity (logD7.4 = -2.7) that shows low brain uptake in animals, which makes it inappropriate for brain PET[58].
Fig. 3.
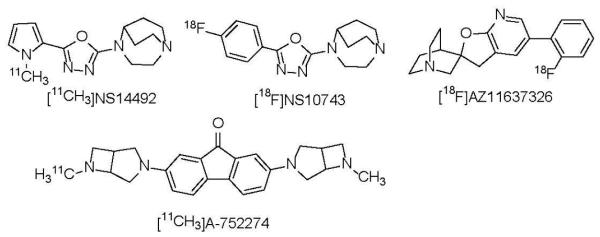
Recent α7-nAChR PET radioligands, [18F]AZ11637326[53, 54], [11C]NS14492[55], [18F]NS10743[56, 57] and [11C]A-752274[58].
3. Development of [18F]ASEM[48, 59]
3.1 Synthesis of ASEM
In 2012 Abbott Laboratories disclosed a number of α7-nAChR ligands that were synthesized as potential drug candidates[60]. One of the compounds of the series was 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)dibenzo[b,d]thiophene 5,5-dioxide (Fig. 4), an α7-nAChR selective ligand with exceptionally high binding affinity, Ki = 0.023 nM, and the ability to penetrate the blood-brain barrier in lab animals[60]. These properties made this dibenzothiophene compound an attractive lead for development of quality α7-nAChR PET tracers.
Fig. 4.
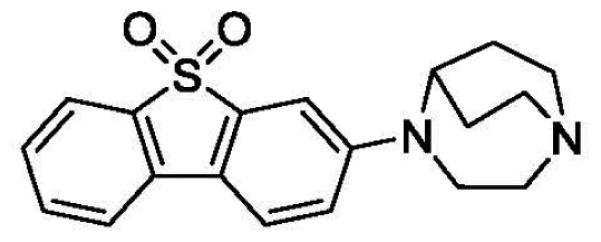
Structure of 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)dibenzo[b,d]thiophene 5,5-dioxide, an α7-nAChR selective ligand with high binding affinity that was developed by Abbott[60].
Even though the Abbott lead (Fig. 4) is difficult to radiolabel with the PET radionuclide 11C, the presence of an electron-withdrawing sulfonyl group opened an opportunity for making [18F]fluoro-derivatives of this compound via the nucleophilc aromatic substitution with [18F]fluoride. PET chemists from JHU synthesized a series of fluoro-derivatives of 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)dibenzo[b,d]thiophene 5,5-dioxide. Within the series, 4-(6- fluorodibenzo[b,d]thiophen-3-yl)-1,4-diazabicyclo[3.2.2]nonane 5,5-dioxide (JHU82132, ASEM) and its para-isomer (JHU82108, para-ASEM) exhibited the best α7-nAChR in vitro binding affinity and high selectivity vs. heteromeric nAChR subtypes or 5-HT3[48, 59] (see Table 1). The abbreviation “ASEM” stands for ALPHA-SEVEN (Α-СЕМЬ) in Greek-Russian as was suggested by Dr. R.F. Dannals from Johns Hopkins University.
Table 1.
Inhibition in vitro binding affinities (Ki, nM) of ASEM, para-ASEM and the Abbott lead toward α7-nAChR, heteromeric nAChR subtypes and 5-HT3 [48].
| Compound | α7-nAChR a | Heteromeric nAChR subtypes b | 5-HT3c | Selectivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α2β2 | α2β4 | α3β2 | α3β4 | α4β2 | α4β4 | α7/α4β2 | α7/5HT3 | |||
|
0.3, 0.5 | - | - | - | - | - | - | 660d | - | - |

|
0.37, 0.45 | >10000 | 4000 | 1000 | 709 | 562 | 1000 | 230 | 1370 | 561 |
|
1.32, 1.35 | 1000 | 8000 | 2000 | 5000 | 885 | 3000 | 505 | 663 | 378 |
Rat cortical membranes, radiotracer [125I]α-bungarotoxin (0.1 nM), KD = 0.7 nM
Inhibition in vitro binding assay of all heteromeric nAChR subtypes was performed with stably transfected HEK293 cells and [3H]epibatidine (0.5 nM), KD = 0.021 nM (α2β2-nAChR), KD = 0.084 nM (α2β4-nAChR), KD = 0.034 nM (α3β2-nAChR), KD = 0.29 nM (α3β4-nAChR), KD = 0.046 nM (α4β2-nAChR), KD = 0.094 nM (α4β4-nAChR).[80]
Human 5-HT3 recombinant/HEK293 cells, radiotracer [3H]GR65630 (0.35 nM), KD = 0.5 nM
the Ki value is taken from[60]
Prior radiolabeling of [18F]ASEM and further animal experiments the JHU group forecasted the PET imaging value of this compound by comparison of its in vitro binding affinity versus the previous best α7-nAChR PET tracers [18F]AZ11637326, [11C]NS14492 and [18F]NS10743(Fig. 3). The binding assay of all four compounds was performed under the same assay conditions (Table 2). This head-to-head comparison demonstrated that the α7-nAChR affinity of ASEM is 1-2 orders of magnitude superior to the previous radiotracers[48] and in vivo specific binding of [18F]ASEM was expected to be proportionally greater.
Table 2.
Comparison of in vitro α7-nAChR inhibition binding affinities of ASEM versus previous PET radioligands NS14492, NS10743, AZ11637326.
The binding assay performed under the same conditions: rat cortical membranes, radiotracer [125I]α-bungarotoxin (0.1 nM), KD = 0.7 nM (commercial assay, CEREP, www.cerep.fr).
In addition to the promising binding affinity, other molecular determinants of ASEM that are important for the blood-brain barrier permeability (molecular weight = 358; lipophilicity logD7.4 = 2.0; polar surface area = 49) are within the optimal range for most brain PET tracers[61, 62].
The appropriate in vitro properties of ASEM for PET and its potential suitability for [18F]-radiolabeling were the driving forces behind the radiosynthesis of [18F]ASEM and further in vivo experiments with this radiotracer. The isomer para-ASEM was selected as a back-up compound since it manifested fairly comparable in vitro binding affinity properties as ASEM (Table 1).
3.2 Radiosynthesis of [18F]ASEM and para-[18F]ASEM
Among the main requirements for [18F]-labeled (half-life t1/2 = 109.77 min) PET tracers is an efficient radiolabeling and its suitability for automation and preparation of the final product with high specific radioactivity, purity and radiochemical yield. The high specific radioactivity of PET radiotracers for neuroreceptors is a general requirement because of the concerns about the binding competition between the [18F] radiotracer and its natural non-radioactive [19F] isotopomer (carrier), which is always present in the radiolabeled product [63]. For reliable quantification analysis and also due to the safety concerns, a dose of PET radiotracer should occupy less than 5% of the available binding sites. This general requirement makes it necessary for a radiotracer to contain a low mass of carrier or, in other words, exhibit high specific radioactivity. Due to the low density of α7-nAChRs in the brain (see above) the radiotracers for this receptor should be used for PET studies only at a specific radioactivity greater than 5000 – 10000 mCi/µmol [35].
[18F]ASEM and para-[18F]ASEM were prepared by a Kryptofix-222® – assisted reaction of the corresponding nitro-precursors, PRE-ASEM and PRE-para-ASEM, with [18F]fluoride (Fig. 5). The radiosynthesis was performed remotely in a PC-controlled radiochemistry synthesis module (Microlab, GE). The final radiolabeled products were purified by preparative high-performance liquid chromatography (HPLC) and solid-phase extraction and formulated as sterile apyrogenic solutions in 7% ethanolic saline. Both radiotracers, [18F]ASEM and para- [18F]ASEM, were prepared with comparable radiochemical yields of 16 ± 6% (n=14) (non-decay-corrected), specific radioactivities in the range of 330 - 1260 GBq/μmol (9-34 Ci/μmol), and a radiochemical purity greater than 99%[48].
Fig. 5.
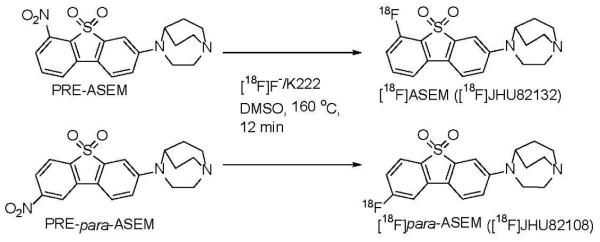
Radiosynthesis of [18F]ASEM and para-[18F]ASEM using a PC-controlled radiochemistry synthesis module (Microlab, GE) [48].
With increased demand for the [18F]ASEM for human and animal PET studies, a fast microwave-assisted synthesis with improved radiochemical yield (25-50%) was further developed[64]. The microwave method allows a routine preparation of about 500 mCi [18F]ASEM per batch with high specific radioactivity (>10000 mCi/µmol) and radiochemical purity (>98%), which means that it can be used for several PET scans within the same day.
4. Pre-clinical studies with [18F]ASEM in mice
4.1 Biodistribution in CD1 mice
After the successful radiosynthesis of [18F]ASEM, the radiotracer biodistribution, in vivo binding specificity, selectivity and brain kinetics were evaluated in CD1 mice [48].
In the ex vivo biodistribution experiments each mouse received an injection of [18F]ASEM into a lateral tail vein [48]. The animals were sacrificed at certain time points (5 – 120 min) and the brain regions were quickly dissected and assayed in a gamma-counter and time-radioactivity curves (Fig. 6) were generated. The study demonstrated that [18F]ASEM readily entered the mouse brain. The peak brain uptake (7.5% injected dose/g tissue) was seen at 5 min post injection, followed by a gradual decline. The time-radioactivity curves showed that the highest accumulation of [18F]ASEM radioactivity occurred in the colliculus, hippocampus and frontal cortex, intermediate radioactivity was observed in the striatum and the rest of the brain and the lowest radioactivity was seen in the cerebellum. This distribution of [18F]ASEM radioactivity is comparable to the in vitro distribution of α7-nAChRs in rodent brain tissue [65, 66].
Fig. 6.
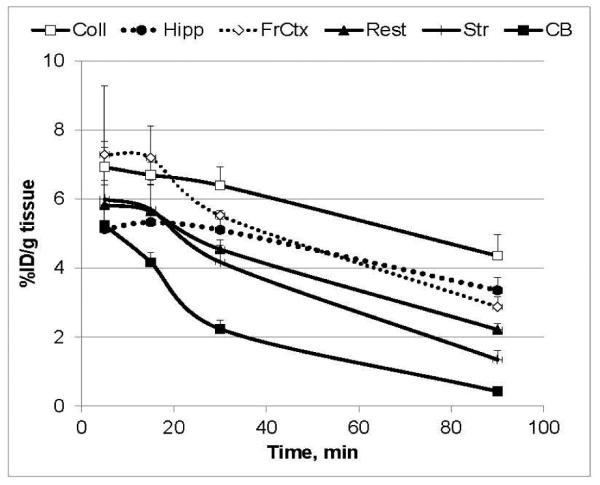
Regional distribution of [18F]ASEM in CD-1 mice. Data: mean %injected dose/g tissue ± SD (n = 3). Abbreviations: Coll = superior and inferior colliculus; Hipp = hippocampus; FrCtx = frontal cortex; Rest = rest of brain; Str = striatum; CB = cerebellum. Reprinted from [48] with permission of Copyright Clearance Center's RightsLink service. The study demonstrated that [18F]ASEM exhibits high brain uptake with regional distribution that matches the distribution of the α7-nAChR in the rodent brain and reversible brain kinetics.
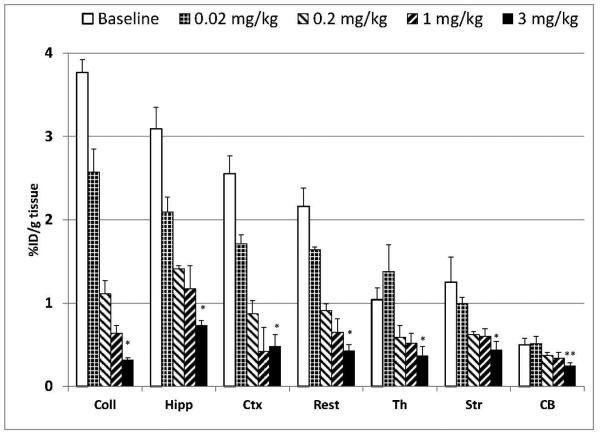
The high α7-nAChR specificity of [18F]ASEM binding was demonstrated by the blocking experiments in CD1 mice with the selective α7-nAChR partial agonist SSR180711 (Fig. 7). For this study each animal was injected with a mixture of [18F]ASEM and SSR180711. As expected, the [18F]ASEM accumulation in the α7-nAChR-rich brain regions was dose-dependently blocked by SSR180711. This result proved that [18F]ASEM uptake in the mouse brain is specific and mediated by α7-nAChRs. The dose escalation blockade also demonstrated that [18F]ASEM is a suitable tool for in vivo evaluation of potential new α7-nAChR drugs.
Fig. 7.
Dose dependent blockade of [18F]ASEM (0.07 mCi, specific radioactivity = 7900 mCi/μmol, i.v.) accumulation by intravenous co-injection of SSR180711, a selective α7-nAChR partial agonist (doses 0.02 mg/kg, 0.2 mg/kg, 1 mg/kg, 3 mg/kg) in the CD-1 mouse brain regions 90 min after the injection. *P ≤ 0.01, significantly different from controls (ANOVA). Data: mean %injected dose/g tissue ± SD (n=3). Abbreviations: Coll = superior and inferior colliculus; Hipp = hippocampus; Ctx = cortex; Str = striatum; Th = thalamus; Rest = rest of brain; CB = cerebellum. Reprinted from [48] with permission of Copyright Clearance Center's RightsLink service
One of the main characteristics of PET radiotracers is binding potential (BPND), which is the ratio of specific-to-nonspecific binding [67]. Good PET tracers are expected to demonstrate BPND>1. The binding potential values of [18F]ASEM in the mouse α7-nAChR – rich regions cortex (5.3), hippocampus, (5.5) and colliculus (8.0) (Table 3)[48] were high and sufficient for the receptor quantification.
Table 3.
Approximate binding potential (BPND) values (unitless) of [18F]ASEM and para-[18F]ASEM in the mouse brain regions. Data: mean ± SD (n = 6) [48].
| Compound | Region | Superior & inferior colliculus |
Hippocampus | Cortex |
|---|---|---|---|---|
| [18F]ASEM | 8.0 ± 1.6 | 5.5 ± 1.7 | 5.3 ± 1.2 | |
| para-[18F]ASEM | 2.0 ± 0.5 | 3.1 ± 0.7 | 2.0 ± 0.3 | |
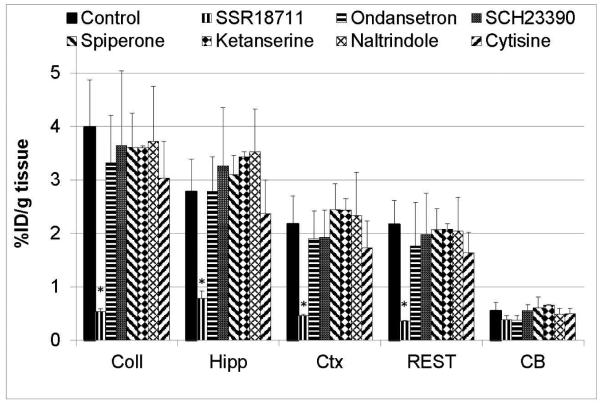
The α7-nAChR in vivo selectivity of [18F]ASEM binding in the mouse brain was tested by the blocking experiments with several non-α7-nAChR CNS drugs (Fig. 8). The drugs were ondansetron (selective 5-HT3 antagonist), SCH23390 (D1- and D5-antagonist and 5-HT1C/2C agonist), ketanserin (5-HT2/5-HT2C antagonist), naltrindole (selective δ-opioid antagonist), cytisine (α4β2-nAChR-selective partial agonist). None of these drugs except the positive control SSR180711 reduced accumulation of [18F]ASEM radioactivity when compared to the baseline controls[48]. The absence of blockade with the α4β2-nAChR-selective cytisine and 5-HT3-selective ondansetron was especially remarkable because α7-nAChR ligands often are not selective and bind at these receptors.
Fig. 8.
Effect of various CNS drugs (2 mg/kg, s.c.) on accumulation of [18F]ASEM in CD-1 mouse brain regions 90 min after injection of tracer expressed as %ID/g tissue. Abbreviations: Coll = superior and inferior colliculus; Hipp = hippocampus; Ctx = cortex; CB = cerebellum; REST = rest of brain. Data are mean ± SD (n=3). *P < 0.01, significantly different from controls. Columns that do not include the asterisk are insignificantly different from controls (P > 0.01) (ANOVA, single-factor analysis). The graph demonstrates that unlike the positive control (SSR180711) all non-α7-nAChR CNS drugs do not have an effect on the cerebral uptake of [18F]ASEM that is α7-nAChR selective in vivo. Drugs: SSR180711 – selective a7-nAChR partial agonist; Ondansetron - selective 5-HT3 antagonist; SCH23390 -D1- and D5-antagonist and 5- HT1C/2C agonist; Ketanserin - 5-HT2/5-HT2C antagonist; Naltrindole - Selective δ-opioid antagonist; Cytisine – α4β2-nAChR agonist [48].
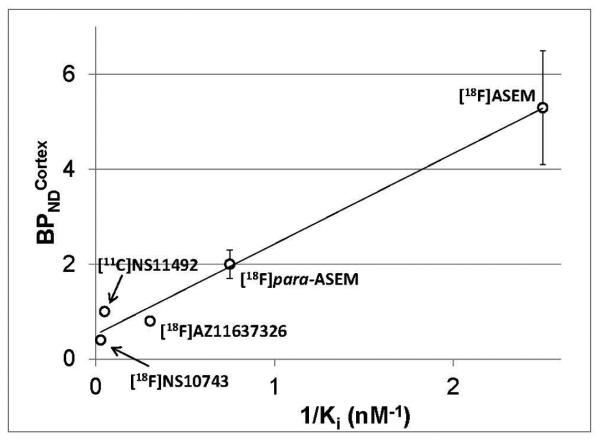
These studies in CD1 mice showed that [18F]ASEM labels α7-nAChR receptors in the mouse brain with high degree of specificity and selectivity [48] (Figs. 6-8). In the opinion of the [18F]ASEM developers the high specific binding of this radiotracer is chiefly attributed to its superior binding affinity versus the previous α7-nAChR radiotracers. This is supported by the correlation of the binding affinity and binding potential of [18F]ASEM and other radiotracers (Fig. 9).
Fig. 9.
Correlation of the BPNDcortex (unitless) vs. 1/Ki (nM−1) of α7-nAChR PET radioligands [18F]AZ11637326, [11C]NS14492, [18F]NS10743, [18F]para-ASEM and [18F]ASEM (y = 1.91x + 0.52, R2 = 0.98). All Ki values were obtained under the same binding assay conditions. Reprinted from [48] with permission of Copyright Clearance Center's RightsLink service
In a parallel set of experiments the para-[18F]ASEM was also tested in CD1 mice. The pattern of regional distribution of para-[18F]ASEM was comparable to that of [18F]ASEM. However, in agreement with its lower binding affinity para-[18F]ASEM exhibited lower specific binding in mice than [18F]ASEM (Tables 1 and 3).
4.2 Radiometabolite analysis of [18F]ASEM in blood and brain
Most PET radiotracers undergo metabolism and generate various radiometabolites that, ideally, do not accumulate in the brain and spoil the quality of the PET image and thus reduce the accuracy of the quantification of the targeted receptor. Conventionally, the brain radiometabolites are considered to be insignificant for accurate quantification if the fraction of the parent radiotracer in the brain is greater than 95%.
The HPLC analysis of blood samples from CD-1 mice, and, also, from baboons[59] and human subjects[68] demonstrated that the parent compound [18F]ASEM was gradually metabolized to the same hydrophilic radiometabolites in all three species. Luckily, only a small fraction of [18F]ASEM radiometabolites penetrates the blood-brain barrier and the main radioactive compound in the brain is the parent [18F]ASEM (>95%), as it is shown by the HPLC analysis of the mouse brain tissue. Because the radiometabolite fraction in the brain tissue is insignificant, mathematical PET modeling of the radiometabolites is not necessary for quantification of α7-nAChRs with [18F]ASEM [59].
4.3 Clinical α7-nAChR drugs block the [18F]ASEM binding in the mouse brain.
Several drugs that target α7-nAChRs are now in the clinical phases of development for treatment of cognitive deficit in various pathologies [27-29]. DMXB-A was the first selective α7-nAChR agonist to demonstrate cognitive enhancement and improvement in negative symptoms in patients with schizophrenia[69, 70]. The more recent drug EVP-6124 (Encenicline) is an α7-nAChR selective partial agonist that is now in a number of clinical trials for treatment of Alzheimer’s disease and schizophrenia (see https://clinicaltrials.gov). It was of interest to test the blocking effect of the clinical doses of the α7-nAChR drugs in the PET experiments with [18F]ASEM.
DMXB-A dose-dependently blocked the [18F]ASEM binding in the α7-nAChR - rich brain regions in mice (Fig. 10a)[68]. The blocking effect was significant when a clinical equivalent dose was used. This result demonstrates the potential feasibility for evaluating the effect of the clinical drug DMXB-A in human subjects with [18F]ASEM, and opens new ways to study the biochemical mechanism of drugs for treatment of cognitive performance in patients with schizophrenia. In addition to DMXB-A, similar [18F]ASEM blocking studies were performed with two other nicotinic drugs [68] in clinical trials that bind at α7-nAChR, EVP-6124[71] and Varenicline (binds at α4β2- and α7-nAChRs [72]) (Figs. 10b,c).
Fig. 10.
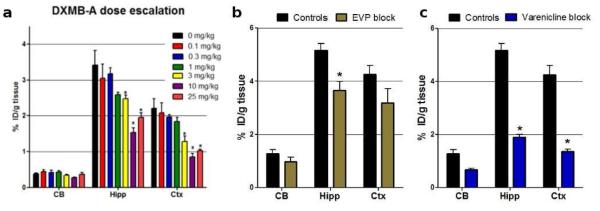
Baseline versus blockade studies of [18F]ASEM with mouse-equivalent doses of clinical α7-nAChR drugs in CD-1 mice. Data: %ID/g tissue ± SD (n = 4). The control mice were treated with vehicle saline. Abbreviations: CB = cerebellum, Hipp = hippocampus; Ctx = cortex. Statistics in all three graphs: *P < 0.01, blockade is significantly different from controls (ANOVA). a: DMXB-A (GTS-21), dose-escalation. Note: a mouse-equivalent dose = 25 mg/kg[81] of the clinical dose (150 mg). 90 min post [18F]ASEM injection. b: EVP-6124, a mouse-equivalent dose (0.18 mg/kg) of the clinical dose (1 mg). 60 min post [18F]ASEM injection. c: Varenicline, a mouse-equivalent dose (0.18 mg/kg) of the clinical dose (1 mg). 60 min post [18F]ASEM injection. The graph demonstrates that in vivo binding of [18F]ASEM in the mouse brain regions enriched with α7-nAChR is significantly blocked by the α7-nAChR drugs DMXB-A, EVP-6124 and varenicline. Reprinted from[68] with permission of Copyright Clearance Center's RightsLink service.
It is noteworthy, that clinical dose equivalents of α7-nAChR drugs EVP-6124 and DMXB-A only partially blocked the binding of [18F]ASEM in the mouse brain (23-28% and 45-55%, respectively) (Fig. 10a,b). This degree of blockade is comparable to the currently accepted degree of α7-nAChR occupancy required to achieve clinical or behavioral efficacy. The α7-nAChR receptors have five binding sites distributed between five α7 subunits. When an agonist is applied to a population of α7-nAChRs, the maximum α7-nAChR receptor activation may occur when two of the five possible binding sites are occupied[73, 74]. At higher concentrations, agonists desensitize the α7-nAChR and may bind to all five binding sites. In agreement with this mechanism, pre-clinical studies and clinical trials suggest that efficacious concentrations of EVP-6124 (Encenicline) is low and sufficient to occupy only one binding site on the α7-nAChRs [71, 75]. It was hypothesized that endogenous acetylcholine (ACh) is required to bind to another site on the α7-nAChR in order to activate the receptor channel opening. Because ACh exhibits low α7-nAChRs binding affinity the complex ACh*α7-nAChR quickly dissociates after the receptor channel opening and, thus, α7-nAChRs are activated by low clinical doses of EVP-6124, but are not desensitized.
This mechanism of action agrees with the observed degree of [18F]ASEM blockade with EVP-6124 and DMXB-A and suggests that in the future human PET studies with clinically efficacious doses of α7-nAChR agonists the binding of [18F]ASEM will not be blocked more than 20% - 40%.
4.4 Distribution of [18F]ASEM in DISC1 mice, a rodent model of schizophrenia.
Post-mortem research demonstrated significantly lower density of α7-nAChRs in the brains of schizophrenia subjects vs. controls (see Introduction for references). Mutant DISC1 mice provide a model for brain and behavioral phenotypes seen in schizophrenia[76]. Based on the favorable imaging properties identified in control CD1 mice, the [18F]ASEM binding was investigated in DISC1 mice. In agreement with the reduced density of α7-nAChRs in the brain tissue of schizophrenic subjects, the [18F]ASEM binding in the α7-nAChR – rich brain regions was significantly lower in the DISC1 mice when compared to control animals (data not shown, see paper[59]). This result emphasizes the potential utility of [18F]ASEM for imaging of α7-nAChRs in schizophrenia.
5. PET-[18F]ASEM imaging of α7-nAChRs in baboons.
The successful brain distribution studies of [18F]ASEM in rodents (see above) provided a foundation for further pre-clinical evaluation in baboons [59]. The main purpose of these studies was to determine if (1) [18F]ASEM exhibits the brain regional distribution that matches the distribution of α7-nAChRs in non-human primates; (2) the binding is α7-nAChR specific; (3) the brain pharmacokinetics of [18F]ASEM are suitable for quantification of α7-nAChRs and (4) to evaluate the PET imaging characteristics of [18F]ASEM by mathematical modeling methods.
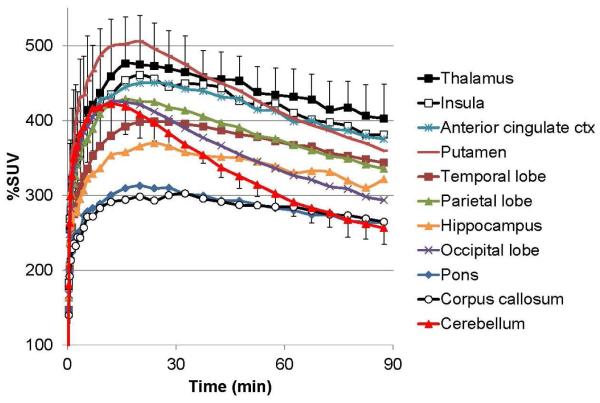
In PET baboon experiments [18F]ASEM exhibited high and reversible brain uptake that peaked (500% standardized uptake value (%SUV)) at 20 min post injection of the radiotracer (Fig. 11)[59]. The distribution pattern of the [18F]ASEM radioactivity was heterogeneous (thalamus > insula > anterior cingulate cortex > putamen > hippocampus > cortical regions > pons ~ cerebellum ~ corpus callosum) and consistent with previously published in vitro distribution of α7-nAChRs in non-human primates [40, 41, 77].
Fig. 11.
Baseline cerebral time-activity curves (TACs) after bolus administration of [18F]ASEM in three baboons. The graph demonstrates a substantial heterogeneous brain uptake of [18F]ASEM that matches the distribution of α7-nAChR in non-human primates[40, 41, 77] and reversible brain kinetics. Data: mean Standardized Uptake Values (%SUV) ± SD (n = 3). Reprinted from[59] with permission of SNMMI.
The α7-nAChR specificity of the [18F]ASEM binding in the baboon brain was established in the blocking PET experiments. The dose-dependent blockade of [18F]ASEM uptake with selective α7-nAChR partial agonist SSR180711 demonstrated that the binding of [18F]ASEM is mediated by α7-nAChR (Fig. 12).
Fig. 12.
Sagittal (Top row), and trans-axial (Middle and Bottom rows) views of VT images of [18F]ASEM in the same baboon for a baseline PET scan (B), and after administration of 0.5 mg/kg (C) and 5 mg/kg (D) of SSR180711, a selective α7-nAChR partial agonist. MR images (A) indicate locations of selected brain structures including the cingulate cortex (Cg), thalamus (Th), and caudate nucleus (CN), which are indicated by + in the VT images (D). The VT images are displayed using the same minimum and maximum values for all scanning conditions. These data demonstrate the dose dependent blockade of [18F]ASEM in baboon brain and provide evidence that [18F]ASEM is specific and mediated by α7-nAChR. The images also suggest that there is no reference region devoid of α7-nAChRs. Reprinted from[59] with permission of SNMMI.
The baseline and blocking PET studies allowed calculation of one of the main PET imaging characteristics of [18F]ASEM in baboon, the binding potential (BPND = 3.9 – 6.6)[59]. The value of BPND is quite high for PET and suitable for reliable quantification of α7-nAChRs. For comparison, all previous α7-nAChR radioligands exhibited BPND < 1[35, 46-48].
6. First-in-human PET - [18F]ASEM imaging of α7-nAChRs[68].
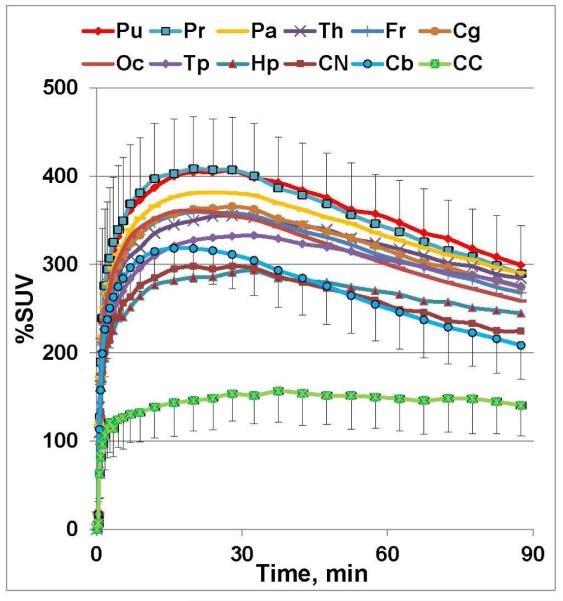
Previously, the lack of a specific PET radioligand has impeded the accurate mapping and quantification of α7-nAChRs in the living human brain. In the first-in-human PET studies in five healthy male subjects under an IND, [18F]ASEM radioactivity peaked in the brain at 20 min after bolus injection with a robust uptake value of 400 %SUV (Fig. 13). The brain pharmacokinetics were reversible and demonstrated a decline of radioactivity concentration after the peak[68]. The highest regional brain uptake (total volume of distribution VT, calculated with plasma reference graphic analysis) was seen in the parietal cortex (VT=22±1.8) ~ putamen (21.8±2.9) > thalamus (20.9±3.0) > cingulate (19.6±2.2) > temporal lobes (19.7±1.8) ~ frontal lobes (19.3±1.9) > hippocampus (VT=17.9±1.9); moderate uptake was found in the cerebellum (15.1±1.6) and brainstem (14.8±1.7); and the lowest uptake was in the corpus callosum (9.9±2.0). The regional distribution of [18F]ASEM in the human brain matches the post-mortem human and non-human primate data [59, 78, 79]. The test-retest variability (TRV) was 10.8±5.1%, which charcterises [18F]ASEM as a reproducible PET radiotracer.
Fig. 13.
Baseline PET/[18F]ASEM TAC’s (%SUV ± SD (n = 5)) in healthy human males. Abbreviations: Pu = putamen; Pr = precuneous; Pa = parietal lobe; Th = thalamus; Fr = frontal lobe; Cg = cingulate; Oc = occipital; Tp = temporal lobe; Hp = hippocampus; CN = caudate nucleus; Cb = cerebellum; CC = corpus callosum. The distribution of [18F]ASEM in the human brain regions is comparable with non-human primate (see for review [34]) and human post-mortem distribution of α7-nAChR [47-48]. The brain kinetics of [18F]ASEM are reversible. Reprinted from[68] with permission of Copyright Clearance Center's RightsLink service.
7. Conclusion
In summary, during the past decade we have witnessed substantial efforts to develop a PET radioligand for quantification of α7-nAChRs in the human brain. Several research groups have radiolabeled a number of α7-nAChR compounds with [18F] and [11C] for PET. Unfortunately, these radioligands did not show suitable specific binding in vivo due to insufficient α7-nAChR binding affinity. The recently developed [18F]ASEM, a highly α7-nAChR specific and selective radiotracer for brain PET, demonstrated excellent in vivo imaging properties in the rodents and baboons and was successfully translated to human subjects. [18F]ASEM opens new horizons for studying α7-nAChRs in the living human brain.
Fig. 14.
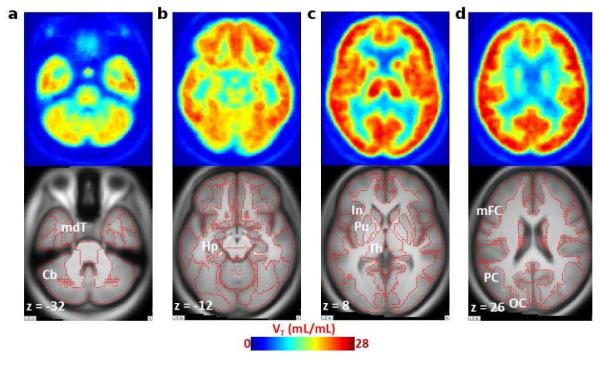
Averaged (n = 5) transaxial images of a spatially normalized VT map of [18F]ASEM and matching MRI in healthy control subjects. Cerebellum (Cb) and medial temporal cortex (mdT; panel a) show relatively low VT values and hippocampus (Hp; panel b) show medium VT values. The insula (In), putamen (Pu), and thalamus (Th) are shown in panel c, and middle frontal (mFC), parietal (PC), and occipital (OC) cortices (panel d) exhibit high VT values in the human brain. Red dots on MRI images indicate outlines of cortical and subcortical structures. Reprinted from[68] with permission of Copyright Clearance Center's RightsLink service.
8. Acknowledgement
The author received NIH grants AG037298 and NS089437 and funds from the Department of Radiology. Special thanks to Drs. Robert Dannals, Yongjun Gao, Hayden Ravert, Dean Wong, Martin Pomper, Hiroto Kuwabara, Mikhail Pletnikov from Johns Hopkins University and Dr. Kenneth Kellar from Georgetown University for fruitful discussions and Julia Buchanan for the editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- [2].Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology (Berl) 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ishikawa M, Hashimoto K. alpha7 nicotinic acetylcholine receptor as a potential therapeutic target for schizophrenia. Curr Pharm Des. 2011;17:121–9. doi: 10.2174/138161211795049561. [DOI] [PubMed] [Google Scholar]
- [4].Parri HR, Hernandez CM, Dineley KT. Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer's disease. Biochem Pharmacol. 2011;82:931–42. doi: 10.1016/j.bcp.2011.06.039. [DOI] [PubMed] [Google Scholar]
- [5].Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woodruff-Pak DS, Gould TJ. Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer's disease and schizophrenia. Behav Cogn Neurosci Rev. 2002;1:5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- [7].D'Hoedt D, Bertrand D. Nicotinic acetylcholine receptors: an overview on drug discovery. Expert Opin Ther Targets. 2009;13:395–411. doi: 10.1517/14728220902841045. [DOI] [PubMed] [Google Scholar]
- [8].Hoffmeister PG, Donat CK, Schuhmann MU, Voigt C, Walter B, Nieber K, et al. Traumatic brain injury elicits similar alterations in alpha7 nicotinic receptor density in two different experimental models. Neuromolecular Med. 2011;13:44–53. doi: 10.1007/s12017-010-8136-4. [DOI] [PubMed] [Google Scholar]
- [9].Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–96. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [10].Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–43. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- [11].Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- [12].Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- [13].Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- [14].Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–33. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- [15].Guan ZZ, Zhang X, Blennow K, Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–82. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- [16].Mathew SV, Law AJ, Lipska BK, Davila-Garcia MI, Zamora ED, Mitkus SN, et al. Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16:2921–32. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- [17].Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- [18].Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–45. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- [19].Sugaya K, Giacobini E, Chiappinelli VA. Nicotinic acetylcholine receptor subtypes in human frontal cortex: Changes in Alzheimer's disease. J Neurosci Res. 1990;27:349. doi: 10.1002/jnr.490270314. [DOI] [PubMed] [Google Scholar]
- [20].Posadas I, Lopez-Hernandez B, Cena V. Nicotinic receptors in neurodegeneration. Curr Neuropharmacol. 2013;11:298–314. doi: 10.2174/1570159X11311030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011;13:701–7. doi: 10.1111/j.1399-5618.2011.00961.x. [DOI] [PubMed] [Google Scholar]
- [22].Besson VC. Drug targets for traumatic brain injury from poly(ADP-ribose)polymerase pathway modulation. Br J Pharmacol. 2009;157:695–704. doi: 10.1111/j.1476-5381.2009.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injuries in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- [24].Verbois SL, Sullivan PG, Scheff SW, Pauly JR. Traumatic brain injury reduces hippocampal alpha7 nicotinic cholinergic receptor binding. J Neurotrauma. 2000;17:1001–11. doi: 10.1089/neu.2000.17.1001. [DOI] [PubMed] [Google Scholar]
- [25].Verbois SL, Scheff SW, Pauly JR. Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J Neurotrauma. 2002;19:1569–85. doi: 10.1089/089771502762300238. [DOI] [PubMed] [Google Scholar]
- [26].Kelso ML, Wehner JM, Collins AC, Scheff SW, Pauly JR. The pathophysiology of traumatic brain injury in alpha7 nicotinic cholinergic receptor knockout mice. Brain Res. 2006;1083:204–10. doi: 10.1016/j.brainres.2006.01.127. [DOI] [PubMed] [Google Scholar]
- [27].Mazurov AA, Speake JD, Yohannes D. Discovery and development of alpha7 nicotinic acetylcholine receptor modulators. J Med Chem. 2011;54:7943–61. doi: 10.1021/jm2007672. [DOI] [PubMed] [Google Scholar]
- [28].Taly A, Charon S. alpha7 nicotinic acetylcholine receptors: a therapeutic target in the structure era. Curr Drug Targets. 2012;13:695–706. doi: 10.2174/138945012800398919. [DOI] [PubMed] [Google Scholar]
- [29].Wallace TL, Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin Ther Tar. 2013;17:139–55. doi: 10.1517/14728222.2013.736498. [DOI] [PubMed] [Google Scholar]
- [30].Lippiello PM, Bencherif M, Hauser TA, Jordan KG, Letchworth SR, Mazurov AA. Nicotinic receptors as targets for therapeutic discovery. Expert Opinion on Drug Discovery. 2007;2:1185–203. doi: 10.1517/17460441.2.9.1185. [DOI] [PubMed] [Google Scholar]
- [31].Kem WR. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer's disease: studies with DMXBA (GTS-21) Behav Brain Res. 2000;113:169–81. doi: 10.1016/s0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- [32].Gatson JW, Simpkins JW, Uteshev VV. High therapeutic potential of positive allosteric modulation of alpha7 nAChRs in a rat model of traumatic brain injury: proof-of-concept. Brain Res Bull. 2015;112:35–41. doi: 10.1016/j.brainresbull.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alavi A, Basu S. Planar and SPECT imaging in the era of PET and PET-CT: can it survive the test of time? Eur J Nucl Med Mol Imaging. 2008;35:1554–9. doi: 10.1007/s00259-008-0813-2. [DOI] [PubMed] [Google Scholar]
- [34].Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- [35].Horti AG, Villemagne VL. The quest for Eldorado: development of radioligands for in vivo imaging of nicotinic acetylcholine receptors in human brain. Curr Pharm Des. 2006;12:3877–900. doi: 10.2174/138161206778559605. [DOI] [PubMed] [Google Scholar]
- [36].Piel M, Vernaleken I, Rosch F. Positron emission tomography in CNS drug discovery and drug monitoring. J Med Chem. 2014;57:9232–58. doi: 10.1021/jm5001858. [DOI] [PubMed] [Google Scholar]
- [37].Halldin C, Gulyas B, Langer O, Farde L. Brain radioligands--state of the art and new trends. Q J Nucl Med. 2001;45:139–52. [PubMed] [Google Scholar]
- [38].Eckelman WC, Reba RC, Gibson RE. Receptor-binding radiotracers: A class of potential radiopharmaceuticals. J Nucl Med. 1979;20:350–7. [PubMed] [Google Scholar]
- [39].Eckelman WC, Kilbourn MR, Mathis CA. Discussion of targeting proteins in vivo: in vitro guidelines. Nucl Med Biol. 2006;33:449–51. doi: 10.1016/j.nucmedbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [40].Kulak JM, Schneider JS. Differences in alpha7 nicotinic acetylcholine receptor binding in motor symptomatic and asymptomatic MPTP-treated monkeys. Brain Res. 2004;999:193–202. doi: 10.1016/j.brainres.2003.10.062. [DOI] [PubMed] [Google Scholar]
- [41].Kulak JM, Carroll FI, Schneider JS. [125I]Iodomethyllycaconitine binds to alpha7 nicotinic acetylcholine receptors in monkey brain. Eur J Neurosci. 2006;23:2604–10. doi: 10.1111/j.1460-9568.2006.04804.x. [DOI] [PubMed] [Google Scholar]
- [42].Horti AG, Gao Y, Kuwabara H, Dannals RF. Development of radioligands with optimized imaging properties for quantification of nicotinic acetylcholine receptors by positron emission tomography. Life Sci. 2009 doi: 10.1016/j.lfs.2009.02.029. doi:10.1016/j.lfs.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Horti AG, Wong DF. Clinical Perspective and Recent Development of PET Radioligands for Imaging Cerebral Nicotinic Acetylcholine Receptors. PET Clin. 2009;4:89–100. doi: 10.1016/j.cpet.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dolle F, Valette H, Hinnen F, Vaufrey F, Demphel S, Coulon C, et al. Synthesis and preliminary evaluation of a carbon-11-labelled agonist of the a7 nicotinic acetylcholine receptor. J Label Compd Radiopharm. 2001;44:785–95. [Google Scholar]
- [45].Pomper MG, Phillips E, Fan H, McCarthy DJ, Keith RA, Gordon JC, et al. Synthesis and biodistribution of radiolabeled alpha 7 nicotinic acetylcholine receptor ligands. J Nucl Med. 2005;46:326–34. [PubMed] [Google Scholar]
- [46].Toyohara J, Wu J, Hashimoto K. Recent development of radioligands for imaging alpha7 nicotinic acetylcholine receptors in the brain. Curr Top Med Chem. 2010;10:1544–57. doi: 10.2174/156802610793176828. [DOI] [PubMed] [Google Scholar]
- [47].Brust P, Deuther-Conrad W. Molecular imaging of alpha7 nicotinic acetylcholine receptors in vivo: current status and perspectives. In: Bright P, editor. Neuroimaging - Clinical Applications. InTech; Rijeka: 2012. pp. 533–58. [Google Scholar]
- [48].Gao Y, Kellar KJ, Yasuda RP, Tran T, Xiao Y, Dannals RF, et al. Derivatives of Dibenzothiophene for Positron Emission Tomography Imaging of alpha7-Nicotinic Acetylcholine Receptors. J Med Chem. 2013;56:7574–89. doi: 10.1021/jm401184f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Toyohara J, Sakata M, Wu J, Ishikawa M, Oda K, Ishii K, et al. Preclinical and the first clinical studies on [11C]CHIBA-1001 for mapping alpha7 nicotinic receptors by positron emission tomography. Ann Nucl Med. 2009;23:301–9. doi: 10.1007/s12149-009-0240-x. [DOI] [PubMed] [Google Scholar]
- [50].Ishikawa M, Sakata M, Toyohara J, Oda K, Ishii K, Wu J, et al. Occupancy of alpha7 Nicotinic Acetylcholine Receptors in the Brain by Tropisetron: A Positron Emission Tomography Study Using [(11)C]CHIBA-1001 in Healthy Human Subjects. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology. 2011;9:111–6. doi: 10.9758/cpn.2011.9.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tanibuchi Y, Wu J, Toyohara J, Fujita Y, Iyo M, Hashimoto K. Characterization of [(3)H]CHIBA-1001 binding to alpha7 nicotinic acetylcholine receptors in the brain from rat, monkey, and human. Brain Res. 2010;1348:200–8. doi: 10.1016/j.brainres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- [52].Ding M, Ghanekar S, Elmore CS, Zysk JR, Werkheiser JL, Lee CM, et al. [3H]Chiba-1001 (methyl-SSR180711) has low in vitro binding affinity and poor in vivo selectivity to nicotinic alpha-7 receptor in rodent brain. Synapse. 2012;66:315–22. doi: 10.1002/syn.21513. [DOI] [PubMed] [Google Scholar]
- [53].Ravert HT, Dorff P, Foss CA, Mease RC, Fan H, Holmquist CR, et al. Radiochemical synthesis and in vivo evaluation of [18F]AZ11637326: An agonist probe for the alpha7 nicotinic acetylcholine receptor. Nucl Med Biol. 2013;40:731–9. doi: 10.1016/j.nucmedbio.2013.04.005. [DOI] [PubMed] [Google Scholar]
- [54].Maier DL, Hill G, Ding M, Tuke D, Einstein E, Gurley D, et al. Pre-clinical validation of a novel alpha-7 nicotinic receptor radiotracer, [3H]AZ11637326: target localization, biodistribution and ligand occupancy in the rat brain. Neuropharmacology. 2011;61:161–71. doi: 10.1016/j.neuropharm.2011.03.024. [DOI] [PubMed] [Google Scholar]
- [55].Ettrup A, Mikkelsen JD, Lehel S, Madsen J, Nielsen EO, Palner M, et al. 11C-NS14492 as a novel PET radioligand for imaging cerebral alpha7 nicotinic acetylcholine receptors: in vivo evaluation and drug occupancy measurements. J Nucl Med. 2011;52:1449–56. doi: 10.2967/jnumed.111.088815. [DOI] [PubMed] [Google Scholar]
- [56].Deuther-Conrad W, Fischer S, Hiller A, Becker G, Cumming P, Xiong G, et al. Assessment of alpha7 nicotinic acetylcholine receptor availability in juvenile pig brain with [18F]NS10743. Eur J Nucl Med Mol Imaging. 2011;38:1541–9. doi: 10.1007/s00259-011-1808-y. [DOI] [PubMed] [Google Scholar]
- [57].Deuther-Conrad W, Fischer S, Hiller A, Nielsen EO, Timmermann DB, Steinbach J, et al. Molecular imaging of alpha7 nicotinic acetylcholine receptors: design and evaluation of the potent radioligand [18F]NS10743. Eur J Nucl Med Mol Imaging. 2009;36:791–800. doi: 10.1007/s00259-008-1031-7. [DOI] [PubMed] [Google Scholar]
- [58].Horti AG, Ravert HT, Gao YJ, Holt DP, Bunnelle WH, Schrimpf MR, et al. Synthesis and evaluation of new radioligands [C-11]A-833834 and [C-11]A-752274 for positron-emission tomography of alpha 7-nicotinic acetylcholine receptors. Nucl Med Biol. 2013;40:395–402. doi: 10.1016/j.nucmedbio.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Horti AG, Gao Y, Kuwabara H, Wang Y, Abazyan S, Yasuda RP, et al. [18F]ASEM ([18F]JHU82132), a radiolabeled antagonist for imaging the a7-nicotinic acetylcholine receptor (α7-nAChR) with positron emission tomography (PET) J Nucl Med. 2014;55:672–7. doi: 10.2967/jnumed.113.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schrimpf MR, Sippy KB, Briggs CA, Anderson DJ, Li T, Ji J, et al. SAR of alpha7 nicotinic receptor agonists derived from tilorone: exploration of a novel nicotinic pharmacophore. Bioorg Med Chem Lett. 2012;22:1633–8. doi: 10.1016/j.bmcl.2011.12.126. [DOI] [PubMed] [Google Scholar]
- [61].Horti AG, Raymont V, Terry GE. PET imaging of endocannabinoid system. In: Dierckx R, Otte A, De Vries EF, Van Waarde A, editors. PET and SPECT of Neurobiological Systems. Springer; Berlin-Heidelberg: 2014. pp. 251–319. [Google Scholar]
- [62].Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5:376–89. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- [63].Dannals RF, Ravert HT, Wilson AA, Wagner HN. Special Problems Associated with the Synthesis of High Specific Activity Carbon-11 Labeled Radiotracers. In: A.M. E, editor. New Trends Radiopharm Synthesis, Quality Assurance and Regulatory Control. Springer US; 1991. pp. 21–30. [Google Scholar]
- [64].Ravert HT, Holt DP, Gao Y, Horti AG, Dannals RF. Microwave-assisted radiosynthesis of [(18) F]ASEM, a radiolabeled alpha7-nicotinic acetylcholine receptor antagonist. J Labelled Comp Radiopharm. 2015;58:180–2. doi: 10.1002/jlcr.3275. [DOI] [PubMed] [Google Scholar]
- [65].Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–15. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Whiteaker P, Davies AR, Marks MJ, Blagbrough IS, Potter BV, Wolstenholme AJ, et al. An autoradiographic study of the distribution of binding sites for the novel alpha7-selective nicotinic radioligand [3H]-methyllycaconitine in the mouse brain. Eur J Neurosci. 1999;11:2689–96. doi: 10.1046/j.1460-9568.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- [67].Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- [68].Wong DF, Kuwabara H, Pomper M, Holt DP, Brasic JR, George N, et al. Human Brain Imaging of Alpha-7 nAChR with [18F]ASEM: a New PET Radiotracer for Neuropsychiatry and Determination of Drug Occupancy. Molecular Imaging and Biology. 2014;16:730–8. doi: 10.1007/s11307-014-0779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- [71].Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, et al. EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- [72].Rollema H, Shrikhande A, Ward KM, Tingley FD, 3rd, Coe JW, O'Neill BT, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–45. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Williams DK, Wang J, Papke RL. Investigation of the molecular mechanism of the alpha7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol. 2011;80:1013–32. doi: 10.1124/mol.111.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol. 2014;89:1–11. doi: 10.1016/j.bcp.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. Journal of psychiatric practice. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- [76].Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–86. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- [77].Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- [78].Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat. 2000;20:281–98. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- [79].Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, et al. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–98. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [80].Xiao Y, Kellar K. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- [81].Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]