Abstract
Host survival of herpes simplex virus type 1 (HSV-1) infection depends on the establishment of latent infections in both peripheral and central nervous systems. Strains of HSV-1 that are successful in escaping the immune response produce a lethal infection. We now report a possible mechanism of immune response evasion used by HSV-1. After intraocular inoculation of mice, HSV-1 strain F established a latent infection in the brain, whereas strain KOS did not. The immune response to HSV-1 infection (strains KOS and F) in the brain was characterized by induction of major histocompatibility complex class II expression and recruitment of CD4+ and CD8+ cells to highly restricted sites of intracerebral viral infection. Major histocompatibility complex class II antigen expression was primarily intracellular in strain KOS infection centers and at the cell surface in strain F infection centers. We propose that major histocompatibility complex class II-restricted viral-antigen presentation to T cells is interrupted during strain KOS infections, thereby allowing KOS infection to evade T-cell-mediated events that would normally protect the host from a lethal infection. Immunocompromised mice (athymic or irradiate mice) could not survive strain F infections; however, latent F infections were established in irradiated mice reconstituted with naive lymph node and spleen cells. These data suggest that class II-restricted presentation of viral antigens is required for the control of HSV-1 infections in the nervous system.
Full text
PDF
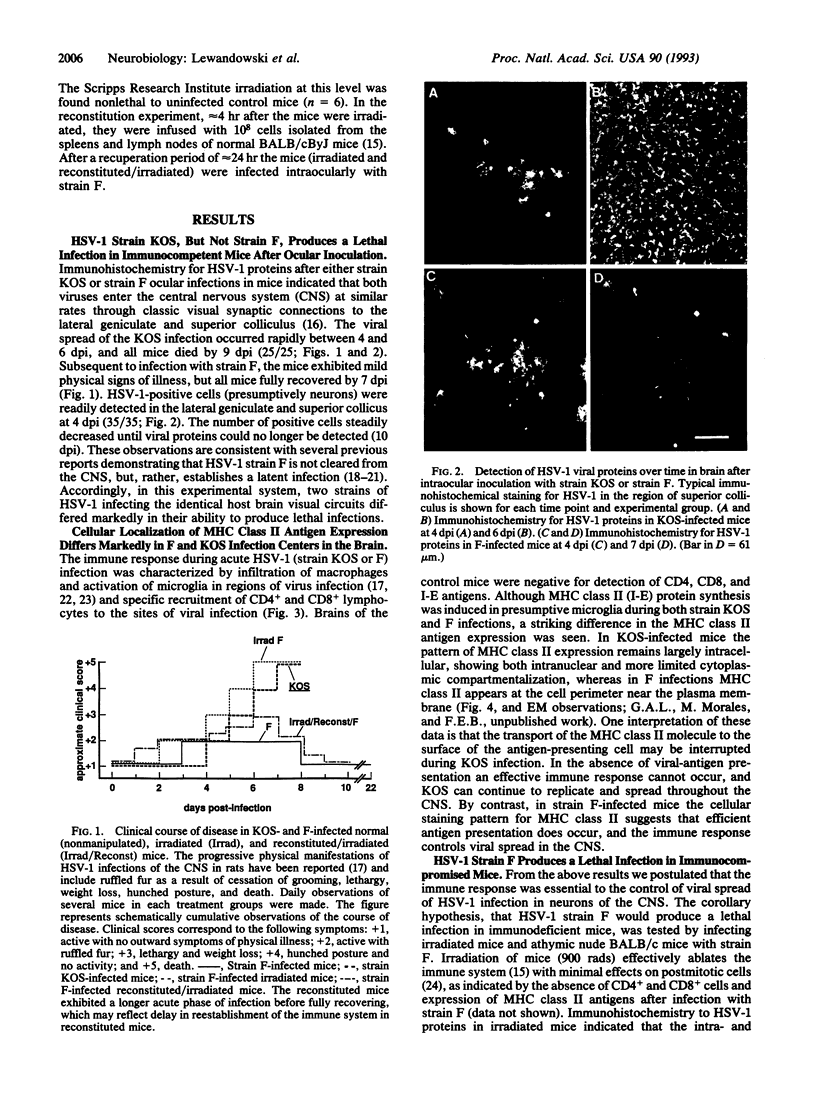

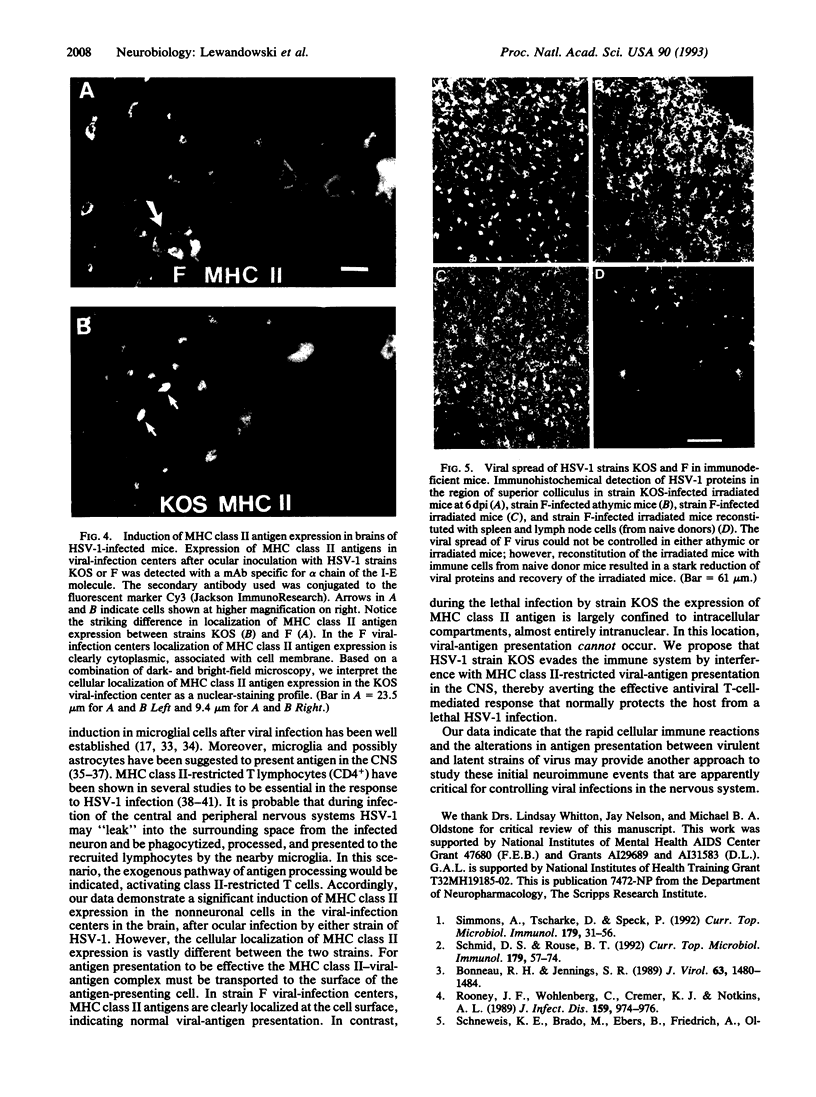

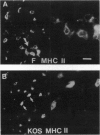
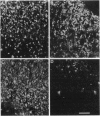
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Battenberg E. L. Immunocytochemistry of endorphins and enkephalins. Methods Enzymol. 1983;103:670–687. doi: 10.1016/s0076-6879(83)03048-7. [DOI] [PubMed] [Google Scholar]
- Bodmer H. C., Bastin J. M., Askonas B. A., Townsend A. R. Influenza-specific cytotoxic T-cell recognition is inhibited by peptides unrelated in both sequence and MHC restriction. Immunology. 1989 Feb;66(2):163–169. [PMC free article] [PubMed] [Google Scholar]
- Bonneau R. H., Jennings S. R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989 Mar;63(3):1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Wohlenberg C., Openshaw H., Rey-Mendez M., Puga A., Notkins A. L. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature. 1980 Nov 20;288(5788):288–290. doi: 10.1038/288288a0. [DOI] [PubMed] [Google Scholar]
- Croen K. D. Latency of the human herpesviruses. Annu Rev Med. 1991;42:61–67. doi: 10.1146/annurev.me.42.020191.000425. [DOI] [PubMed] [Google Scholar]
- Daksis J. I., Preston C. M. Herpes simplex virus immediate early gene expression in the absence of transinduction by Vmw65 varies during the cell cycle. Virology. 1992 Jul;189(1):196–202. doi: 10.1016/0042-6822(92)90695-l. [DOI] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., O'Boyle D. R., 2nd, Fraser N. W. Latent herpes simplex virus type 1 transcripts in peripheral and central nervous system tissues of mice map to similar regions of the viral genome. J Virol. 1988 Mar;62(3):749–756. doi: 10.1128/jvi.62.3.749-756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschl U., Stitz L., Herzog S., Frese K., Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol. 1990;81(1):41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Spivack J. G., Wroblewska Z., Block T., Deshmane S. L., Valyi-Nagy T., Natarajan R., Gesser R. M. A review of the molecular mechanism of HSV-1 latency. Curr Eye Res. 1991;10 (Suppl):1–13. doi: 10.3109/02713689109020352. [DOI] [PubMed] [Google Scholar]
- Gairin J. E., Joly E., Oldstone M. B. Persistent infection with lymphocytic choriomeningitis virus enhances expression of MHC class I glycoprotein on cultured mouse brain endothelial cells. J Immunol. 1991 Jun 1;146(11):3953–3957. [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey W. F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991 Jan;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Ho D. Y. Herpes simplex virus latency: molecular aspects. Prog Med Virol. 1992;39:76–115. [PubMed] [Google Scholar]
- Igietseme J. U., Calzada P. J., Gonzalez A. R., Streilein J. W., Atherton S. S. Protection of mice from herpes simplex virus-induced retinitis by in vitro-activated immune cells. J Virol. 1989 Nov;63(11):4808–4813. doi: 10.1128/jvi.63.11.4808-4813.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H. S., Russell R. G., Rouse B. T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983 Jul;41(1):197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Flavell R. A., Palmiter R. D., Brinster R. L. Tolerance in transgenic mice expressing class II major histocompatibility complex on pancreatic acinar cells. J Exp Med. 1989 Jul 1;170(1):87–104. doi: 10.1084/jem.170.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Kono D. H., Clayton J., Ando D. G., Hinton D. R., Hofman F. M. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Mössner R., Sedgwick J., Flory E., Körner H., Wege H., ter Meulen V. Astrocytes as antigen presenting cells for primary and secondary T cell responses: effect of astrocyte infection by murine hepatitis virus. Adv Exp Med Biol. 1990;276:647–654. doi: 10.1007/978-1-4684-5823-7_88. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Hayashida I., Higa K., Wada T., Mori R. Role of Lyt-1 positive immune T cells in recovery from herpes simplex virus infection in mice. Microbiol Immunol. 1982;26(4):359–362. doi: 10.1111/j.1348-0421.1982.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rooney J. F., Wohlenberg C., Cremer K. J., Notkins A. L. Immunized mice challenged with herpes simplex virus by the intranasal route show protection against latent infection. J Infect Dis. 1989 May;159(5):974–976. doi: 10.1093/infdis/159.5.974. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Rouse B. T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- Schmid D. S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988 May 15;140(10):3610–3616. [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Simmons A., Tscharke D. C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992 May 1;175(5):1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A., Tscharke D., Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Banks M. C. The weakly virulent herpes simplex virus type 1 strain KOS-63 establishes peripheral and central nervous system latency following intranasal infection of rabbits, but poorly reactivates in vivo. J Neuropathol Exp Neurol. 1992 Sep;51(5):550–559. doi: 10.1097/00005072-199209000-00010. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Davey J., Howland K., Rothbard J. B., Askonas B. A. Class I MHC molecules rather than other mouse genes dictate influenza epitope recognition by cytotoxic T cells. Immunogenetics. 1987;26(4-5):267–272. doi: 10.1007/BF00346521. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Anderson C. H., Felleman D. J. Information processing in the primate visual system: an integrated systems perspective. Science. 1992 Jan 24;255(5043):419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Weinstein D. L., Walker D. G., Akiyama H., McGeer P. L. Herpes simplex virus type I infection of the CNS induces major histocompatibility complex antigen expression on rat microglia. J Neurosci Res. 1990 May;26(1):55–65. doi: 10.1002/jnr.490260107. [DOI] [PubMed] [Google Scholar]
- Williamson J. S., Stohlman S. A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990 Sep;64(9):4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984 Nov;133(5):2736–2742. [PubMed] [Google Scholar]
- Yeh H. H., Lin C. S., Woodward D. J. Mossy fiber and Purkinje cell axon collateral arborization patterns in normal and X-irradiated rat cerebellum: a light microscopic study using horseradish peroxidase fiber filling techniques. Brain Res. 1981 Aug;254(1):169–175. doi: 10.1016/0165-3806(81)90068-7. [DOI] [PubMed] [Google Scholar]