Abstract
Concussion, or mild traumatic brain injury (mTBI), can cause persistent behavioral symptoms and cognitive impairment, but it is unclear if this condition is associated with detectable structural or functional brain changes. At two sites, chronic mTBI human subjects with persistent post-concussive symptoms (three months to five years after injury) and age- and education-matched healthy human control subjects underwent extensive neuropsychological and visual tracking eye movement tests. At one site, patients and controls also performed the visual tracking tasks while blood-oxygen-level–dependent (BOLD) signals were measured with functional magnetic resonance imaging. Although neither neuropsychological nor visual tracking measures distinguished patients from controls at the level of individual subjects, abnormal BOLD signals were reliably detected in patients. The most consistent changes were localized in white matter regions: anterior internal capsule and superior longitudinal fasciculus. In contrast, BOLD signals were normal in cortical regions, such as the frontal eye field and intraparietal sulcus, that mediate oculomotor and attention functions necessary for visual tracking. The abnormal BOLD signals accurately differentiated chronic mTBI patients from healthy controls at the single-subject level, although they did not correlate with symptoms or neuropsychological performance. We conclude that subjects with persistent post-concussive symptoms can be identified years after their TBI using fMRI and an eye movement task despite showing normal structural MRI and DTI.
Key words: : behavioral assessments, diffusion tensor imaging, MRI, traumatic brain injury
Introduction
The Centers for Disease Control and Prevention estimates that each year approximately 3.5 million Americans sustain a traumatic brain injury.1 Traumatic brain injuries can be classified into mild, moderate, and severe categories, and about 90% of all TBI cases in the U.S. are classified as mild TBI (mTBI).2
Mild TBI is associated with a host of symptoms and signs: headache, confusion, lightheadedness, dizziness, blurred vision or tired eyes, ringing in the ears, fatigue or lethargy, a change in sleep patterns, behavioral or mood changes (including post-traumatic stress disorder [PTSD] and depression), and problems with memory, concentration, attention and thinking.3–8 It is unknown why a percentage of mTBI individuals (∼10–15% of adults but up to 40% of children)9–11 continue to manifest symptoms at the chronic stage. Conventional structural imaging scans are typically normal.
A key goal of current research is to identify a biomarker of mTBI. While this condition may involve predominantly the white matter, the sensitivity and specificity of diffusion tensor imaging (DTI) methods in mTBI remains relatively low,12,13 although recent DTI studies14–16 have demonstrated promising results. A recent magneto-encephalography (MEG) study reported high accuracy in identifying individual patients with mTBI (96% for blast and 77% for non-blast mTBI),17 suggesting that analyses of functional connectivity may provide a sensitive indicator of white matter injury. However, the literature is mixed concerning functional magnetic resonance imaging (fMRI); task-evoked blood-oxygen-level–dependent (BOLD) responses may be increased or decreased in mTBI patients, with only relatively small group differences, compared with controls.18
One of the most commonly reported symptoms of mTBI is the inability to focus and sustain attention in the presence of distracting information.19,20 Fluctuations in attention may increase performance variability, possibly due to deficits of anticipatory (top-down) prediction21 or an imbalance between goal-driven (top-down) and stimulus-driven (bottom up) attention.22
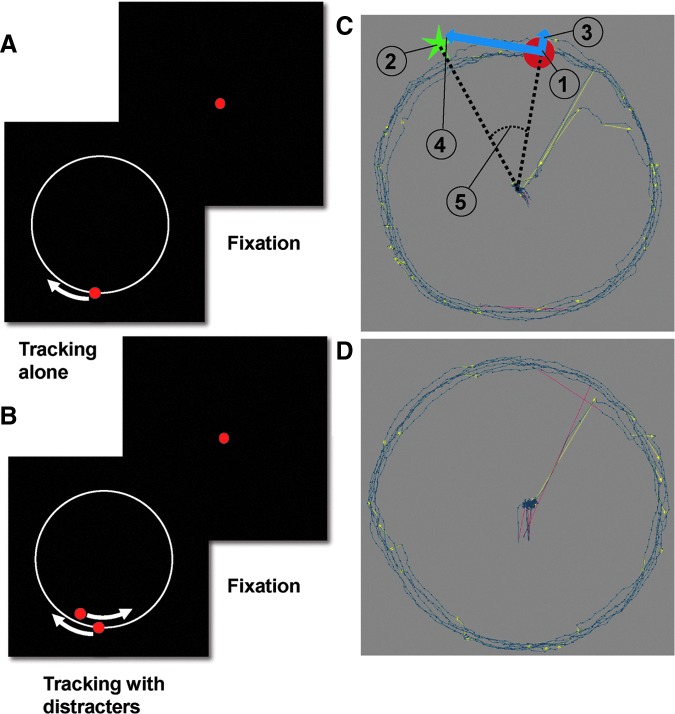
Here, we utilized a battery of neurocognitive and visual tracking tasks to examine anticipatory and/or goal- versus stimulus-driven attention deficits in chronic mTBI individuals with persistent post-concussive symptoms. The “prediction” hypothesis was tested by measuring the accuracy and variability of visual tracking eye movements to a target moving at constant speed in a circular trajectory (“Tracking Alone” condition, TA; Fig. 1A) or when the target disappeared for a short period of time (“Tracking with Gaps,” Gap), tasks that require predictive signals.23–25 The alternative hypothesis of an “imbalance” between goal- versus stimulus-driven attention22 was examined by presenting unexpected distracter stimuli at random locations moving either clockwise or counterclockwise to the target while subjects continued to track the primary visual target. This task required participants to maintain accurate predictive “goal-driven” tracking in the presence of distracting “sensory-driven” input (“Tracking with Distracters,” TD; Fig. 1B).
FIG. 1.
A schematic illustration of eye movement tasks and dependent measures. (A) Tracking Alone task. The red dot indicates the target dot the subject was instructed to follow. The white circle (not visible in actual display) indicates the target path while the white arrow (not visible in actual display) indicates the direction of target motion. The red dot at the center of the Fixation frame indicates the dot on which the subject was instructed to fixate between trials of smooth pursuit tracking. (B) Tracking with Distracters task. The red dot inside the white circle indicates the distracter dot, which in this example moves in the direction opposite to that of the target dot. (C) Sample eye traces (blue lines) for a mild traumatic brain injury patient and schematic display of dependent measures computed from the eye data. Yellow directional lines mark saccades. Red lines mark a blink. (1) The red target that the subject was instructed to track; (2) eye position; (3) radial (4) and tangential vectorial components of eye position error (distance between target position and eye position); and (5) phase error (phase difference between the eye and target positions). (D) Sample eye traces for matched control subject. Color image is available online at www.liebertpub.com/neu
We also used fMRI to measure BOLD signals across the brain in order to identify regions responsive to eye movements and increased metabolic demands. Our results indicate that eye movement measures, BOLD responses in attention-oculomotor regions, and neurocognitive measures do not reliably separate chronic mTBI patients from controls; in contrast, the shape of the BOLD signal during continuous visual tracking tasks in a distinct set of subcortical gray and white matter regions reliably separates chronic mTBI subjects from controls at the individual level.
Methods
Subjects
The data in this report were collected at two sites: Washington University in St. Louis, Missouri, School of Medicine (Wash. U.) and University of California, San Diego (UCSD). Informed consent was obtained in accordance with procedures approved by the local human studies committees.
Based on the literature,26–30 we defined “chronic” mTBI as three months post-injury. The inclusion criteria at both sites were as follows: isolated traumatic brain injury with or without loss of consciousness (LOC) three months to 5.5 years prior to testing; any persistent post-concussive symptoms; any length of post-traumatic amnesia (PTA); Glasgow Coma Scale (GCS) score of 13–15 at time of injury (if available); and age 18–60. Exclusion criteria for both controls and mTBI patients were as follows: neurological or pre-morbid psychiatric disorder (including attention-deficit/hyperactivity disorder [ADHD] and seizure disorder); alcohol/substance abuse; and gross visual (worse than 20/30 corrected) or hearing problems.
Wash. U. site
Twenty chronic mTBI patients (nine males) and twenty-two healthy control subjects (10 males) were enrolled to complete two sessions—one involving psychometric testing and eye movement recordings during visual tracking tasks, and one involving structural and functional MRI. All mTBI patients were enrolled from the Wash. U. Concussion Clinic. As a group (Supplementary Table S1; see online supplementary material at www.liebertpub.com) this sample included a high proportion of professionals with a low incidence of premorbid psychological or psychiatric problems. All patients but one experienced LOC, and all experienced PTA. In three patients, the duration of anterograde PTA (aPTA) was longer than 24 h. Four patients had positive MRI findings, of which only three clearly related to TBI. Two patients with positive radiological findings related to TBI also had >24 h PTA. The great majority of patients returned to work (80%).
Exclusion criteria for MRI included: metal objects in body (except objects proven to be safe for 3 Tesla MRI), pregnancy, and severe claustrophobia. Behavioral data for one chronic mTBI patient were incomplete. Two mTBI patients withdrew from the imaging study, one with >24 h aPTA; one patient with both >24 h aPTA and positive radiology was removed from the imaging study due to excessive movement during scanning.
Therefore, while the group of patients who completed neuropsychological and visual tracking studies included three subjects with prolonged aPTA and three subjects with abnormal radiology (two subjects with both findings, for a total of four subjects), the group that completed the fMRI experiments included only one subject with prolonged aPTA and positive radiology (subject p17) and one subject with positive radiology only (p10). One healthy subject did not participate in the imaging session due to claustrophobia, and one was removed because of excessive movement. Overall, 17 chronic mTBI patients and 20 control subjects were included in the final analysis of the fMRI scans of the visual tracking task. All subjects were compensated for their time ($25/h for MRI sessions and $10/h for the behavioral session).
UCSD site
Twenty-five mTBI (21 males) patients and 25 age- and education-matched healthy control subjects (17 males) were enrolled. Patients were recruited mostly from TBI clinics at UCSD, referrals from neurologists, and other mTBI studies conducted at UCSD. Some patients were recruited from community advertisements. Subjects participated in a neuropsychological testing session, and one session in which eye movements were recorded during visual tracking while subject underwent MEG. In this second sample, 95% of patients reported PTA, and 70% of patients reported LOC. Patients were excluded if they were hospitalized for their injury, had an abnormal computed tomography (CT) or MRI (only for patients who went to the emergency department), were intubated, had multiple TBIs, had loss of job due to the injury, had confirmed use of psychotropic or cognitive enhancing medication, or showed evidence of malingering (Test of Memory Malingering). Admission GCS scores and MRI/CT reports at time of injury were unavailable.
Therefore, the two groups slightly differed in terms of enrollment and severity of mTBI. However, they were similar in terms of level of function (as most patients returned to their original work) and low levels of psychopathology.
Healthy control subjects at both sites were recruited from the universities' research volunteer databases, while some controls subjects at UCSD were recruited from community advertisements. Control subjects were not related to the mTBI patients and were matched with patients for age and education. Control subjects were required to have no history of TBI, closed head injury, or concussion, as confirmed by Brain Injury Screening Questionnaire (BISQ). Control subjects also were required to have no history of depression or PTSD.
Supplementary Table S1 presents information about demographics, socio-economic status, injury variables, and return to work. To insure that the behavioral testing procedure was standardized, the staff performing the measurements at both sites underwent training sessions. The coordinating center (Brain Trauma Foundation) also made a video demonstration of the behavioral battery, which was reviewed by the neuropsychologist at each of the participating sites.
Neuropsychological testing
The following neuropsychological tests were administered at both sites: Head Injury Symptom Checklist (HISC), BISQ, Center for Epidemiologic Studies Depression Scale (CES-D), Conners' Adult ADHD Rating Scales (CAARS), PTSD Checklist (PCL-C), California Verbal Learning Test, Second Edition (CVLT-II), Attention Network Test (ANT), Controlled Oral Word Association Test (COWAT), the Spatial Span subtest of the Wechsler Memory Scale-III, and the Wechsler Test of Adult Reading (WTAR). The Java version of the ANT31 was administered on Windows XP personal computer (Microsoft, Redmond, WA). All other tests were administered using a paper version. Post-concussive symptoms were measured using the HISC and BISQ. The results of several tests—HISC, BISQ, CES-D, PCL-C, and ANT—are reported as raw scores as these measures lack normative scores. CVLT test results were converted into Z-scores based on test norms (except the Immediate Recall subtest, which was converted into T-values). CAARS and COWAT scores are reported as T-scores based on norms. The Spatial Span subtest of the Wechsler Memory Scale-III, and WTAR are reported as standard scores based on the test norms. Test scores not available as standard Z-scores or T-scores (i.e., raw scores for tests without norms) were converted into Z-scores by subtracting our sample mean and dividing the result by the standard deviation of our sample.
Visual tracking tasks
During the Wash. U. behavioral session and UCSD MEG session, subjects were seated in a darkened room, given instructions, and then asked to perform a shortened practice version of each task before testing. The pursuit target was a red disk moving clockwise in a circular trajectory of 10° against a black background (Wash. U. behavioral session and UCSD MEG session) or 6° radius (Wash. U. MRI session) at 0.4 Hz. The eye movement analyses presented in this paper are based on the data collected during the behavioral session. We did not observe any practice effect between the behavioral session and MRI session in the Wash. U. sample.
Three different smooth pursuit tracking tasks were used at both sites (Fig. 1A and 1B). During a TA task, only the target (red circle of 0.5° in Wash. U. behavioral session; supplementary Movie 1; see online supplementary material at www.liebertpub.com) and 0.9° in UCSD MEG session was presented on screen.32 UCSD changed the dot size to achieve a smoother dot motion for the MEG display settings.
During a TD task, a distracter disk (a red disk identical to the target but moving with a slightly different circular trajectory) was occasionally presented for 800–1200 msec with a random inter-stimulus interval (ISI) of 800–1500 msec (supplementary Movie 2; see online supplementary material at www.liebertpub.com). The distracter phase angle always crossed the target phase angle (i.e., the path of the distracter either fell behind or moved ahead of the target), although the target and distracter stimuli always remained distinct throughout the trajectory because of their different radial distances or eccentricities. There were four types of distracter paths, averaged over eccentricities (inside [9.25°] or outside [10.75°] of target trajectory): 1) Initial position (ahead; +2.5° of phase angle), speed (slower; 0.34 Hz), direction (clockwise); 2) Initial position (behind; −2.5° of phase angle), speed (faster; 0.46 Hz), direction (clockwise); 3) Initial position (ahead; +2.5° of phase angle), speed (slower; 0.34Hz), direction (counterclockwise); 4) Initial position (ahead; +2.5° of phase angle), speed (faster; 0.46 Hz), direction (counterclockwise). This task was designed to measure the subject's ability to suppress distracting information, a frequent complaint of mTBI patients.
During a Gap task, the target sometimes disappeared. The subject was instructed to follow the target's movement as closely as possible and anticipate the target's movement if it was not visible. Three gap durations were randomly presented (short, 30° [208 msec]; medium, 45° [312 msec]; and long, 60° [416 msec]), with a random ISI of 1250–3250 msec. This task was designed to measure the subject's ability to maintain predictive signals, which are necessary for accurate tracking, in the absence of sensory input. The behavioral data from the gap task and MEG data will be reported in a separate paper.
During all three tasks, a central red dot was presented when the target was not moving (fixation-only periods). During the Wash. U. behavioral session, the central dot blinked before the target started to move and turned green before the block ended (supplementary Movie 1). During the Wash. U. behavioral session, eight blocks of the eye movement tasks were presented: two blocks of the TA task, three blocks of the TD task, and three blocks of the Gap task. Each block contained three trials, where each trial consisted of six complete cycles around the circle (2.5 sec duration for each cycle, yielding a trial duration of 15 sec). The three tasks were presented in a different random order for each subject for the first set of blocks (1–3). The random task order for the first set of three blocks was reversed for the second set of three blocks (4–6). Blocks 7 and 8, which involved the TD task and Gap task, were presented in random order. About 29 visual distracters were presented per block in the TD task (not counting the distracter presented during the first cycle of each trial), and three blocks were run in each subject; thus, a total of 87 distracters were presented for each subject.
During the Wash. U. MRI session, each eye movement task was performed in three separate scans, where each scan lasted for 2.8 min. The order of scans was determined using a Latin square. Each trial of tracking lasted 15 sec, consisting of six 2.5-sec cycles, and was followed by a fixation period (only central red fixation dot presented) of 9 sec, 11 sec, or 13 sec, randomly determined. A random fixation interval allowed us to estimate the BOLD signal during the visual tracking task without assuming a hemodynamic response function.33,34 Six trials were presented within each scan. Before each MRI scan started, the name of the task was visually presented on screen for several seconds, disappearing with the start of first MRI frame and replaced by the central red fixation dot, which was presented for 8 sec. Eye movements were recorded in all subjects. Eye position signal was of lower quality in two mTBI patients and two controls subjects.
In the UCSD session, all three tasks were presented in the same block. Two orders were used: TA, Gap, TD; or TD, Gap, TA. The order of the tasks was counterbalanced across subjects. Each block consisted of 11 complete cycles of the circle (27.5 sec) and was presented three times with the same task order. About 11 visual distracters were presented per block (not counting the distracter presented during the first cycle), and three blocks were run in each subject; thus, 33 distracters were presented to each subject. A nine-point eye position calibration was performed before each block on both sites in all sessions.
An example recording for an mTBI (Fig. 1C) and a healthy control (Fig. 1D) subject is shown. Eye data were analyzed similarly to previously published data.32 We analyzed the mean and standard deviation (SD) of radial error (RE), mean and SD of tangential error (TE), mean phase error, and saccade parameters, including the number of saccades and the mean saccade duration (Fig. 1C). Calculation of tracking parameters during the TA task was based on the entire period of target motion after the first cycle. In the analysis of the TD task, the 500 msec interval preceding distracter onset was separately analyzed from the 1000 msec interval following the distracter onset. In the Wash. U. behavioral session, the software controlling the TA task was identical to the TD task but distracters were not visible. Therefore, the 500 msec interval before distracter “onset” in the TA task could be processed as in the TD task. Due to technical problems, only 19 mTBI patients and 20 matched controls were included in the analysis comparing the 500 msec interval before distracter presentation in the TA and TD tasks. In the Wash. U. behavioral session, for each block, the eye analysis software analyzed eye movement traces from the eye with the smaller SD of radial error. This procedure minimized the influence of noise on the data and is justified because ocular dominance should have little influence on visual tracking performance35,36; a detailed rationale is described by Maruta and colleagues.36
Apparatus
An infrared eye-tracker (EyeLink 1000; SR Research Ltd, Ontario, Canada) was used to record eye movements binocularly in the Wash. U. behavioral session (sampling at 500 Hz), and monocularly during the Wash. U. MRI (sampling at 500 Hz) and the UCSD MEG sessions (sampling at 1000 Hz). A desktop mount with chin rest was used in the Wash. U. behavioral session, and a long-range mount with head stabilization was used in the Wash. U. MRI and UCSD MEG sessions.
Stimuli were generated on a PC running Windows XP and using Experiment Builder (SR Research Ltd, Ontario, Canada), which allowed online integration with the EyeLink 1000 (SR Research Ltd, Ontario, Canada) eye tracker. Visual stimuli were presented during the Wash. U. behavioral session on a Samsung SyncMaster 2233RZ (Samsung, Ridgefield Park, NJ) LCD monitor (1680×1050 at 120 Hz)37 during the Wash. U. imaging session on a Boxlight CD715X (Boxlight Corporation, WA, USA) digital light processing (DLP) projector (1024×768 at 75 Hz) and rear projection screen, and during the UCSD MEG session on a Panasonic PT-D7700 (Panasonic Corporation of North America, Newark, NJ) DLP projector (1024×768 at 60 Hz).
Eye movement data analysis
Analysis of eye movement data was performed using in-house software (C ++ and scripts based on Matlab (The MathWorks, Natick, MA). The first cycle from each trial was always discarded. Samples that were marked as blinks or saccades were excluded from analysis. We used the EyeLink 1000 saccade and blink detector. Saccades were detected based on the following criteria: saccade motion threshold=0.1°; saccade velocity threshold=30°/sec; saccade acceleration threshold=8000°/sec2. A sample was marked as a blink when the pupil size was either very small, or the pupil either was not detected or was severely distorted by eyelid occlusion (SR Research Ltd manual). All blocks with more than 15% of frames marked as blinks were excluded from the analysis (nine blocks [6%] in mTBI patients and four [2.6%] blocks in controls were excluded from the UCSD data, while no blocks were excluded from the Wash. U. data). Eye data were corrected for hardware delays. Delays were measured by a photodiode that was connected to an amplifier that also received transistor-transistor logic pulses linked to the onset of stimulus generation. The hardware delay was 10 msec for the Wash. U. behavioral session and 36 msec for the UCSD session.
Imaging sessions at Wash. U. site
All MRI scans were collected on a Siemens (Siemens Corporation, New York, NY) 3T Tim-Trio scanner using standard sequences. The first imaging session consisted of structural MRI and DTI scans and roughly 30 min of resting state fMRI scans. The second imaging session consisted of nine (three TA, three TD, three Gap) 5-min scans during which subjects performed the eye movement tasks in a blocked design, with 15-sec visual tracking task periods alternating with variable fixation periods (9, 11, or 13 sec, randomly determined). Structural scans included a sagittal magnetization prepared gradient-echo (MP-RAGE) T1-weighted image (repetition time [TR]=1950 msec; echo time [TE]=2.26 msec; flip angle=9°; voxel size=1.0×1.0×1.0 mm) and a transverse turbo spin-echo T2-weighted image (TR=2500 msec; TE=435 msec; voxel-size=1.0×1.0×1.0 mm). BOLD contrast was measured with a gradient echo echo planar (EPI) sequence (TR=2000 msec; TE=27 msec; 32 contiguous 4 mm slices; 4×4 mm in-plane resolution).
DTI scans consisted of two averages of a 64-direction diffusion tensor imaging sequence (voxel size=2×2×2 mm; TR=9200 msec; TE=92 msec; 9×b-value=0 sec/mm2; the rest b-value=1000 sec/mm2). Because this report does not include results from the resting state scans, we only describe the processing of the task scans and DTI. Functional MRI data were preprocessed using standard methods described previously.38,39 Preprocessing consisted of the following steps: 1) asynchronous slice acquisition was compensated by sinc interpolation to align all slices; 2) elimination of odd/even slice intensity differences resulting from interleaved acquisition; 3) a whole–brain normalization corrected for changes in signal intensity across scans; 4) data were realigned within and across scans to correct for head movement; 5) EPI data were co-registered to the subject's T2-weighted anatomical image, which in turn was co-registered with the T1-weighted MP-RAGE, in both cases using a cross-modal procedure based on alignment of image gradients.40 The MP-RAGE was then transformed to an atlas-space41 representative target using a 12-parameter affine transformation. Movement correction and atlas transformation were accomplished in one resampling step (resulting in an isotropic 3 mm voxel size) to minimize blur and noise. The first four frames of each scan were eliminated to allow steady-state magnetization, and the remaining frames were concatenated.
Similar to the BOLD data, DTI data was preprocessed and transformed into standardized Talairach atlas space. Co-registration of each DTI image set was performed using vector gradient measure maximization.40 The first acquired, unsensitized (b=∼0 sec/mm2) DTI volume was registered to the T2 image; stretch and shear was enabled (9-parameter affine transform) to partially compensate for subject motion and eddy current distortion. For the initial DTI analyses, white matter was segmented using a fractional anisotropy (FA) threshold of 0.2 or higher. Voxelwise statistical analysis of the FA data was performed using Tract-Based Spatial Statistics (TBSS).42 TBSS projects all subjects' FA data onto a mean FA tract skeleton, and measures differences between groups using voxelwise statistics.
The second imaging session consisted of nine scans (83 frames per scan; 2.8 min duration) during which subjects performed the ocular pursuit tasks inside the MRI scanner. fMRI data from the second imaging session were co-registered with the data from the first imaging session. All functional MR frames in the second imaging session (the task imaging session) with a total head movement score of 0.9 mm or higher, including the frame immediately after the frame that exceeded the movement threshold, were removed from analysis. Head movement values were calculated by differentiating head realignment parameters across frames (which yielded a six-dimensional time series that represents instantaneous head motion) and converting them to a single number using a previously-published method.43
The blocked-design task scans were analyzed using an assumed hemodynamic response function (HRF) time-locked to the start of each 15-sec task block33 within a general linear model (GLM). The GLM included separate regressors for task (TA, TD, or Gap), linear trend, and baseline, and provided an estimate of the magnitude of the BOLD response at each voxel for each task. An additional set of GLMs was computed using a finite impulse response model that did not assume a shape for the HRF. This GLM was used to extract time-courses of the BOLD signal.
fMRI classification analyses
To classify healthy controls versus chronic mTBI patients, a leave-one-subject-out (LOSO) analysis was employed in which the BOLD signal magnitude was extracted for each patient or control from a region of interest (ROI) that was determined in a completely separate group of patients and controls.44 To determine the ROIs, we ran 37 voxelwise (17 mTBI, 20 controls) analysis of variance (ANOVA) procedures on the BOLD magnitudes with Task (TA, TD, Gap) and Group (mTBI, controls) as factors, where in each ANOVA one patient or one control subject was excluded. Each of the 37 statistical maps corresponded to the main effect of Group in an ANOVA after correction for multiple comparisons using a high Z-score/low cluster size threshold corresponding to p=0.05 (z value of 4.0 or above, cluster size of 4 voxels). The peak in each ANOVA map with the highest Z-score was selected and used as the ROI in which to measure the BOLD signal magnitude in the patient or control subject that was left out of the ANOVA. This procedure ensures that the classification based on BOLD accuracy was based on an ROI that was created using data that were completely separate from the data for the patient or control subject.
A second analysis evaluated the accuracy of classification obtained from examining individual differences in BOLD signal response of mTBI patients and controls. The BOLD response during visual tracking in each patient (with respect to a fixation baseline) was compared with the BOLD response in a group of healthy controls (17 t-tests; each patient vs. 20 controls); the variability of the response in each control was assessed against the remaining controls (20 t-tests; each control subject vs. the remaining control subjects) following the procedure developed in.45 The resulting z-maps were corrected for multiple comparisons (Monte Carlo correction). We counted the number of positive or negative voxels with a z value ≥2.25 for each patient and control subject within the “abnormal” group ROI (i.e., the ROI in which we find group differences). A binary logistic regression was then computed separately on positive, negative, or both negative and positive voxels.
Statistical analysis
All statistical analysis, except for voxel-wise and regional ANOVAs and t-tests of fMRI data, was performed using IBM SPSS Statistics, v.20 (IBM Corporation, Armonk, NY). We used in-house software to analyze the fMRI data, and results of voxel-wise statistical tests were corrected for multiple comparisons (Monte Carlo correction). Comparisons of eye data parameters and fMRI data were conducted with repeated-measures mixed model ANOVA. A sphericity correction was applied if necessary. The independent samples Mann–Whitney U test was used to compare means for non-normally distributed data, and the independent samples t-test was used to compare normally distributed data. Tests of normality were performed using the Shapiro-Wilk test. Statistical significance was preset at p<0.05.
Results
Neuropsychological tests reveal multiple deficits in mTBI patients
Two separate groups of chronic mTBI patients with persistent post-concussive symptoms (see Methods for inclusion/exclusion criteria) and healthy controls matched for age and education were studied at Wash. U. and UCSD. Patients were on average 14 months (Wash. U.) and 32 months (UCSD) post-injury (Supplementary Table S1). Although months post-injury (MPI) was significantly different between Wash. U. and UCSD, none of the behavioral variables (neuropsychological data and symptoms) correlated with MPI. Supplementary Table S1 presents demographic data, a list of symptoms, and a summary of radiological findings for each mTBI patient.
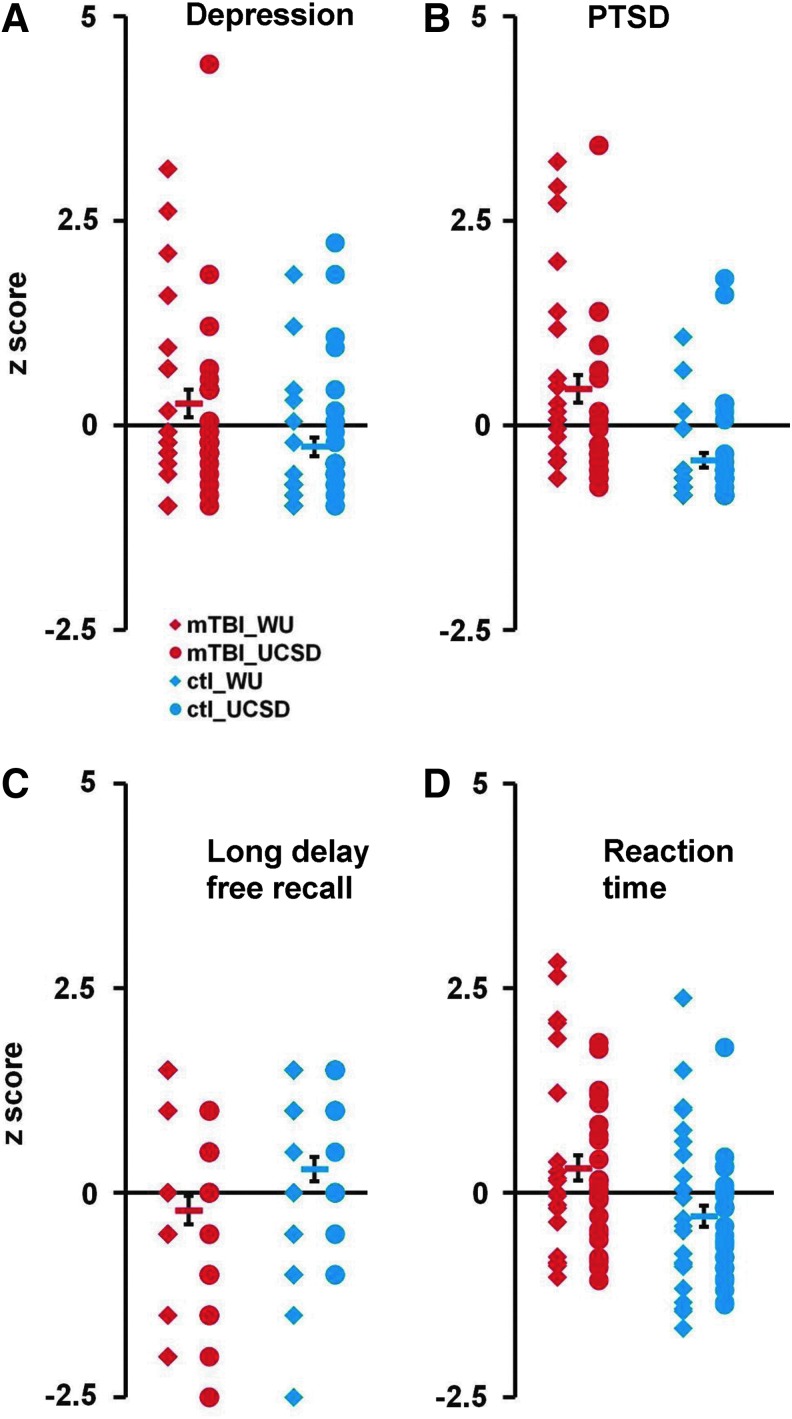
Mild TBI patients typically suffer from somatic problems (e.g. headaches), affective problems (e.g. mood), and cognitive problems (e.g. attention and memory). In our study, 68% of patients reported headache, 18% reported depression, 66% reported trouble concentrating, and 77% reported memory problems. Patients scored higher for depression (CES-D; independent samples Mann–Whitney U test, p<0.008, mTBI [n=45] vs. healthy controls [n=47]; Fig. 2A) and PTSD (PCL-C total; p<0.000; Fig. 2B). Patients also manifested memory deficits on the CVLT-II across multiple sub-scales (Fig. 2C; all, p<0.05). Patients were slower to respond to visual targets (overall reaction time) on the ANT (p=0.005; Fig. 2D), and generated fewer words to a letter (COWAT, p<0.01). Finally, patients demonstrated significantly lower performance on a test of pre-morbid IQ (WTAR; p=0.005). In general, neuropsychological profiles were similar across sites (Fig. 2; Table 1) with a few exceptions (lower scores for WTAR and ANT in the Wash. U. group; all, p<0.05).
FIG. 2.
Neuropsychological test scores in mild traumatic brain injury (mTBI) patients and controls. (A-D) Distribution of test scores (converted into Z-scores) for depression (Center for Epidemiologic Studies Depression scale: A) post-traumatic stress disorder (PTSD; PTSD Checklist scale total); (B) working verbal memory (California Verbal Learning Test-Second Edition long delay free recall score; and (C) and reaction time (Attention Network Test, overall reaction time); (D) Red diamonds display the scores from Washington University (Wash. U.). Mild TBI patients, red circles display the scores from University of California, San Diego (UCSD) mTBI patients; blue diamonds and blue circles display the scores from Wash. U. and UCSD matched control subjects, respectively. Red and blue bars indicate the mean scores for mTBI patients and control subjects, respectively. Error bars represent standard error of the mean. Color image is available online at www.liebertpub.com/neu
Table 1.
Neuropsychological Testing
| Wash. U. | UCSD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | TBI group | Control group | TBI group | Control group | |||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | p ANOVA (main effect of Site) | p ANOVA (main effect of Group) | p ANOVA (Site x Group) | |
| WTAR Premorbid IQ | 104.65 | 1.70 | 111.09 | 1.62 | 110.84 | 1.52 | 113.88 | 1.52 | 0.006 | 0.004 | ns |
| CAARS (ADHD) | 49.15 | 2.15 | 43.73 | 2.05 | 45.24 | 1.92 | 44.24 | 1.92 | ns | ns | ns |
| CESD (depression) | 10.40 | 1.69 | 4.27 | 1.61 | 9.16 | 1.51 | 6.72 | 1.51 | ns | 0.008 | ns |
| PCL-C Total (PTSD) | 33.30 | 1.94 | 20.27 | 1.85 | 26.84 | 1.73 | 21.92 | 1.73 | ns | 0.000 | 0.028 |
| Attention Network Task | |||||||||||
| Alerting | 62.20 | 5.98 | 39.45 | 5.70 | 29.36 | 5.34 | 34.28 | 5.34 | 0.001 | ns | 0.015 |
| Orienting | 43.53 | 5.76 | 41.23 | 5.35 | 39.40 | 5.02 | 35.40 | 5.02 | ns | ns | ns |
| Conflict | 160.05 | 9.03 | 139.55 | 8.39 | 130.28 | 7.87 | 130.20 | 7.87 | 0.021 | ns | ns |
| Response Time | 618.95 | 18.32 | 567.95 | 17.02 | 596.92 | 15.97 | 549.12 | 15.97 | ns | 0.004 | ns |
| Executive Functioning | |||||||||||
| COWAT–Letter Fluency | 44.2 | 1.90 | 49.59 | 1.81 | 47.04 | 1.70 | 51.16 | 1.70 | ns | 0.009 | ns |
| COWAT–Animal Fluency | 52.00 | 2.17 | 52.18 | 2.07 | 46.36 | 1.94 | 50.52 | 1.94 | ns | ns | ns |
| Short and long -Term Verbal memory (CVLT) | |||||||||||
| Immediate recall (T values) | 55.00 | 2.08 | 56.95 | 1.98 | 51.96 | 1.86 | 58.32 | 1.86 | ns | 0.036 | ns |
| Short delay recall | 0.23 | 0.25 | 0.27 | 0.23 | −0.26 | 0.22 | 0.56 | 0.22 | ns | ns | ns |
| Short delay cued recall | 0.33 | 0.23 | 0.36 | 0.22 | −0.40 | 0.21 | 0.46 | 0.21 | ns | 0.039 | ns |
| Long delay recall | 0.18 | 0.24 | 0.07 | 0.23 | −0.52 | 0.21 | 0.48 | 0.21 | ns | 0.048 | 0.015 |
| Long delay cued recall | 0.18 | 0.21 | 0.30 | 0.20 | −0.54 | 0.19 | 0.38 | 0.19 | ns | 0.010 | 0.046 |
| Spatial Working memory | |||||||||||
| Forward Span | 9.55 | 0.67 | 10.23 | 0.64 | 9.24 | 0.60 | 10.16 | 0.60 | ns | ns | ns |
| Backward Span | 9.55 | 0.65 | 8.77 | 0.62 | 8.76 | 0.58 | 9.60 | 0.58 | ns | ns | ns |
| Combined | 9.35 | 0.66 | 9.41 | 0.63 | 8.84 | 0.59 | 9.88 | 0.59 | ns | ns | ns |
Conners' Center for Epidemiologic Studies Depression Scale (CES-D), PTSD Checklist (PCL-C), and Attention Network Test are reported as raw scores as they lack normative scores. CVLT test results were converted into z-scores based on test norms (except the Immediate Recall subtest, which was converted into T-values). The Conners Adult ADHD Rating Scales (CAARS) and Controlled Oral Word Association Test (COWAT) are reported as T-scores based on norms. The Spatial Span subtest of the Wechsler Memory Scale-III and the Wechsler Test of Adult Reading (WTAR) are reported as standard scores based on the test norms.
Wash U., Washington University in St. Louis, Missouri; UCSD, University of California, San Diego, California; TBI, traumatic brain injury; SEM, standard error of the mean; ANOVA, analysis of variance.
Importantly, inspection of the scatter plots for individual tests in which significant group differences were detected (Fig. 2A-D) showed considerable overlap in the distributions of scores for mTBI and control subjects, indicating that the neuropsychological tests did not reliably differentiate control subjects from chronic mTBI patients at the individual subject level.
To examine whether it was possible to differentiate mTBI from healthy controls based on the most informative neuropsychological tests, we averaged the Z-scores for the five variables that showed the strongest significant differences between mTBI patients and controls (CES-D, PCL-C total, overall reaction time on the ANT, letter scale of the COWAT, CVLT-II long delay cued recall standard score; Table 1). A discriminant analysis with “leave-one-out classification” as a cross-validation method was used to quantify the degree to which neuropsychological tests separated mTBI patients from controls. The accuracy of classification was relatively low (62.2%).
No significant group differences in visual tracking performance
Supplementary Movie 3 (see online supplementary material at www.liebertpub.com) presents the actual eye position (left eye: purple dot; right eye: green dot) during the TA task superimposed on eye position traces (blue line) for a chronic mTBI patient.
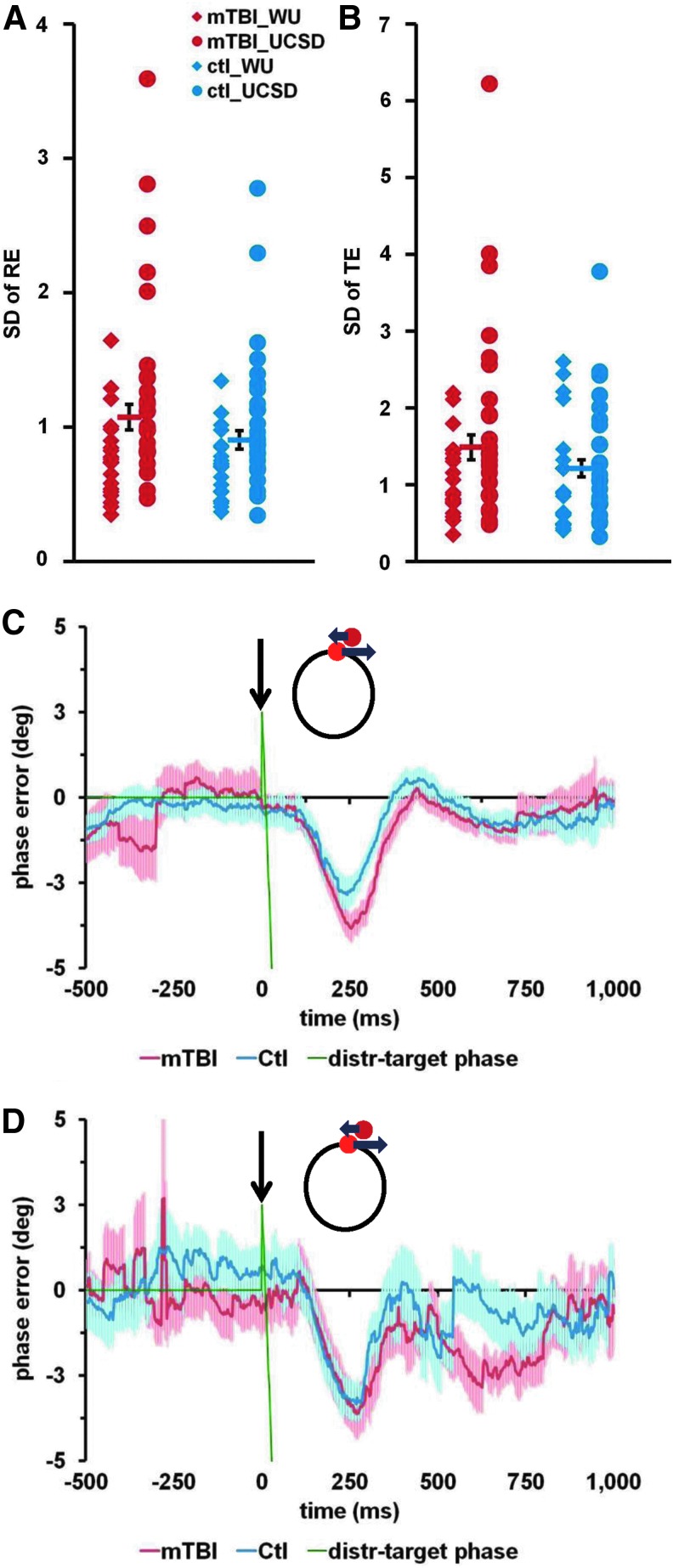
To test for changes in performance variability during predictive tracking, we compared mean eye position error and the variability of the position error averaged over the whole task period in the TA task. Inspection of the scatter plots for mTBI patients and control subjects (separately for Wash. U. and UCSD) for TA (Fig. 3A and 3B) revealed a non-significant trend for increased variability of tracking errors in the mTBI groups, with more variability for participants (both control subjects and mTBI patients) at the UCSD site. These impressions were quantified with separate ANOVAs on the variability of radial error (RE) and tangential error (TE) using as factors Site (Wash. U./UCSD) and Group (mTBI/Control).
FIG. 3.
Visual tracking errors analysis. Distributions of the variability of visual tracking errors and average time-courses of mean phase error. (A) Standard deviation (SD) of radial error (SD of RE; in degrees of visual angle) during Tracking Alone task. (B) SD of tangential error (SD of TE; in degrees of visual angle) during Tracking Alone task. (C and D) Average time-courses of mean phase error during the Tracking with Distracters task. Data are shown for the distracter type with Initial position ahead (+2.5°), speed slower than target (0.34 Hz) and counterclockwise direction, based on the data collected at Washington University (C) and University of California, San Diego (D). The black arrow indicates the time of distracter onset. Green line represents the difference between the distracter phase and target phase. Red and blue lines represent mean phase error for mild traumatic brain injury (mTBI) patients and control subjects, respectively. Red and blue shaded areas represent standard error of the mean for mTBI patients and control subjects, respectively. Color image is available online at www.liebertpub.com/neu
There was greater RE variability at the UCSD than Wash. U. site (UCSD=1.19±0.07 [mean±standard error of the mean], Wash. U.=0.75±0.08; main effect of Site, F[1,88]=16.2; p<0.001). There was only a non-significant trend for overall greater variability in the mTBI group (SD of RE: mTBI=1.05±0.08; control=0.89±0.08; main effect of Group, F[1,88]=1.9; Fig. 3A). No other interactions were significant.
Similar results were obtained for variability of the tangential error (SD of TE; Fig. 3B). There was greater variability at the UCSD site (UCSD=1.55±0.13, Wash. U.=1.11±0.14; main effect of Site, F[1,88]=5.4; p=0.023), and a non-significant trend for overall greater variability in the mTBI patients (mTBI=1.45±0.14; control=1.21±0.13; main effect of Group, F[1,88]=1.6). No other interactions were significant. Null differences between the patients and controls also were obtained for mean eye position errors (radial and phase error). Finally, there were no significant differences between chronic mTBI patients and controls for either average number of saccades per cycle (each cycle lasted 2.5 sec) or for mean saccade duration during the TA task.
To summarize this first set of results, there were only non-significant increases in variability in mTBI patients, against the hypothesis of impaired anticipation of sensory stimuli21 at the chronic stage of injury.
The second hypothesis predicted that mTBI patients would show reduced filtering of irrelevant distracting stimuli, potentially reflecting a deficit in stimulus-driven attention. Therefore, we analyzed the effect of visual distracters on performance by computing effects on eye position error time-locked to the presentation of the distracters.
Tracking performance in the TD and TA tasks differed in two ways. First, the potential presence of a distracter changed how the subjects performed the task, even when a distracter was not present on the screen. We compared the variability of tracking during the 500 msec period prior to the onset of a distracter with a matched 500-msec period in the TA task (the analysis was based on 19 chronic mTBI patients and 20 matched controls collected in Wash. U.; see Methods for details). Both the SD of RE (TA=0.41±0.02; TD=0.37±0.01; main effect of Task, F[1,37]=16.8; p<0.001) and the SD of TE (TA=0.60±0.04, TD=0.52±0.03; main effect of Task, F[1,37]=11.3; p<0.005) were significantly lower in the TD task. Neither the main effect of Group nor the Task×Group interaction were significant for either parameter. The reduction of variability during the pre-distracter baseline in the TD task, relative to the TA task, likely reflected a tonic increase in top-down control in order to prevent interference from the distracters. However, apparently both groups were able to exert stronger attention control.
The second effect of the distracters concerned the eye movement trajectory following distracter onset. Figure 3C (Wash. U.) and Figure 3D (UCSD) present average time-courses of the mean phase error before and after the onset of a distracter that initially appeared ahead of the target and then moved in a direction opposite to the target. Eye movement in both chronic mTBI and healthy subjects was clearly influenced by the onset of the distracter, as their tracking fell behind the target around 100 msec after distracter onset, reaching a maximum deviation at about 250 msec before returning to baseline at around 500 msec after distracter onset.
To quantify the effects of distracters on tracking performance, we compared the mean phase error during the 500 msec pre-distracter baseline period to the same measures during the 50- to 500-msec period following the onset of a distracter. ANOVAs with the factors Distracter type (four distracter types), Site (Wash. U./UCSD), and Group (mTBI/Control), revealed only a main effect of Distracter type (F[2,177]=3.3; p=0.038) due to reduced interference from distracters that initially appeared behind the target and then moved ahead. There was no main effect of Group and no interactions were significant. Thus, the effect of distracters on visual tracking was equivalent for patients and controls, contrary to our hypothesis that patients were more susceptible to distraction.
Therefore, neither neuropsychological nor eye movement measures could be used to accurately determine whether an individual subject was a chronic mTBI patient according to the inclusion criteria for the study. These measures may be more accurate at identifying mTBI patients at the acute stage.
Abnormal BOLD signals in chronic mTBI using visual tracking task
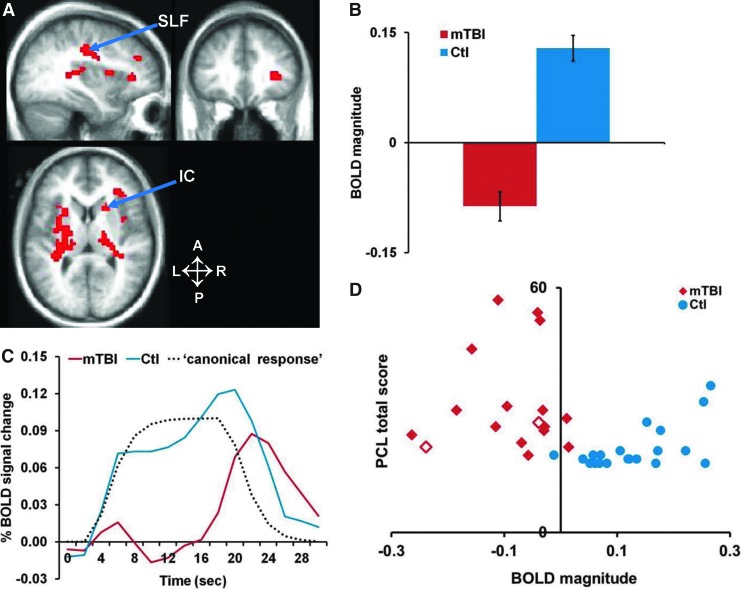
The blocked-design task scans were analyzed using an assumed HRF33 within the GLM. Analysis of the fMRI data for controls from all three visual tracking tasks (TA, TD, and Gap) relative to the fixation baseline (Fig. 4A) revealed strong activation in the dorsal attention system (frontal eye field [FEF], posterior intraparietal sulcus [IPS], ventral IPS, medial temporal complex [MT+]), visual cortex, cerebellum, putamen, and thalamus.22,46–48 In chronic mTBI patients, we observed similar cortical and cerebellar responses, along with decreased activity in different cortical, subcortical, and white matter regions (Fig. 4B). A two-factor voxel-wise ANOVA on BOLD response magnitude with Task (TA, TD, Gap) and Group (mTBI patients, controls) as factors, with correction for multiple comparisons (Monte Carlo correction with a z value of 3.0 or above, with a cluster size of 13 voxels), showed large differences between chronic mTBI patients and controls in right inferior frontal gyrus and underlying white matter, basal ganglia, and several white matter regions (Fig. 4C).
FIG. 4.
Functional magnetic resonance imaging activation during tracking tasks in mild traumatic brain injury (mTBI) patients and matched controls. (A and B) Selected brain slices with overlapping statistical map representing z values of one sample voxelwise t-test that compared tracking (collapsed over tasks) vs. fixation for matched control subjects (A) and for mTBI patients (B). Warm colors indicate activations that are stronger during tracking than during fixation. Cold colors represent activations that are stronger during fixation than during tracking. (C) The same brain slices with overlapping statistical map show z values for the main effect of group from a voxelwise analysis of variance. (Controls vs. mTBI patients, collapsed over task). Color scales indicate z-statistic. WM, white matter; Put, putamen, IC, internal capsule; vCBL, cerebellar vermis; vIPS, ventral IPS; MT+, middle temporal complex; L, left; R, right; A, anterior; P, posterior. Color image is available online at www.liebertpub.com/neu
To examine in greater detail the source of this difference, we created a large “abnormal” ROIs containing all voxels demonstrating significant differences between patients and controls (Fig. 5A). In controls, the BOLD response is positive, while in the mTBI group the response is negative (Fig. 5B). The abnormal response in mTBI was not dependent on the task (ANOVA, Task and Group×Task; p >0.10). Since each task was run in different scans, this result indicates that group differences were robust and reliable.
FIG. 5.
Magnitudes and time-courses from large region of interest (ROI). Magnitudes and time-courses extracted from voxels demonstrating significant differences between patients and controls. (A) Selected brain slices showing voxels with a significantly reduced blood-oxygen-level–dependent BOLD signal in mild traumatic brain injury (mTBI) patients relative to controls. The set of all voxels showing significant differences formed an “abnormal” ROI. L, left; R, right; A, anterior; P, posterior. (B) BOLD magnitudes from the abnormal ROI, averaged across tasks. Error bars represent standard error of the mean. (C) The time-course of the BOLD signal in the abnormal ROI. The canonical hemodynamic response function (HRF) used in the analysis to compute the BOLD magnitudes also is shown (labeled “canonical response”). (D) Scatter plot of BOLD magnitude values from the abnormal ROI for mTBI patients (red diamond) and controls (blue circles) vs. PCL-C (Post-traumatic Stress Disorder Checklist) total score. “Complex mTBI” (mTBI patients with positive radiological findings and/or antegrade post-traumatic amnesia longer than 24 h are indicated by open diamonds. Color image is available online at www.liebertpub.com/neu
The difference in response magnitude (Fig. 5B) reflected a difference in the shape of the BOLD signal time-course evoked by the eye movement task. In healthy controls, the response approximated the standard HRF, while in mTBI the BOLD time-course showed large departures from the canonical HRF with a weak sustained response during the task period and a delayed “off” response at the end of the 15 sec tracking period (Fig. 5C). Signal time-course analysis performed separately for each task (TA, TD, Gap), confirmed that the abnormal BOLD response in chronic mTBI was independent of task. A similar pattern was observed when looking at signals from individual regions contributing to the large ROI, with a decreased sustained response and delayed “off” response (not shown).
Next, we examined individual differences in the abnormal BOLD response between chronic mTBI and control subjects. Figure 5D shows a scatter plot of magnitude values for mTBI and control subjects from the large “abnormal” ROI (the ROI containing all voxels with significantly different magnitudes between patients and controls). A remarkable separation was observed between mTBI and healthy controls. Similar results were obtained for each task separately, or by dividing the data in odd versus even runs (not shown). These control analyses suggest that these results are reliable within this group of subjects.
To examine the accuracy of classification (mTBI vs. healthy) without a bias in ROI selection, we first conducted a LOSO analysis, in which the magnitude of the BOLD signal in a control subject or mTBI patient was determined from an ROI that showed the most reliable group difference between the remaining control subjects and mTBI patients—that is, the LOSO ROI was determined in a group analysis with the patient or control subject “left out”44 (see Methods). Because there were 17 mTBI patients and 20 control subjects, 37 separate ANOVAs were conducted to identify the appropriate unbiased ROI for the 37 participants. Overall, the accuracy of the classification as determined by linear discriminant analysis after the LOSO analysis was 78.4%.
Two locations in the white matter appeared as the most reliable foci in the 37 ANOVAs used to identify the ROIs (i.e., the focus with the highest Z-score for the mTBI vs. control difference in the group ANOVA). Those foci are shown in Figure 5A with arrows. One focus was in the right anterior internal capsule (IC), which had the highest Z-score in 34 of the 37 ANOVAs. A second focus was in the right superior longitudinal fasciculus (SLF), which had the highest Z-score in the remaining three ANOVAs. Similar regions (IC [atlas x,y,z=+21, +08, +16]) and SLF [atlas x,y,z=+31,-22, +36]) were the top two peaks in the group ANOVA map (Fig. 4C and Fig. 5A). BOLD magnitudes extracted from these two regions were significantly correlated (r=0.59; p=0.012) with MPI (i.e., time interval between mTBI injury and testing the subject in months). A similar but non-significant trend was observed for BOLD magnitudes from our larger “abnormal” ROI.
Since BOLD signal decrements were detected only in relatively small “common” regions across subjects, and since TBI may affect different white matter regions depending on the direction of the physical forces applied, a second analysis examined whether BOLD signal abnormalities consistently occurred in the white matter, although their precise location may have differed across subjects. Individual t-tests were run between each patient and the group of healthy controls, and were corrected for multiple comparisons using a Monte Carlo threshold (z=2.25; cluster size, n=53; p<0.05). Figure 6A-C shows three representative subjects in which consistently negative BOLD responses were identified in the white matter, even though their location and extent were variable. This result is consistent with the earlier analysis indicating that only two white matter regions (IC, SLF) showed highly significant and consistent BOLD decreases across subjects.
FIG. 6.
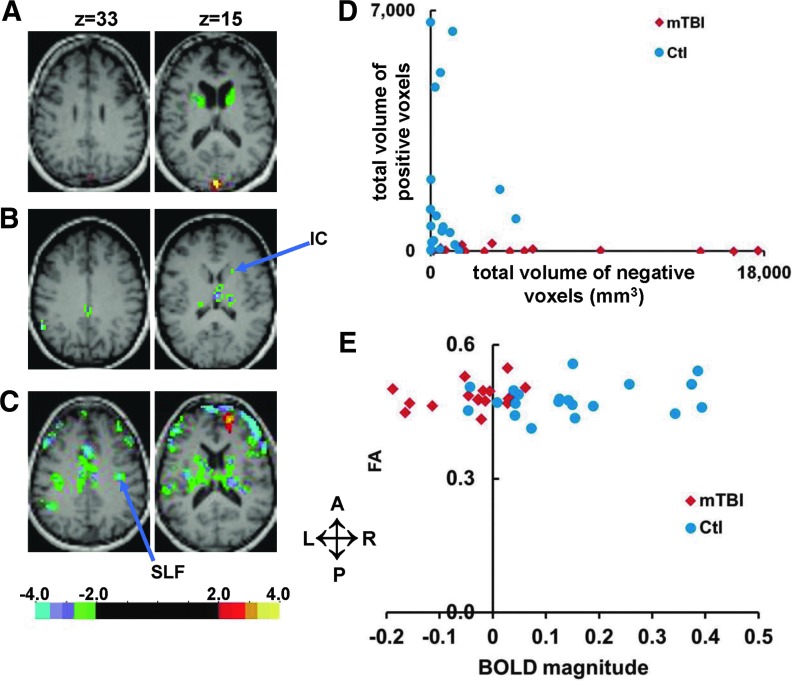
(A-C) Selected transversal slices showing significant (p<0.05; z ≥2.25, cluster size of 53 voxels) voxelwise statistical z-maps for three mild traumatic brain injury (mTBI) patients superimposed on the patients' magnetization prepared gradient-echo. Negative values represent a significantly smaller BOLD magnitude in the mTBI patient, compared with 20 control subjects. L, left; R, right; A, anterior; P, posterior. (D) The total volume (mm3) of significantly (z ≥2.25 without multiple comparison correction) positive (X axis) and negative voxels (Y axis) inside the abnormal region of interest for each patient and control subject. (E) Scatter plots of magnitude values for mTBI patients and controls (X axis) vs. FA values (Y axis) for the same white matter voxels inside right anterior IC. Color image is available online at www.liebertpub.com/neu
Next, for each individual we counted the number of voxels within the original abnormal group ROI (both gray and white matter) with a positive or negative BOLD signal magnitude (with respect to a fixation baseline) corresponding to a z value ≥2.25. For each patient, this Z-score was calculated with respect to the remaining control subjects. (See Methods and Chang and colleagues).45 Figure 6D shows that individual healthy control values were concentrated near zero. In contrast, mTBI subjects showed predominantly negative responses (compared with healthy controls). The number of both positive and negative voxels was significantly different between patients and controls (independent samples Mann–Whitney U test; p<0.002).
In summary, these findings indicate that during visual tracking tasks, BOLD responses in the white matter are abnormally decreased in chronic mTBI. While the location of these decreases varies across subjects, two small regions in the white matter in the IC and SLF seem to be consistently affected. These decreases are moderately predictive of mTBI status.
Abnormal BOLD signals versus fractional anisotropy in white matter
To examine whether BOLD signal decrements corresponded to regions of altered white matter integrity, we compared BOLD magnitude and FA from the same voxels in each subject. The FA map was thresholded at a value of FA=0.2, then masked by the IC and SLF ROIs (i.e., the two top peaks in the group ANOVA map [Fig. 4C and Fig. 5A], and most consistent regions in the LOSO analysis). As a result, for each subject we obtained a region containing only white matter voxels in which the BOLD response was abnormally decreased in mTBI patients. The average volume (SD) of white matter inside the IC ROI was 1474 (±103) mm3 in mTBI patients and 1493 (±132) mm3 for control subjects. The average volume for the SLF ROI was 1817 (±73) mm3 for mTBI patients, and 1796 (±85) mm3 for control subjects. Importantly, the same FA threshold (0.2) yielded ROIs of similar volume in the two groups, indicating that there were no gross FA differences between groups.
Also, importantly, even though the voxels were selected based on the presence of BOLD signals decrements in mTBI, the reported DTI measurements are completely independent and unbiased. Figure 6E shows a scatter plots of magnitude values for mTBI patients and controls (X axis) versus FA values (Y axis) for the same white matter voxels inside the right anterior IC. No significant group differences were found for DTI parameters. A Mann-Whitney U test on FA, axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) values and volume of white matter inside ROIs did not reveal significant group difference. There was no correlation between BOLD magnitudes and DTI values (FA, AD, RD, MD) in either region, except for the correlation of AD with BOLD magnitude in the IC (r=0.7; p=0.002 in mTBI patients only). We also performed a TBSS analysis of FA, AD, RD, and MD. TBSS is a method that allows for a voxel-wise comparison in parts of the white matter that are common across subjects.42 Again no difference was detected. Overall, these analyses indicate that differences of the BOLD signal in IC and SLF during visual tracking were not due to differences in DTI parameters, at least within the limits of our standard acquisition protocol.
Correlation between abnormal BOLD signals and behavior
The BOLD response during the eye movement tasks in the abnormal ROI was not correlated with neuropsychological test scores, behavioral performance, mTBI symptoms (measured by HISQ and BISQ), or mTBI severity (aPTA, etc.). As an example, Figure 5D shows no correlation between BOLD response magnitudes from the abnormal ROI with PCL-C (a test of post-traumatic stress) scores in both patients and controls.
The only neuropsychological variable to show a (weak) correlation with BOLD signal magnitude in the abnormal ROI was the WTAR-estimated premorbid IQ (Pearson r=0.34; p=0.04; n=37). The WTAR-estimated premorbid IQ is often different between healthy controls and chronic mTBI.49,50 While this difference could reflect pre-morbid differences, it is as likely or more likely to reflect reduced verbal memory and fluency in chronic mTBI patients.49
The regression of depression scores (CES-D) or WTAR-estimated premorbid IQ from BOLD magnitudes in LOSO ROI did not reduce the accuracy of discrimination (78.4%; measured by binary logistic regression). Regressing out the PTSD scores (PCL_C) slightly reduced the classification accuracy to 75.7%. Regressing out CES-D and PCL_C, or CES-D, PCL_C and WTAR-estimated premorbid IQ has not changed the accuracy of logistic regression on BOLD magnitudes from LOSO ROI. It is important to note that depression and PTSD are frequent co-morbid factors in mTBI,51 so our sample is comparable to samples in other studies.
One important consideration when comparing BOLD imaging data in clinical populations with healthy subjects is that group differences may be artifactual, reflecting lower SNR or higher movement in the clinical population.43 Statistical analysis (independent samples Mann–Whitney U test) did not reveal any significant differences between patients and controls, based either on total head movements or on percent of BOLD frames skipped. Moreover, there was no significant correlation between magnitude values and total movement or percent of frames skipped.
Normal BOLD signals in eye movement and attention regions for chronic mTBI patients
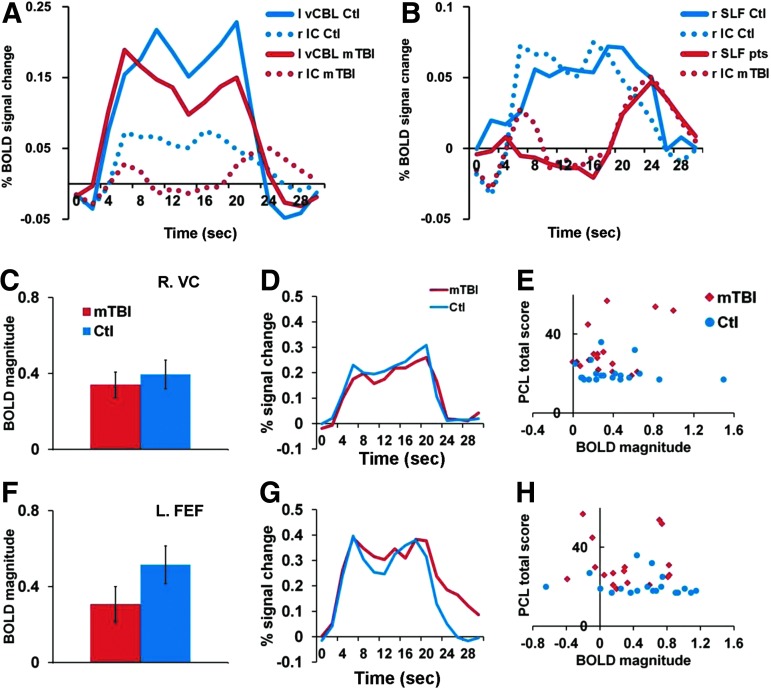
In strong contrast to the abnormal BOLD response in the white matter, the response of oculomotor and attention-related regions was normal in chronic mTBI. A comparison of the response in the right anterior IC versus left cerebellar vermis (Fig. 4A and 4B) in mTBI patients and control subjects is shown in Figure 7A. Both healthy subjects and mTBI patients had a normal-looking BOLD response in the cerebellar vermis (-9, −73, −21), a region involved in oculomotor control. Controls subjects had a much weaker response in the right anterior IC (∼1/4 of the cortical response as reported in a previous study),52 but with a similar time-course to the cortical response. In contrast, mTBI patients had an even more decreased response, with an abnormal time-course in the right anterior IC. The time-course of the BOLD signal from the right anterior IC voxels was similar to both the time-course in our abnormal ROI (Fig. 5C) and in right SLF (Fig. 7B). Similar responses between control and mTBI subjects were observed across many cortical regions involved in visual analysis and oculomotor planning (Fig. 7C, D, F, G). Group-averaged magnitude, response shape, and individual subject magnitude in cortical regions (e.g., right visual cortex; Talairach x, y, z coordinates=+39, −75, −08; Fig. 7 C-E) and left FEF (-38,-06, +53; Fig. 7 F-H) was similar in mTBI patients and controls. The BOLD signals in these regions did not distinguish between chronic mTBI and controls and were not correlated with PTSD scores (Fig. 7E and 7H) or visual tracking performance (not shown).
FIG. 7.
Magnitudes and time-courses extracted from visual and attention regions in patients and controls. (A) The time-courses of the blood-oxygen-level–dependent (BOLD) signal from left cerebellar vermis (vCBL; solid lines) and right anterior IC (dotted lines) in mild traumatic brain injury (mTBI) patients (red lines) and control subjects (blue lines). (B) The time-courses of the BOLD signal from right superior longitudinal fasciculus (SLF; solid lines) and right anterior internal capsule (IC; dotted lines) in mTBI patients (red lines) and control subjects (blue lines). (C, F) BOLD magnitudes averaged across tasks from right visual cortex (C), and left frontal eye field (FEF; F). Error bars represent standard error of the mean. (D, G) BOLD time-courses averaged across tasks from right visual cortex (D) and left FEF (G). (E, H) Scatter plots of BOLD magnitude values for mTBI patients (red diamond) and controls (blue circles) vs. Post-Traumatic Stress Disorder Checklist (PCL) total score from right visual cortex (E) and left FEF (H). Color image is available online at www.liebertpub.com/neu
Based on previous work in normal subjects, a re-orienting response to the distracters should produce greater activity in dorsal frontoparietal regions (IPS, FEF, MT) and the recruitment of a right-lateralized network including right supramarginal gyrus (SMG) and inferior frontal cortex.22 In agreement with this prediction, voxel-wise ANOVAs on the BOLD magnitudes with Task (TA vs. TD) and Group (mTBI patients, controls) showed a stronger response bilaterally in dorsal frontoparietal cortex, and right lateralized in ventral frontoparietal cortex during the presentation of distracters (TD) but this modulation occurred equally in both groups (Supplementary Fig. 1A; see online supplementary material at www.liebertpub.com). These results are in line with the visual tracking results showing a robust effect of distractors on eye movement but no difference between mTBI and control subjects.
Similar results were obtained for the Gap task, in which the disappearance of the target was an infrequent, unexpected transient event that should also drive fronto-parietal regions involved in attention. Voxel-wise ANOVAs on the BOLD magnitudes with Task (TA vs. Gap) and Group (mTBI patients, controls) as factors yielded a significant Task effect (Supplementary Fig. 1B), with stronger responses in right dorsal and ventral frontoparietal regions during the Gap task than TA task.
Overall, the fMRI results indicate differences between chronic mTBI patients and control subjects primarily due to an abnormal BOLD time-course in specific white matter regions. The magnitude of the BOLD response accurately classified individual chronic mTBI patients. Group differences in BOLD signals were observed despite equivalent behavioral performance on the visual tracking tasks and equivalent head stability during the scans. On the other hand, similarities in BOLD activity in oculomotor and attention circuitries in the two groups were in line with the equivalent performance on the visual tracking movement tasks.
Discussion
This study shows that BOLD signals in the white matter of chronic mTBI patients with persistent post-concussive symptoms, obtained during a continuous visual tracking task, are abnormal and could be used to distinguish individual patients from control subjects. Importantly, differences in BOLD signals in white matter were detected in the absence of differences in DTI FA values. Further, the high accuracy of the BOLD signals in discriminating individual patients from controls stands in contrast to the poor accuracy of neuropsychological measures and measures of visual tracking performance.
Shape of BOLD time-course accurately classifies chronic mTBI subjects
Although there was between-subject variability in the location of tissue with decreased BOLD magnitudes in mTBI patients, two regions in the right anterior IC and right SLF showed highly consistent differences between patients and controls. We observed accurate discrimination of chronic mTBI patients from healthy controls when BOLD signals were extracted from the entire abnormal ROI or only from the regions in the IC and SLF. The discrimination of patients from controls was not an artifact of differences between groups in head movement or signal quality. It remained significant when tested on smaller (∼30%) subsets of data created from the separate task conditions (i.e., TA, TD, Gap).
Although we found highly significant group differences in our data, the accuracy of single subject classification using a leave-one-out method was 78.4%. Single-subject analysis revealed individual variability in the location of brain tissue with decreased BOLD magnitudes, along with consistent BOLD abnormalities in a much smaller set of regions (IC, SLF). Therefore, our data indicate the importance of single-subject analyses for properly localizing the entire set of abnormal tissue in an mTBI patient. Consistent with our conclusion, most studies have failed to find mTBI-related damage in consistent regions across all patients.12,16,53 It is important to note that since the prediction values obtained in our study may be sample dependent, our results require replication and validation in a separate sample.
The discrimination of chronic mTBI patients from healthy controls was unrelated to symptoms, neuropsychological, or visual tracking measures. This result is important since group differences in imaging modalities often co-occur with behavioral differences that confound the interpretation of brain signals. The classifier was based on the BOLD response magnitude in the abnormal ROI during the visual tracking tasks, which was obtained by convolving the BOLD signal time series with a canonical HRF based on a model of the task (on for 15 sec, off for a variable interval). The onset of the response in the time-course corresponded to the onset of tracking the target, while the offset corresponded to the disappearance of the target. Figure 5C shows that that the BOLD time-courses in healthy subjects closely approximated the shape of the canonical HRF.
In contrast, chronic mTBI subjects manifested an abnormal shape with an attenuated sustained component and a delayed offset. Importantly, these abnormalities in BOLD response shape did not reflect a generalized problem with neurovascular coupling across the brain, as the shape and magnitude of BOLD signals in most cortical and subcortical regions involved in visual attention and eye movements (visual occipital, cerebellar, and frontoparietal cortex) were normal, both at the group (Fig. 5C and Fig. 7C, 7D, 7F, and 7G) and individual level (Fig.7 E and 7H).
BOLD signals, white matter, and other imaging modalities
Our results are noteworthy in relation to other imaging modalities, such as diffusion imaging, task fMRI studies, and magnetic resonance spectroscopy, which have reported group differences between mTBI and healthy subjects12,13,18,54 but limited success in discriminating individual patients. White matter studies of mTBI using diffusion imaging have shown fairly circumscribed anomalies in subdivisions of the corpus callosum, anterior corona radiata, SLF, and internal capsule.55–59 This distribution generally matched the distribution of BOLD signal anomalies observed in the present study. However, the most recent diffusion imaging studies have shown a widespread loss of white matter integrity in mild TBI12,53,60–65 that is more extensive than the topography of abnormal BOLD signals reported here.
The presence of BOLD signal responses in the white matter is well established in the literature. In general, evoked signals in the white matter are lower in magnitude than cortical responses but have a similar response shape,52,66–74 consistent with our data (Fig. 7A and 7B). The white matter regions that distinguished chronic mTBI patients from controls were part of two of the major white matter tracts frequently damaged in mTBI (IC and SLF).12,55,58–60,63,75 They also matched regions that in simulation studies of head concussion models were strongly affected by physical distortion.76 In addition, these regions are close to a core of damaged tissue, including white matter tracts, basal ganglia, and upper brainstem, that has been found in severe TBI.77 Interestingly, a recent paper78 found that white matter hyper-intensity burden was associated with a BOLD signal decrease in white matter during a finger-tapping task. Therefore, our interpretation is that these regions show an abnormal BOLD signal due to a pathological process caused by the damage inflicted by TBI.
Surprisingly, we did not observe significant differences based on FA values in IC and SLF, while we did observe strong differences between mTBI patients and controls based on BOLD magnitudes from the same white matter voxels. Importantly, we also failed to find a correlation between BOLD magnitudes and FA values. Recent advanced studies of DTI in mTBI suggest that decreases and increases in FA values may reflect different aspects of the mTBI injury53,65,79; therefore, it is logical to suggest that BOLD and DTI may measure different aspects of mTBI and should be used in conjunction to evaluate the full extent of injury after mTBI.80 Future studies will be necessary to evaluate the overlap between abnormalities detected by BOLD signals and DTI.
Unfortunately, only a handful of studies have compared BOLD signals and DTI in humans and animals that have experienced traumatic brain injury.80,81 A study by Niskanen and colleagues in rats with focal TBI showed that decrements of the BOLD response to sensory stimuli are a sensitive physiological marker of behavioral recovery and correlate with loss of myelinated fibers.82 After focal cortical contusion, the shape of the BOLD signal in the affected cortex showed, similarly to our study, a normal onset but a suppressed sustained component (although no offset delay). Critically, BOLD response abnormalities were correlated with decreases in local field potentials but not with changes in cerebral blood volume, a result consistent with alteration of neural activity at the site of damage rather than neurovascular coupling. Finally, the BOLD signals were correlated with a loss of myelinated fibers but not with tissue loss or neurodegeneration. Therefore, the findings of Niskanen and colleagues are consistent with our interpretation that abnormal BOLD responses in our study tracked a pathological process in white matter.
The reduced BOLD response during task performance is consistent with some fMRI studies in chronic mTBI patients.18 However, increased BOLD responses in acute and chronic mTBI patients relative to healthy controls also have been reported. For example, in studies of working memory using the “n-back” task, acute mTBI patients showed greater and less focal activation in prefrontal cortex than controls as memory load increased.18,83,84 Overall, the fMRI literature is mixed concerning whether BOLD activity during task performance is increased or decreased in mTBI patients. Of the roughly 20 studies that have been reported in the literature (the majority involving acute or mixed acute/chronic patients, and only two studies clearly at the chronic stage), about half reported relatively increased or less focal BOLD responses than controls, while about one third reported decreased BOLD responses. The conditions under which mTBI patients show either increased or decreased BOLD activations relative to controls is not well understood.
This is the first fMRI study to report good discrimination at the level of individual subjects, as opposed to group differences. The sensitive results of our study could be due to the continuous nature of the task, which engages both predictive and sensory-driven processes without loading working memory.18 In addition, a recent MEG study at rest17 showed that periods of transiently increased delta power (or abnormal low-frequency magnetic activity, 1–4 Hz) occurred more frequently in chronic mTBI patients than in controls. This measure successfully separated chronic mTBI patients from control subjects with an impressive average accuracy of 87% (96% for the blast and 77% for non-blast injuries).
We can only speculate about the cellular mechanisms underlying BOLD signal decrements. TBI at the acute stage is associated with metabolic changes that involve disturbances of potassium, sodium, and calcium ion balances, as well as hyperglycolysis, glutamate alterations, decreased oxygen, hypometabolism, and apoptosis, as well as altered level of amyloid-β peptide.85–89 Relative alterations in the excitation/inhibition balance of neuronal populations could be responsible at the chronic stage for BOLD signal and neuronal decrements.82 Healthy subjects with higher gamma-aminobutyric acid (GABA) concentration at rest tend to produce BOLD responses of smaller magnitude and longer duration.90 Because the GABAergic complex is very sensitive to damage of the neuronal membrane,91 and GABA signaling is important for axonal remodeling after traumatic brain injury,92 it is possible that relative alterations in GABA content in mTBI patients could affect BOLD signals. Plasticity following TBI6,65,87 may underlie recovery, leaving at the chronic stage reduced or no behavioral deficits, possibly some symptoms, but permanent neural metabolic changes detected as an abnormal BOLD response in selected brain regions.
Poor accuracy of behavioral tests in identifying individual chronic mTBI patients
In contrast to our original hypotheses, we found no significant abnormality in neurocognitive tests, nor in eye movement variability or accuracy during tasks that were strongly dependent on the application of predictive signals (TA task) or that required an interaction between goal-driven and stimulus-driven attention (TD task).21,22 The distracter task induced a reduction of variability prior to distracter onset, which we interpret as an increase in top-down control to limit interference from distracting information, as well as reduced accuracy of tracking following the onset of the distracter.
Notwithstanding the high sensitivity in measuring these dynamic tracking processes, and the effective manipulation of the attentional state of the subjects, tracking behavior was equivalent in chronic mTBI patients and controls. This negative result was validated in two independent samples (UCSD and Wash. U.). Therefore, we conclude that classical measurements of visual tracking and neurocognitive tests did not accurately discriminate symptomatic mTBI patients from controls at the chronic stage. However, they may be helpful in detecting impairments at the acute stage.93
Our chronic mTBI patients showed some affective problems (higher depression and PTSD scores) and cognitive problems (slower reaction time, poorer memory performance, reduced verbal fluency) in the neuropsychological tests, consistent with previously published studies.32,51,75 Analysis of the scatter plots of the neuropsychological scores, however, indicated that the distributions for chronic mTBI patients and matched controls showed considerable overlap, even on tests that revealed significant group differences between patients and controls. Further, there was no correlation (except for WTAR scores) between neuropsychological measures or affective disorders and BOLD signal magnitudes. This result indicates that the focal abnormalities in the white matter were not related to these deficits or disorders. In addition, these disorders do not produce BOLD signal abnormalities comparable to those reported here.78,94–98
Therefore, our results indicate that neither neuropsychological measures nor measures of predictive visual tracking eye movements accurately discriminated individual symptomatic chronic mTBI subjects from controls. These negative results were obtained concurrently with the promising accuracy of classification of chronic mTBI subjects observed using BOLD-fMRI measures in regions of the white matter. The disparity in neurocognitive measures and the BOLD fMRI measures could be explained by the latter being a better marker of the original injury rather than a functional marker. This interpretation would be consistent with the lack of BOLD fMRI correlation with symptoms, which along with the prior history of TBI, completely separates the controls from the chronic mTBI group.
Study limitations
The main limitation of this study is the relatively small sample size. Even though our sample was comparable to that of many studies in the literature, and the level of significance in the voxel-wise statistical maps was corrected for multiple comparisons at the whole–brain level following a random effect statistical model that allowed for generalization at the population level, we consider this study a proof-of-concept that will require replication and validation in a separate and larger independent sample.
The second limitation is the relative heterogeneity between Wash.U. and UCSD samples. There were some differences in enrollment, relative severity of TBI, and exclusion criteria that made the Wash.U. cohort likely to be more severe. This is reflected in the slightly higher scores for depression and PTSD at the group level. There also were differences in the accuracy of eye movement recordings, with more variability in the UCSD subjects, who were recorded within an MEG scanner. These differences would be more problematic if the results and conclusions based on the two cohorts were different, since it would be unclear if the differing results reflected the composition of the two cohorts or inconsistency in the dependent measures. However, the conclusions from the UCSD sample replicated those from the Wash. U. sample in all important respects regarding the poor association of mTBI status with eye movement and neuropsychological measures. Therefore, the similarity of the results from the two cohorts indicates that our conclusions are fairly robust with respect to modest variations in sample composition.
The Wash. U. sample contained three patients with aPTA longer than 24 h, and three patients with radiological abnormalities (of whom two possessed both characteristics; i.e., four more severe patients overall). These patients are defined today as “complex mTBI.”99,100 Their enrollment was due to the use of inclusion criteria, when the research consortium of the Brain Trauma Foundation, UCSD, and Wash. U. became operative in 2009, that are more liberal than current standards. However, even though all four complex mTBI subjects participated in psychometric/visual tracking testing, only two of these patients underwent task fMRI (those patients are indicated by open diamonds on Fig. 5D). Therefore, it is unlikely that the individual differences found in white matter BOLD signals, but not in neuropsychological or visual tracking measures, was related to this factor.
Finally, our extensive analyses using standard DTI did not yield any significant difference. However, it is possible that our differences in white matter BOLD signals might be related to white matter changes as measured with DTI if more advanced methods were employed.101
We conclude that at standard imaging resolutions, BOLD signal changes in the white matter during movement tracking appear to be a promising biomarker for chronic mTBI.
Supplementary Material
Acknowledgments
We gratefully acknowledge Robert Fucetola, PhD, for neuropsychological consultation; A.Z. Snyder, M.D., PhD, for image analysis assistance; K.L. Zinn, P.T., for scheduling patients; David L. Brody, M.D., PhD, for referral of some mTBI subjects; Jerrel Rutlin for TBBS analysis and M.P. McAvoy, PhD, and M.J. Strube, PhD, for statistical advice. Supported by James S. McDonnell Foundation grant for the Attention Dynamics Consortium in Traumatic Brain Injury (ADC-TBI) and National Institute of Child Health and Human Development (NICHD) grant R01 HD061117-05A2.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Coronado V.G., McGuire L.C., Faul M., Sugerman D., and Pearson W. (2012). The Epidemiology and Prevention of TBI (in press) [Google Scholar]
- 2.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B., and Yurkewicz L. (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghajar J. (2000). Traumatic brain injury. Lancet 356, 923–929 [DOI] [PubMed] [Google Scholar]
- 4.Kushner D. (1998). Mild traumatic brain injury: toward understanding manifestations and treatment. Arch. Intern. Med. 158, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 5.Gerber D.J. and Schraa J.C. (1995). Mild traumatic brain injury: searching for the syndrome. J. Head Trauma Rehabil. 10, 28–40 [Google Scholar]
- 6.Chen A.J. and D'Esposito M. (2010). Traumatic brain injury: from bench to bedside [corrected] to society. Neuron 66, 11–14 [DOI] [PubMed] [Google Scholar]
- 7.McDowell S., Whyte J., and D'Esposito M. (1997). Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia 35, 1341–1353 [DOI] [PubMed] [Google Scholar]
- 8.McAllister T.W. (2011). Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 13, 287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikmen S., McLean A., Jr., Temkin N.R, and Wyler A.R. (1986). Neuropsychologic outcome at one-month postinjury. Arch. Phys. Med. Rehabil. 67, 507–513 [PubMed] [Google Scholar]
- 10.Crooks C.Y., Zumsteg J.M., and Bell K.R. (2007). Traumatic brain injury: a review of practice management and recent advances. Phys. Med. Rehabil. Clin. N. Am. 18, 681–710 [DOI] [PubMed] [Google Scholar]
- 11.Thornhill S., Teasdale G.M., Murray G.D., McEwen J., Roy C.W., and Penny K.I. (2000). Disability in young people and adults one year after head injury: prospective cohort study. BMJ 320, 1631–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., and Brody D.L. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 364, 2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellyer P.J., Leech R., Ham T.E., Bonnelle V., and Sharp D.J. (2013). Individual prediction of white matter injury following traumatic brain injury. Ann. Neurol. 73, 489–499 [DOI] [PubMed] [Google Scholar]
- 15.Kim N., Branch C.A., Kim M., and Lipton M.L. (2013). Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PLoS One 8, e59382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorge R.E., Acion L., White T., Tordesillas-Gutierrez D., Pierson R., Crespo-Facorro B., and Magnotta V.A. (2012). White matter abnormalities in veterans with mild traumatic brain injury. Am. J. Psychiatry 169, 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M.X., Nichols S., Robb A., Angeles A., Drake A., Holland M., Asmussen S., D'Andrea J., Chun W., Levy M., Cui L., Song T., Baker D.G., Hammer P., McLay R., Theilmann R.J., Coimbra R., Diwakar M., Boyd C., Neff J., Liu T.T., Webb-Murphy J., Farinpour R., Cheung C., Harrington D.L., Heister D., and Lee R.R. (2012). An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage 61, 1067–1082 [DOI] [PubMed] [Google Scholar]
- 18.McDonald B.C., Saykin A.J., and McAllister T.W. (2012). Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav. 6, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuss D.T., Stethem L.L., Hugenholtz H., Picton T., Pivik J., and Richard M.T. (1989). Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J. Neurol. Neurosurg. Psychiatry 52, 742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson I.H., Manly T., Andrade J., Baddeley B.T., and Yiend J. (1997). ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 35, 747–758 [DOI] [PubMed] [Google Scholar]
- 21.Ghajar J. and Ivry R.B. (2009). The predictive brain state: asynchrony in disorders of attention? Neuroscientist 15, 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbetta M. and Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature reviews. Neuroscience 3, 201–215 [DOI] [PubMed] [Google Scholar]
- 23.Lisberger S.G., Morris E.J., and Tychsen L. (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10, 97–129 [DOI] [PubMed] [Google Scholar]
- 24.Chen Y., Holzman P.S., and Nakayama K. (2002). Visual and cognitive control of attention in smooth pursuit. Prog. Brain Res. 140, 255–265 [DOI] [PubMed] [Google Scholar]
- 25.Collewijn H. and Tamminga E.P. (1984). Human smooth and saccadic eye movements during voluntary pursuit of different target motions on different backgrounds. J. Physiology 351, 217–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boake C., McCauley S.R., Levin H.S., Pedroza C., Contant C.F., Song J.X., Brown S.A., Goodman H., Brundage S.I., and Diaz-Marchan P.J. (2005). Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 17, 350–356 [DOI] [PubMed] [Google Scholar]
- 27.Kovesdi E., Luckl J., Bukovics P., Farkas O., Pal J., Czeiter E., Szellar D., Doczi T., Komoly S., and Buki A. (2010). Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (Wien) 152, 1–17 [DOI] [PubMed] [Google Scholar]
- 28.Leddy J.J., Kozlowski K., Donnelly J.P., Pendergast D.R., Epstein L.H., and Willer B. (2010). A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clin. J. Sport Med. 20, 21–27 [DOI] [PubMed] [Google Scholar]
- 29.McCrea M., Iverson G.L., McAllister T.W., Hammeke T.A., Powell M.R., Barr W.B., and Kelly J.P. (2009). An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin. Neuropsychol. 23, 1368–1390 [DOI] [PubMed] [Google Scholar]
- 30.Yuh E.L., Mukherjee P., Lingsma H.F., Yue J.K., Ferguson A.R., Gordon W.A., Valadka A.B., Schnyer D.M., Okonkwo D.O., Maas A.I., and Manley G.T. (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 73, 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan J., McCandliss B.D., Sommer T., Raz A., and Posner M.I. (2002). Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 14, 340–347 [DOI] [PubMed] [Google Scholar]
- 32.Maruta J., Suh M., Niogi S.N., Mukherjee P., and Ghajar J. (2010). Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 25, 293–305 [DOI] [PubMed] [Google Scholar]
- 33.Boynton G.M., Engel S.A., Glover G.H., and Heeger D.J. (1996). Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 16, 4207–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollinger J.M., Shulman G.L., and Corbetta M. (2001). Separating processes within a trial in event-related functional MRI I. The method. Neuroimage 13, 210–217 [DOI] [PubMed] [Google Scholar]
- 35.Bahill A.T. and McDonald J.D. (1983). Smooth pursuit eye movements in response to predictable target motions. Vision Res. 23, 1573–1583 [DOI] [PubMed] [Google Scholar]
- 36.Maruta J., Heaton K.J., Kryskow E.M., Maule A.L., and Ghajar J. (2013). Dynamic visuomotor synchronization: quantification of predictive timing. Behav. Res. Methods 45, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P. and Nikolic D. (2011). An LCD monitor with sufficiently precise timing for research in vision. Front. Hum. Neurosci. 5, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter A.R., Astafiev S.V., Lang C.E., Connor L.T., Rengachary J., Strube M.J., Pope D.L., Shulman G.L., and Corbetta M. (2010). Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 67, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astafiev S.V., Stanley C.M., Shulman G.L., and Corbetta M. (2004). Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat. Neurosci. 7, 542–548 [DOI] [PubMed] [Google Scholar]
- 40.Rowland D.J., Garbow J.R., Laforest R., and Snyder A.Z. (2005). Registration of [18F]FDG microPET and small-animal MRI. Nucl. Med. Biol. 32, 567–572 [DOI] [PubMed] [Google Scholar]
- 41.Talairach J. and Tournoux P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc.: New York [Google Scholar]
- 42.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 43.Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., and Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esterman M., Tamber-Rosenau B.J., Chiu Y.C., and Yantis S. (2010). Avoiding non-independence in fMRI data analysis: leave one subject out. Neuroimage 50, 572–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang D.J., Zubal I.G., Gottschalk C., Necochea A., Stokking R., Studholme C., Corsi M., Slawski J., Spencer S.S., and Blumenfeld H. (2002). Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia 43, 68–74 [DOI] [PubMed] [Google Scholar]
- 46.Krauzlis R.J. (2005). The control of voluntary eye movements: new perspectives. Neuroscientist 11, 124–137 [DOI] [PubMed] [Google Scholar]
- 47.Petit L., Orssaud C., Tzourio N., Salamon G., Mazoyer B., and Berthoz A. (1993). PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulate cortex involvement. J. Neurophysiol. 69, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 48.Sweeney J.A., Mintun M.A., Kwee S., Wiseman M.B., Brown D.L., Rosenberg D.R., and Carl J.R. (1996). Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J. Neurophysiol. 75, 454–468 [DOI] [PubMed] [Google Scholar]
- 49.Mathias J.L., Bowden S.C., Bigler E.D., and Rosenfeld J.V. (2007). Is performance on the Wechsler test of adult reading affected by traumatic brain injury? Br. J. Clin. Psychol. 46, 457–466 [DOI] [PubMed] [Google Scholar]
- 50.Heitger M.H., Jones R.D., Macleod A.D., Snell D.L., Frampton C.M., and Anderson T.J. (2009). Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132, 2850–2870 [DOI] [PubMed] [Google Scholar]
- 51.Mac Donald C.L., Johnson A.M., Wierzechowski L., Kassner E., Stewart T., Nelson E.C., Werner N.J., Zonies D., Oh J., Fang R., and Brody D.L. (2014). Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol. 71, 994–1002 [DOI] [PubMed] [Google Scholar]
- 52.Fraser L.M., Stevens M.T., Beyea S.D., and D'Arcy R.C. (2012). White versus gray matter: fMRI hemodynamic responses show similar characteristics, but differ in peak amplitude. BMC Neurosci. 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling J.M., Pena A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C., and Mayer A.R. (2012). Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 135, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirov II, Tal A., Babb J.S., Reaume J., Bushnik T., Ashman T., Flanagan S.R., Grossman R.I., and Gonen O. (2013). Proton MR spectroscopy correlates diffuse axonal abnormalities with post-concussive symptoms in mild traumatic brain injury. J. Neurotrauma 30, 1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huisman T.A., Schwamm L.H., Schaefer P.W., Koroshetz W.J., Shetty-Alva N., Ozsunar Y., Wu O., and Sorensen A.G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. A.J.N.R. Am. J. Neuroradiol. 25, 370–376 [PMC free article] [PubMed] [Google Scholar]
- 56.Lipton M.L., Gellella E., Lo C., Gold T., Ardekani B.A., Shifteh K., Bello J.A., and Branch C.A. (2008). Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 25, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 57.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendlin B.B., Ries M.L., Lazar M., Alexander A.L., Dempsey R.J., Rowley H.A., Sherman J.E., and Johnson S.C. (2008). Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage 42, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laitinen T.P., Lopez A.S., Bolkvadze T., Pitkänen A., and Gröhn O.H. (2009). DTI detects FA changes in the internal capsule and thalamus in rat after traumatic brain injury—comparison with histology. In: Proc. Intl. Soc. Mag. Reson. Med [Google Scholar]
- 60.Kinnunen K.M., Greenwood R., Powell J.H., Leech R., Hawkins P.C., Bonnelle V., Patel M.C., Counsell S.J., and Sharp D.J. (2011). White matter damage and cognitive impairment after traumatic brain injury. Brain 134, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Messe A., Caplain S., Paradot G., Garrigue D., Mineo J.F., Soto Ares G., Ducreux D., Vignaud F., Rozec G., Desal H., Pelegrini-Issac M., Montreuil M., Benali H., and Lehericy S. (2011). Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 32, 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Z., Yeo R.A., Pena A., Ling J.M., Klimaj S., Campbell R., Doezema D., and Mayer A.R. (2012). An FMRI study of auditory orienting and inhibition of return in pediatric mild traumatic brain injury. J. Neurotrauma 29, 2124–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morey R.A., Haswell C.C., Selgrade E.S., Massoglia D., Liu C., Weiner J., Marx C.E., Cernak I., and McCarthy G. (2013). Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum. Brain Mapp. 34, 2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E. and Lipton M.L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. A.J.N.R. Am. J. Neuroradiol. 34, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipton M.L., Kim N., Park Y.K., Hulkower M.B., Gardin T.M., Shifteh K., Kim M., Zimmerman M.E., Lipton R.B., and Branch C.A. (2012). Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 6, 329–342 [DOI] [PubMed] [Google Scholar]
- 66.Yarkoni T., Barch D.M., Gray J.R., Conturo T.E., and Braver T.S. (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PLoS One 4, e4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazerolle E.L., Beyea S.D., Gawryluk J.R., Brewer K.D., Bowen C.V., and D'Arcy R.C. (2010). Confirming white matter fMRI activation in the corpus callosum: co-localization with DTI tractography. Neuroimage 50, 616–621 [DOI] [PubMed] [Google Scholar]
- 68.Gawryluk J.R., D'Arcy R.C., Mazerolle E.L., Brewer K.D., and Beyea S.D. (2011). Functional mapping in the corpus callosum: a 4T fMRI study of white matter. Neuroimage 54, 10–15 [DOI] [PubMed] [Google Scholar]
- 69.Mazerolle E.L., Gawryluk J.R., Dillen K.N., Patterson S.A., Feindel K.W., Beyea S.D., Stevens M.T., Newman A.J., Schmidt M.H., and D'Arcy R.C. (2013). Sensitivity to white matter FMRI activation increases with field strength. PLoS One 8, e58130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McWhinney S.R., Mazerolle E.L., Gawryluk J.R., Beyea S.D., and D'Arcy R.C. (2012). Comparing gray and white matter fMRI activation using asymmetric spin echo spiral. Journal of neuroscience methods 209, 351–356 [DOI] [PubMed] [Google Scholar]
- 71.Fabri M., Polonara G., Mascioli G., Salvolini U., and Manzoni T. (2011). Topographical organization of human corpus callosum: an fMRI mapping study. Brain Res. 1370, 99–111 [DOI] [PubMed] [Google Scholar]
- 72.Newman A.J., Supalla T., Hauser P., Newport E.L., and Bavelier D. (2010). Dissociating neural subsystems for grammar by contrasting word order and inflection. Proc. Natl. Acad. Sci. U. S. A. 107, 7539–7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gawryluk J.R., Mazerolle E.L., and D'Arcy R.C. (2014). Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. Neurosci. 8, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gawryluk J.R., Mazerolle E.L., Beyea S.D., and D'Arcy R.C. (2014). Functional MRI activation in white matter during the Symbol Digit Modalities Test. Front Hum Neurosci 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., Suh M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131, 3209–3221 [DOI] [PubMed] [Google Scholar]
- 76.Viano D.C., Casson I.R., Pellman E.J., Zhang L., King A.I., and Yang K.H. (2005). Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery 57, 891–916 [DOI] [PubMed] [Google Scholar]
- 77.Sidaros A., Skimminge A., Liptrot M.G., Sidaros K., Engberg A.W., Herning M., Paulson O.B., Jernigan T.L., and Rostrup E. (2009). Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage 44, 1–8 [DOI] [PubMed] [Google Scholar]
- 78.Patel M.J., Boada F.E., Price J.C., Sheu L.K., Tudorascu D.L., Reynolds III C.F., and Aizenstein H.J. (2012). Association of small vessel ischemic white matter changes with BOLD fMRI imaging in the elderly. Psychiatry Res. 204, 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouix S., Pasternak O., Rathi Y., Pelavin P.E., Zafonte R., and Shenton M.E. (2013). Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PLoS One 8, e66205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmer H.S., Garzon B., Xu J., Berntsen E.M., Skandsen T., and Haberg A.K. (2010). Reduced fractional anisotropy does not change the shape of the hemodynamic response in survivors of severe traumatic brain injury. J. Neurotrauma 27, 853–862 [DOI] [PubMed] [Google Scholar]
- 81.Zhang K., Johnson B., Pennell D., Ray W., Sebastianelli W., and Slobounov S. (2010). Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp. Brain Res. 204, 57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niskanen J.P., Airaksinen A.M., Sierra A., Huttunen J.K., Nissinen J., Karjalainen P.A., Pitkanen A., and Grohn O. (2013). Monitoring functional impairment and recovery after traumatic brain injury in rats by fMRI. J. Neurotrauma 30, 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McAllister T.W., Saykin A.J., Flashman L.A., Sparling M.B., Johnson S.C., Guerin S.J., Mamourian A.C., Weaver J.B., and Yanofsky N. (1999). Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 53, 1300–1308 [DOI] [PubMed] [Google Scholar]
- 84.McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., and Saykin A.J. (2001). Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 85.Giza C.C. and Hovda D.A. (2001). The Neurometabolic Cascade of Concussion. J. Athl. Train. 36, 228–235 [PMC free article] [PubMed] [Google Scholar]
- 86.Lin A.P., Liao H.J., Merugumala S.K., Prabhu S.P., Meehan W.P., 3rd, and Ross B.D. (2012). Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 6, 208–223 [DOI] [PubMed] [Google Scholar]
- 87.DeFIna P., Fellus J., Polito M.Z., Thompson J.W., Moser R.S., and DeLuca J. (2009). The new neuroscience frontier: promoting neuroplasticity and brain repair in traumatic brain injury. Clin. Neuropsychol. 23, 1391–1399 [DOI] [PubMed] [Google Scholar]
- 88.Peskind E.R., Petrie E.C., Cross D.J., Pagulayan K., McCraw K., Hoff D., Hart K., Yu C.E., Raskind M.A., Cook D.G., and Minoshima S. (2011). Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage 54 Suppl 1, S76–S82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brody D.L., Magnoni S., Schwetye K.E., Spinner M.L., Esparza T.J., Stocchetti N., Zipfel G.J., and Holtzman D.M. (2008). Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321, 1221–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muthukumaraswamy S.D., Evans C.J., Edden R.A., Wise R.G., and Singh K.D. (2012). Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum. Brain Mapp. 33, 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heiss W.D. (2010). Reversible dysfunction of receptors in traumatic brain injury? J. Cereb. Blood Flow Metab. 30, 1671–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee S., Ueno M., and Yamashita T. (2011). Axonal remodeling for motor recovery after traumatic brain injury requires downregulation of gamma-aminobutyric acid signaling. Cell Death Dis. 2, e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heitger M.H., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., Ardagh M.W., and Anderson T.J. (2006). Motor deficits and recovery during the first year following mild closed head injury. Brain Injury 20, 807–824 [DOI] [PubMed] [Google Scholar]
- 94.Grimm S., Beck J., Schuepbach D., Hell D., Boesiger P., Bermpohl F., Niehaus L., Boeker H., and Northoff G. (2008). Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol. Psychiatry 63, 369–376 [DOI] [PubMed] [Google Scholar]
- 95.Liotti M. and Mayberg H.S. (2001). The role of functional neuroimaging in the neuropsychology of depression. J. Clin. Exp. Neuropsychol. 23, 121–136 [DOI] [PubMed] [Google Scholar]
- 96.Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., and Castellanos F.X. (2012). Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry 169, 1038–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hull A.M. (2002). Neuroimaging findings in post-traumatic stress disorder. Systematic review. Brit. J. Psychiatry 181, 102–110 [PubMed] [Google Scholar]
- 98.Martin L.F., Olincy A., Ross R.G., Du Y.P., Singel D., Shatti S., and Tregellas J.R. (2011). Cerebellar hyperactivity during smooth pursuit eye movements in bipolar disorder. J. Psychiatr. Res. 45, 670–677 [DOI] [PubMed] [Google Scholar]
- 99.Lewine J.D., Davis J.T., Bigler E.D., Thoma R., Hill D., Funke M., Sloan J.H., Hall S., and Orrison W.W. (2007). Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 22, 141–155 [DOI] [PubMed] [Google Scholar]
- 100.Dempsey K.E., Dorlac W.C., Martin K., Fang R., Fox C., Bennett B., Williams K., and Flaherty S. (2009). Landstuhl Regional Medical Center: traumatic brain injury screening program. J. Trauma Nurs. 16, 6–7, 10–12 [DOI] [PubMed] [Google Scholar]
- 101.Van Essen D.C., Ugurbil K., Auerbach E., Barch D., Behrens T.E., Bucholz R., Chang A., Chen L., Corbetta M., Curtiss S.W., Della Penna S., Feinberg D., Glasser M.F., Harel N., Heath A.C., Larson-Prior L., Marcus D., Michalareas G., Moeller S., Oostenveld R., Petersen S.E., Prior F., Schlaggar B.L., Smith S.M., Snyder A.Z., Xu J., and Yacoub E; WU-Minn HCP Consortium. (2012). The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.