Abstract
The naturally and widely occurring sulfonium compound, S-adenosylmethionine (AdoMet), one of nature's most versatile molecules, is biosynthesized from methionine and ATP by AdoMet synthetase or methionine adenosyltransferase (MAT) in a 2-step reaction in which the energy-rich sulfonium compound is formed by dephosphorylation of ATP. All living cells, with the only exception of some parasites and infectious agents, express MAT.
Keywords: adenosylmethionine synthetase, Archaea, hyperthermophilic enzymes, methionine adenosyltransferase, organic solvent activity and thermostability, Pyrococcus furiosus, S-Adenosylmethionine
Abbreviations
- AdoMet
S-Adenosylmethionine
- BsMAT
MAT from Bacillus subtilis
- DMSO
dimethylsulfoxide; MAT, adenosylmethionine synthetase or methionine adenosyltransferase
- MjMAT
MAT from Methanococcus jannaschii
- PfMAT
MAT from Pyrococcus furiosus
- SsMAT
MAT from Sulfolobus solfataricus
- TkMAT
MAT from Thermococcus kodakarensis
- EcMAT
MAT from E. coli.
In a previous paper we have expressed in E. coli, purified and characterized MAT from the hyperthermophilic archaeon Pyrococcus furiosus (PfMAT) with particular attention to its thermostability and kinetic features. A comparative kinetic investigation showed that PfMAT is the most efficient catalyst for AdoMet synthesis among the characterized MAT from Bacteria and Archaea. In our work we have analyzed the effect of water-miscible organic solvents on PfMAT activity and thermostability to improve the synthesis of AdoMet and AdoMet analogs of biotechnological interest not easily soluble at low temperature or in aqueous systems.
As known, the naturally occurring sulfur-nucleoside, S-adenosylmethionine plays a primary role in cellular metabolism, as it not only represents the major biological donor of methyl groups and the precursor of the polyamines,1 but is also involved in other important processes, such as transsulfuration reactions, synthesis of glutathione, 5'-deoxyadenosyl-5'-radical-mediated biochemical transformations, and synthesis of ethylene in plants.2,3 Recently, it has been reported that AdoMet can induce apoptotic cell death in liver, colon, and breast cancer cells, albeit by different mechanisms.4,5
MAT (EC 2.5.1.6), the only enzyme that catalyzes the synthesis of AdoMet, has been extensively studied and characterized in Bacteria6,7 and Eukarya8 and more recently in hyperthermophilic Archaea9-12 that are worthy of attention for their capacity of survival and reproduction at temperatures near to 100°C.
Enzymes derived from hyperthermophiles, which retain their structure and function at very high temperature, have been the subject of intensive investigations over the years not only for their exceptional stability but also for their unique physicochemical features that have led to many applications of biotechnological interest.13,14 Hyperthermophilic enzymes in fact are superior to the conventional catalysts because they can carry out industrial processes even under severe conditions, in which mesophilic proteins are completely inactive.
Recently, we reported a simple and efficient method for producing high-purity recombinant MAT from the anaerobic hyperthermophilic archaeon Pyrococcus furiosus through thermoprecipitation and a 2-step chromatography process. The enzyme was then extensively characterized. The molecular mass of native PfMAT is 90 kDa. Furthermore, when submitted to SDS PAGE, the purified enzyme shows a single protein band with an estimated molecular mass of 45 kDa. On the basis of these results, PfMAT belongs to the dimeric group of MAT family in contrast to E. coli MAT6 that is a tetrameric protein. The isoelectric point and optimum pH of PfMAT are similar to all the corresponding enzymes from other sources.
As observed in several enzymes from thermophilic microorganisms, the optimum temperature of PfMAT is quite high (90°C), similar to the optimal growth temperature of the bacterium (100°C). Moreover PfMAT shows an Arrhenius plot not linear with a discontinuity around 75°C, suggesting the occurrence of temperature-induced conformational changes that result in enzymatic forms characterized by different catalytic properties. PfMAT is characterized by remarkable thermostability showing a melting temperature of 99°C. Moreover, when the kinetics of inactivation after prolonged incubation at high temperatures were carried out, the enzyme retained fully activity after 1 hour incubation at 70°C and showed half-lives of 23 and 5 minutes when incubated at 95 and 100°C respectively.
PfMAT requires divalent metal ions for activity and is not activated by monovalent cations which activate all AdoMet synthetases so far investigated, or by dimethylsulfoxide (DMSO). Moreover, the enzyme does not require reducing agents for its activity and is not inhibited by alkylating, mercaptide-forming or oxidizing thiol reagents even at relatively high concentrations, suggesting that thiol groups are not involved in the catalytic process.
On the basis of its physico-chemical characterization, reported in the previous work, PfMAT is a thermoactive and thermostable homodimer with features similar to those of other MAT from Archaea such as MAT from Methanococcus jannaschii (MjMAT),9 Thermococcus kodakarensis (TkMAT)10 and Sulfolobus solfataricus (SsMAT).11,12
The comparative kinetic investigation previously reported shows that MAT from P. furiosus is the most efficient catalyst for AdoMet synthesis among the characterized MAT from Bacteria and Archaea exhibiting the highest catalytic power (kcat 7.5 sec−1). Moreover, PfMAT shows a better catalytic efficiency with both substrates of the reaction than the other characterized MAT (kcat/Km values that are from 6 to 58 times higher than those of the other MAT for methionine and from 2.5 to 151 times higher for ATP). These features render PfMAT the most suitable tool for the synthesis of AdoMet and AdoMet analogs of biotechnological interest.
The technological applications for hyperthermophilic enzymes in organic media are attracting more attention.13–15 These enzymes, in fact, acting at harsh conditions are expected to be powerful tools in industrial biotransformation processes. These observations prompted us to investigate the stability of the enzymatic system responsible for the biogenesis of AdoMet in P. furiosus in different water-soluble organic solvents.
PfMAT stability in water-miscible organic solvents at 25°C is somewhat surprising: after 24 h preincubation, the enzyme was fully active in 50% methanol and 50% isopropanol. About 82, 72 and 60% intial activity was still retained after 24 h of preincubation in 50% ethanol, acetonitrile, and DMSO, respectively. In 50% n-propanol after 24 hours, however, the enzyme loses 70% of activity.
To check the effect of various water-miscible organic solvents on the thermostability of PfMAT, the enzyme was incubated in their presence for 15, 30 or 60 min at 50 or 60°C. As shown in Table 1, DMSO does not affect the stability of PfMAT. The thermophilic enzyme shows remarkable stability in 50% methanol, ethanol, and acetonitrile, displaying 100%, 86% and 80% residual activity, respectively, after 60 min preincubation at 50°C, and still retaining 59%, 57% and 73% residual activity, respectively, after 15 min preincubation at 60°C. A high destabilizing effect, to a different extent, was instead observable at 50°C and at 60°C in the presence of the other organic solvents tested.
Table 1.
Effect of several water-miscible organic solvents on the stability of PfMAT at 50°C and 60°C. The enzyme was incubated in stoppered glass tubes in 10 mM Tris-HCl, pH 8.0, with the compounds to be tested. At the time indicated, aliquots were removed from each incubation mixture and assayed for the residual PfMAT activity in standard conditions at 80°C
| Residual activity |
|||||||
|---|---|---|---|---|---|---|---|
| Compound | 50°C |
60°C |
|||||
| |
15 min |
30 min |
60 min |
|
15 min |
30 min |
60 min |
| % | % | ||||||
| None | 100 | 100 | 100 | 100 | 100 | 100 | |
| 50% methanol | 100 | 100 | 100 | 59 | 28 | 16 | |
| 50% ethanol | 118 | 92 | 86 | 57 | 18 | 6 | |
| 50% n-propanol | 36 | 22 | 13 | 33 | 12 | 8 | |
| 50% isopropanol | 66 | 50 | 28 | 26 | 13 | 8 | |
| 50% acetonitrile | 90 | 89 | 80 | 73 | 64 | 42 | |
| 50% DMSO | 100 | 100 | 100 | 100 | 100 | 100 | |
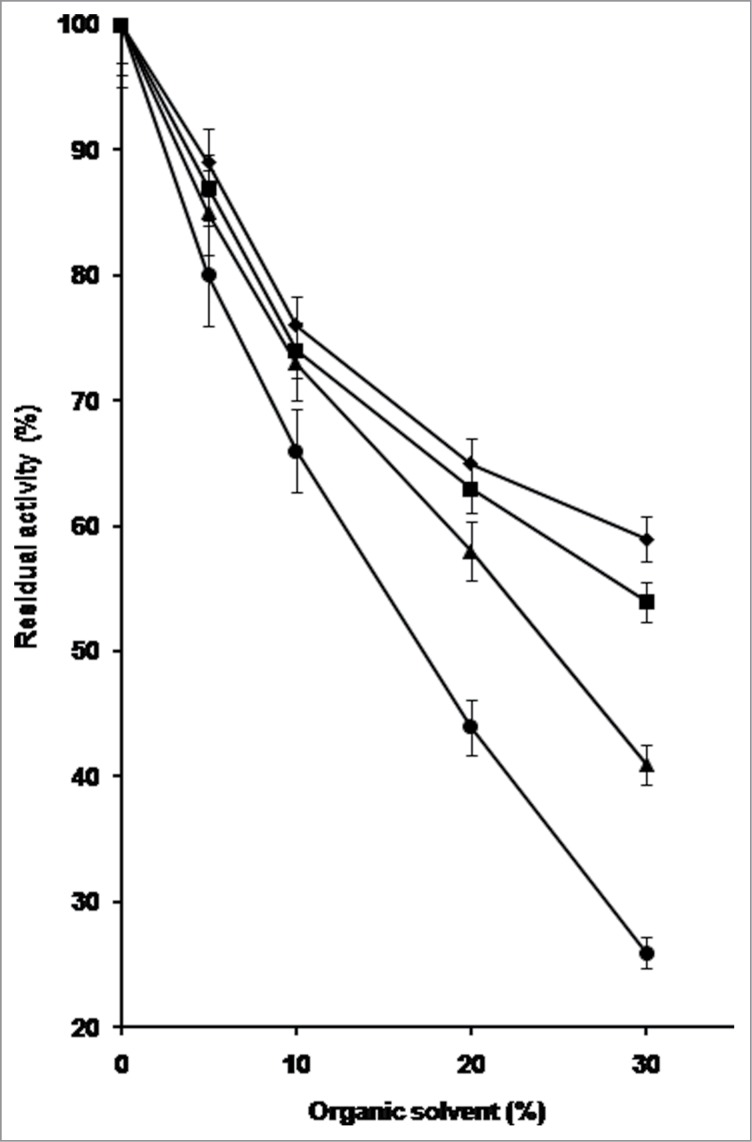
Based on these results PfMAT possesses a high stability at room temperature in water-miscible organic solvents, even when compared with other enzymes from thermophilic sources, suggesting that denaturation phenomena occur slowly in these conditions. Since PfMAT remains almost completely stable at 50°C up to 15 min in the presence of methanol, ethanol, acetonitrile, and DMSO, these conditions have been chosen to teste the effect of such compounds on the catalytic activity of the enzyme at concentrations from 5% to 30%. As shown in Fig. 1, about 40% of PfMAT activity is still observable in the presence of 30% acetonitrile and 20% DMSO and more than 50% in the presence of 30% methanol or ethanol.
Figure 1.
Effect of increasing concentrations of methanol, ethanol, acetonitrile, and DMSO on the acivity of PfMAT at 50°C. The enzyme was assayed in standard conditions for 15 min in the presence of the solvent to be analyzed at different percentage from 5% to 30% (vol/vol). (♦) Methanol; (▪) ethanol; (▴) acetonitrile; (•) DMSO. Each error bar indicates the standard deviation (± SD). Values were the mean of triplicate analyses.
In conclusion, the unique stability features of PfMAT at high temperature, combined with its general remarkable organic solvent tolerance, make PfMAT an excellent catalyst for the production of AdoMet and AdoMet analogs of biotechnological interest.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by grants of Seconda Università di Napoli and of Regione Campania (L.R. n.5/2007).
References
- 1.Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol 2000; 32:391-5; PMID:10762064; http://dx.doi.org/ 10.1016/S1357-2725(99)00139-9 [DOI] [PubMed] [Google Scholar]
- 2.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J 2002; 16:15-26; PMID:11772932; http://dx.doi.org/ 10.1096/fj.01-0401rev [DOI] [PubMed] [Google Scholar]
- 3.Fontecave M, Atta M, Mulliez E. S-Adenosylmethionine: nothing goes to waste. Trends Biochem Sci 2004; 29:243-9; PMID:15130560; http://dx.doi.org/ 10.1016/j.tibs.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Li TWH, Yang H, Peng H, Xia M, Mato JM, Lu SC. Effects of S-adenosylmethionine and metylthioadenosine on inflammation-induced colon cancer in mice. Carcinogenesis 2012; 33:427-35; PMID:22159228; http://dx.doi.org/ 10.1093/carcin/bgr295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu SC, Mato JM. S-Adenosylmethionine in liver health, injury, and cancer. Physiol Rev 2012; 92:1515-42; PMID:23073625; http://dx.doi.org/ 10.1152/physrev.00047.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markham GD, Hafner EW, Tabor CW, Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem 1980; 255:9082-92; PMID:6251075 [PubMed] [Google Scholar]
- 7.Kamarthapu V, Rao KV, Srinivas PN, Reddy GB, Reddy VD. Structural and kinetic properties of Bacillus subtilis S-adenosylmethionine synthetase expressed in Escherichia coli. Biochim Biophys Acta 2008; 1784:1949-58; PMID:18634909; http://dx.doi.org/ 10.1016/j.bbapap.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Kotb M, Geller AM. Methionine adenosyltransferase: structure and function. Pharmacol Ther 1993; 59:125-43; PMID:8278461; http://dx.doi.org/ 10.1016/0163-7258(93)90042-C [DOI] [PubMed] [Google Scholar]
- 9.Lu ZJ, Markham GD. Enzymatic properties of S-adenosylmethionine synthetase from the archaeon Methanococcus jannaschii. J Biol Chem 2002; 277:16624-31; PMID:11872742 [DOI] [PubMed] [Google Scholar]
- 10.Schlesier J, Siegrist J, Gerhardt S, Erb A, Blaesi S, Richter M, Einsle O, Andexer JN. Structural and functional characterization of the methionine adenosyltransferase from Thermococcus kodakarensis. BMC Struct Biol 2013; 13:22-31; PMID:24134203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Singh S, Zhang J, Huber TD, Helmich KE, Sunkara M, Hurley KA, Goff RD, Bingman CA, Morris AJ, Thorson JS, Phillips GN Jr.. Understanding molecular recognition of promiscuity of thermophilic methionineadenosyltransferase sMAT from Sulfolobus solfataricus. FEBS J 2014; 281:4224-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcelli M, Cacciapuoti G, Cartenì-Farina M, Gambacorta A. S-Adenosylmethionine synthetase in the thermophilic archaebacterium Sulfolobus solfataricus. Purification and characterization of two isoforms. Eur J Biochem 1988; 177:273-80; PMID:3142771 [DOI] [PubMed] [Google Scholar]
- 13.Niehaus F, Bertoldo C, Kahler M, Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 1999; 51:711-29; PMID:10422220; http://dx.doi.org/ 10.1007/s002530051456 [DOI] [PubMed] [Google Scholar]
- 14.Egorova K, Antranikian G. Industrial relevance of thermophilic Archaea. Curr Opin Microbiol 2005; 8:649-55; PMID:16257257 [DOI] [PubMed] [Google Scholar]
- 15.Klibanov AM. Improving enzymes by using them in organic solvents. Nature 2001; 409:241-6; PMID:11196652 [DOI] [PubMed] [Google Scholar]