Abstract
Histone deacetylases (Hdacs) are conserved enzymes that remove acetyl groups from lysine side chains in histones and other proteins. Eleven of the 18 Hdacs encoded by the human and mouse genomes depend on Zn2+ for enzymatic activity, while the other 7, the sirtuins (Sirts), require NAD2+. Collectively, Hdacs and Sirts regulate numerous cellular and mitochondrial processes including gene transcription, DNA repair, protein stability, cytoskeletal dynamics, and signaling pathways to affect both development and aging. Of clinical relevance, Hdacs inhibitors are United States Food and Drug Administration-approved cancer therapeutics and are candidate therapies for other common diseases including arthritis, diabetes, epilepsy, heart disease, HIV infection, neurodegeneration, and numerous aging-related disorders. Hdacs and Sirts influence skeletal development, maintenance of mineral density and bone strength by affecting intramembranous and endochondral ossification, as well as bone resorption. With few exceptions, inhibition of Hdac or Sirt activity though either loss-of-function mutations or prolonged chemical inhibition has negative and/or toxic effects on skeletal development and bone mineral density. Specifically, Hdac/Sirt suppression causes abnormalities in physiological development such as craniofacial dimorphisms, short stature, and bone fragility that are associated with several human syndromes or diseases. In contrast, activation of Sirts may protect the skeleton from aging and immobilization-related bone loss. This knowledge may prolong healthspan and prevent adverse events caused by epigenetic therapies that are entering the clinical realm at an unprecedented rate. In this review, we summarize the general properties of Hdacs/Sirts and the research that has revealed their essential functions in bone forming cells (e.g., osteoblasts and chondrocytes) and bone resorbing osteoclasts. Finally, we offer predictions on future research in this area and the utility of this knowledge for orthopedic applications and bone tissue engineering.
I. INTRODUCTION
Bone is a highly dynamic tissue with many shapes and functions. Despite a general recognition that most bone fractures heal completely within a few weeks of time and that genomic material recovered from bones at archeological sites offers valuable clues to evolution of vertebrate life on this planet, the astonishing vitality and regeneration capacity of the bony skeleton are vastly underappreciated. Plasticity in the genome, epigenome, and cellular signaling pathways provides cells of mesodermal origin with the capacity to form bones that are flat, long, and/or curved so that they can protect vital organs and support movement. These processes also temporally and spatially orchestrate timely fracture repair and the production and release of molecules that regulate many physiological processes, including hematopoiesis, metabolism, and bone remodeling (13, 97, 109, 126, 137, 147, 207, 213). Many cellular enzymes drive and reverse the intrinsic chemical reactions that produce collagens, growth factors, hormones, and extracellular matrix proteins characteristic of bone (5, 25, 63). This review focuses on one conserved class of enzymes, histone deacetylases, that control both chromatin structure and signaling events, and thus affect the myriad of activities required for bone formation, repair, and regeneration. Hdacs are clinically relevant because numerous molecules that inhibit their activities are used alone or in combination with other drugs to treat a variety of cancers, and are being considered or tested as treatments for other diseases including arthritis, diabetes, epilepsy, heart disease, HIV infection, neurodegeneration, and numerous aging-related disorders (27, 42, 48, 95, 116, 136, 169).
Histone deacetylases (Hdacs; EC 3.5.1.98) catalyze the hydrolysis of an N6-acetyl-lysine residue of histones and other proteins to yield a deacetylated substrate, an acetyl group and one molecule of H2O. They are thus considered “erasers” of epigenetic marks or posttranslational modifications on chromatin and other intracellular molecular complexes. Hdacs are counteracted by histone/lysine acetyltransferases (HAT/KAT; EC 2.3.1.48), which add acetyl groups to lysines from acetyl coenzyme A (acetyl CoA) and are “writers” of posttranslational modifications. The removal of acetyl groups from lysines by Hdacs restores the positive charge on the side chain and alters the affinity of the modified protein for other molecules, including nucleic acids and proteins with bromodomains or tandem PHD domains (“readers” of acetylated lysines). Histone deacetylation is typically associated with promoter and enhancer inactivity, but deacetylation of non-histone proteins (e.g., transcription factors, DNA repair and replication proteins and chaperones) affects nearly every cellular process by destabilizing proteins or preventing proper cellular trafficking and function (29, 54, 61, 74, 80, 107, 209). A seminal report from 2009 identified 3,600 acetylated residues in over 1,750 proteins by high-resolution mass spectrometry (29). Due to the broad range of substrates, Hdacs are sometimes referred to as lysine deacetylases (Kdacs), but we will refer to them as Hdacs here. In 2011, we reviewed the role of Hdacs in bone development and maintenance and discussed the consequences of Hdac inhibition on bone strength and structure (116). Here we provide an update on research in this area and place the results into the greater context of bone formation, repair, regeneration, aging, and disease. We begin by summarizing general properties of the Hdacs and Sirts, and then discuss their essential functions in bone-forming cells (osteoblasts, chondrocytes, and their mesenchymal progenitors) and bone-resorbing cells (osteoclasts). We will also discuss both the positive and negative clinical implications of pharmaceutically altering Hdac activity by reviewing in vivo studies with Hdac inhibitors and Sirt activators and proposing potential orthopedic applications.
II. HDAC STRUCTURE AND FUNCTION IN BONE
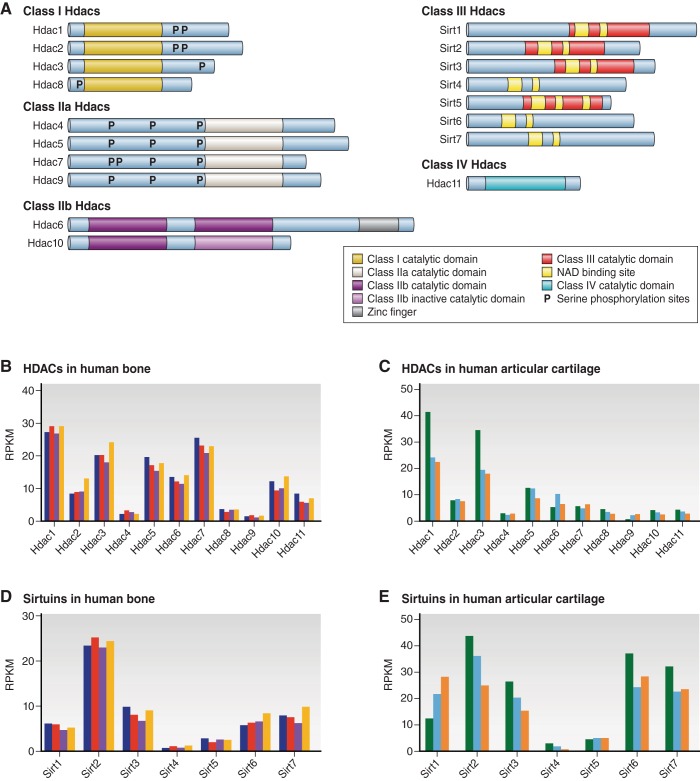
The human and mouse genomes each encode 18 Hdac genes. Hdacs are divided into four families (class I, II, III, and IV) based on their protein structure, function, subcellular localization, and expression (FIGURE 1A). Classes I, II, and IV are zinc-dependent enzymes and will be referred to as Hdacs from here on, while class III Hdacs require NAD+ for enzymatic activity and will be referred to as Sirtuins (Sirts). In a small and preliminary RNA-sequencing study, we observe great variability in expression of HDAC and SIRT transcripts in adult human bone and articular cartilage. HDAC1 and HDAC3 are the most abundant HDACs in both tissues (FIGURE 1, B and C). Adult bone also contains many transcripts for HDAC5, HDAC6, and HDAC7, but relatively few for HDAC4, HDAC8, and HDAC9. In normal human articular cartilage, HDAC2, HDAC5, HDAC6, and HDAC7 are expressed at higher levels than HDAC4, HDAC8, HDAC9, and HDAC10. Among the class III Hdacs, SIRT2 is the most highly expressed in adult bone, whereas several SIRTs are equally expressed in normal human articular cartilage (FIGURE 1, D and E). These expression data provide some context for prioritizing studies on Hdacs and Sirts in a mature skeleton, but as highlighted below, do not necessarily reflect the importance of these enzymes (e.g., Hdac4) in the developing skeleton or in different pathophysiological settings.
Figure 1.
HDAC structure and expression in adult human bone and cartilage. A: HDACs are grouped into four classes based on structural considerations. B–E: HDAC gene expression differs between bone and cartilage as illustrated by RNA-sequencing results (van Wijnen, unpublished data). RNA samples were obtained with Institutional Review Board approval from adult human bone (4 specimens) and articular cartilage (3 specimens) as fresh surgical waste. Each colored bar represents a different patient. Values represents reads per kilobase per million (RPKM).
A. Class I Hdacs
Mammalian Hdacs1, 2, 3, and 8 are structurally similar to the S. cerevisiae protein Rpd3 (34, 174). They contain a conserved deacetylase domain flanked by NH2- and COOH-terminal extensions, are ubiquitously expressed in most tissues, and predominately localize to the nucleus (26, 203) (FIGURE 1A). Class I Hdacs exert high enzymatic activity toward histone substrates and are crucial for gene transcription, as well as for splicing, DNA replication and repair, and cell proliferation and survival (61, 110, 124). Class I Hdacs may be highly expressed in some cancers, but there is no clear evidence that Hdac overexpression is oncogenic. On the other hand, suppression of class I Hdac expression induces cell cycle arrest and other anti-tumor effects (reviewed in Ref. 48). Hdac1 and Hdac2 expression declines during the terminal differentiation of several mesenchymal lineages, including osteoblasts (28, 96).
Class I Hdacs are the enzymatic subunits of several multi-subunit complexes. They contain nuclear localization signals but do not bind chromatin directly. Rather, they are recruited to DNA structures by transcription factors and other proteins. Hdac1 and Hdac2 are highly homologous and can form homo- and heterodimers with each other; thus they are often but not always found together in similar repressive complexes including Sin3, NuRD, CoREST, PRC2, and MiDAC (4, 12, 38, 173, 204). Using a systems biology approach, Joshi et al. (79) described functional interactomes for Hdac1 and other Hdacs. This work demonstrated that the associations between Hdac1 and the above-mentioned chromatin remodeling complexes are relatively stable, as opposed to transient and dynamic interactions with transcription factors (79).
Hdac3 is functionally distinct from Hdac1/2 because it interacts with NCoR-SMRT co-repressor complexes (57, 58, 102, 180, 191). Structural studies on the Hdac3 and SMRT corepressor complex identified an inositol tetraphosphate molecule [d-myo-inositol-(1,4,5,6)-tetrakisphosphate (IP4)] as a crucial functional component of the complex (189). Further analysis found that IP4 binding pockets are also present in Hdac1 and Hdac2 and are necessary for engaging Hdac enzymatic activity (72, 121); however, their role in connecting Hdac activity to cell signaling events has not been elucidated yet. The enzymatic activity of Hdac3 is dependent on the phosphorylation of S424 and inversely correlated with the presence of the serine/threonine phosphatase PP4c (214).
Hdac8 was the last member of class I Hdacs to be cloned. Little is known about its association with cofactor complexes. Durst et al. (45) showed that the fusion protein formed by inv(16) rearrangements in acute myeloid leukemias associates with both mSin3A and Hdac8, but direct associations between Hdac8 and mSin3, NuRD, NCor/Smrt, or any of the aforementioned co-repressor complexes have not been reported. Hdac8 was the first zinc-dependent Hdac to have its crystal structure solved with an Hdac inhibitor (HDI) (183). This structure has been used as model for developing more selective HDIs.
B. Class II Hdacs
Class II Hdacs share structural homology to the yeast protein Hda1 (34) and are divided into two subclasses, class IIa and class IIb. Hdac4, Hdac5, Hdac7, and Hdac9 (alternatively called Hdac7b) comprise class IIa, while Hdac6 and Hdac10 are in class IIb because of the presence of two catalytic domains (Figure 1A). Compared with class I Hdacs, class II Hdacs have large NH2-terminal extensions, which contain conserved binding domains for the myocyte enhancer transcription factor 2 (Mef2) as well as the chaperone protein 14-3-3. Phosphorylation of serine residues in the NH2-terminal extension promotes the binding of 14-3-3 proteins to class II Hdacs, their translocation from the nucleus to the cytoplasm, and subsequent de-repression of target genes (106, 118, 184). The phosphorylation of class II Hdacs is controlled by many extracellular signals that activate multiple intracellular kinases (reviewed in Ref. 139). Generally, class II Hdacs have more temporally and spatially restricted expression patterns and thus have more specialized functions than class I Hdacs (61). Class II Hdacs appear to be important scaffolds for cellular processes, but have drastically reduced intrinsic enzymatic activity than class I Hdacs due to the presence of a histidine residue instead of tyrosine within the catalytic domain (93). The ability of class II Hdacs to recruit class I Hdacs with their conserved COOH-terminal deacetylase domain accounts for the majority of their enzymatic activity (50, 186, 205). This reduced enzymatic activity may explain why global deletion of most class II Hdacs does not cause embryonic lethality.
C. Class III Hdacs/Sirtuins
Class III Hdacs include the sirtuins (Sirts1-7), which are homologues of the yeast protein, silent information regulator (Sir)2. Sirts are dependent on the cofactor nicotinamide adenine dinucleotide (NAD+) and transfer an acetyl group from lysine to NAD+, creating O-acetyl ADP-ribose and nicotinamide, which becomes a feedback inhibitor of the enzymatic reaction. Emerging studies indicate that the enzymatic activity of Sirts may extend to different protein modifications. For example, Sirt6 has relatively low deacetylase activity, but efficiently removes myristoyl groups (long-chain fatty-acyl groups) from lysine residues (7, 75), and Sirt4 is primarily an ADP-ribosyltransferase (62). The subcellular localization of Sirts varies: Sirt1-3, 6, and 7 are found in the nucleus; Sirt1 and 2 are predominantly found in the cytoplasm; and Sirt3-5 are strongly expressed in mitochondria (8, 21, 62, 120, 132, 157, 158, 172). Sirts are of scientific interest because they control energy metabolism, inflammation, genome stability, and aging (reviewed in Refs. 27, 136). Sirts are also important for bone health, as Sirt1 and Sirt6 facilitate endochondral ossification and Sirt activators promote bone formation (see sects. IVB and VII).
D. Class IV Hdac
Hdac11 has a deacetylase domain with small COOH- and NH2-terminal extensions, suggesting structural similarities to both class I and class II Hdacs, but placing it into its own group (class IV) (Figure 1A). Although it is expressed across a variety of tissues, including bone marrow mononuclear cells (152) and adult bone and articular cartilage, little is known about its function other than one report that Hdac11 was unable to suppress Runx2-induced gene transcription (73).
III. HDACS IN BONE DEVELOPMENT AND GENETIC DISEASES
A. Intramembranous Bone Development
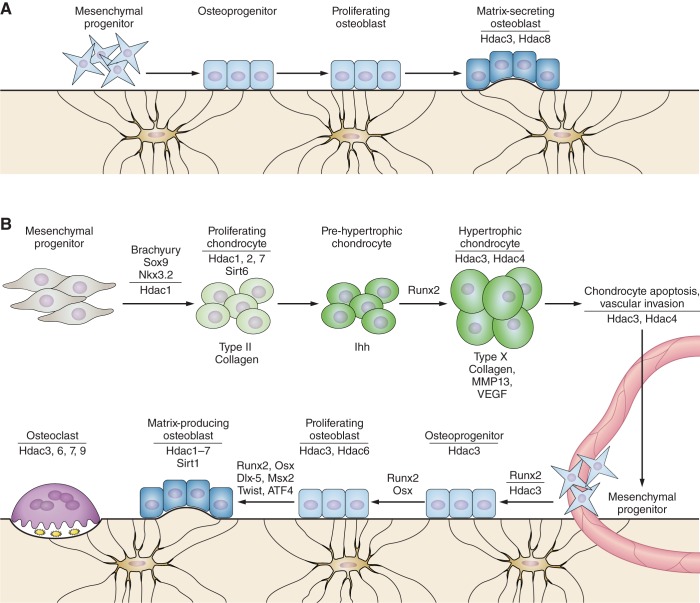
Intramembranous ossification occurs when mesenchymal precursor cells condense within a fibrous connective membrane and directly differentiate into matrix-producing osteoblasts. This process generates many of the flat bones in the skull and the medial portion of clavicles and is reactivated during repair of highly stabile fractures and defects. Direct commitment of mesenchymal cells toward the osteoblast lineage requires the transcription factors Runx2 and Osterix (Osx/Sp7) and is largely influenced by canonical Wnt signaling, which promotes osteoblast differentiation and suppresses chondrogenesis of mesenchymal progenitors. Several Hdacs contribute to intramembranous ossification (Figure 2A). Hdac3 is required for proper craniofacial development, as conditional Hdac3 deletion in the skeletal mesenchyme (with Wnt1-Cre or Pax3-Cre) produced microcephaly, nearly undetectable frontal bone formation, and improper formation of the zygomatic arch (168) (Table 1). This phenotype was ascribed to increased expression of Msx1 and Msx2 and increased apoptosis of cranial neural crest cells. Similarly, deletion of Hdac3 in osteochondral progenitor cells (with Osx1-Cre) decreased calvarial bone thickness and density (150). Calvarial cells from these Hdac3-deficient animals expressed high levels of the cyclin-dependent kinase inhibitor Cdkn1a/p21 and a number of Wnt pathway inhibitors.
Figure 2.
Hdacs in bone and cartilage development. A: Hdacs facilitate intramembranous bone ossification. Mesenchymal cells differentiate directly into osteoblasts during intramembranous bone formation. Deletion of Hdac3 or Hdac8 within the developing skeleton alters intramembranous ossification, skull formation, and bone density. B: during endochondral ossification, mesenchymal condensations give rise to a cartilaginous template for bone formation. Several Hdacs regulate chondrogenesis in the sclerotome, the expression of matrix genes associated with chondrocyte proliferation, and hypertrophy. Hdacs control bone modeling by influencing osteoblast commitment, promoting osteoblast proliferation, regulating osteoblast maturation and matrix deposition, and by facilitating osteoclast resorption activity and motility.
Table 1.
Skeletal phenotypes resulting from altered Hdac and Sirt expression in mice
| Hdac | Genetic Alteration | Cells Targeted | Bone Phenotype | Reference Nos. |
|---|---|---|---|---|
| Hdac1 | KO | All | Embryonic lethal | 92, 122 |
| Hdac2 | KO | All | Reduced body size, smaller vertebrae and pelvis | 178, 218 |
| Hdac3 | KO | All | Embryonic lethal, E9.5 | 6, 123 |
| CKO | Osteochondral progenitors (Osx1-Cre) | Cortical and trabecular bone loss, decreased bone formation rate, fewer osteoblasts, increased marrow adiposity, abnormal chondrocyte hypertrophy | 11, 150 | |
| CKO | Osteoblasts (OCN-Cre) | Age related cortical and trabecular bone loss, decreased bone formation rate, reduced osteoblast numbers | 114 | |
| CKO | Neural crest cells (Wnt1-Cre) | Craniofacial abnormalities | 168 | |
| CKO | Neural crest cells (Pax3-Cre) | Craniofacial abnormalities | 168 | |
| CKO | Chondrocyte (Col2-Cre) | Embryonic lethal | Carpio et al., unpublished data | |
| CKO | Chondrocyte (postnatal Col2-CreERT) | Reduced trabecular bone density, increased cortical bone thickness, short stature, delayed secondary ossification center development, residual hypertrophic cartilage in growth plate, increased osteoclast activity in primary spongiosa | Carpio et al., unpublished data | |
| Hdac4 | KO | All | Ectopic chondrocyte hypertrophy and enhanced endochondral ossification, increased MMP13 expression | 165, 185 |
| CKO | Osteoblast (Runx2-Cre) | Reduced bone density, increased Rankl expression and bone resorption, reduced osteocalcin expression | 108, 133 | |
| Tg | Chondrocytes (Col2a1 promoter) | No endochondral ossification | 185 | |
| Hdac5 | KO | All | No gross skeletal phenotypes at birth | 19 |
| KO | All | Reduced bone density, increased Rankl expression and bone resorption, normal osteocalcin expression | 108, 133 | |
| KO | All | Reduced bone trabecular bone density, reduced bone formation, increased Sost expression | 190 | |
| Hdac6 | KO | All | Increased trabecular bone mineral density | 215 |
| Hdac7 | KO | All | Embryonic lethality | 20 |
| CKO | Chondrocytes (Col2a1-Cre) | Embryonic lethal | 10 | |
| CKO | Chondrocytes (postnatal Col2a1-CreERT) | Reduced trabecular bone volume and trabecular number, increased chondrocyte proliferation | 10 | |
| CKO | Osteoclasts (LysM-Cre) | Reduced bone mass, increased bone resorption, normal bone formation | 76, 170 | |
| CKO | Early osteoclasts, hematopoietic cells (Tie2-Cre) | Embyronic lethality | 76 | |
| CKO | Osteoclasts (PT-Cre) | Reduced bone mass, increased bone resorption, normal bone formation | 76 | |
| Hdac8 | KO | All | Decreased bone mineral density | IMPC* |
| KO | All | Ossification defects in frontal and interparietal bones | 60 | |
| CKO | Neural crest cells (Wnt1-Cre) | Ossification defects in frontal and interparietal bones | ||
| CKO | Osteoblasts (Col1a1-Cre) | None | ||
| CKO | Chondrocytes (Col2a1-Cre) | None | ||
| CKO | Mesenchymal precursors (Twist1-Cre) | None | ||
| Hdac9 | KO | All | Reduced bone mass, increased bone resorption and reduced bone formation | 77 |
| Hdac10 | KO | All | Increased circulating alkaline phosphatase levels in females, no gross phenotype | IMPC* |
| Hdac11 | KO | All | No gross skeletal phenotypes | 152 |
| Sirt1 | KO | All | Shorter stature, craniofacial abnormalities, reduced endochondral ossification, reduced cortical thickness | 51, 119 |
| Het | All | Trabecular bone loss in females, increased osteoclast number, decreased osteoblast numbers, increased marrow adiposity, smaller in size, increased osteoarthritis and articular chondrocyte apoptosis | 30, 51 | |
| CKO | Osteoblasts (2.3Col1a1-Cre) | Decreased bone mass in females, reduced osteoblast differentiation and increased NFκB activity | 47 | |
| CKO | Osteoclasts (LysM-Cre) | Decreased bone mass in females, increased osteoclastogenesis and NFκB acetylation | 47 | |
| CKO | Mesenchymal progenitors (Prx1-Cre) | Cortical bone loss with aging, reduced bone formation | 166 | |
| CKO | Osteochondral progenitors (Osx1-Cre) | Decreased cortical thickness in females, reduced bone formation | 71 | |
| Inducible HDAC | Postnatal-all cells (CreERT2) | Decreased cortical bone thickness | 119 | |
| CKO | Chondrocytes (Col2a1-Cre) | Normal size and long bones growth, increased susceptibility to osteoarthritis | 112 | |
| Sirt2 | KO | All | No gross skeletal phenotype, genomic instability, prone to tumors | 160 |
| KO | All | Increased bone mineral density in males | IMPC* | |
| Sirt3 | KO | All | No gross skeletal phenotype, normal bone mineral density and content | 105 |
| Sirt4 | KO | All | No gross skeletal phenotype | 62 |
| Sirt5 | KO | All | No gross skeletal phenotype | 105 |
| Sirt6 | KO | All | Osteoporosis, shorter long bones, fewer proliferating and hypertrophic cells in growth plates, failed to thrive after 3 wk of age | 125, 143 |
| Sirt7 | KO | All | No reported skeletal phenotype | 182 |
CKO, conditional knockout mice; Het, heterozygous mice; KO, knockout mice; Tg, transgenic mice.
IMPC: International Mouse Phenotyping Consortium (http://www.mousephenotype.org/data/genes/).
Hdac8 is also required for proper skull development. Conditional deletion of Hdac8 within neural crest cells using Wnt1-Cre impaired calvarial development through decreased expression of the transcription factors Otx2 and Lhx1 (60) (Table 1). Loss-of-function mutations in HDAC8 were found in a subset of patients with Cornelia de Lange syndrome (CdLS) and with a condition resembling Wilson-Turner linked mental retardation syndrome (35, 36, 49, 64, 81) (Table 2). In the latter syndrome, mutations alter splicing of HDAC8 and cause premature stops in translation. The CdLS mutations inactivate the deacetylase activity of HDAC8, thus stabilizing the acetylation of the cohesion subunit, structural maintenance of chromosomes 3 (SMC3). Patients with HDAC8 mutations have typical CdLS phenotypes, but delays in anterior fontanel closure are notable because they are highly reminiscent of the Hdac8-deficient mice. Distinct craniofacial dimorphisms and shorter bones characterize both syndromes.
Table 2.
HDAC mutations in human diseases with bone phenotypes
| HDAC (MIM No.) | Disease (OMIM No.) | Mutation/Variant | Bone Phenotype | Reference Nos. |
|---|---|---|---|---|
| HDAC2 (605164) | Interstitial 6q21q22.1 Deletion Syndrome-medial | Deletion of 3–10 genes, including HDAC2 | Hypertelorism, thoracic scoliosis, vertebral rotation | 173a |
| HDAC4 (605314) | Brachydactyly-Mental Retardation Syndrome; aka Albright Hereditary Osteo-Dystrophy-Like Syndrome or 2q37 Microdeletion Syndrome (600430) | Loss of function; deletions result in haploinsufficiency | Distinctive craniofacial features, and skeletal abnormalities, including brachydactyly type E, and shortened metatarsals and metacarpels | 187, 195 |
| HDAC5 (605315) | Bone mineral density quantitative trait locus 15 (613418) | Mutation in miR2861 prevents binding to HDAC5 UTR and increases HDAC5 expression | Low bone mineral density | 101 |
| Osteoporosis (166710) | SNP rs228769 (8 kb upstream of HDAC5) | Low bone mineral density in lumbar spine and femoral neck | 151 | |
| HDAC6 (300272) | Chondrodysplasia with platyspondyly, distinctive brachydactyly, hydrocephaly, and microphthalmia (300863) | Gain of function; mutations in miR binding site in HDAC6 UTR | Distinctive craniofacial features, generalized reductions in bone mineralization, small growth plates | 167 |
| HDAC8 (300269) | Cornelia de Lange Syndrome 5 (300882) | Loss of function; mutations increase acetylation of cohesion subunit SMC3 | Distinctive craniofacial features, delayed fontanel closure, small hands and feet | 35, 81 |
| Wilson-Turner X-linked Mental Retardation Syndrome (309585) | Loss of function; mutations alter splicing and introduce a premature stop codon | Distinctive craniofacial features, short stature | 64 |
MIM, gene locus number for the Mendelian Inheritance in Man catalog; OMIM, Online Mendelian Inheritance in Man; SNP, single nucleotide polymorphism; UTR, untranslated region.
Intramembranous bone defects have not been described in mouse models of altered class II Hdac expression, but other model systems suggest that these Hdacs may also contribute to intramembranous bone formation. Zebrafish embryos injected with hdac4 morpholinos showed improper palate formation and shortened faces (37). Interestingly, deletions or mutations in HDAC2, HDAC4, and HDAC6 are associated with distinctive craniofacial phenotypes or skeletal abnormalities in rare human syndromes; however, a causative role for altered deacetylase function in these phenotypes has not been proven and requires further investigation (Table 2) (167, 173a, 187, 195).
B. Endochondral Bone Development
The majority of bones (including vertebrae and long bones) form and heal by endochondral ossification, where a temporary cartilaginous anlage serves as a template for longitudinal bone growth, repair, and mineralization. During endochondral bone development (Figure 2B), mesenchymal condensations form a growth plate consisting of three zones of resting, proliferative, and hypertrophic chondrocytes. The resting cells are quiescent, but after chondrocytes begin proliferating, they produce a matrix rich in proteoglycan, type IIa collagen, and aggrecan. Chondrocyte maturation through the proliferative zone, into the hypertrophic zone, and subsequently out of hypertrophy is heavily regulated through a feedback mechanism involving parathyroid hormone-related protein (PTHrP) and Indian hedgehog (Ihh) (reviewed in Refs. 83, 91). Hypertrophic chondrocytes downregulate expression of type IIa collagen and begin to synthesize a matrix consisting of type X collagen. In addition, hypertrophic chondrocytes mineralize their surrounding extracellular matrix (131) and promote vascularization of the growth plate through the production of vascular endothelial growth factor (VEGF) and other angiogenic factors. This facilitates the recruitment of myeloid progenitor cells, which differentiate into osteoclasts and chondroclasts that digest the calcified cartilage matrix. Matrix metalloproteinases (MMPs) produced by hypertrophic chondrocytes degrade the matrix within the calcified cartilage matrix. The majority of hypertrophic chondrocytes undergo apoptosis and leave behind a calcified matrix that serves as a scaffold for bone formation, but recent lineage tracing studies provide new support for the hypothesis that some hypertrophic chondrocytes give rise to bone marrow osteoblasts and matrix-residing osteocytes (202, 217). As discussed next, many Hdacs contribute to various steps of the endochondral ossification process.
1. Class I Hdacs and endochondral ossification
a) hdac1/hdac2. Genetic deletion of several class I Hdacs adversely affects endochondral ossification. Loss of hdac1 in zebrafish impaired cartilage formation in zebrafish pectoral fins and produced defective craniofacial cartilage by promoting premature cell apoptosis in some locations (posterior branchial arch) and impairing terminal chondrocyte differentiation in others (anterior mandibular and hyoid arches) (70, 144). Thus far, the role of Hdac1 in postnatal murine bone development has not been determined as Hdac1 KO mice die during early embryogenesis (92, 122). However, in the initial derivation of cartilage from the sclerotome during axial skeleton formation, Hdac1 associates with a crucial transcription factor, Nkx3.2, to confer the repressive activity required for chondrogenesis (85, 128, 211). Wuelling et al. (199) found that the zinc finger nuclear regulator Trps1 promoted chondrocyte mitosis by increasing the activity of Hdac1, as well as Hdac4. Hong et al. (66) found that HDAC1 and HDAC2 levels were elevated in articular cartilage from osteoarthritis patients and that they suppress expression of many matrix proteins through a common domain. They and others documented that Hdac1 repressed expression of cartilage extracellular matrix genes including collagen type 2, aggrecan, and cartilage oligomeric protein (Comp) (66, 68, 103), while Hdac2 suppressed collagen type 2, aggrecan, and collagen type 11 (66). Germline deletion of Hdac2 results in partially penetrant embryonic lethality and decreased survival of postnatal null animals (178, 218). Of the surviving Hdac2 null animals, significant decreases in body size and long bone length were observed with reductions in insulin-like growth factor I (IGF-I) signaling. These data point towards impairment of growth plate development, but this hypothesis has also not yet been tested in depth.
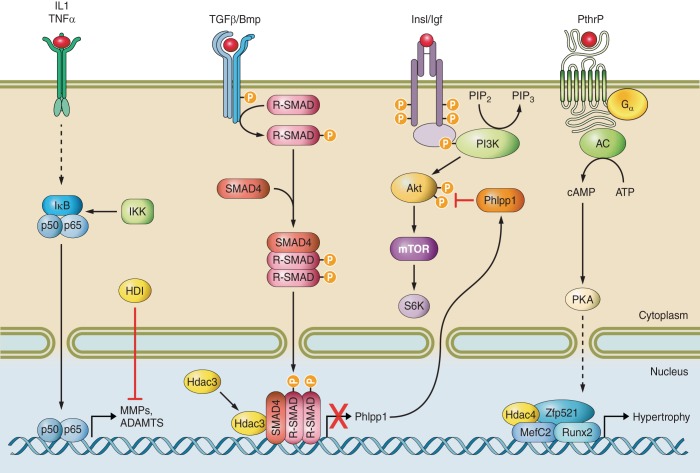
b) hdac3. Compared with Hdac1 and Hdac2, the role of Hdac3 in endochondral ossification has been extensively probed (Table 1). Germline deletion of Hdac3 caused early embryonic lethality (6, 123); however, conditional deletion from Osx1-Cre expressing osteochondroprogenitor cells reduced long bone length, delayed exit from the hypertrophic zone, and produced weak skeletons that were prone to fracture (11, 150). Despite a larger hypertrophic zone, these Hdac3-depleted mice produced less cartilage matrix and had lower expression levels of bone specific genes, aggrecan, Mepe, and osteopontin as well as VEGF (11). The reduced matrix production was at least partially explained by the blunted activation of Akt and its downstream substrates, including the protein translation factor p70 S6 kinase in Hdac3-depleted cells (Figure 3). Akt phosphorylation and activity were reduced in Hdac3-deficient chondrocytes due to increased expression of the phosphatase Phlpp1 (Figure 3) (11). Hdac3 bound to the Phlpp1 promoter in a region containing a Smad3 binding site, indicating that Hdac3 may also repress transforming growth factor (TGF)-β responses (11). More recent studies show that depletion of Hdac3 even earlier in chondrogenesis with Col2a1-Cre produces more severe phenotypes. Hdac3-CKOCol2 animals rarely survived to birth. Those that did survive had severe endochondral bone defects and were hypomorphic for Hdac3 expression (16). Specifically, these animals had decreased femur length, poor trabecular bone architecture, and thin cortical bones. Due to the difficulty in obtaining viable Hdac3-CKOCol2 mice, Hdac3 was also deleted postnatally with a tamoxifen inducible Col2a1-Cre transgene to generate Hdac3-CKOCol2ERT pups. At 8 wk of age (7 wk post induction), Hdac3-CKOCol2ERT animals had decreased cancellous bone and increased cortical bone. The latter phenotype is likely a compensatory adaptation to mechanical loading forces exerted on the long bones. In other words, to compensate for decreased chondrocyte maturation and cancellous bone formation, the cortical bone adapted by increasing in thickness. Mechanistically, Hdac3 deficiency in the Col2-Cre expressing chondrocytes increased production of inflammatory proteins, elevated Stat3 and Erk phosphorylation, and produced subsequent increases in Mmp13 expression in vitro and in vivo. Increased osteoclastogenesis is also apparent in the primary spongiosa of these mice (Carpio and Westendorf, unpublished data). In contrast, conditional loss of Hdac3 in mature osteoblasts and osteocytes expressing OCN-Cre is not associated with growth plate or long bone length defects, but these mice do show an osteopenic phenotype and defective matrix mineralization that worsens with age, leading to increased skeletal fragility (114). Surprisingly, Hdac3 CKO mice made with 2.3Col1a1-Cre transgenic mice were not viable (Westendorf, unpublished data), possibly because early transient expression of this promoter in the embryo caused unintended Hdac3 deletion in vital tissues/organs (104).
Figure 3.
Hdacs in bone and cartilage signaling pathways. Hdacs integrate multiple cytokine signaling cascades to transcription factors and gene transcription during bone and cartilage development.
c) hdac8. As discussed earlier, Hdac8 deficiency primarily altered cranial facial features, but its conditional deletion in either Col2-Cre expressing chondrocytes or Col1a1-Cre expressing osteoblasts produced no obvious long bone phenotypes (60). Further examination of these animals would be warranted in light of discoveries that patients with HDAC8 mutations have shortened long bones (Table 2; Refs. 35, 49, 64, 81).
d) summary of class i hdacs in bone formation. Class I Hdacs (Hdacs 1, 2, 3, and 8) are essential regulators of endochondral ossification. Germline deletion of many of the class I Hdacs results in early embryonic lethality, as does skeletal specific deletion of several class I Hdacs. The requirement of these Hdacs during endochondral bone development may stem from their ability to control the activity of essential cartilage and bone transcription and chromosome maintenance factors.
2. Class II Hdacs and endochondral ossification
a) hdac4. The roles of class II Hdacs in endochondral ossification are under intensive investigation because they integrate extracellular signals required for bone formation with transcriptional regulators (Figure 3). The first evidence that these enzymes contribute to endochondral ossification appeared with generation of mice globally deficient in Hdac4. These mice are viable, but exhibit premature endochondral ossification leading to many skeletal defects including vertebral body fusion, decreased long bone length, and premature ossification of the skull (185). Conversely, transgenic expression of Hdac4 from the Col2a1 promoter inhibited chondrocyte maturation by blocking Runx2 activity (185).
Parathyroid hormone (PTH) and PTHrP are crucial regulators of Hdac4 activity during bone and cartilage development (Figure 2). Shimizu and co-workers (164, 165) showed that PTH induced PKA to phosphorylate Hdac4 at serine 740, which released it from Runx2, induced expression of the Mmp13 promoter, and promoted Hdac4 degradation by the lysosome. PTH also stimulated Hdac4 expression to suppress Mmp13 in a negative feedback loop (165). In another study, PTHrP promoted Hdac4 association with Mef2c, thereby inhibiting the ability of Mef2c to promote chondrocyte hypertrophy (2, 90) (Figure 3). The repressive effects of the Hdac4/Mef2c complex on transcription and chondrocyte hypertrophy were prevented by salt-inducible kinase (SIK)3 and Ca2+/calmodulin-dependent kinase IV, which induced cytosolic localization of Hdac4 (55, 155). PTHrP also stimulated expression of the transcriptional co-repressor Zfp521, which recruited Hdac4 and inhibited Runx2 activity and chondrocyte hypertrophy (31) (Figure 3). MiR365, a microRNA induced in parallel with Ihh signaling in vitro and within the developing growth plate, may promote chondrocyte hypertrophy by decreasing Hdac4 expression (56). A more recent study demonstrated that conditional deletion of Hdac4 in osteoblasts expressing a Runx2-Cre transgene reduced vertebral trabecular bone density in young mice by increasing Rankl expression in osteoblasts, which in turn promoted osteoclastogenesis and bone resorption (133). In this study, PTH promoted the ubiquitination and degradation of Hdac4 in osteoblasts via Smurf2, thereby allowing Mef2c to activate Rankl transcription. Meanwhile, induction of sympathetic signaling in osteoblasts induced Hdac4 nuclear localization and association with the transcription factor Atf4 (133). Osteocalcin expression is enhanced by Atf4, but was reduced in Hdac4-deficient mice, leading the authors to suggest that Hdac4 may support osteocalcin expression through Atf4 (108).
Hdac4 also controls TGF-β and bone morphogenetic protein (BMP) signaling during bone development (Figure 3). Pei et al. (141) found that Hdac4 overexpression enhanced TGF-β-induced chondrogenic differentiation of synovial-derived cells. Hdac4 and Hdac5 bound to Smad3 in response to TGF-β and inhibited Runx2 activity (82). The association of Hdac4 and Hdac5 to TGF-β-activated phospho-Smad2 and 3 complexes may require Ski, which can inhibit the signaling pathway and accelerate chondrocyte differentiation (88). Hdac4 can also deacetylate multiple lysines in Runx2 in response to Bmp2 and promote its degradation via Smurf1 (74).
Loss-of-function, haploinsufficiency-inducing deletions or mutations in HDAC4 were detected in patients with Brachydactyly-Mental Retardation Syndrome (also called 2q37 Microdeletion Syndrome or Albright Hereditary Osteodystrophy-Like Syndrome; OMIM no. 600430) (187, 195) (Table 2). The afflicted individuals have distinctive craniofacial features and shortened metatarsals and metacarpals, consistent with a crucial role for Hdac4 in murine endochondral ossification.
b) hdac5. Hdac5 knockout (KO) mice showed no gross structural patterning or growth defects at birth (19), but two recent and independent studies demonstrated that Hdac5 is required for optimal bone density. Obri et al. (133) reported that Hdac5 KO mice had reduced trabecular bone density at 2-3 mo of age despite modest increases in bone formation rates because Rankl expression was increased in osteoblasts, which stimulated greater bone resorption by osteoclasts. Osteocalcin expression was not changed in this Hdac5 KO colony (108). Using mice from the same strain, Wein et al. (190) found that osteocytes from 8-wk-old Hdac5 KO mice produce more sclerostin, a secreted Wnt/Lrp antagonist, because Mef2c is more active; they did detect increases in osteoclast activity. The discrepancies between these studies might be resolved with tissue-directed depletion studies, which are needed to fully understand Hdac5's role in bone. Hdac5 KO mice are of normal size, but interestingly, crossing them with Hdac9 KO mice, which are also of normal size, decreased long bone length via unknown mechanisms. These results suggest some level of redundancy amongst these Hdacs (19).
HDAC5 was identified as a gene locus affecting bone mineral density (151), and another study showed that mutation in miR2861 prevented its binding to the HDAC5 UTR and increased HDAC5 expression in juveniles with low bone mass (101). HDAC5 can deacetylate multiple lysines in RUNX2 and promote its degradation via Smurf1 (74). RUNX2 levels were suppressed in the juvenile osteoporosis patients, supporting a role for HDAC5 in bone mineral density (101).
c) hdac6. The class IIb Hdac, Hdac6, is intriguing because existing evidence suggests that it has unique roles in endochondral ossification. Hdac6 is best known for its cytoplasmic functions as a tubulin deacetylase and a component of ubiquitin and autophagic complexes (113), but experiments in osteoblasts indicate that it rapidly transits through the nucleus where it can interact with Runx2 (193). Germline deletion of murine Hdac6 modestly increased cancellous bone density (215). The mechanisms contributing to this phenotype are not well understood but are likely to include cytoskeletal changes in osteoclasts and bone resorption (see sect. V). Recent discoveries linking HDAC6 to chondrodysplasia (OMIM no. 300683) should revive interest in this molecule (167) (Table 2).
d) hdac7. Hdac7 is another class IIa Hdac that plays crucial roles in endochondral ossification by suppressing chondrocyte proliferation. Deletion of Hdac7 in all tissues causes embryonic lethality due to vascular dilation and blood vessel ruptures (20). Deletion of Hdac7 in Col2-Cre expressing chondrocytes produced few viable animals, suggesting that Hdac7 is also essential for proper endochondral ossification in utero (10). Postnatal deletion of Hdac7 in the same populations of chondrocytes led to an expansion of the proliferative zone, narrowing of the hypertrophic zone, reduced femur lengths, and decreased trabecular bone density (10). Canonical β-catenin signaling was enhanced in the absence of Hdac7. Cytokines that promote chondrocyte proliferation (insulin and IGF-I) also induced the translocation of Hdac7 from the nucleus to the cytoplasm in wild-type chondrocytes. These results demonstrate that reducing Hdac7 levels may stimulate chondrocyte expansion and cartilage regeneration, but that Hdac7 is necessary for endochondral ossification.
e) summary of class ii hdacs in bone formation. Class II Hdacs are critical regulators of endochondral ossification due to their ability to integrate gene transcription with responses to growth factors and cytokines promoting cartilage and bone formation (Figure 3). Most class II Hdacs bind and regulate the activity of the Mef2 proteins, although it is likely that they control other transcription factors as well. Unlike the class I Hdacs, germline deletion of most class II Hdacs does not cause embryonic lethality, indicating some level of functional redundancy. Class II Hdacs have low intrinsic deacetylase activity and may work through functional complexes involving a class I Hdac. As class II Hdacs shuttle between the nucleus and the cytoplasm, it will be interesting to determine if nontranscriptional functions contribute to endochondral ossification. It will also be important to specifically study the skeletons of class II Hdac KO mice by imaging and histomorphometry because phenotypes may not be readily apparent. Indeed, a recent study identified osteopenia in Hdac9 KO mice (77). This phenotype is discussed in section V because the bone mass alterations were most influenced by increased osteoclastogenesis, although bone formation was also reduced.
3. Class III Hdacs and endochondral ossification
a) sirt1. Several studies have demonstrated that Sirt1 is essential for endochondral ossification in female mice. Germline Sirt1 deficiency increased p53-mediated apoptosis and produced severe developmental defects (24), including shorter stature, craniofacial abnormalities, increased cartilage apoptosis, and reduced endochondral ossification and cortical thickness (51, 119). Trabecular bone loss in Sirt1+/− mice corresponded with fewer osteoblasts, more osteoclasts, increased Sost expression, and greater marrow adiposity (30). Conditional deletion of Sirt1 in committed osteoblasts with the 2.3Col1a1-Cre transgene increased NF-κB activity, which suppressed osteoblast differentiation and decreased trabecular bone mass in female mice (47, 161). Paradoxically, deletion of Sirt1 at earlier stages of osteoblastogenesis with Osx1-Cre and Prx1-Cre, or an inducible CreERT2, did not affect cancellous bone density, but decreased cortical bone thickness in female mice (71, 166). These studies found that Sirt1 promotes cortical bone formation by promoting β-catenin accumulation in the nucleus through deacetylation of K49 and K345 (166) and preventing sequestration by FoxO transcription factors (71). Sirt1 also regulated production of sclerostin by altering histone 3 acetylation of the Sost promoter (1, 30). Sirt1 deletion in chondrocyte lineages did not cause any developmental delays, but made joints more susceptible to osteoarthritis (112). The reason(s) why early deletion of Sirt1 in mesenchymal progenitor cells (and their progeny) causes cortical but not trabecular bone loss, while deletion in committed osteoblasts causes cancellous bone loss is unclear. One possible explanation is that the 2.3Col1a1-Cre transgene is transiently expressed in the early embryo (104) and could prevent skeletal development through other physiological mechanisms.
b) sirt6. Sirt6 facilitates endochondral ossification by controlling chondrocyte proliferation and differentiation. Sirt6 KO mice were born at Mendelian frequencies, but displayed growth retardation shortly after birth, failed to thrive, and died around 3.5 wk of age due to degeneration of multiple organs and genomic instability (125). The Sirt6 KO mice had a 30% reduction in bone mineral density and severe spine curvature. Growth plate chondrocyte proliferation and differentiation were impaired in the Sirt6-deficient animals and linked to reduced Ihh expression and increased cellular senescence (143).
c) summary of class iii hdacs in bone. Thus far, Sirt1 and Sirt6 are the only class III Hdacs that have been specifically shown to have necessary roles in bone. Sirt3 KO mice were found to have normal bone mineral density (105). Interestingly, current information on the International Mouse Phenotyping Consortium's (IMPC) website (http://www.mousephenotype.org/data/genes/) indicates that Sirt2 KO mice may have increased bone mass even though they appear phenotypically normal (160). While the bone cell autonomous roles of Sirt1 have been extensively analyzed genetically (Table 2), much work remains on the other Sirts. Fortunately, mice carrying mutated alleles are available for all of the Sirt genes (Table 2) (62, 105, 160, 182); thus focused studies may reveal bone phenotypes that are not readily apparent.
IV. HDACS IN OSTEOCLASTS AND BONE RESORPTION
Osteoclasts are large, multinucleated cells derived from hematopoietic stem cells and monocyte precursors. These specialized bone-resorbing cells play an essential role in endochondral ossification as they remove calcified cartilage at the growth plate during the initial formation of the mineralized bone (bone modeling), facilitate bone turnover in response to damage or physiological needs (bone remodeling), and clear fracture calluses to aid healing. Osteoclasts remove bone by secreting acids and proteolytic enzymes, such as cathepsin K, into the area of resorption. Osteoclasts also secrete factors that promote the recruitment of preosteoblasts to the resorption site (140, 171). Normally, the amount of bone resorbed by osteoclasts is precisely offset by new bone formation. However, at menopause and in conditions of increased bone resorption (e.g., osteoporosis, Paget's disease, rheumatoid arthritis, and tumor-induced osteolysis), osteoclast-mediated resorption outpaces new bone deposition by osteoblasts and causes net bone loss. The roles of Hdacs in osteoclasts have not been as widely studied as they have been in osteoblasts; however, this is an emerging area of scientific interest.
A. Hdac7 in Osteoclasts
Suppression of the class II Hdac, Hdac7, facilitated osteoclastogenesis and increased osteoclast size (142, 170). This observation was attributed to the ability of Hdac7 to inhibit the transcriptional activity of Mitf, a transcription factor required for Rankl-induced gene expression. The NH2 terminus of Hdac7 was sufficient to bind and inhibit Mitf activity, indicating that the deacetylase domain of Hdac7 was not necessary for this repression (170). In a separate report, conditional deletion of Hdac7 using a LysM-Cre promoted the proliferation of osteoclast precursor cells, and increased Rankl-induced osteoclast differentiation (76). Hdac7 suppressed β-catenin activity and cyclin D1 expression in marrow progenitor populations and differentiated osteoclasts (76).
B. Hdac9
Hdac9 KO mice were originally reported to have no gross phenotypic changes in bone structure up to 3 mo of age (212); however, more focused analyses revealed a large (∼45%) reduction in bone volume in both cancellous and cortical bone (77). Hdac9 levels naturally declined during osteoclastogenesis, and accordingly, osteoclast numbers and bone resorption indexes were elevated in Hdac9 KO mice. Bone formation indexes were also reduced in the Hdac9 KO mice; however, the major driver of the osteopenic phenotype was increased osteoclastogenesis. Bone marrow transplantation experiments showed that Hdac9 depletion in osteoclasts affected bone resorption in a cell autonomous fashion. Thus transplantation of Hdac9 KO bone marrow into wild-type mice was sufficient to cause osteopenia. Conversely, wild-type bone marrow transplantation rescued the osteopenic phenotype of the Hdac9 KO mice. Hdac9 was found to participate in a negative regulatory circuit with Pparγ and Rankl, as both suppressed Hdac9 mRNA levels, and Hdac9 partnered with NCoR and Smrt to suppress Pparγ-dependent transcription. Overall, these studies show that Hdac9 expression suppresses osteoclastogenesis.
C. Other Hdacs in Osteoclasts
Several other Hdacs have been studied in osteoclastic models. Pham et al. (142) demonstrated that shRNA-mediated knockdown of Hdac3 in bone marrow-derived osteoclasts or RAW246.7 cells inhibited osteoclast formation and decreased osteoclast size in response to RANKL. Expression of osteoclast-specific genes, including NFATc1, Ctsk, and DC-Stamp, was also suppressed by Hdac3 knockdown. Meanwhile, Hdac6, a class IIb Hdac and tubulin deacetylase, destabilizes the osteoclast cytoskeleton and inhibits osteoclast migration and podosome formation (40, 146). The class III Hdac Sirt1 also inhibits osteoclast differentiation as conditional deletion of Sirt1 in osteoclasts increased NF-κB activity, osteoclastogenesis, and bone loss in female mice (47, 161).
V. HDAC INHIBITORS
The expanding appreciation that epigenetic events contribute to varied phenotypes and disease etiologies has produced great interest in natural and synthetic compounds that modify the activity of Hdacs and other epigenetic regulators. HDIs are generally classified as short-chain fatty acids, hydroxamic acids, cyclic peptides, or benzamides. Although they have diverse structures and variable specificities, several HDIs with broad HDAC specificity have received Food and Drug Administration approval for clinical use in the United States. The oldest is valproic acid (divalproex sodium; Depakote; valproate), which has been used as an anticonvulsive therapy in epilepsy and a mood stabilizing therapy for bipolar disorder for decades and long before the cloning of Hdacs. Vorinostat (SAHA, Zolinza) and romidepsin (depsipeptide, Istodax) were first approved for treatment of cutaneous T-cell lymphoma, but are now routinely used in combination with other chemotherapeutic agents to treat a variety of hematopoietic and solid tumors. Romidepsin and belinostat (PXD101) are approved for the treatment of peripheral T-cell lymphomas. These and other HDIs, including panobinostat (LBH589), entinostat (MS-275), and givinostat (ITI2357), are in various clinical trials as potential treatments for many diseases including cancer, HIV infection, inflammatory conditions, neurodegeneration, and diabetes (27, 42, 48, 95, 116, 136, 169). The effectiveness of HDIs against cancer is attributed to many mechanisms, including increased acetylation of histones and other proteins, and consequent induction of tumor suppressor genes, stimulation of cell cycle arrest and apoptosis, and inhibition of DNA repair. HDI-induced chromatin relaxation may also make cancerous cells more susceptible to DNA damage from radiation or chemotherapies.
The ubiquitous expression patterns of Hdacs raise important concerns about the safety of HDI-based therapies especially for chronic diseases. Surprisingly, HDIs are fairly well tolerated by cancer patients, possibly because HDIs have short half-lives in serum and their effects can be reversed by acetyltransferases in normal cells that, contrary to tumor cells, are not addicted to oncogenes and have stable genomes (48). Nearly all patients receiving HDIs experience nausea, thrombocytopenia, fatigue, diarrhea, and dehydration, but all of these symptoms are temporary and cease after therapy ends (reviewed in Ref. 95). Some patients experience confusion, vertigo, and other neuropsychological problems or rare pulmonary side effects indicated by coughing and dyspnea. More severe problems, including gastrointestinal toxicity and cardiac conduction abnormalities, have been reported but are rare. Thus far, the effects of HDI therapy on bone density have not been assessed in a prospective randomized trial. Three independent retrospective studies on long-term valproic acid therapy produced varied results, with bone mineral density either decreased or unchanged, and markers of bone resorption increased, decreased, or unchanged (89, 156, 179). However, epidemiological studies indicate that long-term use of HDIs increases fracture risk (a widely accepted surrogate for reduced bone quality) in children and adults and is teratogenic to fetuses (9, 53, 59, 134, 156, 159, 163). Children exposed to the Hdac inhibitor valproic acid in utero developed craniosynostosis, axial skeletal defects, and limb malformations (94, 135, 162, 181). These phenotypes demonstrate the crucial role of Hdac activity in orchestrating human skeletal development. Animal studies have begun to shed light into how HDIs affect the developing and established skeleton.
A. Hdac Inhibitors and the Fetal Rodent Skeleton
Preclinical studies showed that HDIs cause fetal toxicity, growth retardation, and skeletal defects (127, 138, 181, 196). The negative effects of Hdac inhibitors on axial skeletal formation and limb development may in part be due to altered commitment of progenitors to the chondrocyte lineage, as valproate repressed chondrogenesis in ex vivo cultured murine limbs and reduced expression of key endochondral ossification genes, including Sox5, Sox6, Sox9, and type 2 collagen (138). Valproate also reduced expression of type 1 collagen and osteonectin in human fetal osteoblastic precursor hFOB1.19 cells (69). The precise target of the broad-acting HDIs in vivo is unclear; however, the phenotypes of genetically altered mice (reviewed above and in Table 1) indicate that inhibition of any single Hdac or multiple Hdacs may contribute to improper skeletal formation and long bone development in utero.
B. Hdac Inhibitors and the Mature Rodent Skeleton
A number of studies have measured the effects of HDIs on mature rodent skeletons. Depending on dosage and treatment regimen, in vivo treatment with valproate (159) or vorinostat (115, 145) caused bone loss in mouse and rat models. In a study designed to study the effectiveness of vorinostat at reducing tumor burden and associated osteolysis in immunocompromised mice, daily HDI treatment (100 mg/kg ip) for 4 wk prevented bone resorption associated with early stages of tumor growth, but it also caused significant cancellous bone loss in the contralateral non-tumor injected limbs and increased overall osteolysis despite decreasing tumor burden (145). Furthermore, vorinostat increased osteoclast differentiation in immunocompromised mice lacking tumors (145). In an identically designed study, C57Bl/6 mice treated with vorinostat had reduced cancellous bone mineral density, but normal cortical bone density and osteoclast activity (115). Paradoxically, while osteoblast numbers were reduced in HDI-treated C57Bl/6 bones, osteoblast activity (measured by mineral apposition rate) was increased. In vitro studies showed that immature osteoblasts that were not yet making a bone matrix were highly susceptible to DNA damage and cell cycle arrest when cultured in the presence of vorinostat, whereas mature and committed osteoblasts were resistant to the negative effects of HDIs on the cell cycle and possibly produced more extracellular matrix (115, 200).
Several groups found that HDIs like vorinostat, MS-275, and trichostatin (TSA) promote cell cycle arrest, senescence, and apoptosis in stromal cells derived from bone marrow or adipose tissues (41, 43, 98, 111, 115). For example, treatment of human bone marrow-derived mesenchymal stem cells with vorinostat activated caspase 9 and apoptotic pathways and downregulated genes associated with cell stemness (41). Valproate caused G2/M phase arrest of human mesenchymal stem cells (98). Although low concentrations were tolerated, high amounts of vorinostat caused apoptosis of human mesenchymal stromal cells (200). These effects can functionally impair the cells upon further differentiation. For example, pretreatment of human adipose-derived stromal cells with HDI prior to osteogenic differentiation decreased the amount of mineralized matrix formed (111), and murine bone marrow stromal cells exposed to vorinostat at early stages of osteogenic differentiation produce less matrix compared with vehicle-treated cells (115). Histone H4 acetylation patterns throughout the genome were correlated with changes in gene expression in vorinostat-treated MC3T3 osteoblasts, revealing a common mechanism of decreased signaling through the insulin/Akt/FoxO1 pathway in committed osteoblast precursors (44).
The effects of HDIs on bone resorption and osteoclast differentiation warrant further study because interest in their use as treatments for more cancers as well as chronic inflammatory and epigenetic diseases is growing. A body of evidence indicates that HDIs inhibit osteoclasts and joint destruction in animal models of chronic inflammatory diseases, including rheumatoid arthritis and periodontal disease (14). In three preclinical models of inflammatory arthritis, the Hdac inhibitor ITF2357 reduced joint inflammation and cartilage destruction (78). TSA also reduced inflammation and cartilage destruction in inflammatory arthritis (130). In vitro, HDIs reduced inflammatory responses by repressing expression of prostaglandin E2 synthase-1 (mPGES-1) and prostaglandin E2 production in response to interleukin-1 (18, 210).
A number of in vitro studies demonstrated that HDIs directly suppress early osteoclastogenesis. Romidepsin (FR901228, Isodax) induced expression of interferon-β, which decreased expression of two genes, c-Fos and Socs-3, required for osteoclastogenesis (129). MS-275 (Entinostat, SNDX-275) also suppressed osteoclast differentiation by repressing c-Fos expression (87). TSA and sodium butyrate repressed osteoclast formation by blocking the induction of NFATc1 and c-Fos in response to Rankl signaling and inducing the expression of two anti-osteoclastogenic factors, CCAAT enhancer binding protein (C/EBP)-β, and mitogen-activated protein kinase phosphatase (MKP)-1 (148, 194). TSA promoted apoptosis of mature multinucleated osteoclasts by increasing the expression of Cdkn1a/p21WAF (206). Studies on human osteoclasts generated from peripheral blood mononuclear cells indicate that simultaneous inhibition of both class I and II Hdacs may be necessary for osteoclastic suppression by Hdac inhibitors (14). Moreover, Hdac3 and Hdac7 may have distinct if not opposing effects on osteoclastogenesis in vitro (76, 142).
VI. SIRTUIN ACTIVATORS
Because Sirt1 expression lengthens the lifespan of many lower organisms and improves the healthspan of vertebrates, there is great interest in identifying Sirt activating compounds. A number of polyphenols, including resveratrol, are natural Sirt1 activators (39), and numerous studies have documented the benefits of resveratrol on preserving and enhancing bone mass in mice (reviewed in Ref. 177). Resveratrol's antioxidant, proestrogenic, and anti-inflammatory activities both promote bone formation and prevent bone resorption. Several synthetic Sirtuin activating compounds have been tested for their ability to protect bone mass in animal models. SRT2104 increased cancellous bone volume, but not cortical bone mass in male mice subjected to hindlimb suspension (119). In vitro, SRT2104 increased osteoblastic mineralization and inhibited osteoclast formation. Another SIRT activator, SRT3025, increased vertebral bone mass, femoral biomechanical properties, and cortical periosteal mineralizing surface in ovariectomized mice, while suppressing sclerostin expression and increasing β-catenin signaling in osteocytes (3).
VII. HDACS AND ORTHOPEDIC APPLICATIONS
Early in vitro studies with HDIs created a great deal of excitement in the bone field because they pointed to anabolic properties. Indeed, contrary to the studies described above, multiple laboratories throughout the world have reported that HDIs promote maturation of osteoblastic cell lines and calvarial osteoblast cultures in part by enhancing the activity of Runx2 and other transcription factors, as well as by activating BMP signaling pathways and preventing adipogenesis (reviewed in Ref. 116). But as summarized above, in vivo inactivation of Hdacs is generally unfavorable for bone development and maintenance because mesenchymal progenitors are particularly sensitive to Hdac loss of function. While sustained systemic administration of current broad acting HDIs may have adverse effects on the skeleton, these drugs could have some utility for bone tissue engineering (33) and orthopedic applications, such as heterotopic ossification, fracture repair, osteoarthritis, and treatment of bone tumors.
A. Hdacs and Bone Regeneration
Bone fracture healing and repair occur when progenitor cells from the surrounding tissues (e.g., periosteum, marrow, or vasculature) infiltrate the wound site and regenerate new tissue. Rigidly stabilized fractures (e.g., externally fixed breaks, calvarial or single cortex defects) that permit little to no motion of the healing site will heal by intramembranous ossification. Localized HDI treatment can augment these healing processes. For example, addition of the cyclic depsipeptide largazole to a macroporous biphasic calcium phosphate scaffold promoted greater bone formation than scaffold alone in rabbit calvarial bone defects. This was attributed to the induction of Runx2 and BMPs (99). Similarly, treatment of murine critical sized calvarial defects with MS-275 promoted bone formation that exceeded controls and was comparable to the enhanced healing caused by BMPs (86). Thus it has been suggested that HDIs could be developed into adjuvant therapies to promote faster skeletal healing (67). However, given the negative effects of HDI administration on bone mass in vivo and on osteoprogenitor survival in vitro, this concept requires further investigation.
B. Hdacs and Osteoarthritis
Hdacs may also regulate osteoarthritic phenotypes. Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability that poses enormous social and economic burdens in developed countries. Whereas articular chondrocytes are normally maintained in a quiescent or low metabolic state, joint injuries and repetitive use can induce damage and initiate the OA disease process. OA is characterized by thinning of the articular cartilage, induction of chondrocyte proliferation (cloning), aberrant chondrocyte hypertrophy, enzymatic degradation of the cartilaginous matrix, increased matrix calcification, thickening of subchondral bone, and/or inflammation of the joint. Since Hdacs regulate many of the processes involved in OA progression (e.g., inflammation, proliferation, hypertrophy, and matrix synthesis and degradation), their role in controlling OA progression is of interest.
Multiple HDACs are expressed in human articular cartilage (FIGURE 1, D and E), and several were found expressed at higher levels in diseased cartilage from OA joints. HDAC1 and HDAC2 were enriched in chondrocytes obtained from OA cartilage and ectopic expression of these enzymes decreased matrix gene expression (66). Articular cartilage from osteoarthritis patients also has elevated levels of HDAC7 mRNA compared with cartilage obtained from healthy controls (65). HDAC7 was more prevalent in the middle and deep zones of the articular cartilage of OA patients compared with healthy controls. SIRT1 was also elevated and associated with an increased Sox9-driven type 2 collagen expression in human chondrocytes derived from osteoarthritic patients (46); however, in osteoblasts from osteoarthritic joints, SIRT1 expression was reduced and correlated with increased Sost expression (1). Subsequent studies demonstrated that SIRT1 suppression reduced articular chondrocyte matrix gene expression and increased MMP expression and chondrocyte apoptosis (52).
HDIs alter the integrity of the cartilaginous extracellular matrix and OA disease progression. Using a surgical model of OA in the mouse, Culley et al. (32) found that systemic administration of TSA diminished cartilage damage caused by cytokine-induced MMP expression in human articular chondrocytes. Similar results were obtained using a rabbit model of OA where TSA protected cartilage and reduced MMP and cathepsin expression (22, 23, 68, 188); however, TSA also suppressed expression of two major cartilage extracellular components, aggrecan and collagen type 2 (68). Young et al. (208) found that TSA and sodium butyrate inhibited expression of MMPs and aggrecan-degrading enzymes such as ADAMTS5 in human chondrocytes, supporting a potential role for the protective effects of HDIs (208). The effects of Hdac inhibition on MMP production may be mediated through decreased activation of the MAPK pathway as SAHA, TSA, and MS-275 decreased ERK activity in vitro (153, 216) (Figure 3). Thus HDIs have the potential to protect articular cartilage by preventing matrix degradation; however, a role in promoting regeneration in unlikely as HDIs reduced MSC proliferation as well as differentiation into defined lineages, including chondrocytes (98).
C. Hdacs and Bone Tumors
The antiproliferative and chemo/radio-sensitizing properties of HDIs have made them attractive epigenetic therapies for cancers. The relatively modest side effects of these drugs have led to their widespread testing in many clinical trials. Xenograft models of prostate and breast cancer bone metastases indicate that HDIs can access bone marrow and reduce tumor burden (145). The in vivo effectiveness of HDIs against other bone tumors, such as osteosarcoma and myeloma, transplanted into mice is also an active area of investigation (84, 197). The ability of HDIs to kill cells from human and canine osteosarcomas, chondrosarcomas, and other primary bone tumors has been well documented by a number of in vitro studies (15, 175, 176, 197, 198, 201). Sarcoma patients have been included in a number of ongoing clinical trials with HDIs, and time will tell if these cancers are susceptible to HDIs (17, 154). Local application of HDIs may be an effective therapeutic approach for some primary tumors. A recent study demonstrated that HDIs loaded into bone cement scaffolds were released over time in culture and retained their activity (176). But because primary bone tumors are most lethal when they metastasize to the other organs, the effects of HDIs on bone cancer metastases are of interest. One study showed that oral administration of MS-275 caused regression of osteosarcoma lung metastases in a mouse model (149). Thus a number of lines of evidence indicate that HDIs have potential to treat at least some forms of primary and secondary bone tumors.
VIII. SUMMARY AND FUTURE DIRECTIONS
Hdacs have emerged as crucial regulators of both intramembranous and endochondral bone formation. The Zn-dependent Hdacs, especially those in classes I and IIa, appear to be essential for proper skeletal development as their in vivo inhibition generates multiple skeletal defects. This reality is sometimes in conflict with and confused by results from in vitro studies where Hdac inhibition promoted terminal differentiation of committed osteoblasts, but it aligns with data showing that HDIs suppress osteoblastic progenitors. Together, these results suggest that current HDIs may be effective for bone tissue engineering where cells can be coaxed past the proliferative phase before exposure to HDIs, and for some orthopedic applications, such as heterotopic ossification and primary bone tumor inhibition, where local suppression of progenitor cells may be advantageous. However, HDIs may not be effective therapies for systemic and/or aging-related diseases, such as osteoporosis, where stem/progenitor cell pools are essential for new bone formation and where osteoclastogenesis could be enhanced by HDIs. Existing evidence suggests that specific inhibitors of HDAC6 and activators of SIRTs may promote bone formation and/or prevent bone loss, but more studies are needed to understand the mechanisms behind these phenotypes. A challenge for drug studies is deciphering the specificity of HDIs in vivo. Although some HDIs are described or marketed as being specific for certain Hdacs, these conclusions are often based on studies with immunoprecipitated complexes, which may contain multiple Hdacs in mega-Dalton, multiprotein co-repressor complexes. Some evidence indicates the co-repressors can influence HDI specificity for an Hdac (4, 192). It is also possible that the absence of activity towards a particular purified protein in vitro may not represent its activity in vivo or in situ. The rapid emergence of epigenetic inhibitors as potential therapies for a variety of diseases and the advancement of bone imaging technologies should provide opportunities for prospective studies on how Hdac inhibitors, Sirt activators, and modulators of other epigenetic enzymes such as HATs and methyltransferases affect bone quantity and quality in human subjects.
Despite tremendous progress, there is still much to be learned about how Hdacs and Sirts control bone formation and regeneration through mouse genetics. Within the next decade, it is likely that all Hdacs (especially the most highly expressed Hdacs, Hdac1 and Sirt2) will have been individually deleted in bone cells of both the osteoblastic and osteoclastic lineages and that both their unique as well as redundant roles in bone development will be defined. The consequences of altering Hdac activity on chromatin structure and epigenetic memory in osteocytes (the longest living bone cells) and articular chondrocytes will also be of interest. As the crucial roles of Hdacs become more broadly recognized, it is likely that more studies will examine how various stimuli (e.g., Rankl and PTH), mechanical forces, aging, and altered physiological states affect Hdac expression and activity in osteoblasts, osteocytes, and osteoclasts (77, 100). More challenging studies will involve determining their role in adult skeletons and aging. For instance, one interesting question is whether or not deletion of Hdac4 will be as destructive to adult skeletons as it is to developing skeletons given its relatively low expression in adult bone and articular cartilage. Future work will also undoubtedly examine how Hdacs and Sirts expressed in bone regulate (and are regulated by) physiological processes, such as energy metabolism and sympathetic tone. Two recent studies on Hdac3 and Hdac4 CKO offer provocative evidence that Hdacs can control the expression of osteoblastic hormones that regulate systemic metabolism (108, 117). Finally, the mechanisms of Hdac action still need to be defined. A fundamental question is what are the important substrates of Hdacs besides histones. Some studies indicate that deacetylase activity and domains are dispensable for Hdac-mediated suppression and phenotypes, but these mechanisms are not fully understood. Addressing these and other questions are crucial for fully realizing the functions of Hdacs in the skeleton and understanding the advantages and disadvantages of HDI therapies on the skeleton.
GRANTS
The authors receive support from the Mayo Foundation for Education and Research and National Institutes of Health (NIH) Grants AR048069, AR056950, AR060140, AR068103, AR065397, DE20194, and DK050456. The contents of the article are the sole responsibility of the authors and do not represent official views of the Mayo Clinic or NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
We thank Ms. Teresa Hoff for outstanding administrative assistance. Research in this vast field is rapidly expanding and cannot be entirely canvased in a single review. We apologize to authors whose work we inadvertently omitted or were not able to discuss.
E. W. Bradley, L. R. Carpio, and M. E. McGee-Lawrence contributed equally to this work.
Address for reprint requests and other correspondence: J. J. Westendorf, Mayo Clinic, 200 First St. SW, Rochester, MN 55905 (e-mail: Westendorf.jennifer@mayo.edu).
REFERENCES
- 1.Abed E, Couchourel D, Delalandre A, Duval N, Pelletier JP, Martel-Pelletier J, Lajeunesse D. Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/beta-catenin activity. Bone 59: 28–36, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell 12: 377–389, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Artsi H, Cohen-Kfir E, Gurt I, Shahar R, Bajayo A, Kalish N, Bellido TM, Gabet Y, Dresner-Pollak R. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology 155: 3508–3515, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon AM, Schlegl J, Abraham Y, Becher I, Bergamini G, Boesche M, Delling M, Dumpelfeld B, Eberhard D, Huthmacher C, Mathieson T, Poeckel D, Reader V, Strunk K, Sweetman G, Kruse U, Neubauer G, Ramsden NG, Drewes G. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nature Biotechnol 29: 255–265, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Barna M, Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell 12: 931–941, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, Hiebert SW. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell 30: 61–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bheda P, Wolberger C. Biochemistry: sirtuin on a high-fat diet. Nature 496: 41–42, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 73: 417–435, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Boluk A, Guzelipek M, Savli H, Temel I, Ozisik HI, Kaygusuz A. The effect of valproate on bone mineral density in adult epileptic patients. Pharmacol Res 50: 93–97, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bradley EW, Carpio LR, Olson EN, Westendorf JJ. Histone deacetylase 7 (Hdac7) suppresses chondrocyte proliferation and beta-catenin activity during endochondral ossification. J Biol Chem 290: 118–126, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley EW, Carpio LR, Westendorf JJ. Histone deacetylase 3 suppression increases PH domain and leucine-rich repeat phosphatase (Phlpp)1 expression in chondrocytes to suppress Akt signaling and matrix secretion. J Biol Chem 288: 9572–9582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunmeir R, Lagger S, Seiser C. Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53: 275–289, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cantley MD, Fairlie DP, Bartold PM, Rainsford KD, Le GT, Lucke AJ, Holding CA, Haynes DR. Inhibitors of histone deacetylases in class I and class II suppress human osteoclasts in vitro. J Cell Physiol 226: 3233–3241, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Capobianco E, Mora A, La Sala D, Roberti A, Zaki N, Badidi E, Taranta M, Cinti C. Separate and combined effects of DNMT and HDAC inhibitors in treating human multi-drug resistant osteosarcoma HosDXR150 cell line. PLoS One 9: e95596, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpio LR, Bradley EW, McGee-Lawrence ME, Westendorf JJ. Histone Deacetylase 3 Suppresses Erk Phosphorylation and Subsequent Matrix Metalloproteinase (MMP)-13 Activity In Chondrocytes During Endochondral Ossification. In: American Society for Bone and Mineral Research. Houston, TX: Am. Soc. Bone Miner. Res, 2014. [Google Scholar]
- 17.Cassier PA, Polivka V, Judson I, Soria JC, Penel N, Marsoni S, Verweij J, Schellens JH, Morales-Barrera R, Schoffski P, Voest EE, Gomez-Roca C, Evans TR, Plummer R, Gallerani E, Kaye SB, Olmos D. Outcome of patients with sarcoma and other mesenchymal tumours participating in phase I trials: a subset analysis of a European Phase I database. Ann Oncol 25: 1222–1228, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Chabane N, Zayed N, Afif H, Mfuna-Endam L, Benderdour M, Boileau C, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis Cartilage 16: 1267–1274, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol 24: 8467–8476, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell 126: 321–334, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Chen IY, Lypowy J, Pain J, Sayed D, Grinberg S, Alcendor RR, Sadoshima J, Abdellatif M. Histone H2Az is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2alpha. J Biol Chem 281: 19369–19377, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen WP, Bao JP, Hu PF, Feng J, Wu LD. Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep 37: 3967–3972, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Chen WP, Bao JP, Tang JL, Hu PF, Wu LD. Trichostatin A inhibits expression of cathepsins in experimental osteoarthritis. Rheumatol Int 31: 1325–1331, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 100: 10794–10799, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chimal-Monroy J, Diaz de Leon L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. Int J Dev Biol 43: 59–67, 1999. [PubMed] [Google Scholar]
- 26.Chini CC, Escande C, Nin V, Chini EN. HDAC3 is negatively regulated by the nuclear protein DBC1. J Biol Chem 285: 40830–40837, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JE, Mostoslavsky R. Sirtuins, metabolism, and DNA repair. Curr Opin Genet Dev 26C: 24–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone 45: 579–589, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D'Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 152: 4514–4524, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Correa D, Hesse E, Seriwatanachai D, Kiviranta R, Saito H, Yamana K, Neff L, Atfi A, Coillard L, Sitara D, Maeda Y, Warming S, Jenkins NA, Copeland NG, Horne WC, Lanske B, Baron R. Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev Cell 19: 533–546, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culley KL, Hui W, Barter MJ, Davidson RK, Swingler TE, Destrument AP, Scott JL, Donell ST, Fenwick S, Rowan AD, Young DA, Clark IM. Class I histone deacetylase inhibition modulates metalloproteinase expression and blocks cytokine-induced cartilage degradation. Arthritis Rheum 65: 1822–1830, 2013. [DOI] [PubMed] [Google Scholar]
- 33.De Boer J, Licht R, Bongers M, van der Klundert T, Arends R, van Blitterswijk C. Inhibition of histone acetylation as a tool in bone tissue engineering. Tissue Eng 12: 2927–2937, 2006. [DOI] [PubMed] [Google Scholar]
- 34.De Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deardorff MA, Bando M, Nakato R, Watrin E, Itoh T, Minamino M, Saitoh K, Komata M, Katou Y, Clark D, Cole KE, De Baere E, Decroos C, Di Donato N, Ernst S, Francey LJ, Gyftodimou Y, Hirashima K, Hullings M, Ishikawa Y, Jaulin C, Kaur M, Kiyono T, Lombardi PM, Magnaghi-Jaulin L, Mortier GR, Nozaki N, Petersen MB, Seimiya H, Siu VM, Suzuki Y, Takagaki K, Wilde JJ, Willems PJ, Prigent C, Gillessen-Kaesbach G, Christianson DW, Kaiser FJ, Jackson LG, Hirota T, Krantz ID, Shirahige K. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 489: 313–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decroos C, Bowman CM, Moser JA, Christianson KE, Deardorff MA, Christianson DW. Compromised structure and function of HDAC8 mutants identified in Cornelia de Lange Syndrome spectrum disorders. ACS Chem Biol 9: 2157–2164, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeLaurier A, Nakamura Y, Braasch I, Khanna V, Kato H, Wakitani S, Postlethwait JH, Kimmel CB. Histone deacetylase-4 is required during early cranial neural crest development for generation of the zebrafish palatal skeleton. BMC Dev Biol 12: 16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenet 4: 5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denu JM. Fortifying the link between SIRT1, resveratrol, and mitochondrial function. Cell Metab 15: 566–567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci 118: 2901–2911, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Di Bernardo G, Squillaro T, Dell'Aversana C, Miceli M, Cipollaro M, Cascino A, Altucci L, Galderisi U. Histone deacetylase inhibitors promote apoptosis and senescence in human mesenchymal stem cells. Stem Cells Dev 18: 573–581, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med 17: 333–352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudakovic A, Camilleri ET, Lewallen EA, McGee-Lawrence ME, Riester SM, Kakar S, Montecino M, Stein GS, Ryoo HM, Dietz AB, Westendorf JJ, van Wijnen AJ. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. J Cell Physiol 230: 52–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudakovic A, Evans JM, Li Y, Middha S, McGee-Lawrence ME, van Wijnen AJ, Westendorf JJ. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J Biol Chem 288: 28783–28791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durst KL, Lutterbach B, Kummalue T, Friedman AD, Hiebert SW. The inv(16) fusion protein associates with corepressors via a smooth muscle myosin heavy-chain domain. Mol Cell Biol 23: 607–619, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem 283: 36300–36310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, Moore MM, Lwin ST, Yull FE, Mundy GR, Elefteriou F. Silent information regulator (Sir)T1 inhibits NF-kappaB signaling to maintain normal skeletal remodeling. J Bone Miner Res 28: 960–969, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov 13: 673–691, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Feng L, Zhou D, Zhang Z, Liu Y, Yang Y. Exome sequencing identifies a de novo mutation in HDAC8 associated with Cornelia de Lange syndrome. J Hum Genet 59: 536–539, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell 9: 45–57, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Gabay O, Sanchez C, Dvir-Ginzberg M, Gagarina V, Zaal KJ, Song Y, He XH, McBurney MW. Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheumatism 65: 159–166, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabay O, Zaal KJ, Sanchez C, Dvir-Ginzberg M, Gagarina V, Song Y, He XH, McBurney MW. Sirt1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine 80: 613–620, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giavini E, Menegola E. Teratogenic Activity of HDAC Inhibitors. Curr Pharm Des 20: 5438–5442, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene 363: 15–23, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Guan Y, Chen Q, Yang X, Haines P, Pei M, Terek R, Wei X, Zhao T, Wei L. Subcellular relocation of histone deacetylase 4 regulates growth plate chondrocyte differentiation through Ca2+/calmodulin-dependent kinase IV. Am J Physiol Cell Physiol 303: C33–C40, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan YJ, Yang X, Wei L, Chen Q. MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J 25: 4457–4466, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21: 6091–6101, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 14: 1048–1057, 2000. [PMC free article] [PubMed] [Google Scholar]
- 59.Guo CY, Ronen GM, Atkinson SA. Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia 42: 1141–1147, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Haberland M, Mokalled MH, Montgomery RL, Olson EN. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev 23: 1625–1630, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays 22: 138–147, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Harakalova M, van den Boogaard MJ, Sinke R, van Lieshout S, van Tuil MC, Duran K, Renkens I, Terhal PA, de Kovel C, Nijman IJ, van Haelst M, Knoers NV, van Haaften G, Kloosterman W, Hennekam RC, Cuppen E, Ploos van Amstel HK. X-exome sequencing identifies a HDAC8 variant in a large pedigree with X-linked intellectual disability, truncal obesity, gynaecomastia, hypogonadism and unusual face. J Med Genet 49: 539–543, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Higashiyama R, Miyaki S, Yamashita S, Yoshitaka T, Lindman G, Ito Y, Sasho T, Takahashi K, Lotz M, Asahara H. Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis. Mod Rheumatol 20: 11–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong S, Derfoul A, Pereira-Mouries L, Hall DJ. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J 23: 3539–3552, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu N, Wang C, Liang X, Yin L, Luo X, Liu B, Zhang H, Shui W, Nan G, Wang N, Wu N, Chen X, He Y, Wen S, Deng F, Liao Z, Luu HH, Haydon RC, He TC, Huang W. Inhibition of histone deacetylases potentiates BMP9-induced osteogenic signaling in mouse mesenchymal stem cells. Cell Physiol Biochem 32: 486–498, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Huh YH, Ryu JH, Chun JS. Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem 282: 17123–17131, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Humphrey EL, Morris GE, Fuller HR. Valproate reduces collagen and osteonectin in cultured bone cells. Epilepsy Res 106: 446–450, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Ignatius MS, Unal Eroglu A, Malireddy S, Gallagher G, Nambiar RM, Henion PD. Distinct functional and temporal requirements for zebrafish Hdac1 during neural crest-derived craniofacial and peripheral neuron development. PLoS One 8: e63218, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iyer S, Han L, Bartell SM, Kim HN, Gubrij I, de Cabo R, O'Brien CA, Manolagas SC, Almeida M. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing beta-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem 289: 24069–24078, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamaladdin S, Kelly RD, O'Regan L, Dovey OM, Hodson GE, Millard CJ, Portolano N, Fry AM, Schwabe JW, Cowley SM. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc Natl Acad Sci USA 111: 9840–9845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen ED, Schroeder TM, Bailey J, Gopalakrishnan R, Westendorf JJ. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J Bone Miner Res 23: 361–372, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon EJ, Lee KY, Choi NS, Lee MH, Kim HN, Jin YH, Ryoo HM, Choi JY, Yoshida M, Nishino N, Oh BC, Lee KS, Lee YH, Bae SC. Bone morphogenetic protein-2 stimulates Runx2 acetylation. J Biol Chem 281: 16502–16511, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496: 110–113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin Z, Wei W, Dechow PC, Wan Y. HDAC7 inhibits osteoclastogenesis by reversing RANKL-triggered beta-catenin switch. Mol Endocrinol 27: 325–335, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin Z, Wei W, Huynh H, Wan Y. HDAC9 inhibits osteoclastogenesis via mutual suppression of PPARgamma/RANKL signaling. Mol Endocrinol 29: 730–738, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med 17: 391–396, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi P, Greco TM, Guise AJ, Luo Y, Yu F, Nesvizhskii AI, Cristea IM. The functional interactome landscape of the human histone deacetylase family. Mol Syst Biol 9: 672, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juan LJ, Shia WJ, Chen MH, Yang WM, Seto E, Lin YS, Wu CW. Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem 275: 20436–20443, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Kaiser FJ, Ansari M, Braunholz D, Concepcion Gil-Rodriguez M, Decroos C, Wilde JJ, Fincher CT, Kaur M, Bando M, Amor DJ, Atwal PS, Bahlo M, Bowman CM, Bradley JJ, Brunner HG, Clark D, Del Campo M, Di Donato N, Diakumis P, Dubbs H, Dyment DA, Eckhold J, Ernst S, Ferreira JC, Francey LJ, Gehlken U, Guillen-Navarro E, Gyftodimou Y, Hall BD, Hennekam R, Hudgins L, Hullings M, Hunter JM, Yntema H, Innes AM, Kline AD, Krumina Z, Lee H, Leppig K, Lynch SA, Mallozzi MB, Mannini L, McKee S, Mehta SG, Micule I, Mohammed S, Moran E, Mortier GR, Moser JA, Noon SE, Nozaki N, Nunes L, Pappas JG, Penney LS, Perez-Aytes A, Petersen MB, Puisac B, Revencu N, Roeder E, Saitta S, Scheuerle AE, Schindeler KL, Siu VM, Stark Z, Strom SP, Thiese H, Vater I, Willems P, Williamson K, Wilson LC, Hakonarson H, Quintero-Rivera F, Wierzba J, Musio A, Gillessen-Kaesbach G, Ramos FJ, Jackson LG, Shirahige K, Pie J, Christianson DW, Krantz ID, Fitzpatrick DR, Deardorff MA. Loss-of-function HDAC8 mutations cause a phenotypic spectrum of Cornelia de Lange syndrome-like features, ocular hypertelorism, large fontanelle and X-linked inheritance. Hum Mol Genet 23: 2888–2900, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J 24: 2543–2555, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol 25: 629–648, 2009. [DOI] [PubMed] [Google Scholar]
- 84.Kaufman JL, Fabre C, Lonial S, Richardson PG. Histone deacetylase inhibitors in multiple myeloma: rationale and evidence for their use in combination therapy. Clin Lymphoma Myeloma Leuk 13: 370–376, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Kim DW, Lassar AB. Smad-dependent recruitment of a histone deacetylase/Sin3A complex modulates the bone morphogenetic protein-dependent transcriptional repressor activity of Nkx3.2. Mol Cell Biol 23: 8704–8717, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim HN, Lee JH, Bae SC, Ryoo HM, Kim HH, Ha H, Lee ZH. Histone deacetylase inhibitor MS-275 stimulates bone formation in part by enhancing Dhx36-mediated TNAP transcription. J Bone Miner Res 26: 2161–2173, 2011. [DOI] [PubMed] [Google Scholar]
- 87.Kim HN, Lee JH, Jin WJ, Ko S, Jung K, Ha H, Lee ZH. MS-275, a benzamide histone deacetylase inhibitor, prevents osteoclastogenesis by down-regulating c-Fos expression and suppresses bone loss in mice. Eur J Pharmacol 691: 69–76, 2012. [DOI] [PubMed] [Google Scholar]
- 88.Kim KO, Sampson ER, Maynard RD, O'Keefe RJ, Chen D, Drissi H, Rosier RN, Hilton MJ, Zuscik MJ. Ski inhibits TGF-beta/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J Cell Biochem 113: 2156–2166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SH, Lee JW, Choi KG, Chung HW, Lee HW. A 6-month longitudinal study of bone mineral density with antiepileptic drug monotherapy. Epilepsy Behav 10: 291–295, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Kozhemyakina E, Cohen T, Yao TP, Lassar AB. Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2A/histone deacetylase 4/MEF2 pathway. Mol Cell Biol 29: 5751–5762, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kronenberg HM. Developmental regulation of the growth plate. Nature 423: 332–336, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, Koch U, De Francesco R, Steinkuhler C, Gallinari P. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci USA 104: 17335–17340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lajeunie E, Barcik U, Thorne JA, El Ghouzzi V, Bourgeois M, Renier D. Craniosynostosis and fetal exposure to sodium valproate. J Neurosurg 95: 778–782, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Lakshmaiah KC, Jacob LA, Aparna S, Lokanatha D, Saldanha SC. Epigenetic therapy of cancer with histone deacetylase inhibitors. J Cancer Res Ther 10: 469–478, 2014. [DOI] [PubMed] [Google Scholar]
- 96.Lee HW, Suh JH, Kim AY, Lee YS, Park SY, Kim JB. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol 20: 2432–2443, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell 130: 456–469, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee S, Park JR, Seo MS, Roh KH, Park SB, Hwang JW, Sun B, Seo K, Lee YS, Kang SK, Jung JW, Kang KS. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif 42: 711–720, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee SU, Kwak HB, Pi SH, You HK, Byeon SR, Ying Y, Luesch H, Hong J, Kim SH. In vitro and in vivo osteogenic activity of largazole. ACS Med Chem Lett 2: 248–251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li C, Wang W, Xie L, Luo X, Cao X, Wan M. Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression. Ann NY Acad Sci. In press. [DOI] [PMC free article] [PubMed]
- 101.Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 119: 3666–3677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19: 4342–4350, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu CJ, Prazak L, Fajardo M, Yu S, Tyagi N, Di Cesare PE. Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J Biol Chem 279: 47081–47091, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Liu F, Woitge HW, Braut A, Kronenberg MS, Lichtler AC, Mina M, Kream BE. Expression and activity of osteoblast-targeted Cre recombinase transgenes in murine skeletal tissues. Int J Dev Biol 48: 645–653, 2004. [DOI] [PubMed] [Google Scholar]
- 105.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA 97: 4070–4075, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 408: 377–381, 2000. [DOI] [PubMed] [Google Scholar]
- 108.Makinistoglu MP, Karsenty G. The class II histone deacetylase HDAC4 regulates cognitive, metabolic and endocrine functions through its expression in osteoblasts. Mol Metab 4: 64–69, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137, 2000. [DOI] [PubMed] [Google Scholar]
- 110.Marks PA. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim Biophys Acta 1799: 717–725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maroni P, Brini AT, Arrigoni E, de Girolamo L, Niada S, Matteucci E, Bendinelli P, Desiderio MA. Chemical and genetic blockade of HDACs enhances osteogenic differentiation of human adipose tissue-derived stem cells by oppositely affecting osteogenic and adipogenic transcription factors. Biochem Biophys Res Commun 428: 271–277, 2012. [DOI] [PubMed] [Google Scholar]
- 112.Matsuzaki T, Matsushita T, Takayama K, Matsumoto T, Nishida K, Kuroda R, Kurosaka M. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann Rheum Dis 73: 1397–1404, 2014. [DOI] [PubMed] [Google Scholar]
- 113.Matthias P, Yoshida M, Khochbin S. HDAC6 a new cellular stress surveillance factor. Cell Cycle 7: 7–10, 2008. [DOI] [PubMed] [Google Scholar]
- 114.McGee-Lawrence ME, Bradley EW, Dudakovic A, Carlson SW, Ryan ZC, Kumar R, Dadsetan M, Yaszemski MJ, Chen Q, An KN, Westendorf JJ. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone 52: 296–307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McGee-Lawrence ME, McCleary-Wheeler AL, Secreto FJ, Razidlo DF, Zhang M, Stensgard BA, Li X, Stein GS, Lian JB, Westendorf JJ. Suberoylanilide hydroxamic acid (SAHA; vorinostat) causes bone loss by inhibiting immature osteoblasts. Bone 48: 1117–1126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene 474: 1–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGee-Lawrence ME, White TA, LeBrasseur NK, Westendorf JJ. Conditional deletion of Hdac3 in osteoprogenitor cells attenuates diet-induced systemic metabolic dysfunction. Mol Cell Endocrinol 410: 42–51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408: 106–111, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13: 787–796, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16: 4623–4635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV, Fairall L, Schwabe JW. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell 51: 57–67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21: 1790–1802, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 118: 3588–3597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moser MA, Hagelkruys A, Seiser C. Transcription and beyond: the role of mammalian class I lysine deacetylases. Chromosoma 123: 67–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124: 315–329, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Motyl KJ, McCabe LR, Schwartz AV. Bone and glucose metabolism: a two-way street. Arch Biochem Biophys 503: 2–10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murko C, Lagger S, Steiner M, Seiser C, Schoefer C, Pusch O. Histone deacetylase inhibitor Trichostatin A induces neural tube defects and promotes neural crest specification in the chicken neural tube. Differentiation 85: 55–66, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Murtaugh LC, Zeng L, Chyung JH, Lassar AB. The chick transcriptional repressor Nkx3.2 acts downstream of Shh to promote BMP-dependent axial chondrogenesis. Dev Cell 1: 411–422, 2001. [DOI] [PubMed] [Google Scholar]
- 129.Nakamura T, Kukita T, Shobuike T, Nagata K, Wu Z, Ogawa K, Hotokebuchi T, Kohashi O, Kukita A. Inhibition of histone deacetylase suppresses osteoclastogenesis and bone destruction by inducing IFN-beta production. J Immunol 175: 5809–5816, 2005. [DOI] [PubMed] [Google Scholar]
- 130.Nasu Y, Nishida K, Miyazawa S, Komiyama T, Kadota Y, Abe N, Yoshida A, Hirohata S, Ohtsuka A, Ozaki T. Trichostatin A, a histone deacetylase inhibitor, suppresses synovial inflammation and subsequent cartilage destruction in a collagen antibody-induced arthritis mouse model. Osteoarthritis Cartilage 16: 723–732, 2008. [DOI] [PubMed] [Google Scholar]
- 131.Noonan KJ, Hunziker EB, Nessler J, Buckwalter JA. Changes in cell, matrix compartment, and fibrillar collagen volumes between growth-plate zones. J Orthopaedic Res 16: 500–508, 1998. [DOI] [PubMed] [Google Scholar]
- 132.North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol 5: 224, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Obri A, Makinistoglu MP, Zhang H, Karsenty G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J Cell Biol 205: 771–780, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oner N, Kaya M, Karasalihoglu S, Karaca H, Celtik C, Tutunculer F. Bone mineral metabolism changes in epileptic children receiving valproic acid. J Paediatr Child Health 40: 470–473, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Ornoy A. Neuroteratogens in man: an overview with special emphasis on the teratogenicity of antiepileptic drugs in pregnancy. Reprod Toxicol 22: 214–226, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Orozco-Solis R, Sassone-Corsi P. Circadian clock: linking epigenetics to aging. Curr Opin Genet Dev 26C: 66–72, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. Endocrine regulation of male fertility by the skeleton. Cell 144: 796–809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paradis FH, Hales BF. Exposure to valproic acid inhibits chondrogenesis and osteogenesis in mid-organogenesis mouse limbs. Toxicol Sci 131: 234–241, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol 10: 454–460, 2010. [DOI] [PubMed] [Google Scholar]
- 140.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105: 20764–20769, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pei M, Chen D, Li J, Wei L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 78: 260–268, 2009. [DOI] [PubMed] [Google Scholar]
- 142.Pham L, Kaiser B, Romsa A, Schwarz T, Gopalakrishnan R, Jensen ED, Mansky KC. HDAC3 and HDAC7 have opposite effects on osteoclast differentiation. J Biol Chem 286: 12056–12065, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Piao J, Tsuji K, Ochi H, Iwata M, Koga D, Okawa A, Morita S, Takeda S, Asou Y. Sirt6 regulates postnatal growth plate differentiation and proliferation via Ihh signaling. Sci Rep 3: 3022, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pillai R, Coverdale LE, Dubey G, Martin CC. Histone deacetylase 1 (HDAC-1) required for the normal formation of craniofacial cartilage and pectoral fins of the zebrafish. Dev Dyn 231: 647–654, 2004. [DOI] [PubMed] [Google Scholar]
- 145.Pratap J, Akech J, Wixted JJ, Szabo G, Hussain S, McGee-Lawrence ME, Li X, Bedard K, Dhillon RJ, van Wijnen AJ, Stein JL, Stein GS, Westendorf JJ, Lian JB. The histone deacetylase inhibitor, vorinostat, reduces tumor growth at the metastatic bone site and associated osteolysis, but promotes normal bone loss. Mol Cancer Ther 9: 3210–3220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Purev E, Neff L, Horne WC, Baron R. c-Cbl and Cbl-b act redundantly to protect osteoclasts from apoptosis and to displace HDAC6 from beta-tubulin, stabilizing microtubules and podosomes. Mol Biol Cell 20: 4021–4030, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rahman MM, Kukita A, Kukita T, Shobuike T, Nakamura T, Kohashi O. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood 101: 3451–3459, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Rao-Bindal K, Koshkina NV, Stewart J, Kleinerman ES. The histone deacetylase inhibitor, MS-275 (entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Curr Cancer Drug Targets 13: 411–422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PloS One 5: e11492, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41: 1199–1206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sahakian E, Powers JJ, Chen J, Deng SL, Cheng F, Distler A, Woods DM, Rock-Klotz J, Sodre AL, Youn JI, Woan KV, Villagra A, Gabrilovich D, Sotomayor EM, Pinilla-Ibarz J. Histone deacetylase 11: a novel epigenetic regulator of myeloid derived suppressor cell expansion and function. Mol Immunol 63: 579–585, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saito T, Nishida K, Furumatsu T, Yoshida A, Ozawa M, Ozaki T. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthritis Cartilage 21: 165–174, 2013. [DOI] [PubMed] [Google Scholar]
- 154.Sampson VB, Gorlick R, Kamara D, Anders Kolb E. A review of targeted therapies evaluated by the pediatric preclinical testing program for osteosarcoma. Front Oncol 3: 132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sasagawa S, Takemori H, Uebi T, Ikegami D, Hiramatsu K, Ikegawa S, Yoshikawa H, Tsumaki N. SIK3 is essential for chondrocyte hypertrophy during skeletal development in mice. Development 139: 1153–1163, 2012. [DOI] [PubMed] [Google Scholar]
- 156.Sato Y, Kondo I, Ishida S, Motooka H, Takayama K, Tomita Y, Maeda H, Satoh K. Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology 57: 445–449, 2001. [DOI] [PubMed] [Google Scholar]
- 157.Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21: 920–928, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab 7: 104–112, 2008. [DOI] [PubMed] [Google Scholar]
- 159.Senn SM, Kantor S, Poulton IJ, Morris MJ, Sims NA, O'Brien TJ, Wark JD. Adverse effects of valproate on bone: defining a model to investigate the pathophysiology. Epilepsia 51: 984–993, 2010. [DOI] [PubMed] [Google Scholar]
- 160.Serrano L, Martinez-Redondo P, Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB, Kane-Goldsmith N, Tong Q, Rabanal RM, Fondevila D, Munoz P, Kruger M, Tischfield JA, Vaquero A. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev 27: 639–653, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem 286: 11492–11505, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sharony R, Garber A, Viskochil D, Schreck R, Platt LD, Ward R, Buehler BA, Graham JM Jr. Preaxial ray reduction defects as part of valproic acid embryofetopathy. Prenat Diagn 13: 909–918, 1993. [DOI] [PubMed] [Google Scholar]
- 163.Sheth RD, Wesolowski CA, Jacob JC, Penney S, Hobbs GR, Riggs JE, Bodensteiner JB. Effect of carbamazepine and valproate on bone mineral density. J Pediatr 127: 256–262, 1995. [DOI] [PubMed] [Google Scholar]
- 164.Shimizu E, Nakatani T, He Z, Partridge NC. Parathyroid hormone regulates histone deacetylase (HDAC) 4 through protein kinase A-mediated phosphorylation and dephosphorylation in osteoblastic cells. J Biol Chem 289: 21340–21350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shimizu E, Selvamurugan N, Westendorf JJ, Olson EN, Partridge NC. HDAC4 represses matrix metalloproteinase-13 transcription in osteoblastic cells, and parathyroid hormone controls this repression. J Biol Chem 285: 9616–9626, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med 5: 430–440, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simon D, Laloo B, Barillot M, Barnetche T, Blanchard C, Rooryck C, Marche M, Burgelin I, Coupry I, Chassaing N, Gilbert-Dussardier B, Lacombe D, Grosset C, Arveiler B. A mutation in the 3′-UTR of the HDAC6 gene abolishing the post-transcriptional regulation mediated by hsa-miR-433 is linked to a new form of dominant X-linked chondrodysplasia. Hum Mol Genet 19: 2015–2027, 2010. [DOI] [PubMed] [Google Scholar]
- 168.Singh N, Gupta M, Trivedi CM, Singh MK, Li L, Epstein JA. Murine craniofacial development requires Hdac3-mediated repression of Msx gene expression. Dev Biol 377: 333–344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slingerland M, Guchelaar HJ, Gelderblom H. Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors. Anticancer Drugs 25: 140–149, 2014. [DOI] [PubMed] [Google Scholar]
- 170.Stemig M, Astelford K, Emery A, Cho JJ, Allen B, Huang TH, Gopalakrishnan R, Mansky KC, Jensen ED. Deletion of histone deacetylase 7 in osteoclasts decreases bone mass in mice by interactions with MITF. PloS One 10: e0123843, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15: 757–765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282: 6823–6832, 2007. [DOI] [PubMed] [Google Scholar]
- 173.Taplick J, Kurtev V, Kroboth K, Posch M, Lechner T, Seiser C. Homo-oligomerisation and nuclear localisation of mouse histone deacetylase 1. J Mol Biol 308: 27–38, 2001. [DOI] [PubMed] [Google Scholar]
- 173a.Tassano E, Mirabelli-Badenier M, Veneselli E, Puliti A, Lerone M, Vaccari CM, Morana G, Porta S, Gimelli G, Cuoco C. Clinical and molecular characterization of a patient with interstitial 6q21q22.1 deletion. mol cytogenet 8: 31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272: 408–411, 1996. [DOI] [PubMed] [Google Scholar]
- 175.Thayanithy V, Park C, Sarver AL, Kartha RV, Korpela DM, Graef AJ, Steer CJ, Modiano JF, Subramanian S. Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PLoS One 7: e43720, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tonak M, Becker M, Graf C, Eckhard L, Theobald M, Rommens PM, Wehler TC, Proschek D. HDAC inhibitor-loaded bone cement for advanced local treatment of osteosarcoma and chondrosarcoma. Anticancer Res 34: 6459–6466, 2014. [PubMed] [Google Scholar]
- 177.Tou JC. Resveratrol supplementation affects bone acquisition and osteoporosis: pre-clinical evidence toward translational diet therapy. Biochim Biophys Acta 1852: 1186–1194, 2015. [DOI] [PubMed] [Google Scholar]
- 178.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 13: 324–331, 2007. [DOI] [PubMed] [Google Scholar]
- 179.Tsukahara H, Kimura K, Todoroki Y, Ohshima Y, Hiraoka M, Shigematsu Y, Tsukahara Y, Miura M, Mayumi M. Bone mineral status in ambulatory pediatric patients on long-term anti-epileptic drug therapy. Pediatr Int 44: 247–253, 2002. [DOI] [PubMed] [Google Scholar]
- 180.Underhill C, Qutob MS, Yee SP, Torchia J. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J Biol Chem 275: 40463–40470, 2000. [DOI] [PubMed] [Google Scholar]
- 181.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand 112: 137–143, 2005. [DOI] [PubMed] [Google Scholar]
- 182.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102: 703–710, 2008. [DOI] [PubMed] [Google Scholar]
- 183.Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, Paolini C, De Francesco R, Gallinari P, Steinkuhler C, Di Marco S. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci USA 101: 15064–15069, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 24: 8374–8385, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119: 555–566, 2004. [DOI] [PubMed] [Google Scholar]
- 186.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet 19: 286–293, 2003. [DOI] [PubMed] [Google Scholar]
- 187.Villavicencio-Lorini P, Klopocki E, Trimborn M, Koll R, Mundlos S, Horn D. Phenotypic variant of Brachydactyly-mental retardation syndrome in a family with an inherited interstitial 2q37.3 microdeletion including HDAC4. Eur J Hum Genet 21: 743–748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang X, Song Y, Jacobi JL, Tuan RS. Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes. Growth Factors 27: 40–49, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 481: 335–340, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, Nagano K, Baron R, Brooks D, Bouxsein M, Pajevic PD, Kronenberg HM. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res 30: 400–411, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wen YD, Perissi V, Staszewski LM, Yang WM, Krones A, Glass CK, Rosenfeld MG, Seto E. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc Natl Acad Sci USA 97: 7202–7207, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124: 30–39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Westendorf JJ, Zaidi SK, Cascino JE, Kahler R, van Wijnen AJ, Lian JB, Yoshida M, Stein GS, Li X. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol Cell Biol 22: 7982–7992, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Williams PJ, Nishu K, Rahman MM. HDAC inhibitor trichostatin A suppresses osteoclastogenesis by upregulating the expression of C/EBP-beta and MKP-1. Ann NY Acad Sci 1240: 18–25, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams SR, Aldred MA, Der Kaloustian VM, Halal F, Gowans G, McLeod DR, Zondag S, Toriello HV, Magenis RE, Elsea SH. Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am J Hum Genet 87: 219–228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wise LD, Turner KJ, Kerr JS. Assessment of developmental toxicity of vorinostat, a histone deacetylase inhibitor, in Sprague-Dawley rats and Dutch Belted rabbits. Birth Defects Res B Dev Reprod Toxicol 80: 57–68, 2007. [DOI] [PubMed] [Google Scholar]
- 197.Wittenburg LA, Bisson L, Rose BJ, Korch C, Thamm DH. The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemother Pharmacol 67: 83–92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wittenburg LA, Ptitsyn AA, Thamm DH. A systems biology approach to identify molecular pathways altered by HDAC inhibition in osteosarcoma. J Cell Biochem 113: 773–783, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wuelling M, Pasdziernik M, Moll CN, Thiesen AM, Schneider S, Johannes C, Vortkamp A. The multi zinc-finger protein Trps1 acts as a regulator of histone deacetylation during mitosis. Cell Cycle 12: 2219–2232, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu S, De Veirman K, Evans H, Santini GC, Vande Broek I, Leleu X, De Becker A, Van Camp B, Croucher P, Vanderkerken K, Van Riet I. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol Sin 34: 699–709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamamoto S, Tanaka K, Sakimura R, Okada T, Nakamura T, Li Y, Takasaki M, Nakabeppu Y, Iwamoto Y. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Res 28: 1585–1591, 2008. [PubMed] [Google Scholar]
- 202.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA 111: 12097–12102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang WM, Tsai SC, Wen YD, Fejer G, Seto E. Functional domains of histone deacetylase-3. J Biol Chem 277: 9447–9454, 2002. [DOI] [PubMed] [Google Scholar]
- 204.Yang XJ, Seto E. Collaborative spirit of histone deacetylases in regulating chromatin structure and gene expression. Curr Opin Genet Dev 13: 143–153, 2003. [DOI] [PubMed] [Google Scholar]
- 205.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 9: 206–218, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yi T, Baek JH, Kim HJ, Choi MH, Seo SB, Ryoo HM, Kim GS, Woo KM. Trichostatin A-mediated upregulation of p21(WAF1) contributes to osteoclast apoptosis. Exp Mol Med 39: 213–221, 2007. [DOI] [PubMed] [Google Scholar]
- 207.Yin T, Li L. The stem cell niches in bone. J Clin Invest 116: 1195–1201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR, Cawston TE, Clark IM. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther 7: R503–512, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 307: 269–273, 2005. [DOI] [PubMed] [Google Scholar]
- 210.Zayed N, El Mansouri FE, Chabane N, Kapoor M, Martel-Pelletier J, Benderdour M, Pelletier JP, Duval N, Fahmi H. Valproic acid suppresses interleukin-1ss-induced microsomal prostaglandin E2 synthase-1 expression in chondrocytes through upregulation of NAB1. J Rheumatol 38: 492–502, 2011. [DOI] [PubMed] [Google Scholar]
- 211.Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev 16: 1990–2005, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841, 2003. [DOI] [PubMed] [Google Scholar]
- 214.Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, Seto E. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev 19: 827–839, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, Cao C, Li N, Cheng HL, Chua K, Lombard D, Mizeracki A, Matthias G, Alt FW, Khochbin S, Matthias P. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol 28: 1688–1701, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-kappaB nuclear translocation. Int Immunopharmacol 17: 329–335, 2013. [DOI] [PubMed] [Google Scholar]
- 217.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10: e1004820, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, Floss T, Wurst W, Minucci S, Gottlicher M. Reduced body size and decreased intestinal tumor rates in HDAC2-mutant mice. Cancer Res 67: 9047–9054, 2007. [DOI] [PubMed] [Google Scholar]