Abstract
The influenza A virus is a critical public health problem that causes epidemics and pandemics, and occurs widely all over the world. Various vaccines against the virus have not provided a solution to the problem. Different approaches, particularly M2e peptide–based vaccines, are available for developing universal vaccines against influenza A. However, it is important to select a suitable carrier to obtain an effective vaccine. Accordingly, studies on the usage of various carriers are ongoing. Particularly, polymer-based carriers have gained importance due to both drug delivery and adjuvant effects. Therefore, bioconjugate of the M2e protein peptide from the influenza A virus covalent bonded with poly(acrylic) acid was synthesized in our study for the first time. The characterization was performed using size-exclusion chromatography and fluorescence spectroscopy; subsequently, it was found that the bioconjugate of the examined lower doses (0.05 and 0.5 mg/ml) have no toxic effects on human cell lines. These results suggest that, in the future, the poly(acrylic) acid bioconjugate of the M2e peptide should be studied in vivo for universal vaccine development against the influenza A virus.
Keywords: bioconjugate, influenza A virus, M2e, peptide, poly(acrylic) acid, synthetic vaccine, toxicity
Abbreviations
- M2e
The external domain of influenza A M2 protein
- BSA
bovine serum albumin
- PAA
poly(acrylic) acid
- UV
ultraviolet
- RALS
right-angle light scattering
- Trp-W
tryptophan
- Try-Y
tyrosine
- Phe-P
phenylalanine
- SEC
size-exclusion chromatography
- MCF-7
human breast cancer cell
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- DMEM-F12
dulbecco's modified eagle medium F12
- FITC
fluorescein isothiocyanate
- 7-ADD
7-aminoactinomycin D
Introduction
The influenza virus is a critical public health problem that causes epidemics and pandemics. It is known that influenza viruses can be divided into 3 genera—influenza A, B, and C. Influenza A and B cause substantial morbidity and mortality in humans, while influenza A viruses causes greater mortality in most epidemic years.
Up to now, several vaccines, such as inactive or viable attenuated virus, recombinant proteins have been developed against the influenza virus.1 The many subtypes and high mutation rates of the virus and its reassortment are the main problems related to current vaccines. Thus, peptide epitope–based vaccines have gained in importance for the development of efficient influenza vaccines.1 However, most of these types of vaccines have been tested in clinical studies and animal models, but have not been applied in clinical practice.1
M2e is the external domain of the influenza A M2 protein. It is highly conserved in human influenza A viruses; however, its immunogenicity is low. A vaccine based on the M2e protein could be effective against all of the human influenza viruses.2-5 The issue of the low immunogenicity of the M2e peptide can be overcome using a polymeric adjuvant.
Adjuvants and carrier systems are of crucial importance in developing an efficient vaccine to increase the inherently weak immunogenicity of synthetic peptides. Since the beginning of this century, various substances have been added to certain formulations in an attempt to render more effective vaccines.6 Liu et al. have demonstrated that conjugates of peptide sequences with bovine serum albumin (BSA), which are synthesized based on the M2e protein from one of the important surface proteins of the influenza A virus, have high immunogenetic properties in rabbits.2
Despite all this, current studies are investigating more suitable carrier molecules to improve the vaccine against the influenza A virus. In vaccine studies, it has been shown that polymer-based adjuvants conjugated with natural antigens have high immunity.7
Petrov et al. (1985) prepared covalent conjugates of antigen isolated from the influenza A virus and polyelectrolyte, and determined that the conjugates generated an efficient immune response, thereby proving that formulations can be used as new generation artificial vaccines.8 However, there have been no studies in the literature on vaccine designs based on M2e protein peptides from the influenza A virus where polymer is used as a carrier.
Poly(acrylic) acid (PAA) is an anionic polymer with high molecular weight. Muir et al. indicated that PAA has adjuvant effects on humoral and cell-mediated immunity.9-11 Different researchers have shown the adjuvant effect of PAA in several types of infection. A recent study has reported the development of the immunogenic lipophosphoglycan molecule isolated from Leishmania parasites conjugated with PAA.12
The covalent conjugation of peptide and polymer molecules provides the opportunity for next-generation synthetic vaccines by means of modifying the immunogenicity of the peptide antigens. Accordingly, for the first time, we aimed to synthesize, characterize, and determine the toxicity of the bioconjugate of the peptide of the M2e protein covalently bonded with PAA in order to develop a universal synthetic vaccine prototype against influenza A virus.
Results
Characterization of M2e peptide–PAA bioconjugate with size-exclusion chromatography (SEC) and fluorescence spectroscopy
PAA, M2e peptide-PAA bioconjugate synthesized in the presence of carbodiimide, and pure peptide were prepared at pH 7.0. The concentrations of PAA in a polymer mixture and peptide-PAA bioconjugate were constant.
In Fig. 1, the SEC chromatograms of pure peptide, polymer, and peptide-polymer bioconjugate are given. As illustrated in Fig. 1A, the peaks of PAA and pure peptide eluting from the column had different retention times of 10 and 22 min, respectively. As a rule, polymers or bioconjugate with a high molecular weight leave the column first; this is seen clearly in the ultraviolet (UV) chromatograms. Pure peptide molecules leave the column last because their molecular weight is the smallest. After the peptide-PAA bioconjugate is characterized, the peak eluting from the column has a similar retention time to that of the PAA at 10 min. In UV chromatograms, the peak area of peptide-PAA bioconjugate is larger than that of the PAA, which indicates the conjugation of peptides to PAA molecules, as UV absorption is based on the peptides linked to the PAA.
Figure 1.
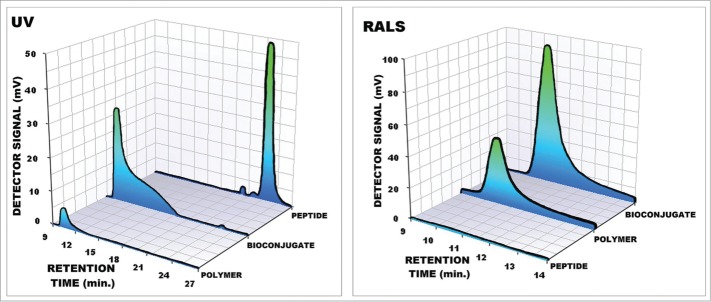
(A) UV (280 nm) and (B) light scattering (RALS) chromatograms of PAA, bioconjugate of peptide-PAA and pure peptide prepared at pH = 7.
As seen in Fig. 1B, the signal of the RALS detector is related to the molecular weight and concentration of the peptide and PAA molecules. As seen from the chromatogram, the retention times are similar to PAA and peptide-PAA bioconjugate at 9.8 min. The peak area of the peptide-PAA bioconjugate is larger than both of the peak areas of PAA and pure peptide. These results also indicate the formation of a peptide-PAA bioconjugate with high molecular weight.
There are 3 aromatic amino acid residues, namely tryptophan (Trp-W), tyrosine (Tyr-Y), and phenylalanine (Phe-F), which contribute to a protein's UV fluorescence. As has been demonstrated in the literature, (Trp-W) emission is extremely dependent on its local environment, and in water, its maximum emission takes place at about 350 nm.13,14
While we investigated the formation reaction of peptide-polymer bioconjugate with fluorescence spectroscopy, conformational changes resulted in changes of fluorescence parameters such as emission maximum (λmax) and fluorescence intensity (Imax). Fig. 2 shows the differences among the fluorescence spectra of PAA, peptide-PAA, and pure peptide. As seen from the spectra, the maximum wavelength of pure peptide was at 353 nm and the maximum fluorescence intensity was 500,000. Due to the covalent conjugation of peptide with the polymer chain, the maximum wavelength of the peptide-PAA bioconjugate shifted to the blue region at 347 nm. Moreover, maximum fluorescence intensity shows a dramatic decrease, at 76,139. These changes indicate that peptide tryptophanyls become less accessible in the solution, which must be the result of tight binding between the polymer and the peptide.
Figure 2.
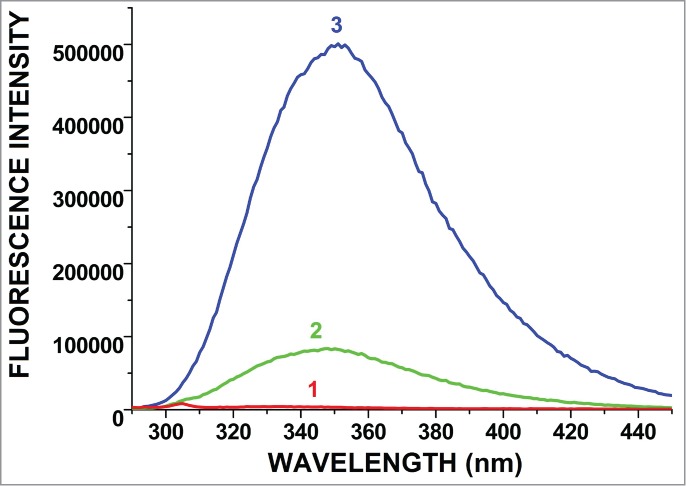
Fluorescence spectra of polymer (PAA) (1), peptide-PAA bioconjugate (2) (λmax = 347 nm), and pure peptide (3) (λmax = 353 nm).
These spectroscopic and chromatographic results prove the formation of bioconjugate between M2e peptide and PAA.
Effect of M2e peptide-PAA bioconjugate on cell morphology and viability
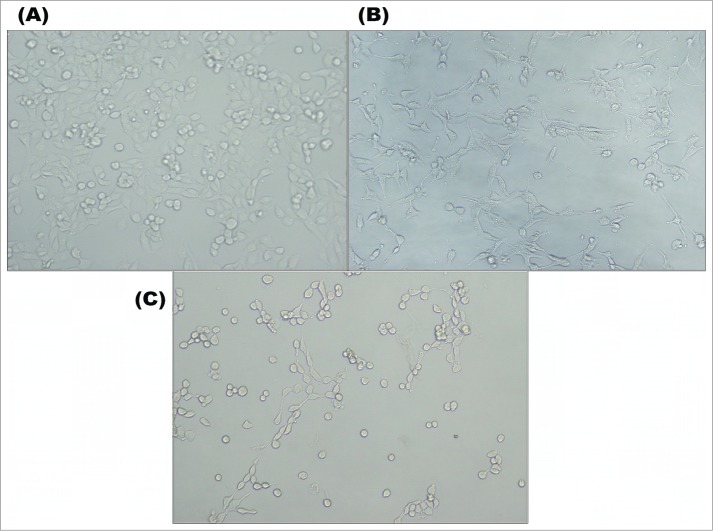
The morphology of MCF-7 cells in a control group is shown in Fig. 3A. In the figure, it can be seen that the MCF-7 cells had elongated shapes that attached to the surface of a microplate. Intercellular cell attachment and cell morphology were typical. The cells exposed to the 1 mg/ml M2e peptide-PAA bioconjugate for 48 h had the morphology both of normal cells and abnormal spherical morphology, losing their elongated shapes (Fig. 3B). Fig. 3B clearly shows the different morphology of some cells, namely, extensive protrusions and inclusions in the cell; this is usually associated with cellular stress. In Fig. 3C, the effect of 5 mg/ml of M2e peptide-PAA bioconjugate emerged on the MCF-7 cells. Cells transformed to an atypical morphology and began to shrink.
Figure 3.
Morphology of MCF-7 cells in control (A) and experimental group of 1 mg/ml (B) and 5 mg/ml (C) of M2e peptide-PAA bioconjugate.
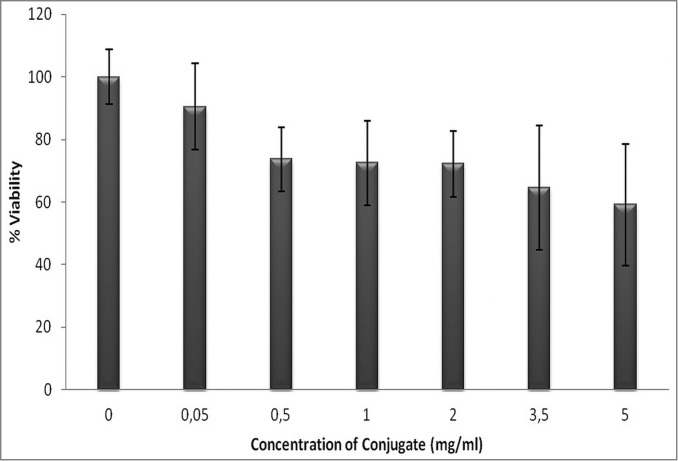
The effects of the different concentrations of influenza A virus M2e peptide-PAA bioconjugate on the viability of MCF-7 cells were analyzed using MTT analysis, based on the ability of viable cells to reduce the tetrazolium salt to an insoluble formazan product. As shown in Fig. 4, cell viability decreased with an increasing concentration of bioconjugate. In particular, cell viability decreased to 1, 2, 3.5, and 5 mg/ml in the bioconjugate group compared with the control group (P < 0.05), with the percentages of viability being 71.4%, 72.1%, 64.5%, and 59.1%, respectively. The analysis showed that there was no significant difference between the control and low concentrations (0.05 and 0.5 mg/ml) of bioconjugate (P < 0.05).
Figure 4.
Percent viability of MCF-7 cells exposed to different concentration the peptide-PAA bioconjugate (error bars ± SD).
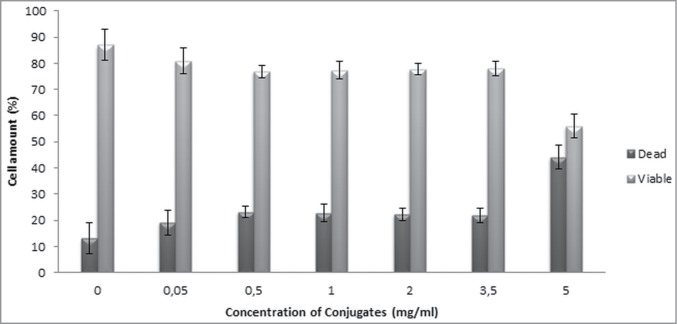
Fig. 5 shows the flow cytometry analysis of cells exposed to the different concentrations of bioconjugate. The numbers of viable and dead cells were calculated, and the results showed that 0.05 and 0.5 mg/ml concentrations of bioconjugate had no adverse effect on MCF-7 cells compared to the control group (P < 0.05). In contrast, cell viability was found to be decreased in concentrations higher than 0.5 mg/ml. The viability of the cells exposed to 5 mg/ml bioconjugates decreased by 55%, probably due to apoptosis. These findings also support the MTT results.
Figure 5.
Viable and dead cell percentages of MCF-7 cells exposed to the peptide-PAA bioconjugate (error bars ± SD).
Discussion
In this study, our aim was to develop a universal synthetic vaccine prototype for the influenza A virus. Thus, we used the M2e protein peptide, which is known to be highly conserved, and whose antigenic region is not affected by viral replication. Accordingly, the bioconjugate of the M2e protein peptide sequence of the influenza A virus and PAA were synthesized using a covalent conjugation method in the presence of water-soluble carbodiimide.
M2e peptide-PAA bioconjugate was characterized by SEC using 2 detectors, namely UV and RALS. Its formation was explicated and clearly demonstrated with the matching results from 2 different detector signals. In addition, it was also characterized by fluorescence spectroscopy. Peptide molecules in the structure of the peptide-PAA bioconjugate were tightly covered by the polymer coil. This structural change was demonstrated by the differentiation of wavelength maximums and fluorescence intensities.
Cell culture tests of bioconjugate of the influenza A virus M2e protein peptide-PAA, which was designed as a model of a synthetic vaccine, were conducted because it is important to ensure that the preparate does not have any toxic effects on living systems. Microscopic images of the cells in the culture medium containing the bioconjugate showed some differences in cell morphology, especially at a high concentration. The lowest concentration caused resulted in some cell difference, but there was no significant effect on cell viability according to the MTT and flow cytometry results. However, at high concentrations, the results revealed changes in the morphology, namely a decrease in the number of extensions and an increase in the number of cells forming round shapes. This indicates a reduction in viability.
The effect of cell viability was measured by flow cytometric detection of dead and living cells, and the results supported those of the MTT method. Flow cytometry studies with an apoptotic marker (Annexin V) showed a decrease in cell viability due to apoptosis. Previous studies have examined the toxicity of PAA at concentrations of 0.1, 5, 10, 15 mg/ml, and showed that the toxic effects emerge at a concentration of 5 mg/ml.15 In our study, the highest concentration of PAA was 5 mg/ml. In future animal experiments, the concentrations will be much lower than this. This study confirmed that the toxicity of the bioconjugate of the M2e protein peptide of the influenza A virus prepared by PAA is not toxic at a suitable concentration for animal studies.
The bioconjugate of influenza A virus M2e peptide-PAA with a lower concentration, designed as a model of a synthetic vaccine, was not shown to have any significant toxic effects on MCF-7 cells in vivo for applications at low concentrations when controlling for the cell viability using MTT experiments, as well as flow cytometry. This study shows that future studies should apply the bioconjugate of the influenza A virus M2e peptide-PAA in vivo to develop an influenza A vaccine.
Materials and Methods
Materials
The peptide sequence of influenza A virus M2e protein was commercially purchased from BioSynthesis Inc. (Texas, USA). The peptide sequence was obtained from NH2 -WETPIRNEWGCRGETPIRNEWGCR- OH. The molecular weight of the peptide was 2946.28 Da. The isoelectric point (pI) of the peptide was 6.4. The peptide was hydrophilic and soluble in aqueous medium; it was dissolved in water.
PAA was obtained from Aldrich (Aldrich, 523925), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide was obtained from Sigma Aldrich (Sigma-Aldrich, E7750). NaH2PO4 (Riedel-de Haën, 04361), Na2HPO4.7H2O (Fluka, 71647), NaCl (Fluka, 71376), and NaOH (Fluka 06203) were obtained from Fluka, and NaN3 was obtained from Applichem (Applichem, A1430). Ultrapure water, obtained from the Millipore MilliQ Gradient System, was used in prepared solutions.
Preparation of the M2e peptide-PAA bioconjugate
The bioconjugate of the peptide sequence of influenza A virus M2e protein and PAA was prepared in aqueous solutions, and the conjugation reaction was performed at pH 5 and pH 7 under ambient temperature, over a short duration to avoid the denaturation of the peptide.16,17 The concentration of the polymer was 0.5 mg/ml in the conjugation procedure.16 Peptide-PAA bioconjugate prepared with a ratio of CPeptide/CPAA = 9 was analyzed by SEC with triple detection.
The polymer (PAA) and peptide were mixed at pH 5, and after stirring for 2 h at room temperature, carbodiimide was added into this mixture and stirred overnight. The bioconjugate was purified using dialysis, and the purified conjugate16 was analyzed using SEC.17
SEC measurements
PAA, influenza A virus M2e protein peptide and peptide-PAA bioconjugate were analyzed using SEC with a triple-detection system. Triple detection consists of refractive index (RI; 660 nm), right-angle light scattering (LS; 670 nm), and UV detectors, which were calibrated using a BSA monomer peak in the mobile phase of phosphate-buffered saline (PBS) with a flow rate of 1.0 ml/min. The dn/dc value and extinction coefficient of BSA were 0.18518,19 and 0.66,19,20 respectively. SEC analyses were performed with a Shimadzu Shim-Pack Diol-300 (500 × 7.9 mm) column at room temperature. PBS (pH = 7.1) was used for the mobile phase and the flow rate was 1.0 ml/min. PBS was prepared using ultrapure water from a Millipore MilliQ Gradient System and consisted of 50 mM phosphate and 150 mM sodium chloride; 0.05% NaN3 of pH 7.0 was added to the mobile phase solution to prevent biological degradation of the columns. Buffer solutions were filtered through 0.45 μm Millipore cellulose nitrate filter and degassed before use.
Fluorescence measurements
Fluorescence emission spectra were obtained using a QM-4/2003 Quanta Master Steady State Spectrofluorimeter (Photon Technology International, Canada) operating in quanta-counting mode. The slits of the excitation and emission monochromators were adjusted to 2 or 3 nm. The excitation was obtained at 290 nm. Interaction with other macromolecules may result in changing of the peptide fluorescence spectra, which is characterized by the wavelength at the maximum emission (λmax) and maximum fluorescence intensity (Imax).
Cell culture studies
MCF-7 cells (105/ml) were cultured in DMEM/F12 medium (Sigma, D5546) supplemented with 2 mM L-glutamine (Biological Industries, 03-020-IC), 100 μg/ml penicillin-streptomycin (Biological Industries, 03-031-IC) and fetal bovine serum (FBS; Seromed, S0115) at 37°C and 5% CO2. Cells were passaged twice a week by trypsin (Biochrom, L2103).
Morphological examination
The effect of the bioconjugate of the influenza A virus M2e peptide with PAA on MCF-7 cells was observed using an inverted microscope (Olympus Microscope CKX41). Cell shape, adhesion properties, and morphology of cells exposed to the bioconjugate and control were examined microscopically. This microscope frame for UIS2 optics with a fixed binocular tube FN20 included 2 WHB10x eyepieces, an integrated quadruple revolving nosepiece, a plain stage (160 × 250 mm), coarse and fine focusing with a tension-adjustment mechanism, an illuminator pillar with a lamp house, a long working distance condenser NA 0.3, WD ¼ 72 mm, aperture stop, Allen key AQ2302, and a transformer integrated in the frame, which further included a lamp socket and 2 halogen bulbs (30 W).
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay
The MTT (Sigma, M5655-1G) assay is a rapid test and gives accurate quantitative results.21 The method is based on the intracellular reduction of tetrazolium salt to a blue-purple formazan product through the mitochondrial dehydrogenase enzyme of viable cells and spectrophotometric measurement of absorbance at a wavelength of 540 nm.22 The effects of the bioconjugate on the viability of cell culture were assessed using the MTT method. Briefly, 104 cells/well of MCF-7 cells were seeded on 96-well polystyrene plates. Ten milliliters of bioconjugate of influenza A virus M2e peptide with a PAA solution (Aldrich, 523925) were added with a ratio of 0.05, 0.5, 1, 2, 3.5, 5 mg/ml after cell adhesion on the wells at 24 h. Cells were incubated in an incubator at 37°C and exposed to humid air for 48 h. The MTT solution was prepared at a concentration of 10 mg/ml and filtered. Ten micrograms of this solution was added to each well and incubated for 4 h. Subsequently, 100 ml of MTT stop solution (DMSO-Sigma D26650) was added to dissolve the formazan crystals in the wells and investigated compounds with DMEM-F12 medium, the same procedure was conducted without cells. The optical density was measured at a wavelength of 570 nm with a microplate reader. The percent viability was calculated as follows:
Flow cytometric measurements of apoptosis and necrosis by staining with annexin V/7-ADD
MCF-7 cells (25 × 104) were cultured with DMEM-F12 (10% FBS) in 6-well plates. Cells were incubated at 37°C and 5% CO2 for 24 h; they were exposed to the influenza A virus M2e peptide with PAA bioconjugate at 37°C and 5% CO2 for 48 h. Cells were trypsinized and washed twice with PBS. Subsequently, 10 μl of Annexin V–fluorescein isothiocyanate (FITC; Invitrogen, IM3546) working solution, 5 μl of 7-AAD (Invitrogen, A07704), and 100 μl of binding solution were added and incubated in dark conditions for 15 min. In the next step, 900 μl of binding solution was added to each sample and analyzed using flow cytometry after 1 h.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). A parametric test (paired t-test) was used to evaluate the significance of the results. Values of P < 0.05 were considered statistically significant.
Authors’ Contributions
YBK: Designing the research, conjugation of influenza A virus M2e protein peptide and PAA, characterizations, data analysis, drafting the manuscript; ZA: designing the research, reviewing the manuscript; RCK: toxicity and flow cytometry studies, drafting the manuscript, reviewing the manuscript; MB: toxicity and flow cytometry studies; AA: designing the toxicity and flow cytometry studies, reviewing and modifying manuscript. All authors read and approved the final manuscript.
Disclosure of Potential Conflicts of Interest
There were no potential conflicts of interest to disclose.
Acknowledgments
In loving memory of Prof. Dr. Mamed Mustafaev, Founder Head of Yıldız Technical University Bioengineering Department, precious man of science. Authors wish to thank Murat Topuzogullari for SEC measurements.
Funding
This research was supported by a grant from T.R. Prime Ministry State Planning Organization (Project No. 25-DPT-07-04-01) and Yildiz Technical University Office of Scientific Research Project Coordination (Project No:29-07-04-ODAP).
References
- 1. Muller CP, Putz MM. Peptide vaccines. In: Topley and Wilson's Microbiology and Microbial Infections. 2010. [Google Scholar]
- 2. Liu W, Li H, Chen YH. N-terminus of M2 protein could induce antibodies with inhibitory activity against influenza virus replication. FEMS Immunol Med Microbiol 2003; 35:141-6; PMID:12628550; http://dx.doi.org/ 10.1016/S0928-8244(03)00009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fiers W, Filette MD, Bakkouri KE, Schepens B, Roose K, Schotsaert M, Birkett A, Saelen X. M2e-based universal influenza A vaccine. Vaccine 2009; 27:6280-3; PMID:19840661; http://dx.doi.org/ 10.1016/j.vaccine.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 4. Filette MD, Jou WM, Birkett A, Lyons K, Schultz B, Tonkyro A, Resch S, Fiers W. Universal influenza A vaccine: optimization of M2-based constructs. Virology 2005; 337:149-61; PMID:15914228; http://dx.doi.org/ 10.1016/j.virol.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 5. Zou P, Liu W, Chen Y-H. The epitope recognized by a monoclonal antibody in influenza A virus M2 protein is immunogenic and confers immune protection. Int Immunopharm 2005; 5:631-5; PMID:15710332; http://dx.doi.org/ 10.1016/j.intimp.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 6. Cox JC, Coulter AR. Adjuvants—a classification and review of their modes of action. Vaccine 1997; 15:248-56; PMID:9139482; http://dx.doi.org/ 10.1016/S0264-410X(96)00183-1 [DOI] [PubMed] [Google Scholar]
- 7. Mustafaev M. Functionally biopolymer systems. J Eng Nat Sci 2004; 4:1-200 [Google Scholar]
- 8. Petrov RV, Khaitov RM, Zhdanov VM, Sinyakov MS, Norimov AS, Nekrasov AV, Podchernyaeva RYa, Kharitonenkov IG, Shchipanova MV. Influenza virus antigens conjugated with a synthetic polyelectrolyte: a novel model of vaccines. Vaccine 1985; 3:392-400; PMID:3936300; http://dx.doi.org/ 10.1016/0264-410X(85)90130-6 [DOI] [PubMed] [Google Scholar]
- 9. Muir W, Bryden W, Husband A. Immunity, vaccination and the avian intestinal tract. Dev Comp Immunol 2000; 24:325-42; PMID:10717296; http://dx.doi.org/ 10.1016/S0145-305X(99)00081-6 [DOI] [PubMed] [Google Scholar]
- 10. Diamantstein T, Wagner B, Beyse I, Odenwald MV, Schulz G. Stimulation of humoral antibody formation by polyanions. I. The effect of polyacrylic acid on the primary immune response in mice immunized with sheep red blood cells. Eur J Immunol 1971; 1:335-40; PMID:4945635; http://dx.doi.org/ 10.1002/eji.18300-10506 [DOI] [PubMed] [Google Scholar]
- 11. Oka T, Honda T, Ohkuma K, Sakoh M, Nonaka S. Influenza vaccine: enhancement of immune response by application of carboxy-vinylpolymer. Vaccine 1990; 8:573-6; PMID:2087878; http://dx.doi.org/ 10.1016/0264-410X(90)90011-A [DOI] [PubMed] [Google Scholar]
- 12. Topuzogullari M, Koc RC, Isoglu SD, Bagirova M, Akdeste Z, Elcicek S, Oztel ON, Yesilkir Baydar S, Canim Ates S, Allahverdiyev AM. Conjugation, characterization and toxicity of lipophosphoglycan-polyacrylic acid conjugate for vaccination against leishmaniasis. J Biomed Sci 2013; 20:1-8; PMID:23731716; http://dx.doi.org/ 10.1186/1423-0127-20-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lakowich JR. Principles of Fluorescence Spectroscopy. New York: Plenum Press; 1986. p. 496-501. [Google Scholar]
- 14. Weinryb I, Steiner RF. The luminecence of the aromatic amino acids. In: Excited State of Proteins and Nucleic Acids. Plenum Press; 1971. p. 277-318. [Google Scholar]
- 15. Cakir R. The new culture methods for investigation polymers toxicity in vitro. Bioengineering Department. Istanbul: Yildiz Technical University; 2008. [Google Scholar]
- 16. Eroglu BI, Kilinc YB, Mustafaeva Z. Bioconjugation of hepatitis B antigenic peptide with polymeric carriers through various carbodiimide chemistry. Turk J Biochem. 2011; 36:222-9 [Google Scholar]
- 17. Budama-Kilinc Y. Investigation of conjugation reaction synthetic peptides of influenza A virus with polyelectrolytes by fluorescence resonance energy transfer and the other methods. Bioengineering Department. Istanbul: Yildiz Technical University; 2013. [Google Scholar]
- 18. Kendrick BS, Kerwin BA, Chang BS, Philo JS. Online size-exclusion high-performance liquid chromatography light scattering and differential refractometry methods to determine degree of polymer conjugation to proteins and protein-protein or protein–ligand association states. Anal Biochem 2001; 299:136-46; PMID:11730335; http://dx.doi.org/ 10.1006/abio.2001.5411 [DOI] [PubMed] [Google Scholar]
- 19. Topuzoğulları M, Çimen NS, Mustafaeva Z, Mustafaev M. Molecular-weight distribution and structural transformation in water-soluble complexes of poly(acrylic acid) and bovine serum albumin. Eur Polym J 2007; 43:2935-46; http://dx.doi.org/ 10.1016/j.eurpolymj.2007.04.025 [DOI] [Google Scholar]
- 20. Wen J, Arakawa T, Philo JS. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal Biochem 1996; 240:155-66; PMID:8811899; http://dx.doi.org/ 10.1006/abio.1996.0345 [DOI] [PubMed] [Google Scholar]
- 21. Muelas-Serrano S, Nogal-Ruiz J, Gómez-Barrio A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol Res 2000; 86:999-1002; PMID:11133116; http://dx.doi.org/ 10.1007/PL00008532 [DOI] [PubMed] [Google Scholar]
- 22. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987; 47:936-42; PMID:3802100 [PubMed] [Google Scholar]