Abstract
South Asians (SA) are at higher risk of cardiometabolic disorders than Europeans (EU), yet the potential determinants of this risk are poorly understood. We tested the hypotheses that 1) South Asians (SA) have greater muscle inflammation compared to Europeans (EU) at similar fat mass 2) differential regional adiposity in SA compared to EU is associated with enhanced muscle inflammation in SA. This cross-sectional study was conducted at a tertiary academic center in Hamilton, Ontario, Canada. The study included 29 EU and 26 SA. Quantitative real-time PCR and western blot were used to measure muscle inflammation. Statistical analysis was done using a General Linear Model. Despite having similar macrophage content to EU, SA muscle had lower levels of chemokine CCL2 compared to EU at gene expression (β -1.099, SE β 0.521, p-value 0.04) and protein (0.84 ± 0.69 versus 1.10 ± 0.60, p-value 0.052) levels. SA had more pronounced abdominal and hepatic adiposity, with smaller Intramyocellular lipid particles compared to EU (0.26 ± 0.12 μm2 versus 0.15 ± 0.06 μm2, p-value 0.02). In conclusion, CCL2 downregulation in SA may be an attempt to protect muscle against macrophage infiltration, and defects in fatty acid partitioning to muscle may lead to the disproportionate adiposity and adverse cardiometabolic profile in SA.
Nearly 2.1 Billion people are overweight or obese worldwide, but obesity affects certain ethnic groups disproportionately1,2. South Asians (SA), encompassing people originating from India, Pakistan, Sri Lanka and Bangladesh with a significant global diaspora3,4,5, have higher rates of obesity, type 2 diabetes and cardiovascular disease at lower body mass index (BMI) compared to Caucasian Europeans (EU)6,7. At similar BMI levels, SA have higher visceral adiposity compared to EU8. In addition, one study suggested that SA have higher Intramyocellular lipids (IMCL) than EU9.
The expansion of different fat depots leads to distinctive consequences on metabolic profiles. Higher visceral adiposity is associated with immune system activation and adipose tissue inflammation10,11,12,13,14,15,16,17,18,19, which can be explained by excess fatty acids, cytokines and chemokines in adipose tissue attracting circulating immune cells, including monocytes, neutrophils, and T-Lymphocytes10,11,12,13,14,15,16,17,18,19. Monocyte attraction into tissues depends on several chemokines, and one of the most important chemokines involved in this process is Chemokine (C-C motif) Ligand 219. Monocytes will sense local adipose tissue milieu and differentiate to macrophages that secrete inflammation-propagating cytokines14,20,21,22. On the other hand, the subcutaneous adipose tissue compartment has been proposed to have favorable metabolic characterisitics, but is SA is characterized by increased adipocyte size and low-grade systemic inflammation with insulin resistance23,24.
While studies have focused on understanding inflammation in adipose tissue10,11,12,13,14,15,16,17,18, and showed an excess of macrophages in obese adipose tissue in EU, there are no data regarding the effect of systemic (total fat mass) and regional adiposity, including visceral adipose tissue (VAT), intrahepatocellular fat, and intramyocellular lipids (IMCL) on inflammation in skeletal muscle in SA compared to EU25,26,27. As muscle is the supreme metabolic tissue for postprandial glucose uptake28,29,30, understanding the mechanisms of muscle inflammation may help manage its adverse effects on muscle insulin signaling and myocellular fat metabolism, and develop better understanding of the potential contribution of inflammation to insulin resistance and excess cardiometabolic risk in SA compared to EU.
In this study, we tested the association of systemic and regional adiposity with muscle inflammation in SA and EU. We hypothesized that 1) SA have greater muscle inflammation compared to EU at similar total fat mass, and 2) Differential regional adiposity in SA is associated with enhanced muscle inflammation compared to EU.
The primary aim of this study is to determine if SA have more muscle inflammation compared to EU. The secondary aims of this study are to determine if SA have differential adiposity patterns compared to EU, and if these depots are associated with a specific inflammatory profile in muscle that differs between SA and EU.
Results
Clinical & biochemical characteristics of participants
Table 1 compares the clinical and biochemical details of SA (n = 26, 7 female) and EU (n = 29, 16 female).
Table 1. Clinical & biochemical characteristics for participants.
| Variable | SA | EU | T-test(p-value) |
|---|---|---|---|
| Total number | 26 | 29 | |
| Age (years) | 35.30 ± 11.10 | 32.20 ± 10.30 | 0.27 |
| Female (n) | 7 | 16 | 0.08 |
| Overweight/Obese (n) | 15 | 22 | |
| Systolic BP (mmHg) | 112.50 ± 10.20 | 110.80 ± 9.50 | 0.49 |
| Diastolic BP (mmHg) | 73.70 ± 9.00 | 73.10 ± 6.50 | 0.65 |
| Body composition & adiposity | |||
| BMI (kg/m2) | 26.20 ± 3.50 | 27.30 ± 5.20 | 0.39 |
| WHR | 0.89 ± 0.09 | 0.84 ± 0.07 | 0.016 |
| Fat mass (kg) | 23.90 ± 8.10 | 25.70 ± 13.10 | 0.53 |
| Fat mass % | 32.0 ± 9.20 | 32.20 ± 13.20 | 0.95 |
| Lean mass (kg) | 50.50 ± 9.50 | 51.90 ± 10.00 | 0.58 |
| Visceral adipose tissue area (cm2, N = 55) | 141.70 ± 15.10 | 66.00 ± 14.10 | 0.001 |
| Subcutaneous adipose tissue area (cm2, N = 38) | 219.10 ± 82.40 | 244.60 ± 105.00 | 0.31 |
| Intrahepatocellular fat % | 9.90 ± 1.70 | 2.50 ± 1.90 | 0.005 |
| Biochemical measurements | |||
| Fasting glucose (mmol/l) | 5.00 ± 0.55 | 4.80 ± 0.44 | 0.06 |
| Fasting insulin (pmol/l) | 8.80 ± 4.10 | 8.00 ± 5.80 | 0.26 |
| HOMA-IR | 2.45 ± 1.70 | 1.70 ± 1.30 | 0.07 |
| Cholesterol (mmol/l) | 4.60 ± 0.99 | 4.80 ± 1.05 | 0.43 |
| Triglycerides (mmol/l) | 1.57 ± 1.16 | 1.12 ± 0.60 | 0.06 |
| HDL (mmol/l) | 1.20 ± 0.27 | 1.39 ± 0.37 | 0.08 |
| LDL (mmol/l) | 2.70 ± 0.80 | 2.80 ± 0.80 | 0.58 |
| Apo B (mmol/l) | 0.91 ± 0.30 | 0.93 ± 0.29 | 0.80 |
SA = South Asian; EU = European; BMI = body mass index; WHR = waist-to-hip ratio; BP = blood pressure; HOMA-IR = Homeostatic model assessment-insulin resistance; HDL = high-density lipoprotein; LDL=low-density lipoprotein; Apo B = Apolipoprotein B; V02 max = maximal oxygen uptake.
Fitness levels were equal in both groups (VO2 (ml/kg) SA 4.14 ± 1.08 versus EU 4.29 ± 1.17, p-value 0.56). SA had a higher trend for fasting blood glucose (5.00 ± 0.55 mmol/l versus 4.80 ± 0.44 mmol/l, p-value 0.06), HOMA-IR (2.45 ± 1.70 versus 1.70 ± 1.30, p-value 0.07), triglycerides (1.57 ± 1.16 mmol/l versus 1.12 ± 0.60, p-value 0.06) and lower HDL (1.20 ± 0.27 mmol/l versus 1.39 ± 0.37 mmol/l, p-value 0.08) compared to EU.
Ethnic differences in fat depots
In the overall molSHARE study (n = 108), SA had greater fat mass, intrahepatic and VAT with higher rates of insulin resistance, lower HDL and higher triglycerides compared to EU8. In the subgroup of participants included in the current study, SA and EU have similar BMI (26.2 ± 3.5 versus 27.3 ± 5.2, p-value 0.39) and fat mass (23.9 ± 8.1 kg versus 25.7 ± 13.1 kg, p-value 0.53) (Table 1), which allows the comparison of the effects of individual fat depots on muscle inflammation between ethnic groups.
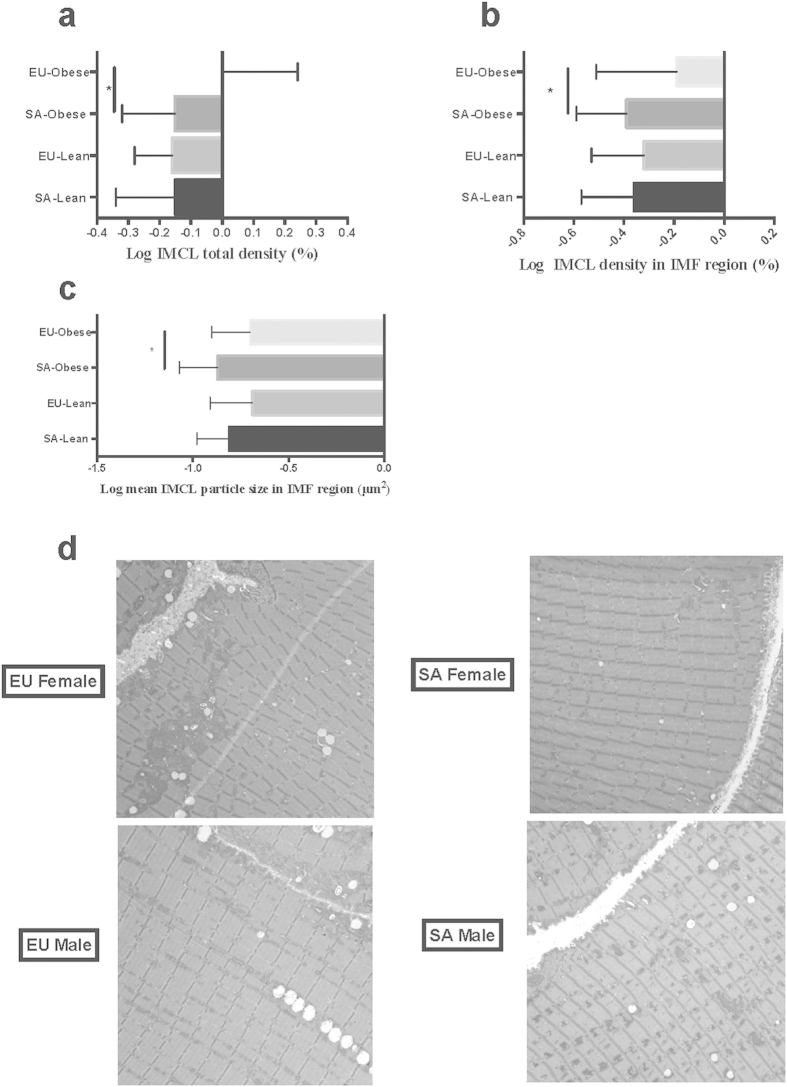
SA participants have larger VAT depot (141.70 ± 15.10 cm2 versus 66.00 ± 14.10 cm2, p-value 0.001), and increased intrahepatocellular fat (9.90 ± 1.70% versus 2.50 ± 1.90, p-value 0.005) compared to EU (Table 1). In contrast to higher visceral and hepatic adiposity, SA have decreased IMCL density in the IMF region compared to EU (0.41±0.24% versus 0.66±0.50%, p-value 0.04; Fig. 1 a & b); this is due to smaller IMCL particles in the IMF region in the overweight/obese SA compared to overweight/obese EU in both sexes (0.15±0.06 μm2 versus 0.26±0.12 μm2, p-value 0.02, Fig. 1c & d), with similar number of particles noted in the IMF region. Lean SA and EU had similar IMCL particle size in IMF region (Table 2). IMCL density in subsarcolemmal region correlated positively with HOMA-IR (p-value 0.04).
Figure 1. Obese SA have smaller IMCL particles in IMF compared to EU.
(a) Log IMCL total density (%); (b) Log IMCL density in IMF region (%); (c) Log mean IMCL particle size in IMF region (μm2); (d) Representative electron microscopy images for IMCL for SA and EU males and females.
Table 2. Log IMCL density, particle size and number in lean and obese SA and EU.
| Log Variable | Lean |
Overweight/obese |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | SD | EU | SD | Mean difference | 95%CI | P-value | SA | SD | EU | SD | Mean difference | 95%CI | P-value | |||
| IMCL density in SS region (%) | 0.74 | 0.15 | 0.62 | 0.18 | 0.12 | −0.03 | 0.27 | 0.11 | 0.69 | 0.29 | 0.82 | 0.27 | −0.13 | −0.33 | 0.07 | 0.19 |
| IMCL density in IMF region (%) | −0.36 | 0.21 | −0.32 | 0.21 | −0.04 | −0.22 | 0.15 | 0.70 | −0.39 | 0.20 | −0.19 | 0.32 | −0.19 | −0.37 | −0.01 | 0.04 |
| IMCL total density (%) | −0.15 | 0.19 | −0.16 | 0.12 | 0.01 | −0.13 | 0.15 | 0.88 | −0.15 | 0.17 | 0.001 | 0.24 | −0.15 | −0.30 | −0.004 | 0.045 |
| Mean IMCL particle size in SS region (μm2) | −0.63 | 0.17 | −0.54 | 0.10 | −0.09 | −0.21 | 0.04 | 0.17 | −0.65 | 0.19 | −0.67 | 0.22 | 0.02 | −0.13 | 0.17 | 0.79 |
| Mean IMCL particle size in IMF region (μm2) | −0.81 | 0.17 | −0.69 | 0.22 | −0.12 | −0.30 | 0.06 | 0.18 | −0.87 | 0.20 | −0.70 | 0.20 | −0.17 | −0.31 | −0.03 | 0.02 |
| Mean total IMCL particle size (μm2) | −0.72 | 0.14 | −0.64 | 0.18 | −0.08 | −0.29 | 0.06 | 0.27 | −0.78 | 0.18 | −0.67 | 0.19 | −0.11 | −0.24 | 0.03 | 0.12 |
| IMCL number of particles in SS region (#/μm2) | −0.58 | 0.15 | −0.65 | 0.11 | −0.08 | −0.22 | 0.06 | 0.23 | −0.64 | 0.18 | −0.64 | 0.14 | −0.01 | −0.12 | 0.10 | 0.90 |
| IMCL number of particles in IMF region (#/μm2) | −1.60 | 0.19 | −1.56 | 0.20 | −0.04 | −0.21 | 0.14 | 0.66 | −1.52 | 0.16 | −1.50 | 0.20 | −0.02 | −0.15 | 0.11 | 0.70 |
| Mean total IMCL particle (#/μm2) | −1.44 | 0.16 | −1.41 | 0.16 | −0.03 | −0.18 | 0.12 | 0.65 | −1.39 | 0.13 | −1.38 | 0.16 | −0.002 | −0.10 | 0.10 | 0.97 |
SS = subsacrcolemmal; IMF = intermyofibrillar; IMCL = intramyocellular lipids.
Ethnic differences in muscle inflammation
To test the effect of ethnic variations on muscle inflammation, gene and protein expression analyses were undertaken to measure cytokine, chemokine/chemokine receptor, TLR, and immune cell markers including total macrophage content (CD68), inflammatory macrophages (CD11c), resident macrophages (MRC1) and neutrophils (MPO). In the unadjusted analyses, fat mass percentage (%FM) correlated with several inflammatory and immune markers (TNFα, CCL2, CCL3, IL-8, CCR2, TLR4, NLRP3, CD68, and MPO; Table 3). Higher VAT content is associated with reduced resident macrophages (MRC1) in muscle, and higher VAT and intrahepatocellular fat content is associated with higher muscle neutrophil content (MPO) (Table 3). Interestingly, IMCL did not correlate with the inflammatory and immune cell markers tested. From this point onwards, we included only the markers that showed correlation with the %FM in further analyses.
Table 3. Unadjusted univariate analysis of muscle gene expression of immune and inflammatory markers correlated with different fat depots.
| Marker | %FM |
IMCL |
VAT |
Intrahepatocellular fat |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE β | P-value | β | SE β | P-value | β | SE β | P-value | β | SE β | P-value | |
| Cytokines | ||||||||||||
| TNFα | −0.014 | 0.006 | 0.02 | 0.073 | 0.139 | 0.60 | 0.001 | 0.001 | 0.92 | 0.000 | 0.009 | 1.00 |
| IL-6 | 0.000 | 0.008 | 0.98 | 0.117 | 0.185 | 0.53 | 0.001 | 0.001 | 0.25 | 0.017 | 0.011 | 0.14 |
| IL-10 | 0.003 | 0.012 | 0.83 | 0.061 | 0.256 | 0.81 | 0.001 | 0.001 | 0.64 | 0.014 | 0.016 | 0.39 |
| Chemokine/receptor | ||||||||||||
| CCL2 | 0.017 | 0.007 | 0.02 | 0.209 | 0.166 | 0.21 | 0.000 | 0.001 | 0.69 | 0.010 | 0.010 | 0.34 |
| CCL3 | −0.019 | 0.010 | 0.08 | 0.349 | 0.228 | 0.13 | 0.001 | 0.001 | 0.50 | 0.006 | 0.014 | 0.66 |
| IL-8 | 0.018 | 0.010 | 0.09 | 0.311 | 0.225 | 0.17 | 0.001 | 0.001 | 0.67 | 0.001 | 0.014 | 0.97 |
| CCR2 | 0.015 | 0.007 | 0.04 | 0.069 | 0.155 | 0.66 | 0.000 | 0.001 | 0.76 | 0.001 | 0.010 | 0.95 |
| TLRs | ||||||||||||
| TLR2 | 0.004 | 0.008 | 0.57 | 0.213 | 0.171 | 0.22 | 0.000 | 0.001 | 0.89 | 0.001 | 0.011 | 0.96 |
| TLR4 | −0.017 | 0.009 | 0.07 | 0.099 | 0.205 | 0.63 | 0.000 | 0.001 | 0.83 | 0.004 | 0.013 | 0.73 |
| Inflammasome | ||||||||||||
| IL-1β | −0.007 | 0.010 | 0.48 | 0.170 | 0.212 | 0.43 | 0.001 | 0.001 | 0.25 | 0.015 | 0.013 | 0.24 |
| IL-18 | −0.009 | 0.012 | 0.45 | 0.076 | 0.264 | 0.78 | 0.002 | 0.001 | 0.13 | 0.004 | 0.016 | 0.82 |
| NLRP3 | −0.023 | 0.010 | 0.02 | 0.279 | 0.225 | 0.22 | 0.001 | 0.001 | 0.34 | 0.018 | 0.014 | 0.19 |
| Immune cells | ||||||||||||
| CD68 | −0.020 | 0.010 | 0.05 | 0.217 | 0.224 | 0.34 | 0.001 | 0.001 | 0.55 | 0.009 | 0.014 | 0.50 |
| MRC1 | −0.010 | 0.008 | 0.23 | 0.223 | 0.185 | 0.23 | 0.003 | 0.001 | 0.01 | 0.007 | 0.012 | 0.55 |
| CD11c | −0.011 | 0.007 | 0.13 | 0.258 | 0.159 | 0.11 | 0.000 | 0.001 | 0.65 | 0.007 | 0.010 | 0.52 |
| MPO | 0.065 | 0.017 | 0.0003 | 0.017 | 0.424 | 0.97 | 0.004 | 0.002 | 0.08 | 0.044 | 0.025 | 0.09 |
In order to determine the factors influencing the relationship of adiposity (%FM) to muscle inflammation, we included TNFα, CCL2, CCL3, IL-8, CCR2, TLR4, NLRP3, CD68, and MPO in General Linear Model and adjusted for age, sex, BMI, HOMA-IR, fitness and ethnicity. SA had higher CD68 (β 1.411, SE β 0.692, p-value 0.047) and lower CCL2 gene expression (β -1.099, SE β 0.531, p-value 0.040) in muscle when compared to EU (Table 4; Fig. 2). There were no significant associations of VAT, hepatic fat and IMCL with TNFα, CCL2, CCL3, IL-8, CCR2, TLR4, NLRP3, CD68, and MPO (data not shown).
Table 4. South Asians have higher muscle CD68 and lower CCL2 gene expression compared to Europeans.
| Marker | β | SE β | P-value |
|---|---|---|---|
| CD68 | 1.411 | 0.692 | 0.047 |
| CCR2 | 0.041 | 0.533 | 0.939 |
| MPO | −0.584 | 1.031 | 0.574 |
| TNFα | −0.284 | 0.408 | 0.491 |
| CCL2 | −1.099 | 0.521 | 0.040 |
| CCL3 | −0.037 | 0.735 | 0.960 |
| IL-8 | −0.651 | 0.795 | 0.417 |
| TLR4 | 0.585 | 0.700 | 0.408 |
| NLRP3 | 0.105 | 0.768 | 0.892 |
General linear model addressing the relationship between inflammatory markers and %FM in South Asians compared to Europeans adjusted for age, sex, BMI, HOMA-IR, fitness and ethnicity.
Figure 2. Gene expression analysis of CD68 and CCL2 for SA and EU.
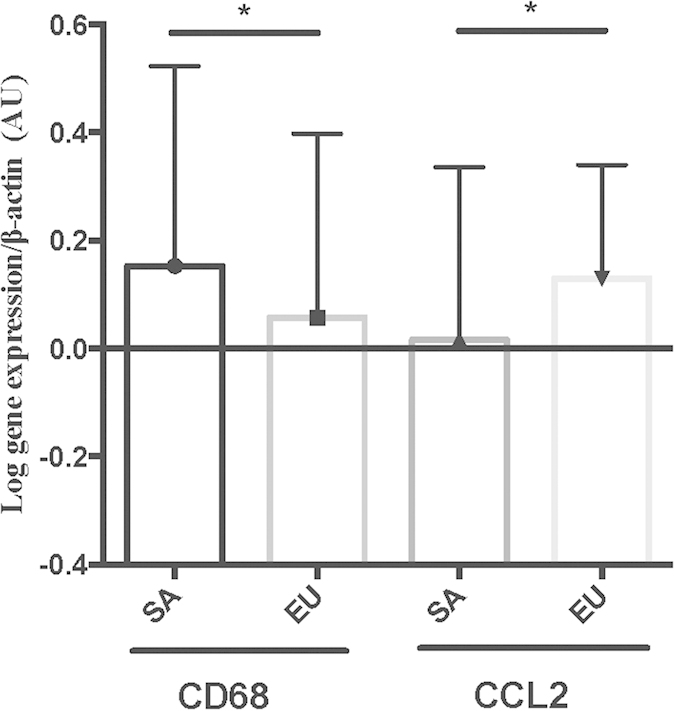
Protein quantification using western blot for CD 68 and CCL2 revealed that CD68 muscle protein content is similar in SA and EU (0.92 ± 0.52 versus 0.98 ± 0.35, p-value 0.63), and there was no correlation between ethnicity and CD68 (p-value 0.37). On the other hand, SA have lower CCL2 muscle protein levels than EU (0.84 ± 0.69 versus 1.10 ± 0.60, p-value 0.052) (Fig. 3a,b). When the whole group is analyzed, neither CD68 nor CCL2 protein correlated with the different fat depots or BMI (data not shown).
Figure 3. Muscle protein expression of CD68 and CCL2 in SA and EU.
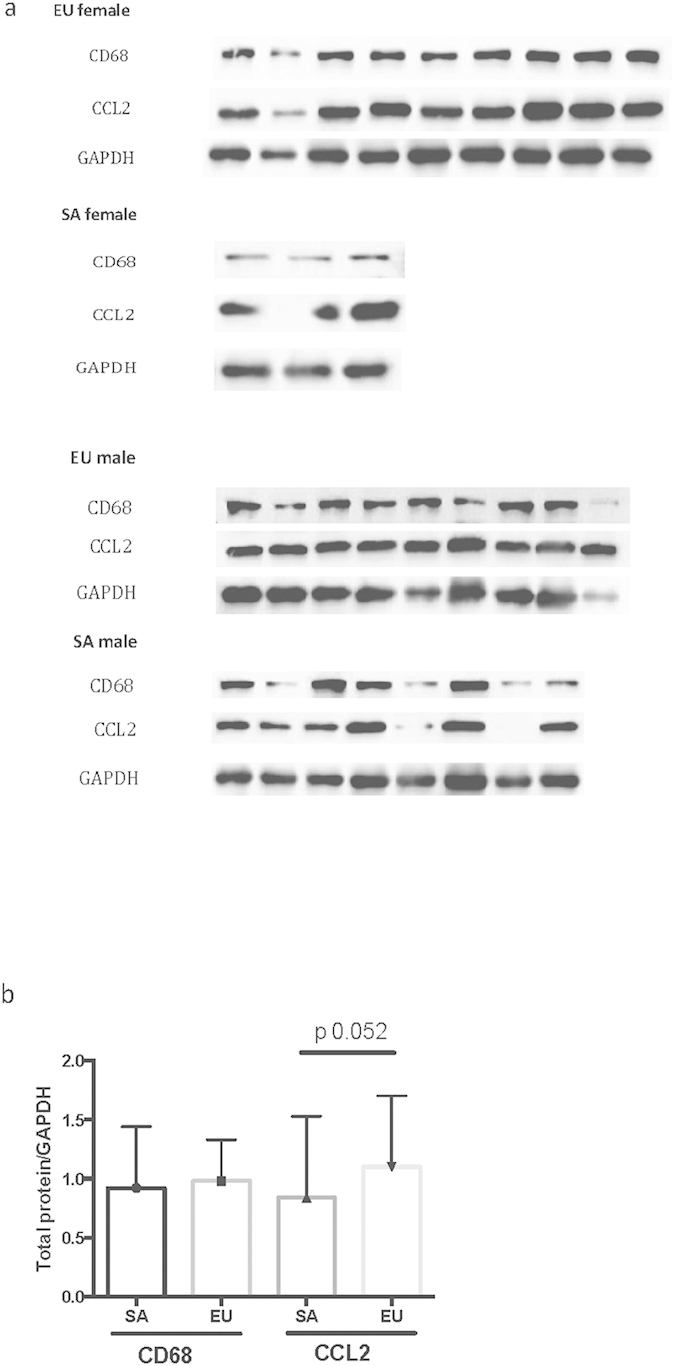
(a) Western blot data for CD68, CCL2, and GAPDH as control. CD68 produces a band at 110 kDa, CCL2 30 kDa, and GAPDH 37 kDa. (b) Quantification of western blot result.
Discussion
In this study of seemingly healthy, non-diabetic subjects with similar age, BMI, and total fat mass, we demonstrate that SA have similar total muscle macrophage content (CD68) and lower monocyte/macrophage chemokine CCL2 compared to EU. SA had smaller IMCL particles in their muscle compared to EU.
In the literature, the evidence describing the relationship of muscle inflammation and adiposity is limited by small sample size, various laboratory techniques used, differences in populations studied, and different statistical methods used for data analysis. Some studies show that muscle macrophages correlate positively with BMI and inversely with insulin sensitivity25,31,32,33. In contrast, the association of muscle macrophages with BMI or adiposity has not been replicated in other studies26,27,34. Our results demonstrate that when samples are pooled in the analysis, muscle CD68 content does not correlate with total adiposity, BMI or the different fat depots.
We predicted that the greater VAT and intrahepatic fat depots in SA are associated with a more prominent inflammatory response in muscle compared to EU. Despite stark differences in regional adiposity patterns, no differences are detected in muscle macrophage content, macrophage subtype and other inflammatory markers between SA and EU when VAT, hepatic fat and IMCL are examined for their association with muscle inflammation. This argues for additional indirect mechanisms through which higher regional adiposity associates with muscle inflammation in different ethnic groups, or that inflammation may play a role later once other risk factors e.g. aging, dyslipidemia or dysglycemia are present, as this group is relatively healthy.
It is intriguing that similar CD68 content in SA muscle is witnessed despite the downregulation of one of the main monocyte/macrophage chemokines (CCL2). Polymorphisms in CCL2 gene in SA are associated with enhanced visceral adiposity35. There are two possibilities that may explain this paradoxical result of equal macrophage content with lower CCL2 in SA compared to EU. There may be additional chemokines that regulate monocyte/macrophage trafficking into SA muscle, so that even when CCL2 is downregulated these chemokines are upregulated to drive monocyte migration into muscle.
Alternatively, the downregulation of CCL2 in SA is an attempt to protect muscle against further monocyte infiltration. The overexpression of CCL2 in adipose tissue in mice is associated with enhanced monocyte recruitment36, and CCL2 knockout mice exhibit reduced adipose inflammation and macrophage infiltration19.
We also demonstrate lower IMCL particle size in IMF region in SA compared to EU. This suggests reduced rates of substrate delivery to muscle that may be due to endothelial dysfunction37,38,39,40,41, and reduced fatty acids available for oxidation. This is consistent with reports of reduced fatty acid oxidation in SA muscle with normal oxidative and lipid metabolism gene expression when compared to EU37. Morbidly obese, insulin resistant subjects have reduced fatty acid oxidation, and this is related to insulin resistance42. These studies are in contrast to other studies that revealed that SA are capable of increased oxidative capacity in association with insulin resistance43. Smaller IMCL particles in SA in our study may explain the differences in ectopic fat deposition between SA and EU. One possibility is that this is an adaptive response by muscle to prevent excess fatty acid supply; the inability to partition excess fatty acids into muscle may then result in larger liver and VAT fat depots, which is associated with adverse cardiometabolic outcomes in SA.
Our results are in contrast to a study that examined IMCL in 20 SA and 20 EU participants by proton magnetic resonance spectroscopy of the soleus muscle, and demonstrated that SA have higher IMCL content than EU9. While SA had higher fat mass and lower insulin sensitivity compared to EU, IMCL did not relate to obesity or insulin sensitivity in SA9. The difference in results between this study and ours may be explained by the difference in the methods used to quantify IMCL, and the different muscles used in this quantification. IMCL is highest in type I fibers (slow twitch, oxidative), and soleous muscle that was used in the above study has more type 1 fibers than the vastus lateralis muscle used in our study9,44,45,46,47. In conclusion, the different muscles studied, different methodologies in quantifying IMCL, and potential differences in the population sampled may explain the differences in these results. One of the major strengths of our study is that we evaluated the effect of regional fat depots, measured using state of the art methods, on muscle directly as the total fat mass, BMI and insulin resistance are similar between SA and EU.
Our study has several additional strengths. The relatively large sample size, well-characterized fat depots, and detailed muscle gene expression analyses of inflammatory markers, and protein analysis of CD68 and CCL2 provide a comprehensive evaluation of muscle inflammation. In addition, our statistical analyses were rigorously performed with a priori selected confounding factors to account for differences between the two ethnic groups.
Measuring muscle cytokines and lipid intermediates including diacylglycerol and ceramide in muscle would have been advantageous, because they may be associated with muscle inflammation. However, this requires significant amounts of muscle tissue that is difficult to access due to the invasive nature of the procedure, and obtaining a large biopsy from healthy donors are significant challenges.
In addition, another fat depot that we did not study is the intermuscular adipose tissue. Skeletal muscle has its own complement of fibro/adipogenic progenitors (FAPs). They represent a pool of cells that has been recently defined in the perivascular region in human muscle48. These cells participate in muscle homeostatic and injury responses in collaboration with myogenic progenitors and macrophages49,50. These cells contribute to intermuscular adipose tissue, which expands in lean and overweight men with the metabolic syndrome regardless of ethnicity51. The intermuscular adipose tissue also expands in other conditions including obesity, excess fatty acids, hypoxia and inflammation30.
In addition, recent evidence suggests that obesity can result in sex-specific muscle fiber organization changes. In obese male mice, the major Type I muscle fiber (slow twitch, oxidative) in soleus muscle rises in proportion to adiposity. There is also transformation of Type I fibers to a hybrid I/IIb type (IIb fast twitch, glycolytic) with male obesity that was not seen in female mice52. While vastus lateralis is mainly composed of Type I fibers, the changes in intermuscular fat and how they impact fiber type organization, and the ethnic differences in these responses will require further study.
In summary, this study reveals that SA have similar muscle macrophage content to EU despite downregulation of CCL2, an important monocyte chemokine. In addition, the noted ethnic differences in fat deposition into muscle may explain the differential patterns of fat accumulation in metabolic organs, which increases the risk of cardiometabolic problems known to affect SA at higher rates than EU.
Further investigation into the genetic, epigenetic, environmental and lifestyle factors influencing fat depot development and immune cell traffic into muscle in different ethnic groups is warranted, to enhance our understanding of the effects of ethnicity on skeletal muscle inflammation and fatty acid partitioning into metabolic organs. This may allow the design of targeted therapies to address inflammation and fatty acid deposition in muscle as potential mechanisms associated with adverse cardiometabolic outcomes in SA.
Methods
This was a cross-sectional study conducted using muscle biopsy samples from the Molecular Study of Health and Risk in Ethnic Groups (Mol-SHARE) cohort, the protocol for which has been previously described8. The primary objective of Mol-SHARE study is to determine the differences in adiposity, adipose tissue inflammation and morphology, and muscle lipid and fatty acid oxidation and mitochondrial function between SA and EU8.
The full study included 18–50 year old subjects (n = 108) who were recruited via mailed notices for the study, and by advertising in temples, Hamilton hospitals, and McMaster University campus. SA and EU subjects who were age, sex and BMI matched, and grouped across different BMI levels (lean = 18.5–25 kg/m2, overweight = 25.1–29.9 kg/m2, and obese = 30–45 kg/m2. We used a subgroup of the total study population for whom muscle tissue was available (SA n = 26, EU n = 29). The Hamilton Health Sciences Research Ethics Board approved the study, and written informed consent was obtained from all participants. The study was conducted in accordance with appropriate clinical practice guidelines and national legal requirements.
Fat depot quantifications
The initial body composition measurement and fat mass determination was done using DEXA scans. To quantify abdominal visceral and subcutaneous adipose tissue, a T1-weighted MRI image at the level of mid-L4 (TR 400 ms, TE 13 ms) was acquired. The volume of subcutaneous and visceral fat was determined by manual tracing of the areas of fat. Intrahepatocellular lipids were measured using single voxel MRS (TR 1500 ms, TE 30 ms, 8 averages, 1024 data points over 1000 Hz spectral width, 1–2 cc voxel volume, and acquisition time 18 s, without water suppression).
The IMCL compartment was measured using electron microscopy53,54. Briefly, freshly biopsied muscle tissue was placed into 2% glutaraldehyde and subjected to transmission electron microscopy for quantification of lipid droplet characteristics. These include the number and density of lipid droplets in subsacrcolemmal (SS) and intermyofibrillar (IMF) regions, mean droplet area, and the percentage of lipids touching mitochondria were measured using image Pro Plus software (Media Cybernetics, Silver Springs, MD). Researchers completing the analyses were blinded to the participants’ ethnicity.
Fitness testing
Fitness testing was done using an electronically braked cycle ergometer (Excalibur Sport, Lode, Groningen, The Netherlands). Subject breathed at rest through a mouthpiece for 5 minutes before commencing the test while seated. Subjects began the cycle test with stage 1 at 15 watts for 5 min, followed by stage 2 at 50 watts for 10 minutes, with total test duration of 15 minutes. Computerized open-circuit gas collection system (Moxus Modulator VO2 system with O2 analyzer S-3 A/I and CO2 analyzer CD-3A, AEI Technologies, Pittsburgh, PA) was used to collect data (Respiratory Exchange Ratio, heart rate, VO2, VCO2) at all time points.
Biochemical Analyses
Participants fasted for 12 hours prior to blood sampling. Enzymatic methods were used to measure total serum cholesterol55 and glucose56. Serum LDL cholesterol was measured using the Friedewald formula57, and a homogenous enzymatic colorimetric assay (ROCHE/Hitachi Modular Package Insert) was used to measure HDL cholesterol. Triglycerides were quantified by the enzymatic colorimetric assay on ROCHE/Hitachi Modular instrument and reagent kit. Insulin was quantified using the Roche Elecsys R 2010 immunoassay analyzer with electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Indianapolis, Indiana, USA). Serum C-Reactive Protein (CRP) was measured using the Roche Hitachi 917 using the Tina-quant R CRP high sensitivity assay.
Gene expression analysis
A Vastus lateralis muscle biopsy was performed as described previously8. Muscle was powdered in liquid nitrogen and added to 1 ml of Trizol. The sample was then homogenized in Trizol and RNA isolation was completed using Qiagen RNAeasy mini kits (Qiagen, Valencia, CA) following manufacturer’s instructions. The quality of the RNA was tested using nanospectrophotometer and all RNA samples had 260/280 ratio of 1.8–2.0. cDNA generation was performed with SuperScript® III reverse transcriptase kit (Invitrogen, Carlsbad, CA) following DNase treatment.
Gene expression analysis was performed for cytokines including Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNFα), Interleukin-10 (IL-10), and chemokines including Chemokine (C-C motif) Ligand 2 (CCL2), Chemokine (C-C motif) Ligand 3 (CCL3), Interluekin-8 (IL-8), and Chemokine (C-C motif) Ligand 20 (CCL20), along with the chemokine receptor for CCL2 called Chemokine (C-C motif) Receptor 2 (CCR2). Innate immune system components were also tested including Toll-Like Receptors 2 & 4 (TLR2 & TLR4).
Innate immune cells including total macrophages (CD68), inflammatory macrophages/dendritic cell (CD11c), resident macrophages (Mannose Receptor, C Type 1, MRC1) and neutrophils (Myeloperoxidase, MPO) were examined. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome and cytokines produced by its activation, including Interluekin-1β (IL-1β) and Interleukin-18 (IL-18) were measured.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) was conducted using TaqMan® Gene Expression Assays (Applied Biosystems; Foster City, CA) using the Rotor-Gene 6000 qRT-PCR machine (Corbett Research; Mortlake, Australia). β-actin was chosen as the reference gene after testing additional reference genes including Ribosomal Protein, Large, P0 (RPLP0) and TATA-box Binding Protein and gene expression was computed using the ΔΔCt method58. The chemokine CCL20 was tested but did not result in a PCR product.
Western blot
The quantification of CD68 and CCL2 protein expression was done using western blot. Briefly, 20 mg of protein were loaded on a 7.5% Tris-Glycine gel using BioRad Mini-PROTEAN Tetra cell electrophoresis chamber. Protein transfer from the gel to nitrocellulose membrane was performed using the BioRad Mini-Transblot Cell running at 100 V for 1 hour at 4 °C. Blots were blocked with 5% Bovine Serum Albumin in TBST buffer with shaking for 1 hour at room temperature. Membranes were incubated with primary CD68 and CCL2 antibodies (Pierce) or GAPDH (Cell Signalling) that were diluted 1:1000 in 5% BSA in TBST overnight at 4 °C. Blots were then washed thrice with TBST, and were then incubated with HRP conjugated secondary antibody (1:1000), with shaking for 1 hour at room temperature and then washed thrice with TBST. Blots were treated with Amersham ECL Western Blotting Detection Reagents for 1 minute and signals detected using Vilber Fusion Imaging machine and software. All bands were quantified using Image J software59.
Statistical analyses
Statistics were performed using SPSS version 20.0 (IBM Corp, Armonk, NY) and Stata/SE 12.1 (Stata Corporation, College Station, TX). Data were examined for normality using the Shapiro-Wilk test, and log transformed if not normally distributed. We imputed missing values of inflammatory markers based on the mean value of the specific inflammatory marker within the corresponding ethnic group. Testing for co-linearity of the cytokine genes and covariates was done using variance inflation factor.
An independent sample t-test was used to examine the differences among clinical variables and metabolic data. A General linear model (GLM) was used to test the association of cytokine gene expression with fat mass percentage (%FM), visceral adipose tissue (VAT), intrahepatocellular fat, and IMCL. The analysis was done initially with no adjustment, and inflammatory and immune markers that correlated with the fat mass with a p-value of 0.1 or less were included in the adjusted analysis, with inclusion of covariates including age, sex, BMI, homeostatic model assessment-insulin resistance (HOMA-IR), fitness levels and ethnicity in the model. Because we had a priori hypothesized the role of the different biomarkers chosen in muscle-immune crosstalk, adjustments for multiple testing are not performed when assessing the significance of these relationships60. Quantification of western blots was adjusted for age, sex and ethnicity. Data are reported as mean ± SD unless otherwise stated, and statistical significance was set at p-value of <0.05.
Additional Information
How to cite this article: Samaan, M.C. et al. Adiposity and immune-muscle crosstalk in South Asians & Europeans: A cross-sectional study. Sci. Rep. 5, 14521; doi: 10.1038/srep14521 (2015).
Acknowledgments
We would like to acknowledge the participants in the study. We also thank Dr. Mohammed Khan (MK) and Ms. Vivian Leong for laboratory technical assistance. MCS is supported by the New Investigator Fund Grant from Hamilton Health Sciences (Grant no. NIF-11272). Dr. Anand is supported by a Canada Research Chair in Ethnicity and Cardiovascular Disease and the Heart and Stroke Foundation Michael DeGroote Chair in Population Health Research. Mol-SHARE study is funded by Heart & Stroke Foundation of Ontario, Canada (Project#: NA5702).
Footnotes
Author Contributions M.C.S., M.A.T. and S.S.A. conceived the study hypothesis. M.C.S., M.A.T., S.S.A., A.M.S. and I.A.S. conceived and designed the experiments. M.C.S., M.K., VL and I.A.S. performed experimental work. M.C.S., J.B., A.B., M.A.T. and S.S.A. analyzed the data and M.C.S. wrote the first draft, and all authors provided feedback to the submitted version of the manuscript.
References
- Campos P., Saguy A., Ernsberger P., Oliver E. & Gaesser G. The epidemiology of overweight and obesity: public health crisis or moral panic? International journal of epidemiology 35, 55–60, 10.1093/ije/dyi254 (2006). [DOI] [PubMed] [Google Scholar]
- Ng M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384, 766–781, 10.1016/S0140-6736(14)60460-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Munter J. S., Agyemang C., van Valkengoed I. G., Bhopal R. & Stronks K. Sex difference in blood pressure among South Asian diaspora in Europe and North America and the role of BMI: a meta-analysis. J Hum Hypertens 25, 407–417, 10.1038/jhh.2010.77 (2011). [DOI] [PubMed] [Google Scholar]
- Madrigal L. et al. Obesity, hypertension, and migration: a meta-analysis of populations of the South Asian diaspora. Human biology 83, 71–86, 10.3378/027.083.0105 (2011). [DOI] [PubMed] [Google Scholar]
- Patel K. C. & Bhopal R. Diabetes epidemic in the South Asian Diaspora: action before desperation. J R Soc Med 100, 115–116, 10.1258/jrsm.100.3.115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S. S. et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). The Lancet 356, 279–284, 10.1016/S0140-6736(00)02502-2 (2000). [DOI] [PubMed] [Google Scholar]
- Yusuf S., Reddy S., Ounpuu S. & Anand S. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 104, 2855–2864, 10.1161/hc4701.099488 (2001). [DOI] [PubMed] [Google Scholar]
- Anand S. S. et al. Adipocyte Hypertrophy, Fatty Liver and Metabolic Risk Factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6, e22112, 10.1371/journal.pone.0022112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouhi N. G. et al. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia 42, 932–935, 10.1007/s001250051250 (1999). [DOI] [PubMed] [Google Scholar]
- Samaan M. C. The macrophage at the intersection of immunity and metabolism in obesity. Diabetology & Metabolic Syndrome 3, 29, 10.1186/1758-5996-3-29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. S. et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30, 1347–1355, 10.1038/sj.ijo.0803259 (2006). [DOI] [PubMed] [Google Scholar]
- Weisberg S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808, 10.1172/JCI19246112/12/1796 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Herrero L. & Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 132, 2169–2180, S0016-5085(07)00585-910.1053/j.gastro.2007.03.059 (2007). [DOI] [PubMed] [Google Scholar]
- Lumeng C. N., Deyoung S. M., Bodzin J. L. & Saltiel A. R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23, 56/1/1610.2337/db06-1076 (2007). [DOI] [PubMed] [Google Scholar]
- Nguyen M. T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282, 35279–35292, 10.1074/jbc.M706762200 (2007). [DOI] [PubMed] [Google Scholar]
- Xu H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112, 1821–1830, 10.1172/JCI19451112/12/1821 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgazar-Carmon V., Rudich A., Hadad N. & Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 49, 1894–1903, M800132-JLR20010.1194/jlr.M800132-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- Kintscher U. et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28, 1304–1310, ATVBAHA.108.165100 10.1161/ATVBAHA.108.165100 (2008). [DOI] [PubMed] [Google Scholar]
- Kanda H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116, 1494–1505, 10.1172/JCI26498 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C. N., DelProposto J. B., Westcott D. J. & Saltiel A. R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246, db08-087210.2337/db08-0872 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B. S. & Roberts L. Relationship between inflammatory markers, metabolic and anthropometric variables in the Caribbean type 2 diabetic patients with and without microvascular complications. J Inflamm (Lond) 3, 17, 10.1186/1476-9255-3-17 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B. S. et al. Association of low serum creatinine, abnormal lipid profile, gender, age and ethnicity with type 2 diabetes mellitus in Trinidad and Tobago. Diabetes Res Clin Pract 91, 342–347, 10.1016/j.diabres.2010.12.017 (2011). [DOI] [PubMed] [Google Scholar]
- Chandalia M. et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One 2, e812, 10.1371/journal.pone.0000812 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab 89, 4206–4210, 10.1210/jc.2004-0631 (2004). [DOI] [PubMed] [Google Scholar]
- Varma V. et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 296, E1300–1310, 90885.200810.1152/ajpendo.90885.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun J. M., Helge J. W., Richelsen B. & Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290, E961–967, 00506.2005 10.1152/ajpendo.00506.2005 (2006). [DOI] [PubMed] [Google Scholar]
- Di Gregorio G. B. et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54, 2305–2313, 54/8/2305 (2005). [DOI] [PubMed] [Google Scholar]
- Petersen K. F. & Shulman G. I. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol 90, 11G–18G (2002). [DOI] [PubMed] [Google Scholar]
- Godoy-Matos A. F. et al. Rosiglitazone decreases intra- to extramyocellular fat ratio in obese non-diabetic adults with metabolic syndrome. Diabetic medicine : a journal of the British Diabetic Association 27, 23–29, 10.1111/j.1464-5491.2009.02868.x (2010). [DOI] [PubMed] [Google Scholar]
- Vettor R. et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297, E987–998, 10.1152/ajpendo.00229.2009 (2009). [DOI] [PubMed] [Google Scholar]
- Kim T. H. et al. IL-6 induction of TLR-4 gene expression via STAT3 has an effect on insulin resistance in human skeletal muscle. Acta diabetologica 50, 189–200, 10.1007/s00592-011-0259-z (2013). [DOI] [PubMed] [Google Scholar]
- Fink L. N. et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 22, 747–757, 10.1002/oby.20615 (2014). [DOI] [PubMed] [Google Scholar]
- Fink L. N. et al. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia 56, 1623–1628, 10.1007/s00125-013-2897-x (2013). [DOI] [PubMed] [Google Scholar]
- Tam C. S. et al. Low macrophage accumulation in skeletal muscle of obese type 2 diabetics and elderly subjects. Obesity (Silver Spring) 20, 1530–1533, 10.1038/oby.2012.24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S. et al. Association of monocyte chemoattractant protein-1-2518 polymorphism with metabolic syndrome in a South Indian cohort. Metabolic Syndrome and Related Disorders 7, 193–198, 10.1089/met.2008.0064 (2009). [DOI] [PubMed] [Google Scholar]
- Kamei N. et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281, 26602–26614, M60128420010.1074/jbc.M601284200 (2006). [DOI] [PubMed] [Google Scholar]
- Hall L. M. L. et al. Fat Oxidation, Fitness and Skeletal Muscle Expression of Oxidative/Lipid Metabolism Genes in South Asians: Implications for Insulin Resistance? PLoS One 5, e14197, 10.1371/journal.pone.0014197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B., Kiens B., Savard G. & Pedersen P. K. Role of hemoglobin and capillarization for oxygen delivery and extraction in muscular exercise. Acta physiologica Scandinavica. Supplementum 556, 21–32 (1986). [PubMed] [Google Scholar]
- Hepple R. T., Mackinnon S. L., Goodman J. M., Thomas S. G. & Plyley M. J. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82, 1305–1310 (1997). [DOI] [PubMed] [Google Scholar]
- Lillioja S. et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80, 415–424, 10.1172/JCI113088 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithell H. et al. Body weight, skeletal muscle morphology, and enzyme activities in relation to fasting serum insulin concentration and glucose tolerance in 48-year-old men. Diabetes 30, 19–25 (1981). [DOI] [PubMed] [Google Scholar]
- Coen P. M. et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 21, 2362–2371, 10.1002/oby.20381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K. S. et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57, 1166–1175, 10.2337/db07-1556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson E., Esbjornsson M., Holm I. & Jacobs I. Increase in the proportion of fast-twitch muscle fibres by sprint training in males. Acta Physiol Scand 140, 359–363, 10.1111/j.1748-1716.1990.tb09010.x (1990). [DOI] [PubMed] [Google Scholar]
- Esbjornsson M. et al. Muscle fibre types and enzyme activities after training with local leg ischaemia in man. Acta Physiol Scand 148, 233–241, 10.1111/j.1748-1716.1993.tb09554.x (1993). [DOI] [PubMed] [Google Scholar]
- Simoneau J. A. & Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol 257, E567–572 (1989). [DOI] [PubMed] [Google Scholar]
- Hwang J. H., Pan J. W., Heydari S., Hetherington H. P. & Stein D. T. Regional differences in intramyocellular lipids in humans observed by in vivo 1H-MR spectroscopic imaging. J Appl Physiol (1985) 90, 1267–1274 (2001). [DOI] [PubMed] [Google Scholar]
- Uezumi A. et al. Identification and characterization of PDGFR[alpha]+ mesenchymal progenitors in human skeletal muscle. Cell death & disease 5, e1186, 10.1038/cddis.2014.161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe A. W. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology 12, 153–163, 10.1038/ncb2015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos D. R. et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med, 10.1038/nm.3869 (2015). [DOI] [PubMed] [Google Scholar]
- Goodpaster B. H. et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165, 777–783, 10.1001/archinte.165.7.777 (2005). [DOI] [PubMed] [Google Scholar]
- Denies M. S. et al. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiological reports 2, e00204, 10.1002/phy2.204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries M. C., Lowther S. A., Glover A. W., Hamadeh M. J. & Tarnopolsky M. A. IMCL area density, but not IMCL utilization, is higher in women during moderate-intensity endurance exercise, compared with men. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 293, R2336–R2342, 10.1152/ajpregu.00510.2007 (2007). [DOI] [PubMed] [Google Scholar]
- Samjoo I. A. et al. Markers of skeletal muscle mitochondrial function and lipid accumulation are moderately associated with the homeostasis model assessment index of insulin resistance in obese men. PLoS One 8, e66322, 10.1371/journal.pone.0066322 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain C. C., Poon L. S., Chan C. S., Richmond W. & Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem 20, 470–475 (1974). [PubMed] [Google Scholar]
- Neeley W. E. Simple automated determination of serum or plasma glucose by a hexokinase-glucose-6 -phosphate dehydrogenase method. Clin Chem 18, 509–515 (1972). [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I. & Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502 (1972). [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Meth 9, 671–675, 10.1038/nmeth.2089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise R. J. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2, 8, 10.1186/1471-2288-2-8 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]