Abstract
Eukaryotic cells have been confronted throughout their evolution with potentially lethal plasma membrane injuries, including those caused by osmotic stress, by infection from bacterial toxins and parasites, and by mechanical and ischemic stress. The wounded cell can survive if a rapid repair response is mounted that restores boundary integrity. Calcium has been identified as the key trigger to activate an effective membrane repair response that utilizes exocytosis and endocytosis to repair a membrane tear, or remove a membrane pore. We here review what is known about the cellular and molecular mechanisms of membrane repair, with particular emphasis on the relevance of repair as it relates to disease pathologies. Collective evidence reveals membrane repair employs primitive yet robust molecular machinery, such as vesicle fusion and contractile rings, processes evolutionarily honed for simplicity and success. Yet to be fully understood is whether core membrane repair machinery exists in all cells, or whether evolutionary adaptation has resulted in multiple compensatory repair pathways that specialize in different tissues and cells within our body.
I. INTRODUCTION
A. The Vulnerability of a Single Membrane Bilayer
Unlike bacterial cells, eukaryotic cells are not protected by a hardened and impermeant cell wall. The “naked” membrane bilayer covering early eukaryotes permitted the evolution of phagocytic vesicles for the uptake of nutrients, and secretory vesicles for the extrusion of waste products, enzymes, and signaling factors. The loss of a cell wall also led to the development of a new internal protective skeleton, the cytoskeleton. Together, cytoskeletal networks working in concert with internal membranes led to the development of the eukaryotic endomembrane system.
However, an unprotected bilayer member renders eukaryotic cells more vulnerable to mechanical and chemical stressors. Consequently, plasma membrane disruption is a common type of cellular injury in eukaryotic cells, and effective membrane repair mechanisms have evolved to rapidly reseal a membrane breach to ensure cell survival. These repair mechanisms utilized the newly evolved endomembrane and cytoskeletal systems. Within this review we outline the subcellular and molecular events that restore bilayer integrity after a membrane disruption injury, highlighting the protein families implicated in membrane repair, and the ancient biology that underpins membrane resealing and cell survival from a membrane breach.
B. Membrane Injury Underlies Many Human Pathologies
Many human pathologies are characterized by membrane injury, and modulation of membrane repair pathways holds tremendous therapeutic potential. Plasma membrane disruptions have been documented under physiological conditions in many mechanically active tissues, such as in the stratified epithelium that covers our body, the endothelia that line our blood vessels, and the epithelial barrier of our gastrointestinal tract (178). Disruptions are especially frequent in skeletal muscle, especially when it undergoes high-force, eccentric contractions (91, 180, 199). In certain forms of muscular dystrophy, the frequency of disruption initiated by physiological contractions is far higher than in normal muscle (54, 180).
Membrane disruptions are also caused by bacterial pore-forming toxins (PFTs) that are potent virulence factors secreted by most pathogenic bacteria (120). As the name suggests, PFTs form stable membrane pores that perforate the plasma membrane of host cells. Pore formation by bacterial pathogens is thought to serve many purposes, the most obvious being lysis and induction of cell death programs in immune cells, to mute immune cell activity and thus facilitate bacterial infection. Pores may also serve as channels for the bacteria to deliver other virulence factors and to access cellular nutrients from infected cells for their own metabolic growth, such as amino acids, ions, and ATP (165). Large pores formed by the cholesterol-dependent cytolysins can span 40 nM (257) and are also permeable to cellular proteins. However, in moderate doses, cells and organisms survive the onslaught of PFT perforation, and we will discuss recent developments regarding membrane repair mechanisms mobilized for survival from bacterial pores.
Cells within our vital organs also suffer membrane damage with ischemia-reperfusion injury, as occurs following heart attack and stroke. Ischemic membrane injury represents a complex cascade of events that results from an interruption to the circulation that feeds an organ oxygen and nutrients. A lack of oxygen causes depletion of ATP. ATP-dependent pumps begin to fail, resulting in disequilibrium in the potassium-sodium gradient, acidosis, and an inability to extrude or sequester calcium. Sodium influx causes cell swelling, and calcium influx induces proteolysis and triggers mitochondrial dysfunction, production of free radicals, and apoptosis. Cell swelling, acidosis, and oxidation compromises the plasma membrane. Membranes become leaky, with breaches sufficiently large to allow the release of cellular enzymes (125). Particularly in the case of contractile cells of the heart, contraction with reperfusion exacerbates membrane injury, and a cascade of necrosis follows. Indeed, traumatic brain injury is also characterized by widespread disruption of neuronal plasma membranes. It has been proposed that these membrane disruption events initiate a “death cascade” that is a major contributor to patient morbidity (39).
C. The Influx of Calcium Through a Membrane Breach Is the Key Trigger for Membrane Repair
Universally accepted within the membrane repair field is the critical role of calcium as an activating trigger for the rapid membrane repair of large lesions. Indeed, initiation of membrane repair may represent one of the most primitive forms of calcium signaling. Eukaryotic cells possess an innate ability to repair very large wounds. Microinjection of human oocytes for in vitro fertilization creates an enormous lesion encompassing hundreds of square micrometers, but is readily survivable. Indeed, sea urchin oocytes can repeatedly reseal sequential wounds encompassing a thousand square micrometers of surface, though they cannot survive a single insult if extracellular calcium is removed (181). Cells can also repair abundant smaller wounds (nanometer diameter), such as those induced by bacterial PFTs, using both calcium-dependent (123, 129) and calcium-independent processes (164).
D. Size Matters With Membrane Injury
1. Spontaneous resealing
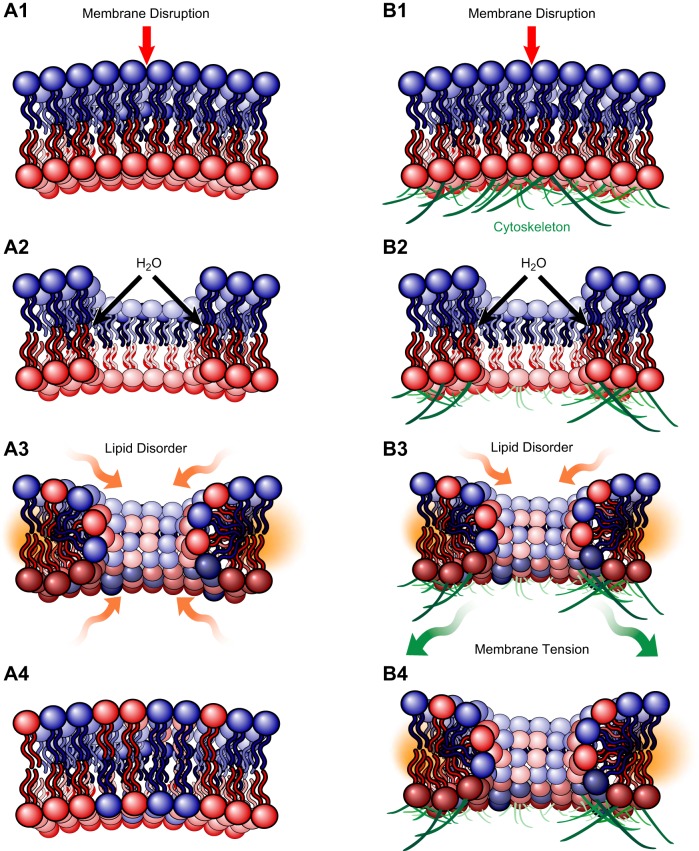
Tiny membrane injuries (less than a nanometer), such as those created by electroporation or proteins that induce lipid disorder, may repair spontaneously. Exposure of hydrophobic domains of lipids rapidly results in diffusion of lipids around the break site to form a curved edge. If the membrane consists only of a phospholipid bilayer, lipid disorder present on the curved edges of a disruption provides a driving force for resealing, and is a function of disruption diameter squared (Figure 1, A1–4). However, when the injured membrane belongs to a eukaryotic cell and the phospholipid bilayer is tethered to an underlying cytoskeleton (Figure 1, B1–4), the opposing force of membrane tension, a function of disruption diameter cubed, prevents spontaneous repair of biological membranes when the disruption exceeds a certain diameter. Based on experimental and theoretical data, that diameter is in the nanometer range (106).
Figure 1.
Spontaneous resealing of plasma membrane injuries in the nanometer range is opposed by the forces of the underlying membrane cytoskeleton. For an injury to a phospholipid bilayer alone (A1-4), the lipid disorder present on the curved edges of the disruption provides the driving force to spontaneously reseal the injury and is a function of disruption diameter squared. However, if the injured phospholipid bilayer is tethered to underlying cytoskeleton (B1-4), the membrane tension from adhesion to the cytoskeleton confers an opposing force for resealing, a function of disruption diameter cubed, and prevents spontaneous repair of membrane disruptions that exceed diameters in the nanometer range (106).
2. Active resealing pathways
Injuries larger than a few nanometers in diameter require the help of an active membrane repair mechanism. For these larger membrane disruptions, the opposing forces of membrane tension preclude spontaneous membrane resealing, and in the case of bacterial pore-forming toxins, the stable proteinaceous structure of the pores cannot be simply resealed.
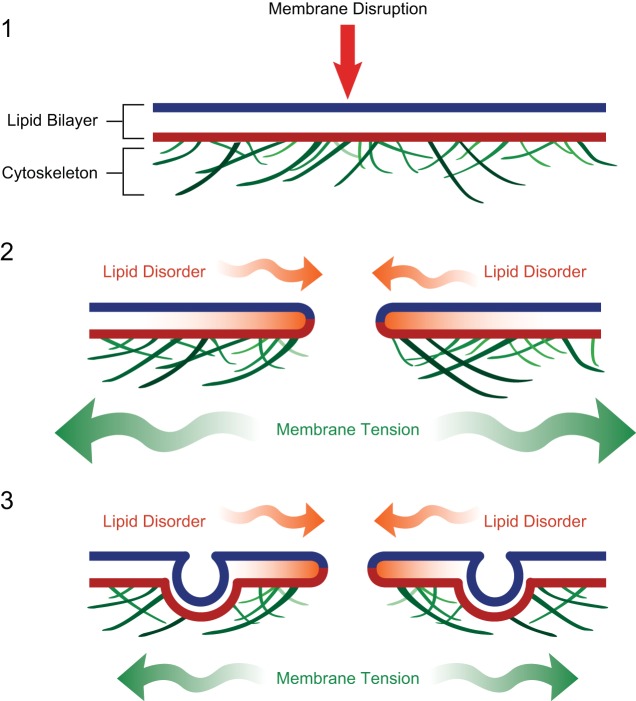
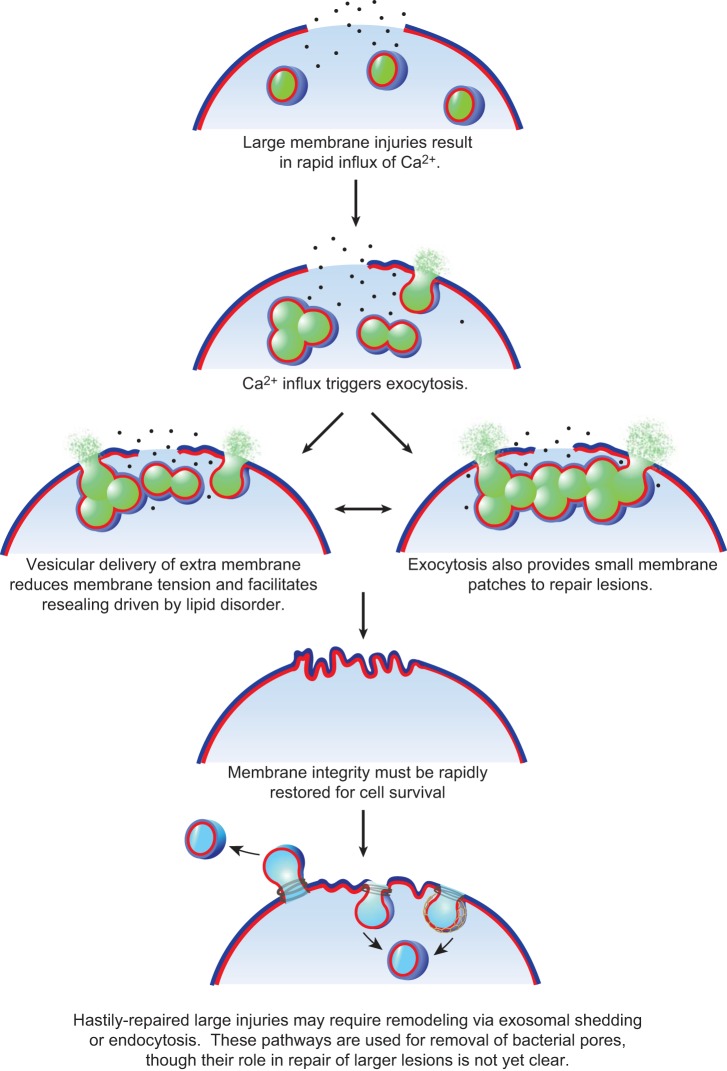
Repair of very large disruptions, hundreds to thousands of nanometers in diameter, requires calcium-dependent exocytosis (27, 267), involving both vesicle-vesicle and vesicle-plasma membrane fusion to crudely “patch” the compromised plasma membrane (285). The sea urchin egg can replace over 2,000 square microns of surface membrane ripped from its surface in <5 s (285), and neurons and muscle cells can survive complete transections (45, 101, 146). In the sea urchin egg, large secretory granules form a membrane patch at large injuries. However, which vesicle population(s) are utilized for membrane patching in different mammalian cells and tissues is not yet clear (see sect. IIIA). Calcium-triggered exocytosis is also thought to reduce membrane tension, which facilitates resealing driven by lipid disorder (Figures 2 AND 3) (292). How exactly the exocytic addition reduces membrane tension is an important unanswered question. The answers likely relate both to the delivery of membrane lipids to reduce lipid packing, as well as the associated remodeling of the submembraneous cytoskeletal that is required for exocytic delivery of vesicles.
Figure 2.
Calcium-activated exocytosis reduces membrane tension and promotes spontaneous repair driven by lipid disorder for injuries hundreds of nanometers in diameter. With larger injuries, the opposing force of membrane tension exceeds the resealing forces of lipid disorder at the edges of the disruption, negating the driving forces of spontaneous membrane resealing. Eukaryotic cells have been shown to utilize calcium-activated exocytosis to reduce membrane tension and promote repair via lipid-disorder driven attractions. The reduction in membrane tension is likely due directly to the addition of phospholipids to reduced lipid packing, as well as due in part to the cytoskeletal remodeling associated with vesicular transport at the plasma membrane.
Figure 3.
Very large plasma membrane disruptions (micron diameter) require membrane patching. The calcium influx of a membrane injury activates vesicular exocytosis and homo- and heterotypic fusion of cytoplasmic vesicles. Exocytic fusion reduces membrane tension, and vesicle-vesicle fusion events provide a patch as a replacement for the membrane barrier missing at the disruption site. The membrane patch may serve only temporarily as a surface barrier replacement that is subsequently remodeled and removed via exocytic and/or endocytic machinery.
Smaller injuries such as those caused by bacterial PFTs (nanometer range) do not appear to utilize “membrane patching” and in fact cannot be repaired but must be removed. Thus exosomal secretion and endosomal uptake are employed for removal of bacterial-lined pores (14, 58, 123, 129, 280) (see sects. IIA and III, D and E). These membrane repair mechanisms are likely also important to remove and remodel hastily repaired larger lesions as part of the membrane repair process (Figure 3).
II. PHYSIOLOGIES OF MEMBRANE INJURY
A. Pore-Forming Toxins
We begin with what was likely an early ancestral challenge requiring membrane repair, eukaryotic cell survival from bacterial PFTs.
1. Evolution and structure
The plasma membrane of a cell represents a remarkable landscape of ordered and partitioned proteins and lipids, working together to maximize cellular signaling and communication. Pathogens have evolved that exploit the predictable clustering of receptors within cholesterol-rich plasma membrane microdomains. These pathogens release pore-forming proteins, named for their capacity to perforate the plasma membrane of their target cell. Pore-forming proteins are best characterized in bacteria (for detailed reviews, see Refs. 30, 105, 120, 213), but are also produced by many higher organisms such as sea anemones and jellyfish (8, 318), earthworms (260), and plants (70). Interestingly, the mammalian immune system has reciprocally adopted a pore-forming strategy to lyse invading pathogens. Several members of complement membrane attack complex function as pore-forming proteins to lyse bacteria and other pathogens (167), and natural killer cells and cytotoxic T-cells expressing the pore-forming protein perforin to provide passage for granzymes into target pathogens to initiate a cell death cascade (19, 149, 239). Mammalian cells also use a pore-forming strategy to activate apoptosis via the Bcl2 protein Bax (145, 217, 251).
PFTs effectively induce lytic death of many cell types in vitro, although the role of pore formation in vivo during infection is more complex (for a comprehensive recent review, see Ref. 165). PFTs specifically target an organism's immune defense by inducing lytic death of immune cells (via pores) as well as inducing cell death programs triggered by potassium efflux, calcium influx, ATP depletion, mitochondrial damage, disrupted ion homeostasis, and swelling. Pores are thought to also provide an entry point for other bacterial virulence factors that aide their infectivity, replication, and ability to escape immune detection. One of the main causes of patient morbidity relates to the deleterious overstimulation of inflammatory pathways that eventually compromises the integrity of epithelial and endothelial barriers, allowing the infection to spread, and disrupting the fidelity of the vasculature (165).
a) pfts are the largest class of virulence-related bacterial toxins. PFTs represent the largest class of bacterial toxins and play a major role in their virulence. Pathogenic bacteria such as Escherichia coli, Pneumococcus, Streptococcus, Staphylococcus, Vibrio cholera, Clostridia, Listeria, and Diphtheria, to name but a few, each produce pore-forming proteins that contribute worldwide to infection-related morbidity and mortality. Pore-forming proteins from different species typically do not share high sequence homology, but are known to assume similar tertiary structures (93, 111, 301, 312) and are thought to function using the same mode of action. There are two main classes of PFTs: the α-PFTs that adopt an α-helical fold for membrane insertion (for example, colicins from Escherichia coli) and β-PFTs that adopt a β-barrel conformation for membrane insertion and pore formation (the most common form of PFTs) (213). PFTs are released as soluble proteins by bacteria and bind to target membranes, recognizing specific GPI-anchored proteins (1, 60, 198) or lipid compartments, such as the cholesterol-dependent cytolysins (240, 257, 296). Binding to specific receptors or lipid raft regions on the plasma membrane increases their local concentration, facilitating their oligomerization, which is an essential step in pore formation. The capacity of PFTs to metamorphosize from their soluble form into oligomers, capable of transmembrane insertion and subsequent formation of a pore, is a truly remarkable feature.
2. The membrane repair response to PFT infection
The response of an intoxicated cell to PFTs depends on the type of toxin, the levels of toxin, and the period of exposure to toxin (for reviews, see Refs. 30, 46). Interestingly, cells require much longer to recover from infection by small-diameter pores (∼2 nm) such as Staphylococcus α-toxin (6 h or more) (118), compared with large-diameter pores (40 nm) such as Streptococcus streptolysin-O (SLO), that are removed by microvesicular shedding and/or endocytosis within minutes (14, 123, 129, 219). The differences in membrane repair response between small and large pores are most likely due to the fact that small pores are not permeable to calcium (305, 321), and thus do not activate rapid calcium-activated exocytic and endocytic responses. Recovery of plasma membrane integrity following infection by bacterial streptolysin-O (SLO) is perhaps one of the most well-characterized examples of how cells are able to utilize both exocytic and endocytic responses to survive a membrane injury.
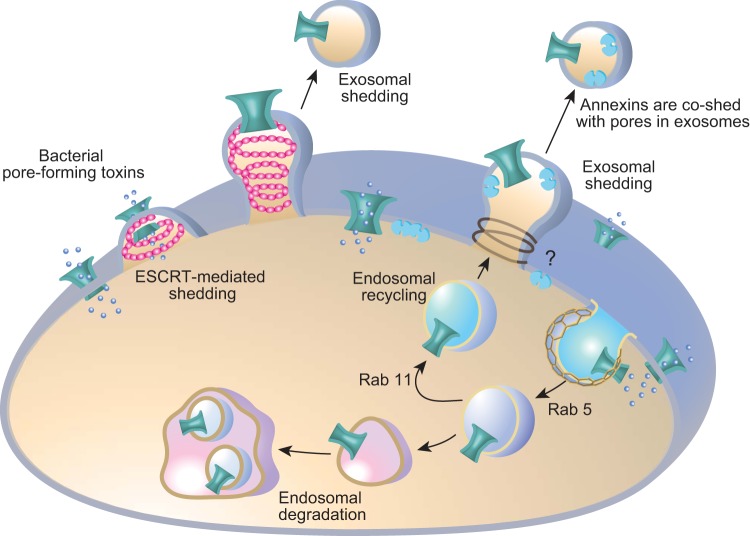
a) survival from pft utilizes both exocytic and endocytic responses. SLO treatment can induce cell blebbing and release of microvesicles containing pores and SLO protein (14, 118, 136) (see Figure 4). However, perforin (287, 288) and SLO (122, 280) have also been shown to induce endocytosis into an abnormally enlarged endosomal compartment. Following pore insertion into the plasma membrane, a local elevation in intracellular calcium induces lysosome fusion with the plasma membrane (123, 229) and release of acid sphingomyelinase (280). It is proposed that hydrolysis of sphingomyelin head groups leads to the formation of ceramide-enriched plasma membrane micro-domains that activate endocytosis, and rapid removal of the SLO pores into the degradative endosomal pathway (280) (Figure 4). Endocytic removal of toxin pores presents its own set of problems, as these pores may remain conductive in the early endosomal pathway and release acid hydrolases into the cellular cytoplasm. However, several PFTs induce endocytic removal from the plasma membrane as part of their pathogenic entry into the cell (96). Thus how endosomal permeability contributes to PFT toxicity is only beginning to be teased out.
Figure 4.
Survival from bacterial pore-forming toxins utilizes both exocytic and endocytic responses. Bacterial pore-forming toxins oligomerize and insert in the plasma membrane of target cells forming a diffusible pore. Evidence suggests these pores are removed both by endosomal degradative pathways (123, 164, 280) and exosomal shedding (14, 118, 136). Shed microvesicles containing streptolysin-O have been shown to also contain annexins A1 and A6 (219). In C. elegans, Rab 5 endocytic and Rab 11 recycling pathways are implicated in pore removal (164). ESCRT machinery (pink helix) has been implicated in exosomal shedding (129), although other exosomal machinery may also be involved (indicated by a question mark).
In vivo studies in C. elegans have addressed the question of how cells escape PFT attack, and also emphasize the unique interplay between exocytic and endocytic aspects of the repair response (118, 164) (Figure 4). PFT infection of C. elegans gut epithelial cells by S. aureus α-toxin (118), B. thuringiensis Cry5B, and V. cholerae cytolysin (VCC) (164) induces rapid endocytosis and also exosomal shedding of pore-containing vesicles. Studies by Los et al. (164) demonstrated these responses were dependent on Rab-5 and Rab-11 (164), master regulators of, respectively, endosome formation and exocytic recycling. RNAi knockdown of Rab-5 or Rab-11 resulted in decreased endocytosis and worm hypersensitivity to PFTs. Importantly, Rab-11 depletion specifically prevented microvillar shedding. The authors propose that the worm's epithelial cells use both routes of PFT elimination. Toxins are directed via Rab-5-based endocytosis into the lysosome and are also shed via Rab-11-based exosomal shedding of microvillar membrane vesicles into the gut lumen (Figure 4).
Data suggest that endocytic and exosomal pathways to remove toxin-lined pores can occur via both calcium-dependent and calcium-independent pathways. α-Toxin, Cry5B, and VCC form small-diameter pores (1-2 nm) (213, 320) and are thought not to be permeable to calcium (305, 321). Moreover, endocytosis and exosomal shedding of Cry5B and VCC following in vivo infection of C. elegans were shown to occur independently of extracellular calcium in the medium (164). In contrast, removal of large calcium-conductive SLO pores in mammalian cells occurs by rapid calcium-dependent endocytosis (123) and calcium-dependent exosomal shedding (14). Additionally, the ESCRT machinery has been recently implicated in the calcium-dependent exosomal shedding of bacterial pore-forming toxins (129), and this is discussed in greater detail in section IIIF. Therefore, whether rapid calcium-dependent or slower calcium-independent pathways are utilized, likely relates to the size of the pores and whether they are conductive to calcium.
B. Muscle Injury and Muscular Dystrophy
1. Eccentric injuries and t-tubules
Muscle fibers are particularly prone to injury when subjected to lengthening contractions, referred to as eccentric damage (180). Eccentric injury occurs because whilst lengthening a muscle, for example, your quadriceps as you stride down hill, you simultaneously ask the muscle to contract against a lengthening stretch, when the membrane tension is significantly increased. The muscle plasma membrane transverse tubule (t-tubule) network is particularly sensitive to eccentric stretch, as this network of small-diameter tubules runs perpendicular to the long axis of contraction. T-tubules are invaginations of the muscle plasma membrane, ∼20–40 nM in diameter (size differs slightly amongst species) (90), that penetrate deep into the interior of the myofiber. T-tubules are anchored at precise intervals along the sarcomere (the contractile unit of muscle) and contain the voltage-gated channels responsible for initiating the wave of calcium release that activates muscle contraction. T-tubules are essential to rapidly conduct the electric impulse from the nerve into the myofiber interior, such that all of the voltage-gated channels open in unison, and contraction occurs simultaneously among all of the bundles of myofibers within a muscle group. However, eccentric stretch can result in disruption of the sarcomeric apparatus (5), and because the t-tubule network is firmly anchored to these contractile units, it too suffers a lateral stretch and gets pulled out of position (221). The fine longitudinal tubules that connect adjacent transverse tubules (80) rapidly and reversibly swell with eccentric stretch, reducing the efficiency of electrical conductivity and contraction.
a) intense eccentric exercise in untrained subjects can kill muscle fibers. When untrained normal healthy controls are subject to shorts bouts of repeated eccentric stretch, for example, 20 min of stepping up and down a stair (stepping down the stair is the eccentric stretch), the muscle membrane is injured and allows the release of the muscle enzyme creatine kinase into the serum over the following hours and days (199). Eccentric muscle damage is characterized by a feeling of weakness and wobbliness immediately after the exercise, with muscles becoming tender, sore, and stiff 1 or 2 days after the injurious event (254). Ultrastructural analysis of muscle biopsies taken from control subjects who have undergone a protocol of eccentric stretch reveals marked disruption in the organization of the skeletal muscle contractile apparatus immediately after the exercise (91, 221). Indeed, the eccentric stretch injury induces such significant damage that over the following days and weeks, the pool of muscle stem cells, called satellite cells, are activated and proliferate to repair and rebuild lethally injured myofibers (91, 131).
2. Persistent membrane injury in muscular dystrophy
In patients with muscular dystrophy, creatine kinase is persistently elevated (regularly hundreds of times higher than control levels) and muscle biopsy samples show histopathological signs of ongoing degeneration and regeneration of muscle fibers. The most common form of muscular dystrophy is Duchenne's muscular dystrophy (DMD), an X-linked inherited disorder affecting ∼1:3,000 boys, due to mutations in the cytoskeletal protein dystrophin (113).
Dystrophin is part of the spectrin superfamily of cytoskeletal proteins and is thus proposed to lend elasticity to the muscle plasma membrane, as well as provide the structural cornerstone of the muscle costamere. The costamere is a focal adhesion-like complex assembled at regular intervals along the sarcomere, providing a stabilizing connection between the cytoskeletal apparatus via the intermediate filament network, through the plasma membrane, and out to the extracellular matrix (85). At costameres, dystrophin forms part of a large transmembrane glycoprotein complex (86, 121). The lack of dystrophin, or other components of the dystrophin-glycoprotein complex, causes different forms of inherited muscular dystrophies (55) and renders muscle much more susceptible to injury with eccentric stretch (216) (for a review, see Ref. 56).
In 1999, a new gene was identified as the cause of a form of inherited muscular dystrophy, dysferlin (22, 161). Dysferlin did not sediment with the dystrophin-glycoprotein complex and instead was shown to be required for acute resealing of laser-injured myofibers (18). Thus, rather than playing a structural role in the sarcolemmal stability, dysferlin was proposed as a key mediator of calcium-dependent muscle membrane repair and is discussed within section IIIB. A primary defect in skeletal muscle membrane repair characterizes dysferlinopathy, but is also a feature of muscle fibers from diabetic mice (116) and cells from patients with lysosomal storage disease (49, 119, 280) (discussed further in sect. IIIF). Furthermore, evidence for t-tubule injury and repair is also a feature in statin myopathy (77, 302). Thus an imbalance in susceptibility to membrane injury, and capacity for membrane repair, may be common in many forms of myopathy.
a) what does muscle membrane injury look like? We do not really know the precise nature of membrane disruptions caused by repeated eccentric stretch, by lengthening strain with a sporting injury, or suffered with more routine physical activity in a patient with muscular dystrophy. What we do know is that the egress of creatine kinase (199) and the uptake of albumin into eccentrically injured muscle fibers (180) indicates sites of membrane permeability are sufficiently large to allow the transfer of large macromolecules. The dimensions of serum albumin are ∼80 Å × 80 Å × 30 Å (272) (roughly 8 nM × 8 nM × 3 nM). Therefore, for significant levels of albumin to enter an injured muscle fiber, there must either be multiple large lesions encompassing hundreds to thousands of square nanometers, a multitude of smaller lesions hundreds of square nanometers, or perhaps a mixture of both. All possibilities are consistent with the notion that mechanical stretch leads to physical disruption of the plasma membrane and t-tubule network (180).
Studies of dystrophin-deficient fibers isolated from the mdx murine model of DMD and loaded with various sodium- and calcium-sensitive dyes could find no evidence for overt membrane tears following eccentric stretch (316). Rather, Yeung et al. (316) provide evidence that dystrophin deficiency alters the properties of stretch-activated calcium channels that leech calcium into the cell after a bout of eccentric injury. The dysregulation in calcium homeostasis leads to calpain overactivity, mitochondrial dysfunction, and oxidative damage (for review, see Ref. 6). Oxidation of membrane lipids and proteins is thought to render muscle fibers more susceptible to injury with subsequent challenges.
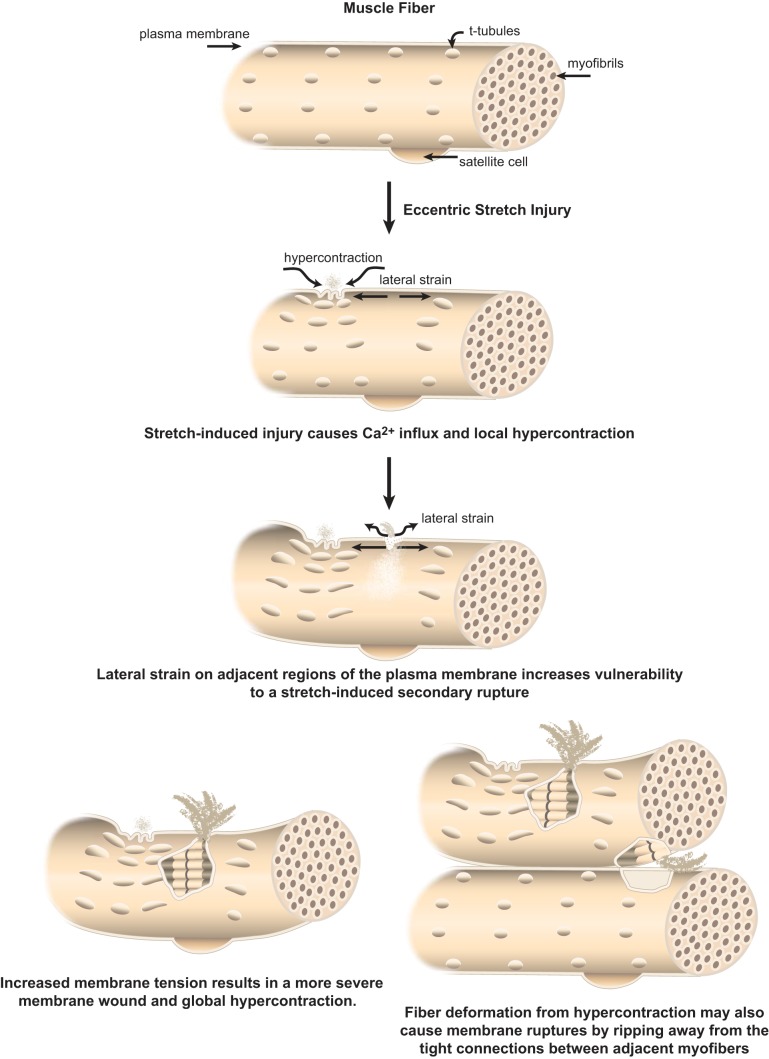
However, these were single fibers studied in isolation. Studies of whole muscles derived from mdx and control mice concurred that calcium appears to “leak” into dystrophin-deficient fibers after bouts of contraction, inducing local hypercontraction. This region of local hypercontraction exerts a lateral strain on adjacent lengthened regions (see Figure 5). These regions then incurred a more significant injury that caused global hypercontraction and “concertina-like” retraction of fibers within the muscle bundle (53). This sort of fiber retraction, ripping away from adjacent muscle fibers, would reasonably cause large membrane injuries, sufficient to explain the large egress of creatine kinase in patients with muscular dystrophy (Figure 5). Even in healthy muscles, an in vivo model of a large strain injury caused membrane injuries of sufficient magnitude to allow uptake of large dextran macromolecules, which are excluded at later time points, but does not activate satellite cell repair (235, 236). This provides direct evidence that an injurious strain to healthy muscle causes membrane injuries that are repaired in a regeneration-independent manner (see sect. IIIB).
Figure 5.
What might membrane injury to muscle fibers look like? Muscle fibers have a complex plasma membrane network with a repeating register of deep plasma membrane invaginations called the t-tubule network. Muscle fibers are subject to huge variations in membrane tension, due to their contractile activity. Repeated eccentric exercise in healthy subjects (i.e., stepping down for 20 min) is known to induce damage so severe that muscle fibers degenerate over the following days and weeks (91, 131, 199). Patients with muscular dystrophy are more susceptible to injury from eccentric stretch (216), with studies in mouse models suggesting susceptibility to injury can escalate with multiple insults (53). One model explaining membrane injury in dystrophin-deficient muscle fibers proposes that an initial injury causes a local influx of calcium and a local region of hypercontraction. These shortened sarcomeres induce a concomitant lengthening of adjacent sarcomeres and increased lateral strain to the plasma membrane. Subsequent insult(s) of eccentric stretch result in a more severe wound and global hypercontraction, producing fiber retraction within the muscle bundle (53). As muscle fibers have strong interfiber connections, muscle injuries may manifest both as shearing of the membrane from increased membrane tension and strain, as well as ripping of plasma membrane regions from fiber retraction or hypercontraction.
C. Ischemic Membrane Injury
1. Ischemia-reperfusion injury is a leading killer in the Western world
Disorders associated with ischemia-reperfusion injury, such as heart attack, stroke, and vascular disease, encompass the largest causes of mortality and morbidity in the Western world. Disruption in the blood flow to a tissue or organ can result in large regions of cell death (infarct), and often occur as a consequence of a clot within a major artery delivering circulation. The size of the infarct is primarily determined by the length of time blood flow is obstructed and the nature of the blood vessel affected. Blood delivers oxygen and glucose to tissues, both of which are required for the production of ATP by the mitochondrial respiratory chain. Thus ischemia induces a rapid loss of cellular ATP, and a complex cascade of events ensues from ATP depletion. For comprehensive reviews in the area of ischemia-reperfusion injury, please refer to References 132, 193, and 247 and references therein.
2. Cells need ATP to maintain ion homeostasis
ATP drives the sodium and potassium exchange pumps that maintain plasma membrane potential, and vitally, the F1/F0-ATPase used to generate mitochondrial membrane potential (Δψ). ATP also drives the pumps that extrude calcium from the cell (sodium-calcium exchanger) and the pumps that drive cytoplasmic calcium back into the endoplasmic or sarcoplasmic reticulum after each contraction in heart and muscle (via sarcoplasmic endoplasmic reticulum calcium ATPase, SERCA). During ischemia, cells are obliged to utilize anaerobic metabolism to produce ATP. This results in a rapid increase in lactate and protons (acidosis), and from here, there is a downhill spiral of compensatory mechanisms that conspire to result in calcium overload and toxicity.
To try and reneutralize normal cytosolic pH, cells furiously pump out the protons in exchange for sodium using the Na+/H+ exchanger (191). These pumps cannot keep up with demand, particularly with low cellular ATP, and the massive influx of sodium ions results in cell swelling. Attempts are also made to extrude the sodium in exchange for calcium using the sodium/calcium exchanger that begins to operate in reverse, allowing more lethal calcium into the cell. Unfortunately, with ATP in short supply, a large problem for contractile heart cells is reuptake of calcium following contractions, whereby ATP-dependent SERCA is compromised, and calcium release by the ryanodine receptor is enhanced (279).
3. Calcium toxicity, cell swelling, and oxidative damage conspire to injure membranes
Reperfusion adds fuel to the fire. Although reestablishing the oxygen supply is essential for cell survival, restoration of extracellular pH to neutrality increases the proton gradient for cells with an acidic intracellular pH; the Na+/H+ exchanger works busily and sodium levels increase, followed by an even larger influx of calcium via the sodium/calcium exchanger (192). Elevated levels of submembraneous calcium activate calpains that cleave focal adhesion complexes and cytoskeletal elements (see sect. IIIC), compromising the fidelity of the plasma membrane. Also with this flush of oxygen, reactive oxygen species (ROS) are produced by the mitochondria, due to damage to the electron transport chain incurred during ischemia, defective transport of electrons, and production of superoxide (10, 94, 322). ROS cause widespread oxidation of both protein and lipids, another insult for the plasma membrane.
Increased levels of cytosolic calcium and ROS can activate a large mitochondrial conductance channel called the mitochondrial permeability transition pore (MPT) (16). The MPT pore allows protons into the mitochondrial matrix, ablating mitochondrial membrane potential, uncoupling the electron transport chain, and inhibiting ATP production. Water also flows through the MPT pore, causing mitochondria to swell and rupture. If a large number of mitochondria within a cell activate the MPT pore, the cell will not be able to synthesize sufficient ATP, ion homeostasis will be lost, the cell will swell, the plasma membrane will rupture, and in many cases the cell will initiate cell death cascades through either necrotic, apoptotic, or autophagic pathways (103, 104).
4. Brain is particularly sensitive to ischemia
Different tissues differ in their sensitivity to ischemic injury, with brain being the most sensitive, then heart, kidney, liver, and lastly skeletal muscle, which is remarkably resistant to ischemia-reperfusion injury. Sensitivity to ischemic injury seems to relate to the capacity of a tissue to transition successfully to anaerobic metabolism, the capacity of its fuel stores, and its intrinsic resistance to oxidative stress (132). Brain is utterly dependent on oxidative metabolism and has very low levels of stored glycogen, in contrast to liver and skeletal muscle, for example. Brain also has lower levels of antioxidants such as superoxide dismutase, glutathione peroxidase (4), and heme oxygenase (68). Collectively, these attributes render the brain particularly sensitive to ischemic injury, with stroke being a leading cause of worldwide morbidity and mortality.
5. What does ischemic membrane injury look like?
The precise structural form of ischemic membrane injury is not known. Membrane permeability most likely occurs as a combination of effects related to calcium toxicity, calpain activation, cell swelling due to altered sodium/potassium homeostasis, oxidative damage, and in the case of cardiac myocytes, contraction-induced mechanical injury of a damaged plasma membrane. The heart cannot stop beating. Like skeletal muscle injury, ischemic membrane permeability is inferred by the egress and uptake of large macromolecules such as lactate dehydrogenase and albumin that are unable to cross an intact lipid bilayer. Thus we know the membrane is permeable, and permeable enough to allow high levels of macromolecular passage, but we do not know exactly what is the size, number, or disposition of the membrane breaches.
III. THE PROTEIN MACHINERY OF ACUTE MEMBRANE REPAIR
A. SNAREs
1. Evolution and structure
The SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) comprise three families of proteins that interact to form the core machinery of vesicle fusion: syntaxins, SNAPs (soluble N-ethylmaleamide attachment proteins), and VAMPs (vesicle associated membrane proteins). SNARE proteins have ancient eukaryotic origins and are present in protozoans, early unicellular eukaryotes, fungi, yeast, and animals, and are central players in the evolution of the eukaryotic endomembrane system (65–67).
a) snare complex and how vesicle fusion happens. Three proteins form a SNARE complex: two proteins from the target membrane, syntaxin (26) and SNAP (207), and one from the vesicle membrane, VAMP (295). Most SNARE proteins are tail-anchored transmembrane proteins, with the “business side” of the protein facing the cytoplasm, and only a short luminal or extracellular tail (see Figure 6). A common feature of all SNAREs is the presence of a cytoplasmic α-helical heptad repeat domain, consisting of ∼67 amino acids, often positioned just before the transmembrane domain. This α-helical sequence is referred to as the SNARE motif and facilitates interaction of the three SNARE proteins into a parallel coil-coiled structure (275). The quaternary α-helical complex formed by assembly of syntaxin, SNAP, and VAMP is extremely stable and resistant to heating (but not boiling) in sodium dodecyl sulphate (SDS) lysis buffer. These ternary complexes self-assemble to form a ring (50), zippering from the NH2 terminus to the COOH terminus to draw the opposing membranes of the vesicle and target membranes together for fusion (for recent reviews, see Refs. 210, 234).
Figure 6.
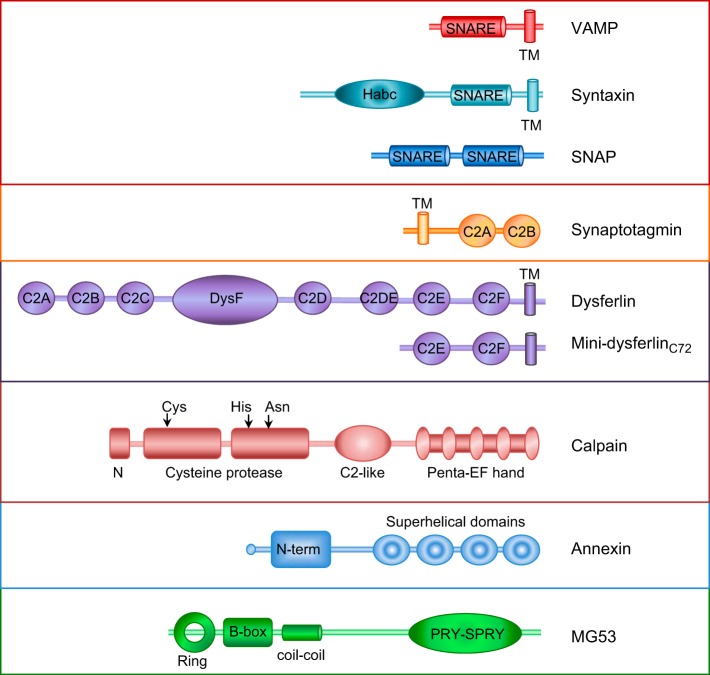
Schematic representation of the structural features of the protein families implicated in membrane repair.
The surface of exocytic vesicles is negatively charged due to polar phosphate headgroups, providing a natural vesicle-vesicle and vesicle-plasma membrane repulsion that must be overcome for membrane fusion. Thus membrane fusion is thought to be driven by the strong association of the SNARE components, the binding of positively charged calcium to neutralize the negative repulsion and repel water between the fusing membranes (128), and local disruption of the membrane curvature of the opposing bilayers by the transmembrane domains of the SNARE proteins (266; for recent reviews, see Refs. 127, 133).
b) synaptotagmins are late evolutionary arrivals to the snare complex. At neural synapses, synaptotagmins 1 and 2 trigger the calcium-activated fusion of neurotransmitter-containing vesicles (35, 38, 81) through interaction with SNARE vesicle fusion machinery (253). Synaptotagmins 1 and 2 localize to secretory vesicles and are type I transmembrane proteins with two, tandem cytoplasmic C2 domains and show calcium-regulated interaction with members of the SNARE complex. However, the precise mechanism by which synaptotagmins facilitate or accelerate calcium-regulated exocytic fusion remains the topic of intense debate. It is thought that calcium-binding to synaptotagmin triggers phospholipid binding of its C2 domains to the vesicle and plasma membrane, as well as binding to the SNARE complex, releasing complexins, which negatively regulate SNARE assembly (282).
Interestingly, phylogenetic analyses reveal that synaptotagmins originated in multicellular eukaryotes (61) evolutionarily postdating syntaxins (65, 66), suggesting core SNARE machinery could once function independently of synaptotagmin in primitive cells, or alternately worked in concert with a more evolutionary ancient predecessor. The synaptotagmin gene family rapidly expanded in metazoans, with correlation between multicellular complexity and the number of synaptotagmin paralogs particularly apparent in the evolution of green plants (248). In humans, there are 16 synaptotagmins (61) and 15 syntaxins (284), each displaying a distinct pattern of subcellular localization to mediate the trafficking of vesicular cargo between different intracellular destinations. Synaptotagmins-1 and -2 are essential for rapid, synchronous neurotransmission at vertebrate synapses and induce neonatal lethality in knockout mice (97, 209).
2. Role in membrane repair
a) parallels between membrane repair and synaptic exocytosis. Steinhardt et al. (267) significantly advanced our understanding of how membrane repair works, when they showed that membrane resealing utilized a process with parallels to synaptic neurotransmission. Confocal microscopy of damaged sea urchin embryos or unfertilized eggs showed that a membrane puncture induced the exocytic fusion of secretory yolk granules (27, 267). This injury-induced exocytic fusion was wholly dependent on calcium, not activated by other cations, and antagonized by magnesium, with each property also being a feature of synaptic exocytosis. Larger membrane disruptions required vesicle-vesicle fusion to form a patch, whereby steps of “vesicle-to-vesicle” fusion, and integration of fused “patches” into the plasma membrane, were both strictly dependent on extracellular calcium (179, 285). These studies also showed that successful membrane repair correlated with the degree of exocytic fusion and that membrane repair was blocked or severely inhibited by clostridial neurotoxins in sea urchin eggs (docked cortical granules), embryos (undocked cortical granules), and mammalian Swiss 3T3 cells (nonsecretory cells) (27, 267). The clostridial neurotoxins are proteases that specifically cleave SNARE proteins and induce paralysis in infected victims (29, 252, 314). Further experiments using recombinant fragments of synaptotagmins or SNAREs, or inhibitory antibodies recognizing synaptotagmins or SNAREs, collectively implicated SNARE proteins in the vesicle fusion of membrane repair (72, 291).
b) a role for synaptotagmin vii and lysosomal exocytosis for membrane repair? In 2001, Reddy et al. (229) reported that membrane repair in nonsecretory skin fibroblasts was mediated by synaptotagmin VII (SytVII) via calcium-activated lysosomal exocytosis (229). Membrane injury induced the calcium-dependent appearance of the luminal epitope of lysosomal associated membrane protein (LAMP-1) on the cell surface of wounded skin fibroblasts, and treatment with anti-SytVII antibodies, recombinant SytVII C2A, or anti-LAMP-1 antibodies impaired lysosomal exocytosis and membrane repair (229). A SytVII knockout mouse was then generated and showed normal growth and development, but developed an inflammatory myopathy with elevated creatine kinase and muscle weakness with other autoimmune symptoms, such as dermatomyositis and an antinuclear antibody response (47). Embryonic fibroblasts from SytVII knockout mice were shown to be defective in lysosomal exocytosis and membrane resealing after wounding (47). The authors later showed that loss of SytVII resulted in fewer lysosomal fusion events overall and the properties of the fusion events were different (126). In wild-type cells, calcium influx triggers lysosomal fusion events characterized by a small fusion pore and minimal diffusion of lysosomal transmembrane proteins into the plasma membrane. In contrast, SytVII knockout fibroblasts showed more complete lysosomal fusion events with merging of lysosome membrane contents with the plasma membrane, suggesting SytVII regulates and restricts fusion pore formation (126). Further studies are required to reconcile how these aberrations in lysosomal exocytosis relate to failed repair in SytVII null cells.
More detailed studies of the effect of recombinant domains of synaptotagmin I and VII on membrane repair showed that the C2A domain of SytVII had no effect on membrane resealing, but did impair the facilitated response to a second injury at the same site (258). Interestingly, treatment with recombinant C2B of SytVII inhibited both initial membrane resealing and the capacity of an injured cell to more readily repair a second injury. Similar inhibition of membrane resealing was observed with recombinant C2B of synaptotagmin 1. Inhibitory effects were dependent on the calcium sensitivity of the C2 domains, with no effects elicited by calcium-binding mutants of each domain. However, the authors stress that treatment with recombinant C2 domains of synaptotagmins likely indirectly impacts membrane repair by promiscuously binding SNARE machinery and target phospholipids (258).
3. Perspectives
Evidence suggests that lysosomes are not membrane repair organelles and do not directly contribute to the exocytic formation of “membrane patches” for lesion repair. Although lysosomal exocytosis was shown to occur in response to ballistics injuries in skeletal muscle cells, these fusion events occurred distal to the injury site, and lysosomal markers did not demark vesicles recruited and enriched at injury sites labeled by dysferlin (150). Similarly, zebrafish lysosomal membrane proteins did not accumulate at injury sites in zebrafish larval muscle cells, although lysosomes were observed to fuse with the plasma membrane when near an injury site (238). However, there is an expanding body of evidence that lysosomal exocytosis occurs in response to the acute elevation in intracellular calcium caused by membrane injury and that machinery of the late endosomal pathway play key roles in membrane repair. Specific roles in membrane repair for late endosomal machinery ESCRT (129, 250) and mucolipin-1 (49) are discussed in section IIIF. Exocytic fusion of late endosomes/lysosomes may function to reduce membrane tension, to deliver protein machinery required for vesicle formation and exosomal shedding (129, 250), as well as to release enzymes such as acid sphingomyelinase to activate endocytosis (280).
Collectively, there is a strong body of evidence showing that membrane repair is inhibited by clostridial neurotoxins, recombinant protein fragments of SNARE proteins and synaptotagmin C2 domains, and by antibodies that functionally inhibit SNAREs and synaptotagmins. Thus, although SNARE machinery is squarely implicated in the vesicle fusion of membrane repair, it is yet to be determined whether there is an ancient and preserved set of core SNARE machinery for membrane repair, or whether there have been evolutionary adaptations in the repair mechanism to best suit the types of injury encountered, and the available endogenous vesicle fusion machinery, in different cell lineages.
While the extremely high concentration of local calcium around a wound site is not altogether dissimilar from an active synaptic zone, or directly adjacent to the calcium-release channels of the triad junction in skeletal muscle, this level of intracellular calcium would be an unusual event in a nonsecretory cell and would certainly create a unique and very active local environment. The flood of extracellular calcium thought the breached plasma membrane will promiscuously initiate calpain-mediated proteolysis, calcium second messenger signaling, and calcium-regulated fusion of any nearby primed or “primable” vesicles. In the setting of a membrane injury where it is repair or die, the evolutionary pressure on the effectiveness of membrane repair means that each of these cascades must play an important and interrelated role. It is difficult to reason whether it is more likely that a core, evolutionary ancient membrane repair machine exists in all cells, or whether promiscuity in the fusion machinery and vesicle populations utilized for membrane repair is central to the survival response itself.
B. Dysferlin
1. Evolution and structure
Dysferlin is a large (∼240 kDa) tail-anchored transmembrane protein that bears the unique feature of seven tandem C2 domains within its large cytoplasmic domain, the most of any protein family. C2 domains are independently folding protein motifs comprised of ∼110–130 amino acids, originally identified in protein kinase C (202). C2 domains mediate lipid and protein binding, often regulated by coordination of calcium ions within a negatively charged binding pocket comprised of highly conserved acidic residues (usually aspartate) (51). The crystal structures of many C2 domains have been solved and feature a folded sandwich of two β-sheets, each containing four anti-parallel β-stands (87, 274). Clustered at the end of the β-sheet sandwich reside three variable connecting loops that, in the case of calcium-sensitive C2 domains, contain the highly conserved acidic residues that form the binding pocket for multiple calcium ions. The binding of calcium within this pocket directly facilitates membrane interaction (196), with the amino acid composition of the loop region shown to influence phospholipid selectivity for targeting specific membrane compartments (51).
a) dysferlin belongs to an ancient family of vesicle fusion proteins. There are six mammalian ferlin proteins: dysferlin (Fer1L1), otoferlin (Fer1L2), myoferlin (Fer1L3), Fer1L4, Fer1L5, and Fer1L6, each characterized by a cytoplasmic domain bearing between five and seven tandem C2 domains anchored by an extreme COOH-terminal transmembrane domain (for a recent review, see Ref. 151). Our phylogenetic studies have revealed that ferlins have ancient origins in eukaryotic biology (152). Ferlins are detected in all eukaryotic kingdoms, including unicellular phytoplankton and in protozoans, indicating origins predating evolutionary branching. Ferlins have not yet been identified in fungi or plants, suggesting they may have been lost from these evolutionary lineages. Invertebrate and vertebrate animal models of ferlin deficiency are united by pathologies linked to defective calcium-activated vesicle fusion (3, 18, 59, 205, 242). Thus it is proposed that ferlins are a family of calcium-binding vesicle fusion proteins for regulated exocytosis, with evidence suggesting more ancient evolutionary origins than the classical mediators of vesicle fusion, the synaptotagmins (see section IIIA) (151).
2. Localization
Dysferlin is expressed ubiquitously in mammalian tissues, with high levels in skeletal muscle and heart (9). Dysferlin localizes to the skeletal muscle plasma membrane, also called the sarcolemma (9, 218), to the invaginating t-tubule network (7, 135, 138, 163). Dysferlin has also been shown to localize to the apical plasma membrane of syncytiotrophoblasts (297), suggesting a role in polarized membrane trafficking in the placenta. Studies of dysferlin trafficking in transfected C2C12 mouse myoblasts reveal that dysferlin shuttles between the plasma membrane and the endo-lysosomal pathway (88).
3. Role in human disease
Dysferlin mutations underlie a form of autosomal recessive inherited muscular dystrophy, called limb girdle muscular dystrophy type 2B (LGMD2B) (22, 161). Dysferlinopathy is a late-onset form of muscular dystrophy, manifesting in older teenagers or adults, and is characterized by absence or marked reduction of dysferlin protein in the skeletal muscle of affected patients. Curiously, prior to their presentation, dysferlinopathy patients show no evidence for subclinical muscle weakness as children, with many patients reporting sporting distinction in their youth. This differs from other later onset muscular dystrophies and myopathies, in which there is often a long history of poor sporting performance and avoidance of strenuous physical activities. In dysferlinopathy, physically able teenagers often suffer an injury that is difficult to recover from, and begin to experience unexplained fatigue and muscle pain, followed by progressive muscle weakness. Unfortunately, once dysferlin muscular dystrophy manifests, the physical decline can be rapid, from being able-bodied to nonambulant (unable to walk) in 4–8 yr (200). It is not understood why dysferlin deficiency does not clinically affect young muscles of affected children yet results in severe weakness and muscle degeneration in adulthood, with some patients also presenting with mild cardiac involvement (143, 310).
4. Role in membrane repair
a) dysferlin plays a key role in calcium-dependent membrane repair. Dysferlin-deficient mice also exhibit a late-onset progressive muscular dystrophy (18, 32, 112). A seminal study by Bansal et al. in 2003 (18) revealed that muscle fibers lacking dysferlin did not show the same membrane fragility as dystrophin-deficient fibers (the basis of Duchenne muscular dystrophy, the most common human muscular dystrophy), and instead demonstrated defects in calcium-activated membrane resealing following laser injury. Control muscle fibers were shown to effectively reseal a laser-induced plasma membrane injury and exclude the styryl dye FM1-43 within 30 s, whereas dysferlin-null mouse muscle fibers showed increased and prolonged entry of FM1-43 up to 2 min following the laser injury. Indeed, the kinetics and magnitude of FM1-43 dye entry in injured dysferlin-null fibers resembled results from wild-type fibers damaged in the absence of calcium. Given the homology of dysferlin to the C. elegans protein Fer-1, previously shown to regulate calcium-activated vesicle fusion (3, 307), dysferlin was therefore proposed to be a key mediator of calcium-activated vesicle fusion for muscle membrane repair.
A role for mammalian ferlins in calcium-activated vesicle fusion was further strengthened by the discovery that otoferlin, the genetic basis of a form of inherited nonsyndromic deafness in humans (315), was due to defective calcium-activated auditory neurotransmission at the cochlear inner hair cell synapse (242). It remains debated whether the role of otoferlin in the cochlear relates solely to functions as a calcium-activated trigger for synaptic vesicle exocytosis, or whether its role may instead/also relate to endocytosis and recycling of synaptic vesicles to restock the ready releasable pool of neurotransmitter containing vesicles required for sustained, high-frequency firing of the highly demanding inner hair cell synapse (211).
Although dysferlin is expressed ubiquitously, the skeletal muscles are particularly affected by loss of dysferlin. This may relate to the specialized architecture of the skeletal muscle t-tubule membrane network and its vulnerability to eccentric stretch. Dysferlin is abundantly expressed within the t-tubule network, and dysferlin-deficient muscle fibers show major t-tubule abnormalities after a bout of in vivo lengthening strain injuries (135). Indeed, dysferlin-deficient fibers suffer significant damage after long strain injury, requiring satellite cell-mediated repair pathways to rebuild necrosing muscle fibers following a stretch protocol (236). In contrast, wild-type fibers readily survive a lengthening strain injury without evidence for necrosis, and without requiring satellite-cell mediated repair (236). These data suggest dysferlin is particularly important for repair and remodeling of t-tubule membranes.
b) dysferlin translocation to injury sites. Bansal et al. (18) studied the localization of dysferlin in myofibers injured via aspiration through an 18-gauge needle, using fluorescent dextran to demark injured fibers. Confocal microscopy revealed intensely labeled “patches” of dysferlin that appeared to correlate with potential sites of plasma membrane disruption, inferred by brightfield images showing small regions of thickened and nonuniform sarcolemma, visually discontinuous with an otherwise uniform section of myofiber sarcolemmal membrane, and consistent with a small repair patch. These regions showed reduced or absent labeling for constitutively expressed sarcolemmal proteins caveolin-3 and δ-sarcoglycan, consistent with recently repaired injury “patches” derived from nonsarcolemmal membrane sources. Dysferlin is also observed to intensely label the fine, meshlike network of longitudinal tubules of the t-tubule network that become vacuolized and injured with over-stretch (304).
Many groups have attempted to study the recruitment of heterologously expressed dysferlin to sites of membrane injury in cultured myotubes. Klinge et al. (138) suggested that an NH2-terminal EGFP-dysferlin fusion protein showed calcium-dependent and injury-dependent plasma membrane enrichment in C2C12 myotubes injured by rolling glass beads. Dysferlin enrichment appeared broad and generalized over large areas of a myotube, in stark contrast to the tightly refined patches of endogenous dysferlin recruited to proposed injury sites in mature skeletal myofibers reported by Bansal et al. (18). This may reflect immaturity of the myogenic model employed by Klinge et al. (138), comparatively larger areas of membrane injury induced by the rolling beads, or perhaps disruption of normal dysferlin behavior through epitope-tagging. Arguably however, the precise site of membrane injury could not accurately be determined in either case, and therefore, it is difficult to qualify whether areas of dysferlin enrichment represent injury sites, or not.
Live cell imaging experiments in transfected murine C2C12 myoblasts indicated that EGFP-dysferlin is recruited to sites of laser injury or needle microinjection only when coexpressed with the muscle-specific membrane repair cofactor mitsigumin-53 (MG53, discussed in detail in sect. IIIE) (42). However, zebrafish dysferlin expressed as a COOH-terminal fusion with monomeric teal fluorescent protein showed rapid recruitment to injury sites during in vivo imaging of laser-injured zebrafish myofibers, and zebrafish do not express a MG53 paralog (238). Thus whether MG53 is required for injury recruitment of dysferlin in muscle cells remains unclear. The sophisticated in vivo imaging experiments described by Roostalu and Strahle (238) revealed that a short dysferlin COOH-terminal fragment (the extracellular domain of 22 amino acids, transmembrane domain, and only 29 of the ∼2,000 amino acids of the cytoplasmic domain) was sufficient to confer targeting to recruited repair membranes. In contrast, truncated dysferlin NH2-terminal domains were unable to be effectively recruited, suggesting cellular targeting of dysferlin by its transmembrane domain to appropriate membrane compartments is vital for its mobilization and recruitment to injury sites.
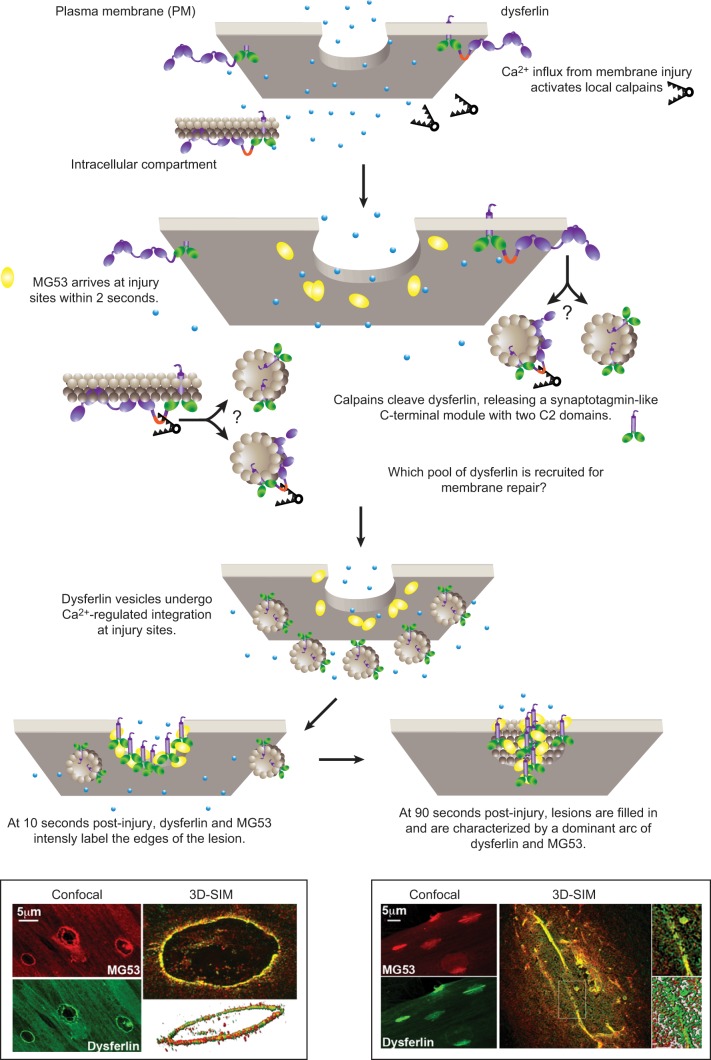
Lek et al. (150) recently developed a ballistics model of membrane injury in cultured human myotubes, producing readily identifiable enface injuries, suitable for high-resolution imaging. Three-dimensional structured illumination microscopy (3D-SIM) was used to reconstruct the recruitment of dysferlin to injury sites (150). Super-resolution imaging of injury sites resolved the rapid recruitment of dysferlin-containing cytoplasmic vesicles to sites of membrane injury within 10 s, undergoing calcium-dependent integration into plasma membrane compartments decorated by MG53 (Figure 7). This process is surprisingly consistent with the mechanism proposed by Steinhardt et al. (267) nearly 20 years ago entitled: “membrane repair occurs via a process analogous to synaptic exocytosis.”
Figure 7.
A cartoon depicting the potential role of dysferlin-mediated vesicle fusion in membrane repair. Membrane injury causes a local influx of calcium and activation of calpains. MG53 (40) shows diffuse enrichment at injury sites within 2 s of membrane injury in a calcium-independent manner (150). The signal to activate recruitment of MG53 to injury sites is not clear, but may relate to its role as a ubiquitin ligase to target substrate(s) damaged as a consequence of the membrane injury. Dysferlin is not detected at injury sites until 10 s postinjury, a delay we attribute to an intermediary step involving calpain cleavage. Activated calpains cleave dysferlin within a motif specifically encoded by alternately spliced exon 40a (230). As dysferlin may only be detected at injury sites with antibodies recognizing COOH-terminal epitopes, and not several antibodies to NH2-terminal or central domains (150), data suggest the COOH-terminal cleaved fragment termed mini-dysferlinC72 is the form specifically recruited to injury sites. It remains uncertain whether full-length dysferlin is also present at injury sites but is structurally precluded from antibody labeling and whether the dysferlin recruited for membrane repair is derived from plasma membrane or intracellular membrane compartments. Super-resolution 3-dimensional structured illumination microscopy (3D-SIM) reveals dysferlin-laden vesicles undergo calcium-dependent integration at injury sites (150). At 10 s postinjury, dysferlin and MG53 form a lattice that intensely labels the edges of the lesions. Lesions expand and are filled in by a dysferlin and MG53 lattice. At 90 s postinjury, filled lesions are characterized by a dominant arc of dysferlin and MG53 labeling we believe represents the original edges of the lesion that have been drawn together by cytoskeletal motors for resealing. [3D-SIM images from Lek et al. (150), with permission from The Journal of Neuroscience.]
Immediately following ballistics injury, dysferlin specifically and intensely labeled the exposed phospholipids encircling the ballistics injuries (10 s post injury) (150). At later time points, dysferlin formed an intricate lattice (with MG53) labeling the broader surrounds of the membrane lesions (20–60 s post injury). One to two minutes after injury, dysferlin labeled a bright arc of two closely opposed membranes lying in a bed of dysferlin and MG53 lattice, positioned parallel to the long axis of the cultured myotubes (Figure 7). These observations were consistent with a model whereby dysferlin initially labels the periphery of the lesion, forms a repair lattice that infiltrates the lesion surrounds as new membrane is delivered, and cytoskeletal motors “zipper” the ballistics lesions together by drawing opposing membranes together lengthwise along the long axis of the myotube.
c) injury-activated calpain cleavage of dysferlin: emergency production of a synaptotagmin-like vesicle fusion module for membrane repair? Perplexingly, dysferlin could only be detected at injury sites using an antibody recognizing the extreme COOH terminus of the dysferlin cytoplasmic domain, Hamlet-1, and not with three other anti-dysferlin antibodies recognizing more NH2-terminal epitopes (150). Biochemical analyses subsequently revealed that dysferlin was cleaved by activated calpains with membrane injury, releasing a COOH-terminal fragment of 72 kDa, termed mini-dysferlinC72. Interestingly, mini-dysferlinC72 bears the last two most ancestrally conserved C2 domains and transmembrane domain (152), with structural parallels to the classical vesicle fusion proteins, the synaptotagmins. Results therefore suggest that it may not be full-length dysferlin that is recruited to injury sites in cultured human myotubes, but a COOH-terminal fragment of dysferlin, mini-dysferlinC72.
We have subsequently shown that calcium-dependent cleavage of dysferlin is mediated by the ubiquitous calpains (calpains-1 and -2) via a cleavage motif encoded by an alternately spliced exon, exon 40a (230). Thus not all dysferlin isoforms may be cleaved by calpains in response to injury. Interestingly, other members of the ferlin family are also cleaved by calpains to release similar COOH-terminal modules (230). Evolutionary conservation of this feature implies that calpain cleavage of ferlins bestows an important functional modification in settings of intense calcium signaling.
Interestingly, while dysferlin transcripts bearing exon 40a are abundantly expressed in many human tissues (40–60% of all transcripts in kidney, lung, liver, placenta, and pancreas) (230), somewhat counterintuitively, only ∼15% of dysferlin transcripts in skeletal muscle contain exon 40a (220, 230). Thus not all dysferlin isoforms in skeletal muscle can be cleaved by calpains. Further studies are needed to clarify the respective roles of full-length dysferlin (without exon 40a) and cleaved mini-dysferlinC72 for membrane repair of skeletal muscle and other tissues.
d) a patient mini-dysferlin restores membrane repair, but not dystrophic pathology. Further evidence that cleaved mini-dysferlinC72 plays a specialized role in membrane repair has been provided serendipitously, through studies of a naturally occurring truncated dysferlin based on a patient with a genomic deletion within the dysferlin gene, that is very similar to calpain-cleaved mini-dysferlinC72 (140). In this patient, a genomic region encompassing exons 2–40 of dysferlin are deleted, and the patient employs a cryptic splice site to express a truncated dysferlin with 13 amino acids at the NH2 terminus derived from intronic sequences, followed by exons 41–55. Calpain-cleaved mini-dysferlinC72 bears approximately seven residues of exon 40a (although calpains do not strictly cleave at one site, and may cleave either side of their preferred site), followed by residues encoded by exons 41–55. The patient presents with a mild-moderate dysferlinopathy and transgenic expression of the patient mini-dysferlin in dysferlin-null muscle fibers restored normal membrane repair (140). These data support our proposal that calpain-cleaved mini-dysferlinC72 is an important mediator of membrane repair. However, transgenic expression of the patient mini-dysferlin did not prevent development of a dystrophic pathology in dysferlin-null mice (140, 166). Thus defective membrane repair does not appear the sole factor underlying the pathology of dysferlinopathy.
e) which pool of dysferlin is cleaved? A recent study created a transgenic mouse model expressing dysferlin (without exon 40a) with an extracellular pHluorin tag (172). pHluorin is a genetically modified form of GFP that is pH sensitive (184), in this case showing reduced fluorescence in acidic compartments. McDade et al. (172) showed that immediately following membrane injury, dysferlin-pHluorin fluorescence diminished in regions surrounding, and distal to, the injury site, and cytoplasmic accumulations of dysferlin formed in regions distal to the injury site. These results suggest dysferlin may initially be endocytosed into acidic compartments in response to membrane injury. Is it endocytosed dysferlin that is cleaved by calpains, then vesicles laden with cleaved mini-dysferlinC72 exocytosed at injury sites (Figure 7)?
5. Perspectives
It is clear that dysferlin plays a key role in membrane repair. However, exactly what dysferlin does to mediate membrane repair is only beginning to be teased out. From the perspective of the pathogenesis of dysferlinopathy, collective evidence suggests defective membrane repair may be only one contributing factor to disease pathology. Dysferlin deficiency also affects the trafficking and signaling of growth factor receptors (71) and adhesion molecules (256). Muscle injury may therefore present a “perfect storm” for dysferlinopathy patients, where a defective response to an acute membrane injury collides with a static defect in day-to-day trafficking of dysferlin-specific cargo, affecting muscle, vascular, and immune cells and producing a poor regenerative environment. Given the late presentation of dysferlin disease, one can only assume there are intrinsic differences in requirements for dysferlin-dependent trafficking and membrane repair pathways between growing muscle fibers of children versus fully mature adult myofibers.
Our recent discovery revealing activated calpains specifically cleave dysferlin in response to membrane injury provides the first step linking membrane repair roles separately established for dysferlin and calpains (discussed in detail in sect. IIIC). It remains to be experimentally determined whether mini-dysferlinC72 possesses specialized vesicle fusion activity that is important for membrane repair, and whether dysferlin-laden cytoplasmic vesicles interact with classical SNARE fusion machinery. Also yet to be determined is the role of the cleaved dysferlin NH2-terminal domain. Modal functions of different dysferlin C2 domains makes sense in light of the evolutionary preservation of each of the ferlin C2 domains, that are highly divergent from one another, but very similar to the analogous C2 domain in other ferlins. This tells us that each C2 domain is functionally specialized.
Endocytosis, calpain cleavage, then reexocytosis seems a labored path for a rapid emergency response. However, evidence that dysferlin may participate in both endocytic and exocytic pathways during the membrane repair response is consistent with the dual exocytic and endocytic recycling roles proposed for otoferlin in auditory neurotransmission (211, 242). Determination of the nature and the cargo contained within dysferlin-laden vesicles recruited to injury sites may provide valuable clues: are these purely a source of membrane lipids to patch a hole, or are soluble and transmembrane proteins codelivered that play an integral role in the repair and remodeling process?
C. Calpains
1. Evolution and structure
Calpains are an ancient family of thiol-proteases, present in protozoa, plantae, and eukaryota kingdoms (62, 84). The ubiquitous calpains, calpain-1 and calpain-2, typically exist as heterodimers, with a large catalytic subunit of ∼80 kDa and a smaller regulatory subunit of 28 kDa (for comprehensive review, see Ref. 102). The large catalytic subunit of calpain consists of four different domains (115, 270). Domain I is an α-helical domain comprised of 10–20 amino acids and is divergent among calpain orthologs and paralogs; with some isoforms bearing Zn-finger or transmembrane helices within domain I (263). Proteolytic removal of this NH2-terminal helix plays a role in the activation of some calpains (137). Domain II forms the catalytic core, rich in essential catalytic residues cysteine, histidine, and asparagine. Domain III consists of an eight β-strand sandwich with structural resemblance to a C2 domain, and thought to confer calcium and phospholipid binding. Domain IV bears five tandem calcium-binding EF hand domains (33, 159). The presence of a calmodulin-like penta-EF-hand module characterizes the “typical calpains” such as ubiquitous calpain-1 and -2 and occurred late in calpain phylogeny, present only in metazoans (animal lineage). Protozoan, plant, and fungal calpains do not possess an EF-hand containing domain IV; moreover, several “atypical” mammalian calpains also lack this domain (62, 102).
a) calpains selectively modify target substrates. Calpains target selective substrates and use complex substrate recognition motifs dictated both by primary and secondary protein structure (162, 294). Calpains are not terminal degradative enzymes but, rather, selectively modify their target substrate. More than 100 proteins have been identified as calpain substrates; many are cytoskeletal proteins, such as vimentin, talin, desmin, troponin, dystrophin, and spectrin, but substrates also include receptors, ion channels, transcription factors, signaling proteins, and enzymes (102).
2. Expression and localization
There are 14 genes encoding large catalytic calpain subunits in humans. Calpain-1 and -2 are ubiquitously expressed and are also referred to as micro (μ)- and milli (m)-calpain, respectively, thus named according to their activating calcium concentration for proteolytic activity: 10–50 μM for μ-calpain and 0.25–0.35 mM for m-calpain. Many calpains show tissue-specific expression, for example, calpain-3a in skeletal muscle, calpain-6 in placenta, calpain-8 and -9 in the gastrointestinal tract, and calpain-11 in testis (see reviews in Refs. 102, 276).
The ubiquitously expressed calpain-1 and -2 are the best studied of the calpains. In cultured cells, calpains show cytosolic and membrane localization and are widely described to translocate to the plasma membrane in response to cellular signaling by calcium (see, for example, Refs. 99, 100) and with growth factor receptor activation (153, 255). The localization of calpains has been widely studied in skeletal muscle fibers, with most labeling detected within the myofibrillar apparatus (142). More recent studies in skinned skeletal muscle fibers show that calpain-1 can freely diffuse out of the myofibrillar compartment at resting calcium levels, but becomes tightly bound within skinned fibers with increased cytosolic calcium (20 μM) (195). Muscle cells present a complex environment when considering calpain activity; the calcium transients of muscle contraction would theoretically provide sufficient cytosolic calcium to activate proteolytic activity of μ-calpain but, fortunately for muscle fibers, does not. It is therefore proposed that calpain activity in skeletal muscle is tightly regulated by calpastatin (an abundantly expressed natural and specific inhibitor of calpain-1 and -2), their localization, and accessibility to their discreet repertoire of protein substrates.
3. Role in human disease
Calpains play a key role in development, with targeted knockout of calpain-2 resulting in embryonic lethality due to an implantation defect (11, 79). Although calpain-1 and -2 cleave many of the same substrates in vitro, knockout of calpain-1 produces viable mice that are morphologically normal but show defects in platelet function (13). This highlights different functional roles for calpain-1 and calpain-2, whereby calpain-1 is unable to compensate for calpain-2 deficiency during early embryonic development.
Disturbances in calpain behavior are implicated in numerous human pathologies (see review and articles within Ref. 317). The crux of the problem in most cases stems from calpain “overactivity” due to aberrant calcium handling and is often associated with pathologies of membrane injury, such as muscular dystrophy (262, 289), cardiac ischemia-reperfusion injury (124), traumatic brain injury (243) and stroke (17, 64), Alzheimer's disease (273), multiple sclerosis (259, 271), and cataract formation (31). Moreover, calpains are becoming increasingly implicated in cancer biology, with roles in pro-survival or apoptotic decision-making, and cytoskeletal remodeling and migration, highly relevant to tumorigenesis and metastasis (268).
A polymorphism within intron 3 of calpain-10 has been implicated as a susceptibility factor for type 2 diabetes (114). However, a direct role for calpains in human disease is best described by a monogenic form of autosomal recessive limb girdle muscular dystrophy (LGMD2A) due to mutations in the gene encoding calpain 3a (CAPN3) (233). The mechanism underpinning LGMD2A remains relatively poorly understood. Calpain-3, although abundant, is extremely labile and partitions to the myofibrillar compartment of skeletal muscle (194). The current paradigm for the pathogenesis of LGMD2A centers upon a role for calpain-3 as a “sarcomeric remodeler” (24, 283), although different studies have separately implicated roles in muscle maturation (264), myonuclear apoptosis (15), and maintenance of the costameric dystrophin-associated complex via targeted cleavage of filamen-C and δ- and γ-sarcoglycan (107).
4. Role in membrane repair
a) activation of calpain is vital for the acute membrane repair response. Interestingly, modulating calpain activity bestows both a blessing and a curse for cellular survival following membrane injury. Although treatment with calpain inhibitors improves physiological outcomes and the degree of cell and tissue death following pathologies of membrane injury such as ischemia-reperfusion injury in the heart, stroke, and traumatic brain injury (317), activation of calpain is vital for the survival of an acute membrane injury (183).
In 1991, Xie and Barrett (313) presented evidence showing that calcium-activated proteases facilitated membrane resealing of transected mammalian neurites; calpain inhibitors strongly inhibited resealing of severed axons, suggesting a requirement of calpain activity for neurite sealing. A role for calpains in neurite sealing was confirmed by Godell et al. (101), who demonstrated that application of exogenous calpain restored the resealing capacity of crayfish medial giant axons (MGAs) in calcium-free media, and could also induce sealing in transected squid giant axons (GAs) that otherwise do not seal (with or without calcium). Godell et al. (101) showed that both crayfish MGAs and squid GAs recruited vesicles to the transected end of the axon. In the presence of calcium, vesicles recruited to the end of transected crayfish MGAs fuse to form a dye-impermeable barrier, whereas vesicles in squid GAs do not. Treatment of transected MGA neurites with calpain inhibitors did not attenuate vesicle formation or recruitment, but prevented their fusion, in some cases inducing the formation of abnormally large vesicles that failed to fuse with the cut axonal end. The authors therefore proposed that calpains enhanced the fusion of recruited vesicles to reseal transected neurites and suggested squid GAs possess all of the machinery for effective resealing, although must lack sufficient active calpain to facilitate vesicle fusion.
Calpains have also been shown to be vital for membrane resealing of mammalian somatic cells. In the acute setting of membrane scrape-injury, treatment with calpain inhibitors markedly impairs cell survival of immortalized mouse embryonic fibroblasts (MEFs) and primary human skin fibroblasts and neonatal rat cardiomyocytes (182, 183). Using mouse embryonic fibroblasts with targeted knockout of the CAPNS1 regulatory subunit of calpain-1 and -2 (11), Mellgren et al. (183) were able to show that calpain-1 and/or calpain-2 were required for acute membrane repair (183). In contrast, calpain-3a did not contribute to survival after scrape damage of skeletal myoblasts or to acute repair of a laser damaged membrane in skeletal myotubes derived from CAPN3 knockout mice (182).
b) the puzzle: how do calpains underpin the calcium-dependence of membrane repair? Significantly, these studies suggested that calpains were primary mediators of the calcium dependence of membrane repair. CAPNS1-deficient MEFs did not show improved membrane repair outcomes in the presence of calcium, in contrast to wild-type MEFs, or virally rescued CAPNS1 knockout MEFs. The calcium dependence of membrane repair is a central dogma within the membrane repair field, historically attributed to a requirement for calcium-dependent exocytosis (27, 267) and vesicle-vesicle fusion to facilitate membrane “patching” of plasma membrane disruptions (181, 285). Thus how do calpains regulate calcium-dependent exocytic fusion underpinning the membrane repair paradigm?
A role for calpain-1 and -2 in membrane repair of adult cardiomyocytes was recently confirmed using a cardiac-specific knockout of CAPNS1 (281). Cardiac CAPNS1 null mice showed normal heart histology and function at baseline, although developed symptoms of congestive heart failure following a surgical protocol to constrict the aorta and induce pressure overload to the heart. Cardiac CAPNS1-null mice showed increased levels of fibrosis, poorer cardiac contractility, and greater permeability to Evan's Blue dye uptake following the hemodynamic stress protocol. Isolated cardiomyocytes subjected to the two-photon laser damage assay (18) showed a significant defect in plasma membrane repair, although whether CAPNS1-null cardiomyocytes still possessed calcium-dependent membrane repair was not examined (281).
Although calpain is known to cleave numerous substrates, the functional specialization that calpain cleavage imparts upon target substrates is largely unknown, with few exceptions. Recent results from our own research illuminate dysferlin as a specific substrate of injury-induced calpain activation, with a clear link to membrane repair. Since dysferlin is proposed to be the “calcium-sensing vesicle fusion protein” for exocytic membrane repair (18, 181), could calpain cleavage of dysferlin to release a synaptotagmin-like module, mini-dysferlinC72, explain how calpains may regulate the exocytic fusion of membrane repair?
5. Perspectives
Calpain inhibition is being explored as a therapeutic strategy in numerous pathologies (see Ref. 44). The challenge with emerging calpain-inhibitor-based therapies is to specifically target the appropriate calpain and to define the therapeutic window that permits the acute resealing response, but protects against the uncontrolled calpain activity that follows, leading to a negative spiral of deleterious calpain-mediated degradation of cellular proteins that results in cytotoxicity.
The superphysiological activating calcium concentration for m-calpain has puzzled biochemists for the last two decades, and much effort has been devoted to deciphering molecular modifications that occur in vivo to lower its required calcium concentration for activation. Membrane injury seems tailor-made for m-calpain; full activating calcium concentrations, discrete substrate specificity, and a repertoire of protein substrates that one can easily fit into a model for membrane repair: 1) dysferlin and voltage-gated calcium channels for vesicle fusion; 2) matrix proteins and submembraneous cytoskeleton to allow delivery of exocytic vesicles for patch-repair as well as internalization of endocytic vesicles to remove oxidized lipids and damaged receptors; 3) dystrophin, talin, and spectrin to severe adhesion anchors and improve membrane flexibility to allow remodeling and resealing of large injuries; and 4) annexins to compartmentalize and order the resulting mish-mash of lipid membranes delivered in the emergency response to restore membrane integrity.
On balance, several lines of evidence are converging to triangulate calpains as central regulators of membrane repair. One begins to question whether dysferlin is indeed a master regulator of membrane repair, or one of several effectors activated by calpain during its boisterous response to the unregulated influx of calcium that uniquely characterizes the local environs of a membrane injury.
D. Annexins
1. Evolution and structure
The annexins are an ancient family of calcium-sensitive phospholipid binding proteins (98). There are 12 annexins in chordates (A1-A11, A13), with more than 500 members found across all major eukaryotic phyla (190). Like dysferlin (152), annexins are also present in primitive unicellular protists and are absent from prokaryotes and yeast (190).
There are three major classes of structurally diverse calcium-binding domains found in eukaryotic proteins: the β-sheet sandwich of the C2 domain, the helix-loop-helix module of EF hands, and the superhelical annexin calcium binding domain. Annexins bear a conserved domain structure of a variable NH2-terminal domain and a COOH-terminal core domain of four, tandem, calcium-binding repeats (eight in annexin VI) (98). Each repeat is ∼70 amino acids in length, forming a superhelix of 5 inter-wound α-helices (158). The four superhelical domains of annexin pack into a compact disc, slightly convex on one side and slightly concave on the other side. The calcium-coordinating residues lie on the convex face of the disc and are highly evolutionarily conserved (190). Thus the annexin core functions as a four-domain module that binds negatively charged phospholipids via its calcium-bound convex surface, with its concave surface facing the cytoplasm.
The annexin NH2-terminal domains vary in length and are thought to lie on the concave side of the core module where they confer specificity for different binding partners. In the case of annexin A1, the NH2-terminal domain was shown to be buried within the core of the molecule and released in a conformational shift with calcium binding (241). Thus calcium coordination by annexin A1 simultaneously mediates phospholipid binding of the convex face, and, effector interaction by the NH2 terminus on the cytoplasmic concave face. For annexins A1 and A2, the unique NH2-terminal domains also convey specificity for binding the EF-hand accessory proteins S100A10 (annexin II) and S100A11 (annexin I) (156).
2. Expression and localization
Many annexins are ubiquitously expressed (A1, 2, 4, 5, 6, 7, 11), highly abundant proteins, although several annexins show tissue-specific expression; for example, A3 in neutrophils, A8 in placenta and skin, A9 in the tongue, and A10 and A13 in the gastrointestinal tract (98). The term annexin is derived from annexare (Latin) and annexer (French) meaning to join, to connect, to attach; the primordial role of all annexins is thought to relate to their capacity to aggregate and organize membranes in response to calcium.
Annexins generally show a cytoplasmic distribution, but partition to discrete membrane compartments upon calcium signaling. Studies of GFP-annexin fusion proteins have revealed that different annexins target different subcellular membrane compartments with calcium signaling, and indeed, respond to different activating calcium concentrations (78, 188). Annexins A1 and A2 shuffle between plasma membrane and endosomal compartments (170, 231), with emerging evidence for extracellular activities in anti-inflammatory and anticoagulation pathways (108, 214). Annexin 5 localizes to several different compartments of the biosynthetic pathway, including the endoplasmic reticulum, Golgi, late endosomes, and nuclear envelope (21, 73, 225). Annexin A5 is also widely used as a marker of apoptotic cells via its specificity for calcium-dependent binding to exposed phosphatidlyserine (139).
3. Role in membrane repair
Annexins are becoming widely implicated in different aspects of membrane compartmentalization, stabilization, and remodeling associated with membrane repair (76). Annexin A1 interacts with dysferlin (154) and was the first of the annexins to be implicated in membrane repair (176). Annexin A1 translocates to the plasma membrane with injury (14, 176), and an anti-annexin A1 blocking antibody, a small peptide inhibitor, and a calcium-binding annexin A1 mutant were each shown to inhibit resealing in HeLa cells subjected to mechanical scrape injury or laser irradiation (176). Levels of annexin A1 are upregulated in patients with muscular dystrophy (304), and annexin A1 labels vacuolized longitudinal membranes of the t-tubule network (a response to stretch injury) in patients with muscular dystrophy (304) and statin myopathy (302).
a) annexins incrementally respond to different degrees of calcium influx. Subsequent studies of SLO perforated HEK293 cells have revealed unique interplay between annexins 6 and 1 following membrane injury (14, 219). Annexin 6 shows higher sensitivity to elevations in intracellular calcium, and rapidly and reversibly translocates to the plasma membrane in cells suffering minor SLO perforation, inducing local hot spots of elevated calcium in the range of 5–10 μM. Annexin 6 specifically targets these injured regions of the plasma membrane and is encapsulated together with SLO perforated membranes into microvesicles that are then shed by the cell. With more severe SLO perforation, cytosolic [Ca]i exceeds 10 μM and activates the less sensitive annexin A1, which translocates to the nuclear envelope and plasma membrane and is associated both with microvesicle shedding of perforated membranes as well as internalization of ceramide platforms (14). The careful work of this research group has revealed cooperative roles of annexins A1 and A6 that can incrementally respond to different degrees of membrane damage according to local elevations in [Ca]i, and either package small lesions into shed microvesicles, or alternately internalize larger areas of membrane damage demarked by ceramide plaques (76).
b) annexin a5: a shield for injury sites? A recent study has shown that calcium-dependent binding of annexin A5 to exposed phosphatidylserine plays a biological role in the membrane repair response of damaged perivascular cells (37). Annexin A5 is known to self-assemble into trimers that interconnect to form two-dimensional structural arrays once bound to negatively charged phospholipids (206). This property plays an important role in its anti-thrombolytic and anticoagulant activity (237, 278, 286). Maternal anti-annexin A5 autoantibodies that disrupt this crystalline annexin A5 anticoagulant shield that covers the placenta is thought to be the basis for thrombosis and pregnancy loss in human anti-phospholipid syndrome (226).
Annexin A5 displays rapid recruitment to injury sites in damaged C2C12 myoblasts (40) and perivascular cells (37). Moreover, exogenous application of recombinant annexin A5 improves membrane repair in wild-type perivascular cells and rescues the membrane repair defect in annexin A5 null perivascular cells (37). Through design of an annexin A5 mutant unable to form two-dimensional arrays from assembled trimers, Bouter et al. (37) were able to demonstrate that formation of crystalline arrays is crucial for the role of annexin A5 in membrane repair.
c) annexin a6 participates in skeletal muscle membrane repair. Intravital imaging of annexins in the zebrafish model of sarcolemmal membrane repair also identified a role for annexin A6 (238). Annexin A6 showed immediate (10–20 s), dysferlin-independent translocation to injury sites, temporally preceding recruitment of annexin 11a (40 s), annexin 2a (60–80 s), and annexin 1a (200–240 s). This is consistent with the temporal progression of annexin recruitment to injury sites in SLO-treated HEK293 cells (219).
Morpholino knockdown of annexin A6 resulted in similar, though more mild, symptoms to dysferlin morphants, with a curved trunk, myofibrillar misalignment, and a reduction in birefringence (238). Double knockdown of both dysferlin and annexin A6 caused a severe phenotype, with all animals presenting with a curved trunk, severe cardiac edema, myofibrillar disorganization, and almost absent birefringence (238). Knockdown of annexin A6, dysferlin, or both prevented injury-activated accumulation of annexin A1, and slowed recruitment of annexin A2, with more profound effects observed with the double knockout. Collectively, results suggest that dysferlin and annexin A6 are independently recruited to injury sites, and work cooperatively to facilitate the subsequent accumulation of slower responding annexins A1 and A2. These data are supported by studies in human myotubes, where dysferlin rapidly recruits to injury sites and specifically labels the edges of the lesion, whereas annexins A1 and A2 do not show enrichment until 30 s postinjury, and do not specifically label injury sites but instead show more generalized enrichment in a large zone around the ballistics lesion (150).
Recently, annexin A6 has been identified as a potential disease modifier of muscular dystrophy, which is characterized by a high degree of pathological variability in human patients. A truncated version of A6 was found in a genome-wide scan of mice, and this abnormal A6 was shown to interfere with membrane repair by disrupting the recruitment of normal A6 to disruption sites (277).
4. Perspectives
The ancient phylogeny of the annexins, their high abundance, and their ability to stabilize and order phospholipid membranes makes them good candidates as primordial mediators of a membrane repair response. It makes sense that cells will activate and employ different coping mechanisms, depending on the location and extent of the membrane injury, whether that be rapid shedding of microvesicles or internalization of larger sections of the bilayer with more substantial damage. Ceramide accumulation at annexin A1-labeled injury sites, or crystalline annexin A5 arrays may function threefold: to provide a mechanism for the cell to recognize a damage site and target endocytic machinery, to provide a scaffold to aide in the recruitment and assembly of other repair and remodeling factors, and, more intriguingly, perhaps to provide a temporary shield to delay an immune or apoptotic response to allow time for repair.
Although calcium signaling may provide the ultimate “membrane injury” signal to activate a tailored response by different cytoplasmic annexins in many cells and tissues throughout the body, it is difficult to reconcile how such a mechanism works effectively in the muscle myoplasm. The cytoplasm of contractile muscle cells is routinely flooded with extraordinarily high levels of calcium during contraction; ∼1–3 μM cytoplasmic calcium with ∼100 μM locally at the triad junction (23, 148). These cytoplasmic calcium concentrations would rarely be encountered in other cell types, except in the local environs of specific calcium signaling or a small membrane rupture. Perhaps it is this specific property of muscle that confounds an ancestral membrane repair response, which perhaps requires further refinement by muscle-specific effectors such as MG53.
E. TRIM72/MG53
1. Evolution, structure, and localization
Mitsigumin-53 (MG53) was first identified as part of an immunoproteomic study to derive monoclonal antibodies against skeletal muscle triad proteins, mitsigumin being the Japanese word for triad. MG53 is highly expressed in skeletal and cardiac muscle and immunolocalizes to the t-tubule/sarcoplasmic reticulum triad junction in murine muscle (308), with more variable cytosolic, sarcolemmal, and t-tubule labeling in human skeletal muscle fibers (304). MG53 is a member of the TRIM family of E3 ubiquitin ligases and is also known as TRIM72.
There are more than 70 TRIM family proteins in mammals, thus named due to their characteristic NH2-terminal tripartite motif, a RING finger domain conferring ubiquitination activity, one or two zinc-binding B-box domains; TRIM-family specific sequences thought to mediate protein-protein interactions, and a coiled-coiled domain that mediates hetero- and homo-oligomerization (130, 232). TRIM proteins play diverse roles in development, differentiation, oncogenesis, and immunity (175, 197, 208). Phylogenetic studies of TRIM-family specific B-box sequences reveal the presence of this domain in protozoa and unicellular eukaryotes, but the tripartite motif that characterizes the TRIM family E3 ligases is exclusive to multicellular metazoans (249).
The NH2-terminal tripartite motif is generally thought to collectively mediate E3 ligase activity of TRIM proteins, whereas the COOH-terminal motif is though to confer substrate specificity and varies throughout the TRIM protein family. MG53 (TRIM72) is a group 2 TRIM protein bearing a COOH-terminal PRY-SPRY domain (249). This class of TRIM proteins are evolutionary newcomers, present only in chordates and showing rapid gene expansion in humans. The PRY-SPRY domain of MG53 has been crystallized, revealing a compact globular structure characterized by a convex surface on one side and a concave surface on the other that includes a large binding pocket (212).
2. Role in human disease
a) mg53. MG53 has not been implicated in human muscle disease but shows upregulation in patients with muscular dystrophy (304). Recently, MG53 has been implicated in the metabolic syndrome that results in type II diabetes and insulin resistance (261). Levels of MG53 were elevated in animal models with insulin resistance and metabolic syndrome, as well as in obese humans. Upregulation of MG53 in skeletal muscle via transgenic overexpression caused whole body insulin resistance and metabolic syndrome, whereas mice deficient in MG53 were protected from the metabolic effects of high fat feeding. Biochemical studies showed that MG53 overexpression induced ubiquitination and degradation of the insulin receptor and the insulin receptor substrate IRS1, therefore functioning as a muscle-specific negative regulator of insulin signaling (261).
b) other trim family proteins. Mutations in several other TRIM-family ubiquitin ligases cause different forms of inherited disorders in humans. MID1 (midline-1) causes Opitz Syndrome (223), and TRIM37 causes Mulibrey (muscle-liver-brain-eye) nanism (12); both are developmental disorders affecting several organs and tissues. Interestingly, another TRIM protein is implicated in the pathogenesis of muscular dystrophy in humans. Mutations in TRIM32 cause an autosomal recessive muscular dystrophy (LGMD2H) (92), although the disease mechanism is unknown. TRIM32 belongs to a more ancient subgroup of TRIM proteins present in invertebrates, but shows only weak amino acid identity to MG53 and does not contain the COOH-terminal PRY-SPRY domain. It remains to be determined whether TRIM32 mutations impact skeletal muscle membrane repair in affected patients.
3. Role in membrane repair
In 2009, a MG53 knockout mouse was derived and found to show exercise intolerance with downhill running and increased muscle damage detected by Evans Blue dye uptake (40). Closer examination revealed histopathological signs of a mild, progressive muscular dystrophy. Membrane damage assays using laser irradiation and microinjection revealed that MG53 was rapidly recruited to sites of membrane injury in a calcium-independent manner and that MG53−/− null myofibers and myotubes possessed a primary defect in membrane resealing. MG53 was shown to oligomerize via cysteine 242, with mutation of C242 to alanine ablating MG53 injury recruitment and membrane repair activity (40). MG53 was thus proposed to be an oxidative sensor of membrane injury, forming an oligomerized scaffold for assembly of the membrane repair complex (for review, see Ref. 177).
MG53 was shown to coimmunoprecipitate dysferlin and the muscle-specific caveolin-3 (42). Caveolins are the major protein constituents of membrane caveolae, and mutations in caveolin-3 cause a form of autosomal dominant muscular dystrophy (LGMD1C) (186). A Golgi-retained muscular dystrophy mutant of caveolin-3 (P104L) caused coaccumulation of dysferlin and MG53 in the Golgi apparatus of skeletal myofibers, resulting in defective membrane repair (42). Overexpression of MG53 in C2C12 mouse myoblasts was shown to activate membrane exocytosis, inducing masses of filopodic extensions (41), a process reversible by coexpression of caveolin-3. Collectively, these findings implicated functional interplay between MG53, caveolin-3, and dysferlin in membrane trafficking events required for membrane repair. MG53 recruitment and anchorage at sites of membrane injury have since been to shown to require the cytoskeletal motor nonmuscle myosin IIA (160) and caveolae regulatory protein PTRF (polymerase I and transcript release factor, also known as cavin-1) (319).
Application of recombinant MG53 can improve resealing outcomes when applied to the extracellular media bathing dysferlin-deficient myofibers and can improve dystrophic pathologies when systemically delivered to the mdx mouse, a model of Duchenne muscular dystrophy (309). MG53 has also been implicated in the response to membrane injury in the heart (43, 306).
4. Perspectives
MG53 is expressed only in chordates, with no ortholog identified in chicken or zebrafish (249). As membrane repair is a feature of both vertebrate and invertebrate cells, one must consider that MG53 is not an ancestral membrane repair component, although it may have assumed the function of an evolutionarily more ancient predecessor or perhaps lent a tissue-specific specialization to the membrane repair response. In the setting of a membrane injury with unregulated calcium influx, many plasma membrane proteins and receptors will be damaged by oxidation and calpain cleavage. Therefore, it seems likely that the trigger for MG53 recruitment relates to its role as a ubiquitin ligase, utilizing existing trafficking machinery that ubiquitin ligases transit to rapidly label target substrate(s) for endocytosis and degradation.
The recent discovery of the insulin receptor as a specific substrate of MG53 ubiquitin ligase activity (261) is not easily reconciled with the role of MG53 in membrane repair (40). It is possible that MG53 has several ubiquitination targets and that one of these additional targets plays an important role in the response to, and the signaling of, a membrane injury. Given the ancient eukaryotic origins of other proteins implicated in membrane repair (SNAREs, ferlins, annexins, and calpains), one might suppose that the ubiquitination target of MG53 belongs to an ancient receptor or channel family, which has perhaps evolved a chordate-specific or tissue-specific isoform that MG53 has recently evolved to specifically target.
F. Late Endosomal Pathway And Membrane Repair
Recent research is expanding the body of evidence implicating machinery of the late endosomal pathway in membrane repair, including components of the endosomal sorting complex ESCRT-III (129, 250) and an endolysosomal transient receptor potential (TRP) cation channel, mucolipin-1 (TRPML-1) (49).
1. ESCRT
a) evolution, structure, and localization. ESCRT (endosomal sorting complexes required for transport) plays a key role in the endocytic trafficking and lysosomal targeting of ubiquitinylated transmembrane receptors and proteins (for recent reviews, see Refs. 110, 224). ESCRT proteins were first identified in yeast, as class E vacuolar protein sorting (Vps) genes, required for sorting of transmembrane proteins into intraluminal endosomal vesicles (228, 298). The ESCRT complex is comprised of five subcomplexes: ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and the VPS4 ATPase, and it works in concert with a wide range of adaptor proteins. Components of ESCRT complexes-I, -II, -III, and the VPS4 ATPase are conserved throughout eukaryotic evolution, present in protozoan parasites through to metazoa (155). Furthermore, there is evidence for ancestral ESCRT-III proteins and the VPS4 ATPase in archae-bacteria (89, 203). However, ESCRT-0 is found only in Opisthokonts (animal and fungi kingdoms) and is not present in primitive eukaryotes (155).
The ESCRT complexes are best known for their role in endosomal sorting, whereby ubiquitinylated receptors are endocytosed into clathrin-coated endosomes, clustered within the endosomal membrane, then partitioned into membrane compartments that invaginate and bud-off into the endosomal lumen to form multivesicular bodies (MVBs) (204). MVBs then fuse with lysosomes, resulting in the eventual degradation of the ubiquitinylated cargo (117). ESCRT-0 bears a ubiquitin-interacting domain and a FYVE domain that specifically recognizes phosphatidylinositol 3-phosphate lipids highly enriched within the endosomal membrane (95, 144). ESCRT-0 is thought to recognize the ubiquitinylated cargo within the endosomal membrane and facilitate recruitment of ESCRT-I, which in turn mediates interaction with ESCRT-II. The serial assembly of ESCRT-0, -I and -II complexes provides the platform for assembly of ESCRT-III, the core workhorse of the ESCRT machinery that forms filamentous circular arrays to drive deformation of the endosomal membrane and formation of intraluminal endosomal vesicles (109, 224). The VPS4 ATPase disassembles the ESCRT-III machinery and facilitates recycling of its protein constituents (244).
In addition to a role in endosomal sorting, components of ESCRT play key roles in viral budding (269, 303) and the abscission step of cytokinesis (189, 246, 265) (for recent reviews, see Refs. 171, 174, 245).
b) role in membrane repair. Recently, membrane fission roles of the ESCRT complex have been implicated in the closure of small wounds to the plasma membrane (129) (Figure 4). Using several modes of membrane injury (microinjection, laser ablation injury, pore formation), Jimenez et al. (129) show recruitment to injury sites of several members of the ESCRT-III complex (CHMP4, 3, 2A, and 2B), the Vsp4 ATPase, and the adaptor protein ALIX known to facilitate assembly of ESCRT-III in the absence of ESCRT-I and II complexes. Interestingly, recruitment of ESCRT proteins was calcium-dependent, but not accompanied by vesicular recruitment of late endosomes/lysosomes, and was not blocked by microtubule deploymerization. These data suggest ESCRT proteins are not delivered via vesicular transport but are recruited as soluble, cytoplasmic proteins.
Using quantitative analyses of live imaging data combined with scanning electron microscopy, the authors revealed the ESCRT proteins play a role in repair of small lesions <100 nM, including small lesions made by bacterial pore-forming toxins. Repair and removal of the bacterial pores or small microinjuries are related to outward budding of the decorated wounds and pinching-off via exocytic shedding (129) (see Figure 4). The capacity to survive the membrane injury and shed ESCRT-positive exosomes were both ATP-dependent, suggesting the Vsp4 ATPase may be required to disassemble the ESCRT-III complexes so the protein constituents can be recycled, and/or play a direct role in the pinching off the exocytic buds (129).
A subsequent study used a proteomics approach to identify proteins exocytosed to the plasma membrane after an acute elevation in submembraneous Ca2+ (using the Ca2+ ionophore ionomycin), and highlighted several components of the ESCRT-III machinery (250). Importantly, Scheffer et al. (250) established that the ALIX-binding protein ALG-2 (apoptosis-linked gene-2), a penta-EF hand protein, provides the Ca2+-dependent component for sequential recruitment and assembly of the ESCRT-III complex (250). Using siRNA knockdown of ALG-2, ALIX, and Vsp4 ATPase, they demonstrated each of these components was required for repair, closure, and exosomal shedding of plasma membrane injuries. By studying the capacity of each knockdown cell line to recruit the different components of the ESCRT-III machinery, a model emerged whereby ALG-2 responds to the Ca2+ influx and facilitates recruitment of its binding partner ALIX. In turn, ALIX mediates the sequential assembly of CHMP proteins, then the Vsp4 ATPase (250). The ESCRT-III machinery deforms the plasma membrane into outwardly forming buds that are pinched off to close and/or remodel the wound, with abscission steps involving the ESCRT Vsp4-ATPase (129, 250).
c) perspectives. Interestingly, although data presented by Jimenez et al. (129) suggest ESCRT-III machinery does not influence the closure of wounds larger than 100 nM, Scheffer et al. (250) found cells with >90% knockdown of ALG-2, ALIX, and Vsp4 ATPase were more vulnerable to larger laser injuries (∼1 μM) than control cells. Thus whether ESCRT-III plays a greater role in repair of smaller versus larger injuries requires further clarification. Importantly, Scheffer et al. (250) showed ESCRT-III plays a role in membrane repair in C2C12 myoblasts and primary myofibers, and thus is a ubiquitous response, and not a peculiarity of the immortal HeLa cell line. Notably, both studies carefully demonstrated that recruitment of ESCRT-III machinery was not accompanied by lysosomal exocytosis at the site of injury, although lysosomal exocytosis was observed to occur distally in response to the membrane injury (129, 250).
Although one begins to sniff a tantalizing potential link between the E3 ubiquitin-ligase MG53 (TRIM72) and ESCRT machinery in membrane repair, immunolabeling for polyubiquitination of plasma membrane proteins following injury suggested assembly of ESCRT-III preceded polyubiquitination events (129). Again, Ca2+ provides a key trigger for ESCRT-III exosomal shedding for membrane repair, via ALG-2. On this basis, it will be important to determine whether other settings of ESCRT-mediated fission are also Ca2+-dependent and, furthermore, whether the concentration of calcium required to activate recruitment of ESCRT-III machinery to injury sites is consistent with concentrations required to activate calcium-dependent membrane repair in mammalian cells [∼150 μM in human muscle cells (230) and ∼300 μM in 3T3 fibroblasts (267)].
Yet to be determined and vital for this field are the connections and interplay between the rapid Ca2+-dependent exocytosis triggered in the immediate surrounds of a membrane injury (<10 s; Ref. 150), the rapid compensatory endocytosis (57, 123, 173), the slower phase of lysosomal exocytosis at regions distal to the injury site (126, 229), and the vesicle-independent accumulation of ESCRT-III machinery for exosomal shedding of the injured membrane (49, 129).
2. Mucolipin-1
a) evolution, structure, and localization. Mucolipin-1 (also known as TRPML-1) is one of three members of the vertebrate mucolipin-subfamily of the TRP (transient receptor potential) cation channels, a superfamily of nonselective cation channels bearing six transmembrane domains (S1–S6) (299). Functional TRP channels are homo- or hetero-oligomers of four subunits, with a pore domain located between transmembrane domains S5 and S6 consisting of negatively charged glutamate and aspartate residues to confer cation selectivity (157). TRP channels are best known for their roles as sensory channels for photoreception, proprioception, mechanosensation, nociception, and thermosensation (299). Members of the TRP superfamily are present in yeast through to metazoa, and although TRP-like genes are identified in unicellular phytoplankton, TRP-like genes are absent in plants (201). The mucolipin subfamily of TRP channels emerged as a single gene in invertebrates, with gene duplication events producing three mucolipin genes in vertebrates (201). The mucolipin-1 channel localizes to Rab-7-positive late endosomal and lysosomal compartments and is permeable to Ca2+ and Fe2+ (75).
b) role in disease. Mutations in the mucolipin-1 gene (MCOLN-1) cause an autosomal recessive lysosomal storage disease, mucolipidosis type IV (20). Mucolipidosis type IV is a devastating neurodegenerative disorder characterized by progressive intellectual disability and motor dysfunction, muscle weakness, retinal degeneration and clouding of the cornea, and anemia due to deficient acid secretion in the gut (acid is required for iron absorption), likely confounded at the cellular level by defects in iron-conducting properties of the mucolipin-1 channel itself. Abnormally swollen and enlarged lysosomal compartments filled with membrane and protein aggregates are a feature of patients with mucolipidosis type IV, suggesting abnormal maturation and fusion of endolysosomal organelles (48, 147).
c) role in membrane repair. Somewhat unexpectedly, the major presentation of a mouse knockout model of mucolipin-1 is an early-onset progressive muscular dystrophy, characterized by abundant internal nuclei, fibrotic and inflammatory infiltrate, and elevated serum creatine kinase (49). Dystrophic pathology is apparent as early as 1 mo of age, preceding overt histopathological symptoms of lysosomal storage disease or neuronal degeneration (apparent >5 mo of age) (49). Skeletal muscle fibers isolated from mucolipin-1−/− mice show a major defect in Ca2+-dependent membrane repair, characterized by defective lysosomal exocytosis. Similarly, when mucolipin-1 channels are blocked in C2C12 myoblasts or MEFs using synthetic inhibitors, membrane repair is inhibited and lysosomal exocytosis is reduced. Thus lysosomal Ca2+ flux via mucolipin-1 is an important event for both membrane repair and for lysosomal exocytosis. The importance of lysosomal Ca2+ stores was supported through treatment with glycyl-l-phenylalanine 2-naphthylamide (a substrate hydrolysed by cathepsin C leading to lysosomal osmotic injury and depletion of Ca2+), and BAPTA-AM (a cell-permeable, rapid, and highly effective Ca2+ chelator). Both treatments inhibited membrane repair, suggesting intracellular Ca2+, and lysosomal Ca2+ in particular, is important for membrane repair. Lastly, electrophysiological analyses of wild-type cells reveals an increase in whole cell mucolipin-1 currents following membrane perforation with SLO, implying mucolipin-1 channels become incorporated into the plasma membrane following lysosomal exocytosis in injured cells.
c) perspectives. This study provides additional evidence that lysosomal exocytosis plays an important role in membrane repair. Mucolipin-1 mediates lysosomal Ca2+ release in response to injury, and this Ca2+ release is required for normal lysosomal exocytosis and membrane repair. In the context of membrane repair, it is not yet clear whether lysosomal Ca2+ release mediated by mucolipin-1 is required for hetero- or homotypic fusion of endolysosomal compartments (215, 222) that precede lysosomal exocytosis, or whether it provides a specific Ca2+ flux important for docking and fusion of lysosomes with the plasma membrane. Notably, mucolipin-1 is a binding partner of ALG-2 (300), potentially providing a key link to the ESCRT-III membrane repair pathway.
In addition to the endoplasmic reticulum, mitochondria and lysosomes are major reservoirs of Ca2+, with intraluminal lysosomal Ca2+ around 0.4–0.6 μM (52). One provocative possibility is whether the dystrophic pathology of mucolipin-1−/− mice may relate to defects in Ca2+ sequestration, or more intriguingly, Ca2+ extrusion. Persistent elevation of intracellular Ca2+ in skeletal muscle, induced by transgenic overexpression of the stretch-activated TRPC3 channel, induces a severe muscular dystrophy phenotype (185). This study shows that development of a muscular dystrophy phenotype is strongly related to unregulated Ca2+ influx. If, like mitochondria, lysosomes play a role in Ca2+ sequestration following membrane injury, then levels of lysosomal Ca2+ will increase in the seconds and minutes following membrane injury. Despite the vast Ca2+-handling capabilities of the sarcoplasmic reticulum, it is conceivable that lysosomal exocytosis triggered by membrane injury may also play some role in Ca2+ extrusion, to help purge the cell of unwanted Ca2+, protect mitochondria from Ca2+ overload, and protect a cell from deleterious calpain overactivity.
G. Cytoskeletal Networks
1. Structure and evolution
Eukaryotic cells are differentiated from their prokaryotic and archaeal ancestors not only by their endomembrane system, but also by their complex cytoskeleton. Prokaryotic cells do not possess direct homologs of the major cytoskeletal proteins or tubulin or actin, although they express divergent filamentous proteins with remarkable structural similarity to eukaryotic tubulin (bacterial FtsZ; Refs. 69, 227) and actin (bacterial MreB; Ref. 36). A dynamic cytoskeleton underpins eukaryotic cell division, cell shape, motility, as well as the capacity to phagocytose nutrients for growth and secrete signals for cellular communication.
Isoforms of tubulin and actin are present in every phyla of the eukaryotic kingdom, together with their molecular motors kinesin/dynein and myosin (for reviews, see Refs. 82, 311). Tubulin microfilaments possess an inherent instability intrinsic to their dynamic function. Mature microtubules typically consist of 13 protofilaments that interact to form a hollow cylinder. Each protofilament results from the polymerization of GTP-bound α- and β-tubulin heterodimers; free GTP at the growing (plus) end of the filament promotes further polymerization, whereas hydrolysis of GTP to GDP-tubulin exerts a structural and energetic change that renders the microtubule prone to deploymerization (83). Dynamic microtubules form mitotic spindles for cell division and cytoskeletal highways for vesicular transport via their molecular motors dynein and kinesin.
Actin is one of the most highly conserved eukaryotic proteins, showing ∼85% identity between human, yeast, and plant actin isoforms. Actin filaments consist of two strands of polymerized actin, interwound into a tight right-handed helix (74). Actin filaments are not as intrinsically unstable as microtubules, but nevertheless are capable of rapidly polymerizing and depolymerizing through an active treadmilling process where GTP-bound actin monomers are added to the plus end of the growing filament and removed from the minus end of the filament, regulated by conserved families of monomer-binding factors, capping proteins, filament-stabilizing proteins, and actin-severing proteins. Actin-myosin contractile cycling is best characterized in skeletal and cardiac muscle, with nonmuscle isoforms of actin and myosin playing vital roles in cytokinesis, cell motility, and endomembrane transport (34).
Myosins are also an evolutionary ancient gene family, characterized by the myosin head domain that encompasses a powerful ATPase that utilizes ATP hydrolysis to power movement along an actin filament. Myosin head proteins are present in virtually all eukaryotes, as are microtubule-dependent motors, the kinesins. Indeed, structural similarity between that catalytic core of myosin and kinesin suggests a common evolutionary ancestor of both ATPase motor domains (141).
2. Role in human disease
Mutations in tubulin, actin, and myosin underpin a broad spectrum of inherited disorders in humans. Tubulin mutations have recently been implicated in inherited neurodegenerative disorders, consistent with the vital role of the microtubule cytoskeleton for neuronal function (134, 290). Mutations in members of the actin and myosin gene families cause a wide range of clinical defects, typically affecting tissues and organs where they are dominantly expressed (63). For example, mutations in skeletal actin (ACTA1) and myosin (MYH7 and MYH2) cause a skeletal myopathy, mutations in cardiac actin (ACTC1) and myosin (MYH7) cause cardiomyopathy, and mutations in smooth muscle actin (ACTA2) and myosin (MYH11) cause thoracic aortic aneurysms and dissections. Mutations within nonmuscle members of the actin and myosin gene families can induce deafness, blindness, and other neurological and immune cell dysfunction, depending on the isoform involved. Currently, no pathology caused by mutations in cytoskeletal proteins has been linked directly to defects in membrane repair.
3. Role in membrane repair
A role for cytoskeletal motors in membrane repair was proposed by Xie and Barrett (313) in the early 1990s, who revealed that microtubule destabilizing agents (colchicine) promoted resealing of transected neurons, whereas microtubule stabilizing agents (taxol) inhibited resealing. Discreet roles for microtubule/kinesin and actin/myosin motors for membrane repair was elaborated upon in greater detail by Bi et al. (28), who presented evidence for three phases of vesicle fusion associated with membrane repair of embryonic sea urchin cells: immediate, fast, and slow phases. Immediate exocytosis (<5 s) that occurred in response to membrane wounding was insensitive to both kinesin and myosin inhibitors, and presumed to involve predocked vesicles that did not require kinesin or myosin motors for delivery or fusion. The next “fast” phase (5–15 s) of vesicle fusion was sensitive to myosin inhibitors, but not kinesin inhibitors, suggesting a local pool of vesicles that can quickly respond to the calcium flux of membrane injury. The third “slow” phase (20–60 s post injury) of vesicle fusion was sensitive to kinesin inhibition, and also to myosin inhibition, suggestive of longer distance recruitment of vesicles trafficked along microtubules toward the site of membrane injury.
Inhibition of kinesin motor activity was similarly shown to impair membrane resealing of mammalian Swiss 3T3 fibroblasts (267). Treatment with the actin depolymerizing agent DNAse I improved resealing of gastric epithelial cells, whereas actin filament stabilizing agents phalloidin and jasplakinolide strongly inhibited resealing (187). There is some discrepancy regarding the effects of cytochalasin D, an actin severing agent, shown to exert inhibitory (urchin embryos, Ref. 28), facilitative (3T3 fibroblasts, Refs. 123, 292), or no effect (gastric epithelia, Ref. 187) on membrane resealing from a single wound. However, collectively, evidence using different actin stabilizing and destabilizing agents in different models suggests local deploymerization of subcortical actin facilitates the vesicle fusion of membrane repair.
Further research later refined a specific role for nonmuscle myosin IIB in wound-activated exocytosis and membrane repair of injured 3T3 fibroblasts (293). Knockdown of nonmuscle myosin IIA did not affect exocytosis or membrane repair, but rather inhibited facilitated resealing, a more rapid resealing response to a second injury at the same site due to vesicle formation and recruitment from endomembrane organelles such as the trans-Golgi network (TGN).
a) an acto-myosin ring for membrane repair. A series of elegant imaging experiments in Xenopus oocytes revealed evidence for complex interplay between acto-myosin and microtubule cytoskeletal motors for wound repair (25, 168, 169). Xenopus oocyte injury sites were shown to be encircled by a ring of nonmuscle myosin II positioned at the inside edge of a wider band of actin. This acto-myosin ring was surrounded by a radial array of microtubules that were integrally associated with actin filaments and indeed appeared to be pulled into the active zone of actin polymerization by the acto-myosin motors themselves (168). In turn, the microtubule networks controlled the breadth of the zone of actin assembly, facilitating formation of the actin-myosin II contractile ring that gradually contracts to seal the wound. Inhibition of either acto-myosin or microtubule motor systems impaired wound healing and cell survival. Importantly, wounds made in calcium-free medium failed to reseal, with injected fluorophores leaking out and failing to successfully label cytoskeletal compartments. This suggests that formation of the acto-myosin contractile ring occurs after formation of a membrane barrier that is dependent on extracellular calcium.
These studies were mirrored in wounded early Drosophila embryos that do not undergo cytokinesis for the first few nuclear divisions and exist as large syncytial cells. Both actin and myosin showed rapid accumulation at injury sites (from ∼30 s after injury), aligning in concentric circles that gradually contract to reseal the injury (2). Resealing was shown to occur via discrete stages of expansion, contraction and closure. Immediately after wounding, the lesions expand until actin is observed to accumulate at the periphery. Myosin II accumulated at the wound edge forms a contractile actomyosin ring mediating wound closure. Interestingly, this study revealed evidence for periodic connections between the actomyosin ring and the circumference of the wound sites through interactions mediated by DE-cadherin, suggesting that final stages of wound closure and remodeling following injury occurs via tethering of the contractile machinery to stable adhesive connections with the plasma membrane.
4. Perspectives
The importance of both the actin cytoskeleton (172) and microtubule/kinesin motors (173) has been confirmed for membrane resealing of murine skeletal myofibers, and it is very likely that acto-myosin contractile rings and microtubule transport networks underpin plasma membrane repair and remodeling in all of eukaryota. Whereas endocytosis and exocytic shedding provide an effective means for removal and repair of small injuries (14, 129, 164), larger injuries present a more complicated problem for resealing. As one reflects upon the central themes of membrane repair–calcium, vesicle fusion, calpains, cytoskeletal remodeling, contractile rings–there is remarkable complementarity and consistency among major findings by different research groups, despite huge variance in experimental models and approaches. Acknowledging the oversimplification of what is a complex and incompletely understood pathway, as well as the caveats of comparison between different model systems, the timelines separately proposed by individual research groups fit surprisingly well into a collective model for membrane repair and makes for interesting contemplation.
IV. LITERATURE OVERVIEW: A COLLECTIVE MODEL FOR REPAIR OF LARGE MEMBRANE INJURIES
A. 0–10 s
Calcium floods down a steep concentration to create a microenvironment of high local intracellular calcium, activating calcium-activated signaling molecules and calpains, which cleave dysferlin and other cytoskeletal and plasma membrane substrates. In skeletal muscle cells, MG53 responds to an unknown signal and is rapidly recruited to injury sites. The immediate and fast phases of vesicle fusion occur, utilizing predocked and local vesicle pools, including nearby lysosomes. Dysferlin is endocytosed from regions distal to the injury site, and dysferlin is delivered to the plasma membrane in cytoplasmic vesicles, perhaps triggering their fusion, and upon integration into the plasma membrane intensely labels the circumference of injury sites. There is reduced labeling for filamentous cortical actin surrounding the membrane injury, due to proteolytic cleavage of membrane tethers by calpain and actin deploymerization. Lesions expand.
B. 10–30 s
The slow phase of vesicle fusion begins, requiring cytoskeletal transport of vesicles upon microtubule and actin-myosin motors. ALG-2 and annexins A6 and A11 are delivered to injury sites. Calcium influx continues to trigger exocytic fusion of lysosomes in zones surrounding the lesion site. Calpains cleave cytoskeletal networks and adhesion proteins. Lesions continue to expand.
C. 30–60 s
Actin accumulates at the wound periphery, forming a dynamic zone of rapidly polymerizing and depolymerizing actin. Actin filaments interconnect with local polymerizing microtubule filaments, pulling them toward the wound site and anchoring these microtubule highways in position for rapid transport of vesicles, signaling proteins and cytoskeletal remodeling proteins. Microtubules in turn stabilize the active zone of actin polymerization to facilitate formation of an acto-myosin contractile ring. ALIX, CHMP, and Vsp4 ATPase ESCRT-III machinery sequentially accumulate at injury sites. Acid sphingomyelinase released by lysosomal exocytosis hydrolyzes sphingomyelin to form ceramide-rich platforms to activate endocytosis. Damaged plasma membrane is removed by endocytosis and exosomal shedding. Lesions contract.
D. 60–240 s
The acto-myosin ring gradually contracts and closes, reestablishing the network of cytoskeletal connections beneath the wounded plasma membrane. Annexins A1 and A2 accumulate at injury sites. Phases of exocytosis, endocytosis and exosomal shedding remodel the hastily repaired plasma membrane barrier, replacing damaged proteins and receptors, and reconstituting the normal repertoire of plasma membrane lipids and microdomains.
V. CONCLUDING REMARKS
It seems no accident that membrane repair uses ancient and robust molecular machinery, such as contractile rings and vesicle fusion; these are fundamental biological processes vital for early eukaryotic life. Along these lines, one suspects the vesicle fusion of membrane repair may be more rudimentary than that of synaptic neurotransmission, evolving 500 million years later. Importantly for the membrane repair field, the biology of vesicle fusion and cytokinesis are well defined, each with established pharmacological modifiers. The key now is to connect each research field implicated in membrane repair and better elucidate the molecular signaling governing the temporal progression of each stage in the membrane repair process. It is only then we will be able to strategize novel interventions so desperately needed to treat the huge spectrum of human disorders characterized by membrane injury.
GRANTS
S. T. Cooper is supported by an Australian National Health and Medical Research Council (NHMRC) Career Development Fellowship APP1048816, NHMRC Project Grant APP1048814, and funding from the Jain Foundation. P. L. McNeil was supported by National Institutes of Health Grants AR-060565 and DK-090703.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: S. T. Cooper, Discipline of Paediatrics and Child Health, Univ. of Sydney, Institute for Neuroscience and Muscle Research, The Children's Hospital at Westmead, Locked Bag 4001, Sydney, NSW 2145, Australia (e-mail: Sandra.cooper@sydney.edu.au).
REFERENCES
- 1.Abrami L, Fivaz M, Glauser PE, Parton RG, van der Goot FG. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol 140: 525–540, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol 193: 455–464, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci 110: 1073–1081, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal 12: 125–169, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand 171: 311–319, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88: 83–91, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol 24: 134–144, 2005. [PubMed] [Google Scholar]
- 8.Anderluh G, Macek P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 40: 111–124, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, Johnson MA, Bashir R, Britton S, Keers S, Argov Z, Mahjneh I, Fougerousse F, Beckmann JS, Bushby KM. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet 8: 855–861, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Arroyo CM, Kramer JH, Dickens BF, Weglicki WB. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett 221: 101–104, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol 20: 4474–4481, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avela K, Lipsanen-Nyman M, Idanheimo N, Seemanova E, Rosengren S, Makela TP, Perheentupa J, Chapelle AD, Lehesjoki AE. Gene encoding a new RING-B-box-Coiled-coil protein is mutated in mulibrey nanism. Nature Genet 25: 298–301, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Azam M, Andrabi SS, Sahr KE, Kamath L, Kuliopulos A, Chishti AH. Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol Cell Biol 21: 2213–2220, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differentiation 16: 1126–1134, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N, Hay RT, Chemaly R, Halaby G, Loiselet J, Anderson LV, Lopez de Munain A, Fardeau M, Mangeat P, Beckmann JS, Lefranc G. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A. Nature Med 5: 503–511, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol 104: 181–188, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke 38: 674–676, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Baran K, Dunstone M, Chia J, Ciccone A, Browne KA, Clarke CJ, Lukoyanova N, Saibil H, Whisstock JC, Voskoboinik I, Trapani JA. The molecular basis for perforin oligomerization and transmembrane pore assembly. Immunity 30: 684–695, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G. Identification of the gene causing mucolipidosis type IV. Nature Genet 26: 118–123, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Barwise JL, Walker JH. Subcellular localization of annexin V in human foreskin fibroblasts: nuclear localization depends on growth state. FEBS Lett 394: 213–216, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nature Genet 20: 37–42, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Baylor SM, Hollingworth S. Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J Gen Physiol 139: 261–272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckmann JS, Spencer M. Calpain 3, the “gatekeeper” of proper sarcomere assembly, turnover and maintenance. Neuromuscular Disorders 18: 913–921, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol 9: 579–587, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257: 255–259, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol 131: 1747–1758, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi GQ, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol 138: 999–1008, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269: 1617–1620, 1994. [PubMed] [Google Scholar]
- 30.Bischofberger M, Gonzalez MR, van der Goot FG. Membrane injury by pore-forming proteins. Curr Opin Cell Biol 21: 589–595, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Biswas S, Harris F, Dennison S, Singh J, Phoenix DA. Calpains: targets of cataract prevention? Trends Mol Med 10: 78–84, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nature Genet 23: 141–142, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard H, Grochulski P, Li Y, Arthur JS, Davies PL, Elce JS, Cygler M. Structure of a calpain Ca2+-binding domain reveals a novel EF-hand and Ca2+-induced conformational changes. Nature Struct Biol 4: 532–538, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94: 235–263, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature 363: 163–165, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci USA 89: 7290–7294, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouter A, Gounou C, Berat R, Tan S, Gallois B, Granier T, d'Estaintot BL, Poschl E, Brachvogel B, Brisson AR. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature Commun 2: 270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256: 1021–1025, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Buki A, Povlishock JT. All roads lead to disconnection? Traumatic axonal injury revisited. Acta Neurochir 148: 181–193, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 11: 56–64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem 284: 3314–3322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem 284: 15894–15902, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 121: 2565–2574, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharmaceutical Design 12: 615–638, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Casademont J, Carpenter S, Karpati G. Vacuolation of muscle fibers near sarcolemmal breaks represents T-tubule dilatation secondary to enhanced sodium pump activity. J Neuropathol Exp Neurol 47: 618–628, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Cassidy SK, O'Riordan MX. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins 5: 618–636, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol 162: 543–549, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci USA 95: 6373–6378, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, Garrity AG, Wang X, Ferrer M, Dowling J, Xu L, Han R, Xu H. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nature Med 20: 1187–1192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho SJ, Kelly M, Rognlien KT, Cho JA, Horber JK, Jena BP. SNAREs in opposing bilayers interact in a circular array to form conducting pores. Biophys J 83: 2522–2527, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta 1761: 838–849, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115: 599–607, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Claflin DR, Brooks SV. Direct observation of failing fibers in muscles of dystrophic mice provides mechanistic insight into muscular dystrophy. Am J Physiol Cell Physiol 294: C651–C658, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Clarke MS, Khakee R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci 106: 121–133, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve 23: 1456–1471, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Cooper ST, Head SI. Membrane injury and repair in the muscular dystrophies. The Neuroscientist. In press. [DOI] [PubMed] [Google Scholar]
- 57.Corrotte M, Almeida PE, Tam C, Castro-Gomes T, Fernandes MC, Millis BA, Cortez M, Miller H, Song W, Maugel TK, Andrews NW. Caveolae internalization repairs wounded cells and muscle fibers. eLife 2: e00926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corrotte M, Fernandes MC, Tam C, Andrews NW. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic 13: 483–494, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Covian-Nares JF, Koushik SV, Puhl HL 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci 123: 1884–1893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowell S, Aschauer W, Gruber HJ, Nelson KL, Buckley JT. The erythrocyte receptor for the channel-forming toxin aerolysin is a novel glycosylphosphatidylinositol-anchored protein. Mol Microbiol 25: 343–350, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC Genomics 5: 43, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol 8: 218, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cruz M, Kaemd L. Actin-Binding Proteins and Disease. Berlin: Springer, 2008, p. 348. [Google Scholar]
- 64.Czogalla A, Sikorski AF. Spectrin and calpain: a “target” and a “sniper” in the pathology of neuronal cells. Cell Mol Life Sci 62: 1913–1924, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dacks JB, Doolittle WF. Novel syntaxin gene sequences from Giardia, Trypanosoma and algae: implications for the ancient evolution of the eukaryotic endomembrane system. J Cell Sci 115: 1635–1642, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci 120: 2977–2985, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Dacks JB, Peden AA, Field MC. Evolution of specificity in the eukaryotic endomembrane system. Int J Biochem Cell Biol 41: 330–340, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Damle SS, Moore EE, Babu AN, Meng X, Fullerton DA, Banerjee A. Hemoglobin-based oxygen carrier induces heme oxygenase-1 in the heart and lung but not brain. J Am Coll Surg 208: 592–598, 2009. [DOI] [PubMed] [Google Scholar]
- 69.De Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature 359: 254–256, 1992. [DOI] [PubMed] [Google Scholar]
- 70.De Sousa MV, Morhy L. Enterolobin, a hemolytic protein from Enterolobium contortisiliquum seeds (Leguminosae–Mimosoideae). Purification and characterization. Anais da Academia Brasileira de Ciencias 61: 405–412, 1989. [PubMed] [Google Scholar]
- 71.Demonbreun AR, Fahrenbach JP, Deveaux K, Earley JU, Pytel P, McNally EM. Impaired muscle growth and response to insulin like growth factor 1 in dysferlin mediated muscular dystrophy. Hum Mol Genet 20: 779–789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Detrait E, Eddleman CS, Yoo S, Fukuda M, Nguyen MP, Bittner GD, Fishman HM. Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J Neurobiol 44: 382–391, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Diakonova M, Gerke V, Ernst J, Liautard JP, van der Vusse G, Griffiths G. Localization of five annexins in J774 macrophages and on isolated phagosomes. J Cell Sci 110: 1199–1213, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys 40: 169–186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455: 992–996, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Draeger A, Monastyrskaya K, Babiychuk EB. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol 81: 703–712, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Draeger A, Sanchez-Freire V, Monastyrskaya K, Hoppeler H, Mueller M, Breil F, Mohaupt MG, Babiychuk EB. Statin therapy and the expression of genes that regulate calcium homeostasis and membrane repair in skeletal muscle. Am J Pathol 177: 291–299, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Draeger A, Wray S, Babiychuk EB. Domain architecture of the smooth-muscle plasma membrane: regulation by annexins. Biochem J 387: 309–314, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dutt P, Croall DE, Arthur JS, Veyra TD, Williams K, Elce JS, Greer PA. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol 6: 3, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edwards JN, Launikonis BS. The accessibility and interconnectivity of the tubular system network in toad skeletal muscle. J Physiol 586: 5077–5089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell 72: 153–159, 1993. [DOI] [PubMed] [Google Scholar]
- 82.Erickson HP. Evolution of the cytoskeleton. BioEssays 29: 668–677, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erickson HP, O'Brien ET. Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct 21: 145–166, 1992. [DOI] [PubMed] [Google Scholar]
- 84.Ersfeld K, Barraclough H, Gull K. Evolutionary relationships and protein domain architecture in an expanded calpain superfamily in kinetoplastid parasites. J Mol Evol 61: 742–757, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell 66: 1121–1131, 1991. [DOI] [PubMed] [Google Scholar]
- 87.Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature 380: 595–602, 1996. [DOI] [PubMed] [Google Scholar]
- 88.Evesson FJ, Peat RA, Lek A, Brilot F, Lo HP, Dale RC, Parton RG, North KN, Cooper ST. Reduced plasma membrane expression of dysferlin mutants is attributed to accelerated endocytosis via a syntaxin-4-associated pathway. J Biol Chem 285: 28529–28539, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Field MC, Dacks JB. First and last ancestors: reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr Opin Cell Biol 21: 4–13, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Franzini-Armstrong C, Landmesser L, Pilar G. Size and shape of transverse tubule openings in frog twitch muscle fibers. J Cell Biol 64: 493–497, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 4: 170–176, 1983. [DOI] [PubMed] [Google Scholar]
- 92.Frosk P, Weiler T, Nylen E, Sudha T, Greenberg CR, Morgan K, Fujiwara TM, Wrogemann K. Limb-girdle muscular dystrophy type 2H associated with mutation in TRIM32, a putative E3-ubiquitin-ligase gene. Am J Hum Genet 70: 663–672, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galitsky N, Cody V, Wojtczak A, Ghosh D, Luft JR, Pangborn W, English L. Structure of the insecticidal bacterial delta-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallographica Sect D Biol Crystallogr 57: 1101–1109, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res 61: 757–760, 1987. [DOI] [PubMed] [Google Scholar]
- 95.Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature 394: 432–433, 1998. [DOI] [PubMed] [Google Scholar]
- 96.Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infection Immunity 55: 1641–1646, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79: 717–727, 1994. [DOI] [PubMed] [Google Scholar]
- 98.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev 82: 331–371, 2002. [DOI] [PubMed] [Google Scholar]
- 99.Gil-Parrado S, Popp O, Knoch TA, Zahler S, Bestvater F, Felgentrager M, Holloschi A, Fernandez-Montalvan A, Auerswald EA, Fritz H, Fuentes-Prior P, Machleidt W, Spiess E. Subcellular localization and in vivo subunit interactions of ubiquitous mu-calpain. J Biol Chem 278: 16336–16346, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Glaser T, Schwarz-Benmeir N, Barnoy S, Barak S, Eshhar Z, Kosower NS. Calpain (Ca2+-dependent thiol protease) in erythrocytes of young and old individuals. Proc Natl Acad Sci USA 91: 7879–7883, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Godell CM, Smyers ME, Eddleman CS, Ballinger ML, Fishman HM, Bittner GD. Calpain activity promotes the sealing of severed giant axons. Proc Natl Acad Sci USA 94: 4751–4756, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- 103.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32: 37–43, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Golstein P, Kroemer G. A multiplicity of cell death pathways. Symposium on apoptotic and non-apoptotic cell death pathways. EMBO Reports 8: 829–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B. Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci 65: 493–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gozen I, Dommersnes P. Pore dynamics in lipid membranes. Eur Phys J Spec Top 223: 1813–1829, 2014. [Google Scholar]
- 107.Guyon JR, Kudryashova E, Potts A, Dalkilic I, Brosius MA, Thompson TG, Beckmann JS, Kunkel LM, Spencer MJ. Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans. Muscle Nerve 28: 472–483, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Hajjar KA, Krishnan S. Annexin II: a mediator of the plasmin/plasminogen activator system. Trends Cardiovasc Med 9: 128–138, 1999. [DOI] [PubMed] [Google Scholar]
- 109.Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol 180: 389–402, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 21: 77–91, 2011. [DOI] [PubMed] [Google Scholar]
- 111.Hilsenbeck JL, Park H, Chen G, Youn B, Postle K, Kang C. Crystal structure of the cytotoxic bacterial protein colicin B at 2.5 A resolution. Mol Microbiol 51: 711–720, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH Jr. Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet 13: 1999–2010, 2004. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928, 1987. [DOI] [PubMed] [Google Scholar]
- 114.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nature Genet 26: 163–175, 2000. [DOI] [PubMed] [Google Scholar]
- 115.Hosfield CM, Elce JS, Davies PL, Jia Z. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel mode of enzyme activation. EMBO J 18: 6880–6889, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howard AC, McNeil AK, Xiong F, Xiong WC, McNeil PL. A novel cellular defect in diabetes: membrane repair failure. Diabetes 60: 3034–3043, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20: 4–11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett 583: 337–344, 2009. [DOI] [PubMed] [Google Scholar]
- 119.Huynh C, Roth D, Ward DM, Kaplan J, Andrews NW. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci USA 101: 16795–16800, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iacovache I, van der Goot FG, Pernot L. Pore formation: an ancient yet complex form of attack. Biochim Biophys Acta 1778: 1611–1623, 2008. [DOI] [PubMed] [Google Scholar]
- 121.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702, 1992. [DOI] [PubMed] [Google Scholar]
- 122.Idone V, Tam C, Andrews NW. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol 18: 552–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180: 905–914, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res 96: 23–31, 2012. [DOI] [PubMed] [Google Scholar]
- 125.Ishiharajima S, Aida T, Nakagawa R, Kameyama K, Sugano K, Oguro T, Asano G. Early membrane damage during ischemia in rat heart. Exp Mol Pathol 44: 1–6, 1986. [DOI] [PubMed] [Google Scholar]
- 126.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol 2: E233, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jena BP. Role of SNAREs in membrane fusion. Adv Exp Med Biol 713: 13–32, 2011. [DOI] [PubMed] [Google Scholar]
- 128.Jeremic A, Kelly M, Cho JA, Cho SJ, Horber JK, Jena BP. Calcium drives fusion of SNARE-apposed bilayers. Cell Biol Int 28: 19–31, 2004. [DOI] [PubMed] [Google Scholar]
- 129.Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science 343: 1247136, 2014. [DOI] [PubMed] [Google Scholar]
- 130.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102: 549–552, 2000. [DOI] [PubMed] [Google Scholar]
- 131.Jones DA, Newham DJ, Round JM, Tolfree SE. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol 375: 435–448, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298: 229–317, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92: 1915–1964, 2012. [DOI] [PubMed] [Google Scholar]
- 134.Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnar Z, Pinon MC, Dear N, Valdar W, Brown SD, Davies KE, Rawlins JN, Cowan NJ, Nolan P, Chelly J, Flint J. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 128: 45–57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kerr JP, Ziman AP, Mueller AL, Muriel JM, Kleinhans-Welte E, Gumerson JD, Vogel SS, Ward CW, Roche JA, Bloch RJ. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proc Natl Acad Sci USA 110: 20831–20836, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keyel PA, Loultcheva L, Roth R, Salter RD, Watkins SC, Yokoyama WM, Heuser JE. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J Cell Sci 124: 2414–2423, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitagaki H, Tomioka S, Yoshizawa T, Sorimachi H, Saido TC, Ishiura S, Suzuki K. Autolysis of calpain large subunit inducing irreversible dissociation of stoichiometric heterodimer of calpain. Biosci Biotechnol Biochem 64: 689–695, 2000. [DOI] [PubMed] [Google Scholar]
- 138.Klinge L, Laval S, Keers S, Haldane F, Straub V, Barresi R, Bushby K. From T-tubule to sarcolemma: damage-induced dysferlin translocation in early myogenesis. FASEB J 21: 1768–1776, 2007. [DOI] [PubMed] [Google Scholar]
- 139.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84: 1415–1420, 1994. [PubMed] [Google Scholar]
- 140.Krahn M, Wein N, Bartoli M, Lostal W, Courrier S, Bourg-Alibert N, Nguyen K, Vial C, Streichenberger N, Labelle V, DePetris D, Pecheux C, Leturcq F, Cau P, Richard I, Levy N. A naturally occurring human minidysferlin protein repairs sarcolemmal lesions in a mouse model of dysferlinopathy. Science Transl Med 2: 50ra69, 2010. [DOI] [PubMed] [Google Scholar]
- 141.Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 380: 550–555, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE. Localization of the Ca2+-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec 232: 60–77, 1992. [DOI] [PubMed] [Google Scholar]
- 143.Kuru S, Yasuma F, Wakayama T, Kimura S, Konagaya M, Aoki M, Tanabe M, Takahashi T. [A patient with limb girdle muscular dystrophy type 2B (LGMD2B) manifesting cardiomyopathy]. Rinsho Shinkeigaku Clin Neurol 44: 375–378, 2004. [PubMed] [Google Scholar]
- 144.Kutateladze TG, Ogburn KD, Watson WT, de Beer T, Emr SD, Burd CG, Overduin M. Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol Cell 3: 805–811, 1999. [DOI] [PubMed] [Google Scholar]
- 145.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342, 2002. [DOI] [PubMed] [Google Scholar]
- 146.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119: 624–635, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.LaPlante JM, Ye CP, Quinn SJ, Goldin E, Brown EM, Slaugenhaupt SA, Vassilev PM. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun 322: 1384–1391, 2004. [DOI] [PubMed] [Google Scholar]
- 148.Launikonis BS, Stephenson DG, Friedrich O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol 587: 2299–2312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Law RH, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, D'Angelo ME, Orlova EV, Coulibaly F, Verschoor S, Browne KA, Ciccone A, Kuiper MJ, Bird PI, Trapani JA, Saibil HR, Whisstock JC. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468: 447–451, 2010. [DOI] [PubMed] [Google Scholar]
- 150.Lek A, Evesson FJ, Lemckert FA, Redpath GM, Lueders AK, Turnbull L, Whitchurch CB, North KN, Cooper ST. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci 33: 5085–5094, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lek A, Evesson FJ, Sutton RB, North KN, Cooper ST. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic 13: 185–194, 2012. [DOI] [PubMed] [Google Scholar]
- 152.Lek A, Lek M, North KN, Cooper ST. Phylogenetic analysis of ferlin genes reveals ancient eukaryotic origins. BMC Evol Biol 10: 231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leloup L, Shao H, Bae YH, Deasy B, Stolz D, Roy P, Wells A. m-Calpain activation is regulated by its membrane localization and by its binding to phosphatidylinositol 4,5-bisphosphate. J Biol Chem 285: 33549–33566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH Jr. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem 278: 50466–50473, 2003. [DOI] [PubMed] [Google Scholar]
- 155.Leung KF, Dacks JB, Field MC. Evolution of the multivesicular body ESCRT machinery: retention across the eukaryotic lineage. Traffic 9: 1698–1716, 2008. [DOI] [PubMed] [Google Scholar]
- 156.Lewit-Bentley A, Rety S, Sopkova-de Oliveira Santos J, Gerke V. S100-annexin complexes: some insights from structural studies. Cell Biol Int 24: 799–802, 2000. [DOI] [PubMed] [Google Scholar]
- 157.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504: 107–112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liemann S, Huber R. Three-dimensional structure of annexins. Cell Mol Life Sci 53: 516–521, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin GD, Chattopadhyay D, Maki M, Wang KK, Carson M, Jin L, Yuen PW, Takano E, Hatanaka M, DeLucas LJ, Narayana SV. Crystal structure of calcium bound domain VI of calpain at 1.9 A resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nature Struct Biol 4: 539–547, 1997. [DOI] [PubMed] [Google Scholar]
- 160.Lin P, Zhu H, Cai C, Wang X, Cao C, Xiao R, Pan Z, Weisleder N, Takeshima H, Ma J. Nonmuscle myosin IIA facilitates vesicle trafficking for MG53-mediated cell membrane repair. FASEB J 26: 1875–1883, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH Jr. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nature Genet 20: 31–36, 1998. [DOI] [PubMed] [Google Scholar]
- 162.Liu Z, Cao J, Gao X, Ma Q, Ren J, Xue Y. GPS-CCD: a novel computational program for the prediction of calpain cleavage sites. PloS One 6: e19001, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lo HP, Cooper ST, Evesson FJ, Seto JT, Chiotis M, Tay V, Compton AG, Cairns AG, Corbett A, MacArthur DG, Yang N, Reardon K, North KN. Limb-girdle muscular dystrophy: diagnostic evaluation, frequency and clues to pathogenesis. Neuromuscular Disorders 18: 34–44, 2008. [DOI] [PubMed] [Google Scholar]
- 164.Los FC, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 9: 147–157, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev 77: 173–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lostal W, Bartoli M, Roudaut C, Bourg N, Krahn M, Pryadkina M, Borel P, Suel L, Roche JA, Stockholm D, Bloch RJ, Levy N, Bashir R, Richard I. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PloS One 7: e38036, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lovelace LL, Cooper CL, Sodetz JM, Lebioda L. Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J Biol Chem 286: 17585–17592, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol 13: 1096–1105, 2003. [DOI] [PubMed] [Google Scholar]
- 169.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol 154: 785–797, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mayran N, Parton RG, Gruenberg J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J 22: 3242–3253, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annu Rev Biochem 82: 663–692, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McDade JR, Archambeau A, Michele DE. Rapid actin-cytoskeleton-dependent recruitment of plasma membrane-derived dysferlin at wounds is critical for muscle membrane repair. FASEB J 28: 3660–3670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McDade JR, Michele DE. Membrane damage-induced vesicle-vesicle fusion of dysferlin-containing vesicles in muscle cells requires microtubules and kinesin. Hum Mol Genet 23: 1677–1686, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McDonald B, Martin-Serrano J. No strings attached: the ESCRT machinery in viral budding and cytokinesis. J Cell Sci 122: 2167–2177, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McNab FW, Rajsbaum R, Stoye JP, O'Garra A. Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol 23: 46–56, 2011. [DOI] [PubMed] [Google Scholar]
- 176.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem 281: 35202–35207, 2006. [DOI] [PubMed] [Google Scholar]
- 177.McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol 11: 7–9, 2009. [DOI] [PubMed] [Google Scholar]
- 178.McNeil PL. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol 3: 302–307, 1993. [DOI] [PubMed] [Google Scholar]
- 179.McNeil PL, Baker MM. Cell surface events during resealing visualized by scanning-electron microscopy. Cell Tissue Res 304: 141–146, 2001. [DOI] [PubMed] [Google Scholar]
- 180.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140: 1097–1109, 1992. [PMC free article] [PubMed] [Google Scholar]
- 181.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nature Rev Mol Cell Biol 6: 499–505, 2005. [DOI] [PubMed] [Google Scholar]
- 182.Mellgren RL, Miyake K, Kramerova I, Spencer MJ, Bourg N, Bartoli M, Richard I, Greer PA, McNeil PL. Calcium-dependent plasma membrane repair requires m- or mu-calpain, but not calpain-3, the proteasome, or caspases. Biochim Biophys Acta 1793: 1886–1893, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem 282: 2567–2575, 2007. [DOI] [PubMed] [Google Scholar]
- 184.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195, 1998. [DOI] [PubMed] [Google Scholar]
- 185.Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA 106: 19023–19028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco M, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nature Genet 18: 365–368, 1998. [DOI] [PubMed] [Google Scholar]
- 187.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci 114: 3487–3494, 2001. [DOI] [PubMed] [Google Scholar]
- 188.Monastyrskaya K, Babiychuk EB, Hostettler A, Rescher U, Draeger A. Annexins as intracellular calcium sensors. Cell Calcium 41: 207–219, 2007. [DOI] [PubMed] [Google Scholar]
- 189.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26: 4215–4227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moss SE, Morgan RO. The annexins. Genome Biol 5: 219, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Murphy E, Cross H, Steenbergen C. Sodium regulation during ischemia versus reperfusion and its role in injury. Circ Res 84: 1469–1470, 1999. [DOI] [PubMed] [Google Scholar]
- 192.Murphy E, Cross HR, Steenbergen C. Is Na/Ca exchange during ischemia and reperfusion beneficial or detrimental? Ann NY Acad Sci 976: 421–430, 2002. [DOI] [PubMed] [Google Scholar]
- 193.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Murphy RM. Calpains, skeletal muscle function and exercise. Clin Exp Pharmacol Physiol 37: 385–391, 2010. [DOI] [PubMed] [Google Scholar]
- 195.Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of mu-calpain in rat skeletal muscle. J Physiol 576: 595–612, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nalefski EA, Falke JJ. Location of the membrane-docking face on the Ca2+-activated C2 domain of cytosolic phospholipase A2. Biochemistry 37: 17642–17650, 1998. [DOI] [PubMed] [Google Scholar]
- 197.Napolitano LM, Meroni G. TRIM family: pleiotropy and diversification through homomultimer and heteromultimer formation. IUBMB Life 64: 64–71, 2012. [DOI] [PubMed] [Google Scholar]
- 198.Nelson KL, Raja SM, Buckley JT. The glycosylphosphatidylinositol-anchored surface glycoprotein Thy-1 is a receptor for the channel-forming toxin aerolysin. J Biol Chem 272: 12170–12174, 1997. [DOI] [PubMed] [Google Scholar]
- 199.Newham DJ, Jones DA, Edwards RH. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve 9: 59–63, 1986. [DOI] [PubMed] [Google Scholar]
- 200.Nguyen K, Bassez G, Krahn M, Bernard R, Laforet P, Labelle V, Urtizberea JA, Figarella-Branger D, Romero N, Attarian S, Leturcq F, Pouget J, Levy N, Eymard B. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol 64: 1176–1182, 2007. [DOI] [PubMed] [Google Scholar]
- 201.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 12: 218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665, 1988. [DOI] [PubMed] [Google Scholar]
- 203.Obita T, Saksena S, Ghazi-Tabatabai S, Gill DJ, Perisic O, Emr SD, Williams RL. Structural basis for selective recognition of ESCRT-III by the AAA ATPase Vps4. Nature 449: 735–739, 2007. [DOI] [PubMed] [Google Scholar]
- 204.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95: 847–858, 1998. [DOI] [PubMed] [Google Scholar]
- 205.Ohsako T, Hirai K, Yamamoto MT. The Drosophila misfire gene has an essential role in sperm activation during fertilization. Genes Genet Syst 78: 253–266, 2003. [DOI] [PubMed] [Google Scholar]
- 206.Oling F, Bergsma-Schutter W, Brisson A. Trimers, dimers of trimers, and trimers of trimers are common building blocks of annexin a5 two-dimensional crystals. J Struct Biol 133: 55–63, 2001. [DOI] [PubMed] [Google Scholar]
- 207.Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109: 3039–3052, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ozato K, Shin DM, Chang TH, Morse HC 3rd. TRIM family proteins and their emerging roles in innate immunity. Nature Rev Immunol 8: 849–860, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Sudhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci 26: 13493–13504, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22: 496–505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pangrsic T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, Frank T, Tarantino LM, Bailey JS, Strenzke N, Brose N, Muller U, Reisinger E, Moser T. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat Neurosci 13: 869–876, 2010. [DOI] [PubMed] [Google Scholar]
- 212.Park EY, Kwon OB, Jeong BC, Yi JS, Lee CS, Ko YG, Song HK. Crystal structure of PRY-SPRY domain of human TRIM72. Proteins 78: 790–795, 2010. [DOI] [PubMed] [Google Scholar]
- 213.Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol 88: 91–142, 2005. [DOI] [PubMed] [Google Scholar]
- 214.Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci 18: 60–64, 2003. [DOI] [PubMed] [Google Scholar]
- 215.Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396: 575–580, 1998. [DOI] [PubMed] [Google Scholar]
- 216.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci USA 98: 3012–3017, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Piccolo F, Moore SA, Ford GC, Campbell KP. Intracellular accumulation and reduced sarcolemmal expression of dysferlin in limb–girdle muscular dystrophies. Ann Neurol 48: 902–912, 2000. [PubMed] [Google Scholar]
- 219.Potez S, Luginbuhl M, Monastyrskaya K, Hostettler A, Draeger A, Babiychuk EB. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J Biol Chem 286: 17982–17991, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pramono ZA, Tan CL, Seah IA, See JS, Kam SY, Lai PS, Yee WC. Identification and characterisation of human dysferlin transcript variants: implications for dysferlin mutational screening and isoforms. Hum Genet 125: 413–420, 2009. [DOI] [PubMed] [Google Scholar]
- 221.Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537: 333–345, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Quaderi NA, Schweiger S, Gaudenz K, Franco B, Rugarli EI, Berger W, Feldman GJ, Volta M, Andolfi G, Gilgenkrantz S, Marion RW, Hennekam RC, Opitz JM, Muenke M, Ropers HH, Ballabio A. Opitz G/BBB syndrome, a defect of midline development, is due to mutations in a new RING finger gene on Xp22. Nature Genet 17: 285–291, 1997. [DOI] [PubMed] [Google Scholar]
- 224.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458: 445–452, 2009. [DOI] [PubMed] [Google Scholar]
- 225.Rambotti MG, Spreca A, Donato R. Immunocytochemical localization of annexins V and VI in human placentae of different gestational ages. Cell Mol Biol Res 39: 579–588, 1993. [PubMed] [Google Scholar]
- 226.Rand JH, Wu XX, Lapinski R, van Heerde WL, Reutelingsperger CP, Chen PP, Ortel TL. Detection of antibody-mediated reduction of annexin A5 anticoagulant activity in plasmas of patients with the antiphospholipid syndrome. Blood 104: 2783–2790, 2004. [DOI] [PubMed] [Google Scholar]
- 227.RayChaudhuri D, Park JT. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature 359: 251–254, 1992. [DOI] [PubMed] [Google Scholar]
- 228.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell 3: 1389–1402, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106: 157–169, 2001. [DOI] [PubMed] [Google Scholar]
- 230.Redpath G, Woolger N, Piper A, Lemckert F, Lek A, Greer P, North K, Cooper S. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol Biol Cell 25: 3037–3048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rescher U, Zobiack N, Gerke V. Intact Ca2+-binding sites are required for targeting of annexin 1 to endosomal membranes in living HeLa cells. J Cell Sci 113: 3931–3938, 2000. [DOI] [PubMed] [Google Scholar]
- 232.Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, Guffanti A, Minucci S, Pelicci PG, Ballabio A. The tripartite motif family identifies cell compartments. EMBO J 20: 2140–2151, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81: 27–40, 1995. [DOI] [PubMed] [Google Scholar]
- 234.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nature Struct Mol Biol 15: 665–674, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roche JA, Lovering RM, Bloch RJ. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport 19: 1579–1584, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roche JA, Lovering RM, Roche R, Ru LW, Reed PW, Bloch RJ. Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries. Am J Physiol Cell Physiol 298: C298–C312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Romisch J, Seiffge D, Reiner G, Paques EP, Heimburger N. In-vivo antithrombotic potency of placenta protein 4 (annexin V). Thromb Res 61: 93–104, 1991. [DOI] [PubMed] [Google Scholar]
- 238.Roostalu U, Strahle U. In Vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell 22: 515–529, 2012. [DOI] [PubMed] [Google Scholar]
- 239.Rosado CJ, Buckle AM, Law RH, Butcher RE, Kan WT, Bird CH, Ung K, Browne KA, Baran K, Bashtannyk-Puhalovich TA, Faux NG, Wong W, Porter CJ, Pike RN, Ellisdon AM, Pearce MC, Bottomley SP, Emsley J, Smith AI, Rossjohn J, Hartland EL, Voskoboinik I, Trapani JA, Bird PI, Dunstone MA, Whisstock JC. A common fold mediates vertebrate defense and bacterial attack. Science 317: 1548–1551, 2007. [DOI] [PubMed] [Google Scholar]
- 240.Rosado CJ, Kondos S, Bull TE, Kuiper MJ, Law RH, Buckle AM, Voskoboinik I, Bird PI, Trapani JA, Whisstock JC, Dunstone MA. The MACPF/CDC family of pore-forming toxins. Cell Microbiol 10: 1765–1774, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rosengarth A, Luecke H. A calcium-driven conformational switch of the N-terminal and core domains of annexin A1. J Mol Biol 326: 1317–1325, 2003. [DOI] [PubMed] [Google Scholar]
- 242.Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 127: 277–289, 2006. [DOI] [PubMed] [Google Scholar]
- 243.Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics 7: 31–42, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci 32: 561–573, 2007. [DOI] [PubMed] [Google Scholar]
- 245.Samson RY, Bell SD. Ancient ESCRTs and the evolution of binary fission. Trends Microbiol 17: 507–513, 2009. [DOI] [PubMed] [Google Scholar]
- 246.Samson RY, Obita T, Freund SM, Williams RL, Bell SD. A role for the ESCRT system in cell division in archaea. Science 322: 1710–1713, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 301: H1723–H1741, 2011. [DOI] [PubMed] [Google Scholar]
- 248.Sanderfoot A. Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol 144: 6–17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sardiello M, Cairo S, Fontanella B, Ballabio A, Meroni G. Genomic analysis of the TRIM family reveals two groups of genes with distinct evolutionary properties. BMC Evol Biol 8: 225, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, Jaiswal JK. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nature Commun 5: 5646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA 94: 5113–5118, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett 335: 99–103, 1993. [DOI] [PubMed] [Google Scholar]
- 253.Schiavo G, Stenbeck G, Rothman JE, Sollner TH. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci USA 94: 997–1001, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exercise 15: 51–56, 1983. [PubMed] [Google Scholar]
- 255.Shao H, Chou J, Baty CJ, Burke NA, Watkins SC, Stolz DB, Wells A. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol 26: 5481–5496, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sharma A, Yu C, Leung C, Trane A, Lau M, Utokaparch S, Shaheen F, Sheibani N, Bernatchez P. A new role for the muscle repair protein dysferlin in endothelial cell adhesion and angiogenesis. Arterioscler Thromb Vasc Biol 30: 2196–2204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shatursky O, Heuck AP, Shepard LA, Rossjohn J, Parker MW, Johnson AE, Tweten RK. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99: 293–299, 1999. [DOI] [PubMed] [Google Scholar]
- 258.Shen SS, Tucker WC, Chapman ER, Steinhardt RA. Molecular regulation of membrane resealing in 3T3 fibroblasts. J Biol Chem 280: 1652–1660, 2005. [DOI] [PubMed] [Google Scholar]
- 259.Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci USA 96: 11486–11491, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shogomori H, Kobayashi T. Lysenin: a sphingomyelin specific pore-forming toxin. Biochim Biophys Acta 1780: 612–618, 2008. [DOI] [PubMed] [Google Scholar]
- 261.Song R, Peng W, Zhang Y, Lv F, Wu HK, Guo J, Cao Y, Pi Y, Zhang X, Jin L, Zhang M, Jiang P, Liu F, Meng S, Zhang X, Jiang P, Cao CM, Xiao RP. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 494: 375–379, 2013. [DOI] [PubMed] [Google Scholar]
- 262.Sorimachi H, Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc Res 96: 11–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sorimachi H, Suzuki K. The structure of calpain. J Biochem 129: 653–664, 2001. [DOI] [PubMed] [Google Scholar]
- 264.Spencer MJ, Guyon JR, Sorimachi H, Potts A, Richard I, Herasse M, Chamberlain J, Dalkilic I, Kunkel LM, Beckmann JS. Stable expression of calpain 3 from a muscle transgene in vivo: immature muscle in transgenic mice suggests a role for calpain 3 in muscle maturation. Proc Natl Acad Sci USA 99: 8874–8879, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Spitzer C, Schellmann S, Sabovljevic A, Shahriari M, Keshavaiah C, Bechtold N, Herzog M, Muller S, Hanisch FG, Hulskamp M. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 133: 4679–4689, 2006. [DOI] [PubMed] [Google Scholar]
- 266.Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature 460: 525–528, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263: 390–393, 1994. [DOI] [PubMed] [Google Scholar]
- 268.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nature Rev Cancer 11: 364–374, 2011. [DOI] [PubMed] [Google Scholar]
- 269.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114: 689–699, 2003. [DOI] [PubMed] [Google Scholar]
- 270.Strobl S, Fernandez-Catalan C, Braun M, Huber R, Masumoto H, Nakagawa K, Irie A, Sorimachi H, Bourenkow G, Bartunik H, Suzuki K, Bode W. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci USA 97: 588–592, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci 233: 3–13, 2005. [DOI] [PubMed] [Google Scholar]
- 272.Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Engineering 12: 439–446, 1999. [DOI] [PubMed] [Google Scholar]
- 273.Supnet C, Bezprozvanny I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer's disease. J Alzheimer's Dis 20 Suppl 2: S487–498, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80: 929–938, 1995. [DOI] [PubMed] [Google Scholar]
- 275.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395: 347–353, 1998. [DOI] [PubMed] [Google Scholar]
- 276.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes 53 Suppl 1: S12–18, 2004. [DOI] [PubMed] [Google Scholar]
- 277.Swaggart KA, Demonbreun AR, Vo AH, Swanson KE, Kim EY, Fahrenbach JP, Holley-Cuthrell J, Eskin A, Chen Z, Squire K, Heydemann A, Palmer AA, Nelson SF, McNally EM. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci USA 111: 6004–6009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tait JF, Cerqueira MD, Dewhurst TA, Fujikawa K, Ritchie JL, Stratton JR. Evaluation of annexin V as a platelet-directed thrombus targeting agent. Thrombosis Res 75: 491–501, 1994. [DOI] [PubMed] [Google Scholar]
- 279.Talukder MA, Yang F, Nishijima Y, Chen CA, Kalyanasundaram A, Periasamy M, Zweier JL. Reduced SERCA2a converts sub-lethal myocardial injury to infarction and affects postischemic functional recovery. J Mol Cell Cardiol 46: 285–287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189: 1027–1038, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Taneike M, Mizote I, Morita T, Watanabe T, Hikoso S, Yamaguchi O, Takeda T, Oka T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Takeda J, Mochizuki N, Komuro I, Otsu K. Calpain protects the heart from hemodynamic stress. J Biol Chem 286: 32170–32177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126: 1175–1187, 2006. [DOI] [PubMed] [Google Scholar]
- 283.Taveau M, Bourg N, Sillon G, Roudaut C, Bartoli M, Richard I. Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol Cell Biol 23: 9127–9135, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Teng FY, Wang Y, Tang BL. The syntaxins. Genome Biol 2: 3012, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol 139: 63–74, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Thiagarajan P, Benedict CR. Inhibition of arterial thrombosis by recombinant annexin V in a rabbit carotid artery injury model. Circulation 96: 2339–2347, 1997. [DOI] [PubMed] [Google Scholar]
- 287.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping IS, Bleackley RC, Kirchhausen T, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nature Immunol 12: 770–777, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Thiery J, Keefe D, Saffarian S, Martinvalet D, Walch M, Boucrot E, Kirchhausen T, Lieberman J. Perforin activates clathrin- and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood 115: 1582–1593, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int J Biochem Cell Biol 32: 1–5, 2000. [DOI] [PubMed] [Google Scholar]
- 290.Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG, Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcategui CE, de Uzcategui N, Collins ML, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM, Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Moller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML Jr, Pellman D, Engle EC. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140: 74–87, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci 112: 719–731, 1999. [DOI] [PubMed] [Google Scholar]
- 292.Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell 11: 4339–4346, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell 15: 688–695, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, Hudecz F, Friedrich P. On the sequential determinants of calpain cleavage. J Biol Chem 279: 20775–20785, 2004. [DOI] [PubMed] [Google Scholar]
- 295.Trimble WS, Cowan DM, Scheller RH. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci USA 85: 4538–4542, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tweten RK, Parker MW, Johnson AE. The cholesterol-dependent cytolysins. Curr Top Microbiol Immunol 257: 15–33, 2001. [DOI] [PubMed] [Google Scholar]
- 297.Vandre DD, Ackerman WEt Kniss DA, Tewari AK, Mori M, Takizawa T, Robinson JM. Dysferlin is expressed in human placenta but does not associate with caveolin. Biol Reprod 77: 533–542, 2007. [DOI] [PubMed] [Google Scholar]
- 298.Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol 119: 773–786, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Vergarajauregui S, Martina JA, Puertollano R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J Biol Chem 284: 36357–36366, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Vetter IR, Parker MW, Tucker AD, Lakey JH, Pattus F, Tsernoglou D. Crystal structure of a colicin N fragment suggests a model for toxicity. Structure 6: 863–874, 1998. [DOI] [PubMed] [Google Scholar]
- 302.Voigt T, Sebald HJ, Schoenauer R, Levano S, Girard T, Hoppeler HH, Babiychuk EB, Draeger A. Annexin A1 is a biomarker of T-tubular repair in skeletal muscle of nonmyopathic patients undergoing statin therapy. FASEB J 27: 2156–2164, 2013. [DOI] [PubMed] [Google Scholar]
- 303.Von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell 114: 701–713, 2003. [DOI] [PubMed] [Google Scholar]
- 304.Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, Lek A, Street NE, Lin P, Clarke NF, Landstrom AP, Ackerman MJ, Weisleder N, Ma J, North KN, Cooper ST. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropathol Exp Neurol 70: 302–313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun 61: 4972–4979, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circulation Research 107: 76–83, 2010. [DOI] [PubMed] [Google Scholar]
- 307.Washington NL, Ward S. FER-1 regulates Ca2+-mediated membrane fusion during C. elegans. J Cell Sci 119: 2552–2562, 2006. [DOI] [PubMed] [Google Scholar]
- 308.Weisleder N, Takeshima H, Ma J. Immuno-proteomic approach to excitation-contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium 43: 1–8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science Translational Med 4: 139ra185, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Wenzel K, Geier C, Qadri F, Hubner N, Schulz H, Erdmann B, Gross V, Bauer D, Dechend R, Dietz R, Osterziel KJ, Spuler S, Ozcelik C. Dysfunction of dysferlin-deficient hearts. J Mol Med 85: 1203–1214, 2007. [DOI] [PubMed] [Google Scholar]
- 311.Wickstead B, Gull K. The evolution of the cytoskeleton. J Cell Biol 194: 513–525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wiener M, Freymann D, Ghosh P, Stroud RM. Crystal structure of colicin Ia. Nature 385: 461–464, 1997. [DOI] [PubMed] [Google Scholar]
- 313.Xie XY, Barrett JN. Membrane resealing in cultured rat septal neurons after neurite transection: evidence for enhancement by Ca2+-triggered protease activity and cytoskeletal disassembly. J Neurosci 11: 3257–3267, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem 269: 12764–12772, 1994. [PubMed] [Google Scholar]
- 315.Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nature Genet 21: 363–369, 1999. [DOI] [PubMed] [Google Scholar]
- 316.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562: 367–380, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zatz M, Starling A. Calpains and disease. N Engl J Med 352: 2413–2423, 2005. [DOI] [PubMed] [Google Scholar]
- 318.Zhang M, Fishman Y, Sher D, Zlotkin E. Hydralysin, a novel animal group-selective paralytic and cytolytic protein from a noncnidocystic origin in hydra. Biochemistry 42: 8939–8944, 2003. [DOI] [PubMed] [Google Scholar]
- 319.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem 286: 12820–12824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zitzer A, Palmer M, Weller U, Wassenaar T, Biermann C, Tranum-Jensen J, Bhakdi S. Mode of primary binding to target membranes and pore formation induced by Vibrio cholerae cytolysin (hemolysin). Eur J Biochem 247: 209–216, 1997. [DOI] [PubMed] [Google Scholar]
- 321.Zitzer A, Wassenaar TM, Walev I, Bhakdi S. Potent membrane-permeabilizing and cytocidal action of Vibrio cholerae cytolysin on human intestinal cells. Infect Immun 65: 1293–1298, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84: 1404–1407, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]