Abstract
Anhedonia, the diminished anticipation and pursuit of reward, is a core symptom of major depressive disorder (MDD). Trait behavioral activation (BA), as a proxy for anhedonia, and behavioral inhibition (BI) may moderate the relationship between MDD and reward-seeking. The present studies probed for reward learning deficits, potentially due to aberrant BA and/or BI, in active or remitted MDD individuals compared to healthy controls (HC). Active MDD (Study 1) and remitted MDD (Study 2) participants completed the modified monetary incentive delay task (mMIDT), a behavioral reward-seeking task whose response window parameters were individually titrated to theoretically elicit equivalent accuracy between groups. Participants completed the BI Scale and BA Reward-Responsiveness and Drive Scales. Despite individual titration, active MDD participants won significantly less money than HCs. Higher Reward-Responsiveness scores predicted more won; Drive and BI were not predictive. Remitted MDD participants’ performance did not differ from controls’, and trait BA and BI measures did not predict r-MDD performance. These results suggest that diminished reward-responsiveness may contribute to decreased motivation and reward pursuit during active MDD, but that reward learning is intact in remission. Understanding individual reward processing deficits in MDD may inform personalized intervention addressing anhedonia and motivation deficits in select MDD patients.
Keywords: Depression, Remitted MDD, Reward Processing, Behavioral Activation, Behavioral Inhibition
Study 1
1. Introduction
Depression is the second leading cause of disability in the world (Ferrari et al., 2013) and carries a 16.5% lifetime prevalence rate in American adults (NIMH, 2013). Such high rates of occurrence nationally and globally demonstrate the critical need for continuing research into the etiology and treatment of this disorder. Anhedonia, the reduced anticipation of pleasurable stimuli and blunted responsiveness to reward, is one of the core symptoms of major depressive disorder (MDD) in the DSM-5 (American Psychiatric Association, 2013) and has been shown to be a predictor of antidepressant efficacy (Keedwell et al., 2005). Anhedonia contributes to reward-processing deficits in depression (Treadway and Zald, 2011) and can be studied using paradigms that assess both anticipatory and consummatory processes.
It is not yet clear whether anhedonia in MDD is a trait that can be exacerbated during active states of illness, or whether it is a transient marker associated with acute disease. One way to probe this particular question is to determine whether trait personality markers of reward anticipation and pursuit are lowered in active and remitted states of MDD. The Behavioral Activation Scale is a personality trait scale that probes the stability of desire for and pursuit of hedonic goals. The BAS measures traits related to feelings of elation and desire through incentivized pursuit of rewards and goals, and has three subcomponents: Reward-Responsiveness, Drive, and Fun-Seeking. We focused specifically on Reward-Responsiveness and Drive, which are more specifically related to anhedonia. The BAS also has a parallel scale, the Behavioral Inhibition Scale, measuring anxiety and over-reactive inhibition due to sensitivity to threat cues and punishment (Carver and White, 1994; Johnson et al., 2003).
Individuals with MDD have lowered BA Reward-Responsiveness and Drive, which may impair functioning in goal pursuit and may predict symptom change over time (Kasch et al., 2002). Lowered BA may result in a decreased advantageous response bias, in that those with MDD may have difficulty modulating their behavior to respond positively to ambiguous cues in the context of reward contingencies (Pizzagalli et al., 2009a). This diminished responsiveness to reinforcement may lead to decreased drive towards, learning of, and engagement in pleasurable activities and rewards (Pizzagalli et al., 2009a). Indeed, participants with MDD exhibit reduced reward responsiveness by failing to modify their responding in order to maximize gains during a behavioral reward-seeking task (Henriques and Davidson, 2000). These deficits could be due to difficulty incorporating internal feedback (perceived error or discrepancy between desired goal and actual attainment), or affective interference with goal pursuit. It may also be a response to external feedback (failed goal attainment), interfering with learning and behavior modification (Holmes and Pizzagalli, 2007). These disruptions in individuals with depression could be a result of BA dysfunction resulting in low motivation and pursuit and/or increased BI over-function interfering with such processes.
Particularly when considering BI, patients with MDD seem to perceive punishment more readily and intensely than non-MDD individuals, which could trigger negative thoughts, strengthen BI, and interfere with the successful pursuit of reward (Eshel and Roiser, 2010). Task paradigms that include punishment trials in addition to reward trials attempt to tease apart hypoactive BA functioning from hyperactive BI functioning, with the consideration that the BI may suppress BA in punishment trials. In support of this hypothesis, individuals with subthreshold MDD are more willing to classify ambiguous stimuli as cues for punishment rather than reward, demonstrating increased sensitivity to aversive stimuli and decreased reward-responsiveness (Henriques et al., 1994). Similarly, MDD patients are more likely to classify ambiguous facial expressions as negative versus positive (Bouhuys et al., 1999), providing further evidence that individuals with MDD systematically interpret ambiguous stimuli with a negative bias. Furthermore, the degree to which r-MDD patients (currently euthymic individuals who have experienced one or more episodes of depression) perceive negative emotions in ambiguous faces predicts relapse (Bouhuys et al., 1999). It is important to better understand this negative bias in MDD, whether it is related to BI, and how it may interfere with MDD individuals seeking and deriving pleasure. This may be particularly true in contexts where there are potential rewards to be gained but the cues are ambiguous or certainty of reward is unclear.
The present investigation sought to evaluate differences in reward-seeking between individuals with active MDD (a-MDD) and never-depressed individuals (healthy controls). Additionally, we investigated whether BAS and BIS scores predict performance on a reward-learning task. BI was operationalized as the BIS score and BA was characterized as BAS Drive and BAS Reward-Responsiveness scores (Carver and White, 1994). Reward learning and seeking was assessed using the modified Monetary Incentive Delay Task (mMIDT), a measure of reward sensitivity.
In Study 1, we first hypothesized that a-MDD participants, relative to never-depressed participants (healthy controls; HC), would have lower accuracy (win less money) in the baseline portion of the behavioral mMIDT. To bolster the ability of a-MDD and HC to perform between 50% and 80% accuracy, we modified the MIDT (Knutson et al., 2000) by adding between-run modulation of response time. This was accomplished by individually adjusting response time windows during the baseline and first half of the task. Response time windows were lengthened to accommodate participants with slower responses and shortened to challenge those with faster responses. In the second half of the task, response windows were not further adjusted. Given this titration procedure, our second hypothesis was that between-groups performance differences observed in the baseline portion of the task would decrease and no longer be significant during the second half of the task. Third, we hypothesized that for a-MDD individuals, the BIS/BAS scales, especially BAS Reward-Responsiveness, would predict performance on the baseline and titrated mMIDT. Due to a restricted range of BIS/BAS scores in the HC group, we did not make a hypothesis about the scale predicting the HC group’s performance. We predicted that the BIS/BAS scales would predict the titrated mMIDT performance more strongly because the titration procedure would control for individual response time variance, potentially strengthening the observed relationship between performance and affective personality traits.
2. Methods
2.1 Participants
Participants were 18–55 years old and were free of any chronic or serious medical condition. Participants with MDD met DSM-IV criteria for current MDD and were free of psychotropic medication in the past three months. Anxiety disorders were allowed. HC participants had no personal or family history of any psychiatric disorder. Exclusionary criteria included current or past psychotic symptoms, current or past bipolar disorder or mania, a family history of psychosis, a history of suicidal attempts or ideation in the past six months, regular tobacco use (more than 10 cigarettes per week), and presence of alcohol or substance abuse in the last six months. See Table 1 for demographic information.
Table 1.
Participant Characteristics, Neuropsychological Variables, and Predictors in Study 1
| Measure | a-MDD (n = 27) M (SD) |
HC (n = 27) M (SD) |
t | p |
|---|---|---|---|---|
| Gender (% female) | 74.1 | 77.8 | χ2 = 0.101 | 0.75 |
| Age | 27.07 (6.73) | 30.96 (10.17) | 1.66* | 0.11 |
| Educationa | 15.80 (1.63) | 15.31 (1.74) | −1.04 | 0.30 |
| Shipley Estimated IQa | 111.58 (6.31) | 102.28 (23.98) | −1.63 | 0.11 |
| HAM-Da | 18.48 (4.24) | 0.15 (0.46) | −21.48* | <0.001 |
| HAM-Aa | 18.55 (7.69) | 0.16 (0.50) | −11.19* | <0.001 |
| No. of Depressive Episodesb | 4.30 (6.67) | 0.00 (0.00) | −2.89* | 0.009 |
| BAS-RRc | 15.38 (2.97) | 17.00 (1.59) | 2.13* | 0.04 |
| BISc | 23.90 (3.85) | 17.19 (3.02) | −5.76 | <0.001 |
| BAS-Dc | 8.76 (2.33) | 11.31 (1.40) | 3.85 | <0.001 |
| Go Target Reaction Time (ms)b | 438.79 (47.19) | 409.54 (32.12) | −1.96 | 0.06 |
| Go Target Accuracy (%)b | 0.95 (0.06) | 0.98 (0.03) | 1.65* | 0.12 |
| Digit Span (scaled) a | 10.86 (2.64) | 11.0 (3.02) | 0.15 | 0.88 |
| Digit Symbol (scaled) a | 11.35 (3.30) | 13.44 (2.89) | 2.07 | 0.05 |
| Purdue Pegboard (right hand) (s) a | 14.55 (4.110) | 15.50 (2.33) | 0.61 | 0.55 |
Levene’s test indicated equal variances not assumed.
Missing data for up to 9 participants per group.
Missing data for 13 a-MDD participants.
Sample size reduced because not all participants completed the BIS/BAS (a-MDD = 21, HC = 16)
2.2 Measures
2.2.1 Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS)
The BIS/BAS is a 20-item self-report measure that assesses trait inhibition and appetitive motivation (Carver and White, 1994). We used two of the three BAS subscales: Drive (BAS-D) and Reward-Responsiveness (BAS-RR). The BAS-D assessed the degree to which an individual will persistently pursue a desired goal (“I go out of my way to get things I want”). The BAS-RR probed positive responses to the anticipation or occurrence of reward (“When I get something I want, I feel excited and energized”). The third BAS subscale, Fun Seeking, measured impulsivity and the desire for novelty-seeking. Because Fun Seeking is less relevant to the construct of diminished reward learning and pursuit being tested here (Taubitz et al., 2015), it was not included as a predictor variable. In contrast, the BIS measured punishment anticipation, sensitivity to anxiety-provoking circumstances (“Criticism or scolding hurts me quite a bit”), and conflict generation and resolution (McNaughton and Gray, 2000). Items are rated on a Likert scale (1 = strongly disagree; 4 = strongly agree). The BIS/BAS has appropriate divergent and convergent validity, test-retest correlations ranging from 0.59 to 0.69 (Carver and White, 1994), good psychometric properties, high internal consistency, moderate intercorrelation of the BAS subscales, modest inverse correlation of the BAS and BIS scales, and high long-term reliability in assessing stable characteristics in a depressed sample (Kasch et al., 2002).
2.2.2. Modified Monetary Incentive Delay Task (mMIDT)
The mMIDT was a 24-minute reward-processing task in which participants responded to a simple visual stimulus (target) with an index-finger button-press within a predefined response window. The task was completed during fMRI. There were three types of trials: win, neutral, and loss trials. At the beginning of each trial, the type of trial upcoming and amount of money at stake was indicated by a cue: “win $5” or “win $0.20” in a red circle, “don’t lose $5” or “don’t lose $0.20” in a blue square, or “no money at stake” in a green triangle. The cue then disappeared and, after a variable delay, a white square (the target) flashed on the screen. Upon seeing the target, participants had to press the button within the response window in order to win $0.20 or $5 (on win trials) or avoid losing $0.20 or $5 (on loss trials). On neutral trials, no money was at stake, no matter how quickly participants respond. After the target disappeared, they received feedback as to whether they won or lost money. The three types of trials yielded nine possible outcomes: small win ($0.20/none), big win ($5/none), small loss (none/-$0.20), big loss (none/-$5), or no money at stake ($0). The inter-trial interval was jittered, resulting in an average trial duration of 2000ms. Each run contained 25 trials (5 per type) and lasted about 6 minutes.
Before completing runs 1 – 4, participants completed a 25-trial baseline run. Besides acquainting participants with the task, the purpose of the baseline task (with a fixed 250 ms response time) was to measure each participant’s reaction time to the target stimulus and then titrate the actual task to that individualized response window. For example, if a participant’s average reaction time to the target during the baseline is 220 ms with a standard deviation of 30 ms, the initial response window is set to 265 ms for run one (mean plus 1.5*SD). If performance in the next run is lower than 50%, we make the task slower; if subsequent performance is better than 80% we make the task faster, in increments of 0.5 SD. The individual titration process should result in each participant making a correct response above 50% and less than 80% of the time. Titration adjustments were also made after the first and second runs of the fMRI task based upon performance, which was tracked by the experimenter and kept blind to the participant. Participants were told that only their performance on runs three and four would count towards their total earnings (up to $52 more than the base compensation) and that no money would be taken away if their final performance was below $0. The titration procedure adapts the task to the participant’s advantage so they can win a majority of the trials. Titration also standardizes the task by removing the effect of each participant’s individual psychomotor ability.
Groups did not differ on the how much the response window was adjusted from the start of run 1 to the start of run 3, t = 0.67, p = 0.52. There were no differences between groups in the response window for runs 3 and 4, t = 0.77, p = 0.45. In the a-MDD group, the final response window length was not correlated with any of the following variables: amount won in runs 3 and 4, r = −0.04, p = 0.83; win trial accuracy in runs 3 and 4, r = −0.01, p = 0.98; loss trial accuracy in runs 3 and 4, r = 0.06, p = 0.79; or null trial accuracy in runs 3 and 4, r = −0.26, p = 0.25. These variables were also not associated with the final response window length for the HC group, all p’s > 0.05.
Baseline psychomotor speed and working memory differences were evaluated using Digit Symbol and Digit Span WAIS subtests (Wechsler, 1997), Go response time and accuracy for level 1 of the Parametric Go/No-go test (Langenecker et al., 2007), and the Purdue Pegboard test (Tiffin and Asher, 1948; to assess dominant hand dexterity/speed; see Table 2).
Table 2.
Predicting Amount of Money Won in Runs 3 & 4 in Study 1
| Both Groups | a-MDD Only | |||||
|---|---|---|---|---|---|---|
| Predictor | Stand. β | t | p | Stand. β | t | p |
| Intercept | - | 2.88 | <0.01 | - | −0.38 | 0.71 |
| Diagnostic Group | −0.48 | −2.25 | 0.03 | - | - | - |
| BAS-RR | 0.51 | 3.01 | <0.01 | 0.65 | 2.89 | 0.01 |
| BIS | 0.11 | 0.58 | 0.57 | 0.25 | 1.33 | 0.20 |
| BAS-D | −0.19 | −0.99 | 0.33 | −0.13 | −0.57 | 0.58 |
| Interactions | ||||||
| BAS-RR*aMDD | 0.48 | 1.38 | 0.18 | - | - | - |
| BIS*aMDD | 0.44 | 1.62 | 0.12 | - | - | - |
| BAS-D*aMDD | 0.25 | 0.76 | 0.45 | - | - | - |
| Age | −0.14 | −0.78 | 0.44 | −0.12 | −0.35 | 0.73 |
| HAM-D | - | - | - | 0.08 | 0.22 | 0.83 |
| HAM-A | - | - | - | 0.16 | 0.59 | 0.57 |
| No. Depressive Episodes | - | - | - | 0.28 | 1.38 | 0.19 |
Computerized tasks were presented in E-Prime (Version 2.0, Psychology Software Tools Inc., Pittsburgh PA, USA).
2.3 Procedures
Participants were recruited from the community and initially screened over the phone by a trained research assistant. All study procedures were approved by the University of Michigan IRB, and participants provided informed consent consistent with the Declaration of Helsinki. A trained doctoral-level interviewer conducted the Structured Clinical Interview for DSM-IV (SCID) to confirm a diagnosis of major depressive disorder. The present study was part of a larger protocol that included a comprehensive battery of neuropsychological tests, self-report measures (Behavioral Inhibition System/Behavioral Activation System Scales [BIS/BAS]), and structural and functional magnetic resonance imaging (fMRI), which will be reported elsewhere. Participants were compensated $30 for the SCID intake, and $100 for the fMRI session, with an additional $0–52 for their performance on the final two runs of the mMIDT.
2.4 Statistical Analyses
In SPSS (IBM SPSS Statistics for Macintosh, Version 22.0), repeated measures ANOVA was used to compare groups on the net amount of money won (AMW), with AMW in the baseline task and AMW in the titrated task (runs 3 and 4) as the repeated dependent variable. This repeated measures design was intended to obtain the difference in performance when psychomotor speed was not controlled for (baseline) versus controlled for (runs 3 and 4). Runs 1 and 2 were not analyzed because the titration procedure was still in progress during those runs. Hierarchical linear multiple regressions were used to predict AMW in runs 3 and 4 using diagnostic group, BAS-RR, BAS-D, and BIS as predictors. One regression was run with the entire sample, and a second was computed with the a-MDD group only. In the first regression, we entered diagnostic group in the first block, BAS-RR, BAS-D, and BIS in the second block, interaction terms for the BIS/BAS in the third block, and age as a covariate in the fourth block. Non-significant variables were then removed and a reduced model with only diagnostic group and the BIS/BAS variables was run. In the second regression with a-MDD only, we entered the BIS/BAS variables in one block. HAM-A, HAM-D, and number of depressive episodes violated assumptions of equal variance and were not used for the first regression, but were included in an additional regression with a-MDD only. Each variable was grand mean centered for Study 1 and for Study 2. All predictor variables were normally distributed.
3. Results for Study 1
Group means of the predictor variables, as well as measures of reaction time, target accuracy, working memory, attention, and motor dexterity were compared to determine whether a-MDD and healthy control groups were comparable in baseline performance (Table 1). The a-MDD group performed significantly poorer in one measure of processing speed, Digit Symbol.
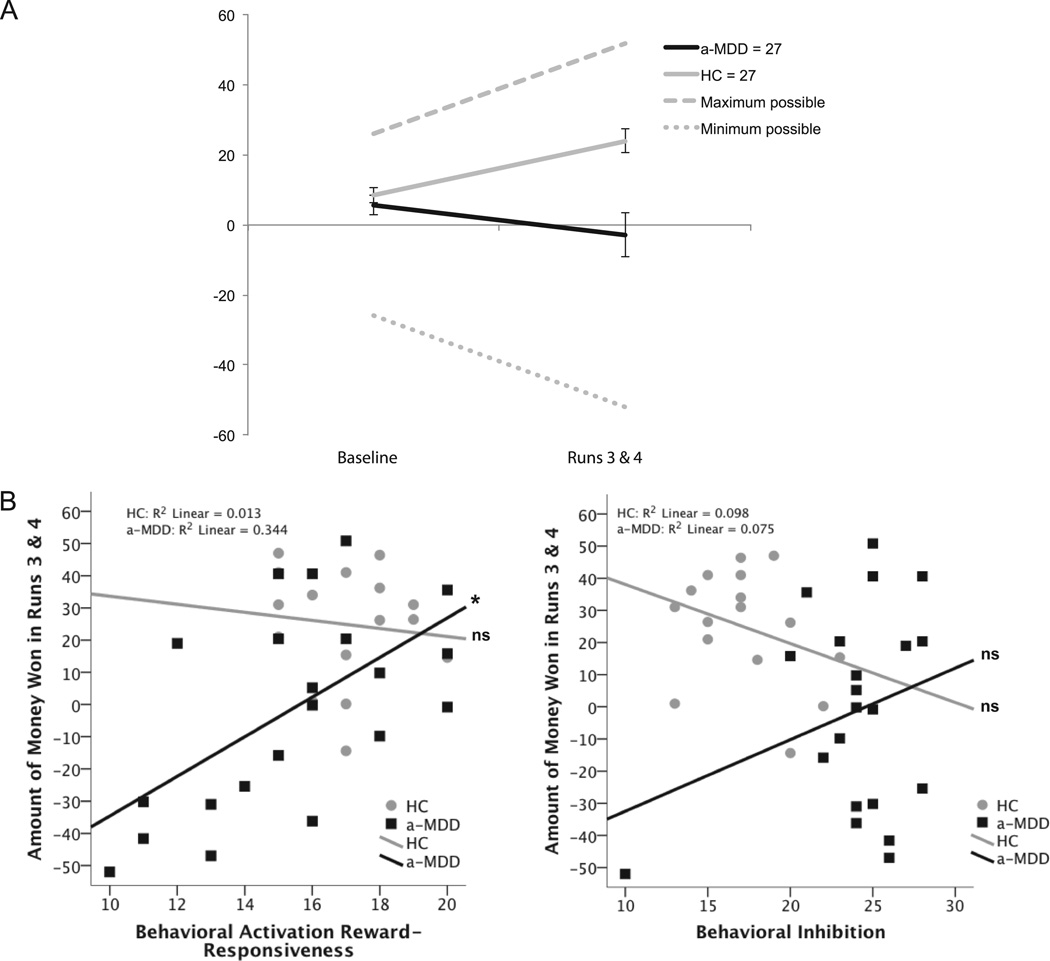
A repeated measures ANOVA revealed that a-MDD participants won less money than HC participants overall, F(1, 52) = 11.42, p = 0.001. There was also a significant interaction of diagnosis and time, F(1, 52) = 12.08, p = 0.001, such that groups were equivalent at baseline, F(1, 52) = 0.64, p > 0.05, but then HC participants gradually won more money while a-MDDs lost money or broke even throughout the task, F(1, 52) = 14.42, p < 0.001 (see Figure 1). In posthoc paired t tests, both groups had greater accuracy in win relative to null and loss trials (p’s < 0.05), and in loss trials relative to null trials (p’s < 0.01).
Fig. 1.
Panel A: Amount of money won in both groups during baseline and in titrated runs 3 and 4 of the mMIDT. Error bars represent standard error of the mean. Panel B: Scatterplots illustrating amount of money won in runs 3 and 4 in relation to BA Reward-Responsiveness and BI in a-MDD and HC groups.
Two hierarchical multiple linear regressions were computed. The first was used to establish group differences in the relationship between the dimensional measures of BA and BI with AMW. Each of these was then run using a reduced model, i.e. excluding non-significant variables. For the entire sample in the full and reduced models, diagnostic group and the BAS Reward-Responsiveness scale predicted AMW (see Table 2). The sample sizes are slightly lower in the regression analysis than the ANOVA because some participants completed the mMIDT but not the BIS/BAS.
The second regression examined individual differences in the a-MDD group only. In the second full model analysis with the a-MDD group only, BAS-RR predicted AMW (see Table 2). This analysis included HAM-D, HAM-A, and number of depressive episodes as covariates, but none were significant and were excluded in a reduced model that was run subsequently. The relationship between BAS-RR and BIS with AMW in the titrated task is displayed in scatterplots (Figure 1).
As a follow-up analysis, we looked to determine whether the baseline difference in Digit Symbol was related to AMW, finding that Digit Symbol and AMW were not correlated in the entire sample or in the a-MDD group alone (r = 0.28, p = 0.09; r = 0.14, p = 0.55, respectively).
4. Study 1 Discussion
The main goal of Study 1 was to evaluate the sensitivity of individuals with MDD to reward-based tasks and whether measures of BA and BI were predictive of performance. We hypothesized that the a-MDD group would win less money in the baseline mMIDT than the HC group, and that a-MDD and HC groups would win equivalent amounts of money in the titrated condition. Contrary to both hypotheses, performance accuracy for the HC and a-MDD groups did not differ significantly in the baseline mMIDT but did differ in the titrated portion. We also hypothesized that the BIS/BAS scale (especially BAS-RR) would predict the mMIDT performance, and our results did support this hypothesis. The primary finding was that although titration appeared to help the HCs improve performance, the a-MDD group did not benefit from titration and failed to earn and avoid losing money, thus earning less than HCs in the titrated portion of the task.
Groups were not significantly different on most neuropsychological tests, suggesting that group differences on the mMIDT cannot be attributed to baseline differences in attention, working memory, and psychomotor ability. Active MDD and HC differed only on Digit Symbol, a measure of attention, processing speed, and working memory, which is consistent with the literature (Gotlib and Joorman, 2010; Snyder, 2013). Follow-up analyses suggested that the difference in Digit Symbol did not affect the titrated mMIDT results.
Surprisingly, even though the task was titrated to optimize performance and indeed equalize task performance between groups, the a-MDD participants performed at the low end of the accuracy range. In spite of group differences in the titrated task, there were no significant group differences in accuracy in the baseline portion, possibly suggesting that the a-MDD group grew fatigued as the task went on. Also possible is that HCs are more attentive and responsive to environmental changes (i.e., task parameter titration). The a-MDD group may also have had trouble sustaining engagement with the task, both sustained neural processing resources and psychomotor ability. Posthoc, we indirectly assessed fatigue by looking at reaction time across runs over the course of the task, and whether it varied differentially between groups. We assessed sustained engagement by inspecting reaction time standard deviation across runs and between groups. There were no interactions between diagnostic group and RT or SD, meaning that these variables did not change differentially between groups over the course of the task. However, there was significant missing data for reaction time because response time was not captured if participants responded after the cue had disappeared from the screen. Given the missing data we can only speculate that fatigue and sustained attention are not alternate explanations for the findings.
Inability to earn money as the task progressed may reflect an inability to sustain positive emotion over time (Heller et al., 2009). In another study using an emotion regulation task, in which depressed participants were instructed to up-regulate their response to positive images, nucleus accumbens activation decreased over time, suggesting that individuals with MDD may have difficulty sustaining positive affect and reward-related neural activity (Heller et al, 2009).
Given the differences after titration, we investigated whether BA and BI trait factors moderated the effect of the titration procedure differently for each group. In support of our third hypothesis, a-MDD participants’ BAS Reward-Responsiveness scores predicted AMW in the titrated condition, whereas this was not the case in the baseline condition. BAS-RR scores capture an inclination of attention toward and enjoyment of positive experiences. Although it is possible that this trait characteristic is unrelated to psychomotor speed and pursuit of reward, controlling for psychomotor speed (in the titrated task runs) made the relationship between Reward-Responsiveness scores and task performance stronger. The BAS Drive scale did not predict total amount won, possibly because the mMIDT does not involve a voluntary initiation of the pursuit of reward and participants are told that a set amount of money is available to them to earn.
The BIS scale also did not predict total amount won. Nonetheless, because people with MDD tend to be hypersensitive to punishment (Henrique et al., 1994; Eshel and Roiser, 2010), losses early in the task could have triggered rumination and feelings of hopelessness about doing well on the rest of the task. This cognitive-emotional interference with motivation (Papageorgiou and Siegle, 2003) could result in slower reaction times and less money won in the a-MDD group. Failing to win money and increased loss of money could confirm depressed participants’ pathologically negative self-image and interfere with their ability to achieve and sustain good performance (Eshel and Roiser, 2010). In contrast, in the context of reward HC participants may be more resilient to initial difficulty and persist in learning and adapting.
Study 2
5. Introduction
Although previous research has suggested that depressed patients are able to recover select neuropsychological functions in remission (Lin et al., 2014), few studies have looked at reward-processing in r-MDD individuals. Studying reward-processing in remitted MDD allows for better focus on trait or scar effects of illness, given the minimal effects of depressive symptoms. In one study of remitted depressed (r-MDD) individuals, participants exhibited neural hyperactivation and slowed responses during the anticipation of reward and hypoactivation when receiving feedback, relative to HC (Dichter et al., 2012). Remitted MDD individuals may need to over-recruit neural resources to attain rewards (Dichter et al., 2012). Despite being euthymic, r-MDD participants may engage in excessive rumination about the prior trial, which could interfere with anticipation and preparation for the upcoming trial, disrupting the motoric component of reward seeking and resulting in slower behavioral responses (Dichter et al., 2012). It is also possible that cognitive interference between anticipation of reward and estimation of success may result in slower responses. In contrast, another study found hypoactivation of reward- and error-related brain regions in response to primary rewarding stimuli in an r-MDD sample (McCabe et al., 2009). Additionally, as compared to healthy individuals, r-MDD participants failed to develop a response bias towards a more frequently rewarded stimulus, even when controlling for residual anhedonic symptoms (Pechtel et al., 2013). Together, r-MDD individuals seem to experience reward-seeking deficits due to underlying neural or cognitive trait vulnerabilities that persist in remission. Alternatively, these differences in reward learning and seeking could be scar effects of previous episodes.
The results of Study 1 highlighted three important limitations that needed to be addressed to clarify the role of BA reward-responsiveness in MDD individuals. First, because trait BA and BI can be altered in the context of active MDD, current MDD symptoms may have interfered with performance and estimation of trait-performance relationships. Put another way, it is unclear whether the observed differences in reward-seeking were driven by trait or state factors. Second, it is possible that chronic recurrent MDD results in diminished reward learning and pursuit due to scar effects of multiple episodes (Kerestes et al., 2012). Younger, remitted MDD individuals without significant recurrence of illness might not exhibit similar deficits to older participants with a longer MDD history. Further, examining reward-related deficits earlier in the course of illness may also help us predict the recurrence of MDEs in early adulthood. Third, the distracting fMRI scanner environment may have contributed to greater heterogeneity of performance by inducing psychomotor slowing (Gutchess and Park, 2006), preventing the a-MDD group from optimizing their performance. This third limitation could be addressed by running the task outside the scanner. Each of these limitations was addressed in the design of Study 2.
Study 2 aimed to test whether disrupted reward learning and pursuit persist independent of active MDD symptoms and if BAS-RR is predictive. First we hypothesized that the r-MDD and HCs would perform equally on the baseline mMIDT, similar to the results with the a-MDD group. Second, we expected that after titration the HC but not r-MDD group would optimize performance, which would suggest trait reward-seeking deficits in remitted MDD. Third, we hypothesized that the BIS/BAS scales would predict performance on the titrated mMIDT in r-MDD individuals, similar to that observed in a-MDD.
6. Methods
6.1. Participants
Participants (ages 17–23) were enrolled under very similar inclusionary and the same exclusionary criteria as Study 1. No participants in Study 1 participated in Study 2. Remitted MDD participants had between one and three prior episodes of MDD but no major depressive episode within the last month. Remitted MDD participants could have a family history of depression or anxiety, could have a comorbid anxiety diagnosis, and were free of psychotropic medication use in the past three months. See Table 3 for demographics.
Table 3.
Participant Characteristics, Neuropsychological Variables, and Predictors in Study 2
| Measure | r-MDD (n = 37) M (SD) |
HC (n = 23) M (SD) |
t | p |
|---|---|---|---|---|
| Gender (% female) | 70.3 | 52.2 | χ2 = 2.00 | 0.16 |
| Age | 21.19 (1.79) | 21.34 (1.82) | 0.33 | 0.74 |
| Education | 14.30 (1.58) | 14.83 (1.53) | 1.28 | 0.21 |
| Verbal IQa | 104.29 (9.01) | 102.82 (9.37) | −0.59 | 0.56 |
| HAM-D | 2.62 (2.94) | 0.43 (1.04) | −4.13* | <0.001 |
| HAM-A | 2.92 (3.02) | 0.83 (1.80) | −3.36* | 0.001 |
| No. of Depressive Episodesa | 2.24 (2.11) | 0.00 (0.00) | −6.11* | <0.001 |
| BAS-RR b | 16.85 (2.24) | 16.94 (1.89) | 0.14 | 0.89 |
| BIS b | 20.12 (3.37) | 19.71 (2.47) | −0.45 | 0.66 |
| BAS-D b | 10.62 (2.00) | 10.82 (1.47) | 0.38 | 0.71 |
| Go Target Reaction Time (ms) a | 443.82 (38.97) | 427.62 (31.76) | −1.57 | 0.12 |
| Go Target Accuracy (%) a | 0.96 (0.04) | 0.98 (0.03) | 1.36 | 0.18 |
| Digit Symbol (scaled) a | 10.92 (1.98) | 10.90 (2.95) | −0.03 | 0.98 |
| Purdue Pegboard (Dominant hand)a | 14.95 (1.76) | 15.25 (1.74) | 0.62 | 0.54 |
Levene’s test indicated equal variances not assumed.
Missing up to 4 participants per group.
Sample size reduced because not all participants completed the BIS/BAS (rMDD = 34, HC = 17)
6.2 Measures
The administration of the mMIDT (including titration procedures) and BIS/BAS were identical in Study 2 compared to Study 1. To avoid any diagnosis-by-magnet interactions, the mMIDT was not administered during fMRI. Baseline psychomotor speed, attention, and working memory differences were evaluated using the Digit Symbol WAIS subtest (Wechsler, 1997), Go response time and accuracy for level 1 of the Parametric Go/No-go test (Langenecker et al., 2007), and the Purdue Pegboard test (Tiffin and Asher, 1948).
6.3 Procedures
Participants were recruited from the community and initially phone screened by a trained research assistant. Participants gave informed consent consistent with the Declaration of Helsinki. A trained doctoral-level interviewer conducted the Diagnostic Interview for Genetic Studies, Hamilton Anxiety Rating Scale (HAM-A), and Hamilton Depression Rating Scale (HAM-D). Participants completed a neuropsychological testing battery that assessed memory, visuospatial and motor skills, inhibitory control, attention, and reward processing (the mMIDT). Participants completed the BIS/BAS and other self-report questionnaires. Participants were compensated $120 for completion of the neuropsychological battery, and had the opportunity to earn an additional $52 for the titrated portion of the mMIDT. All study procedures were approved by the University of Illinois at Chicago IRB.
Statistical analyses were identical to those used in Study 1, now with r-MDD instead of a-MDD.
7. Results for Study 2
We present the group means of the predictor variables in Table 3. Groups did not differ on measures of reaction time, target accuracy, working memory, attention, or motor dexterity (see Table 3).
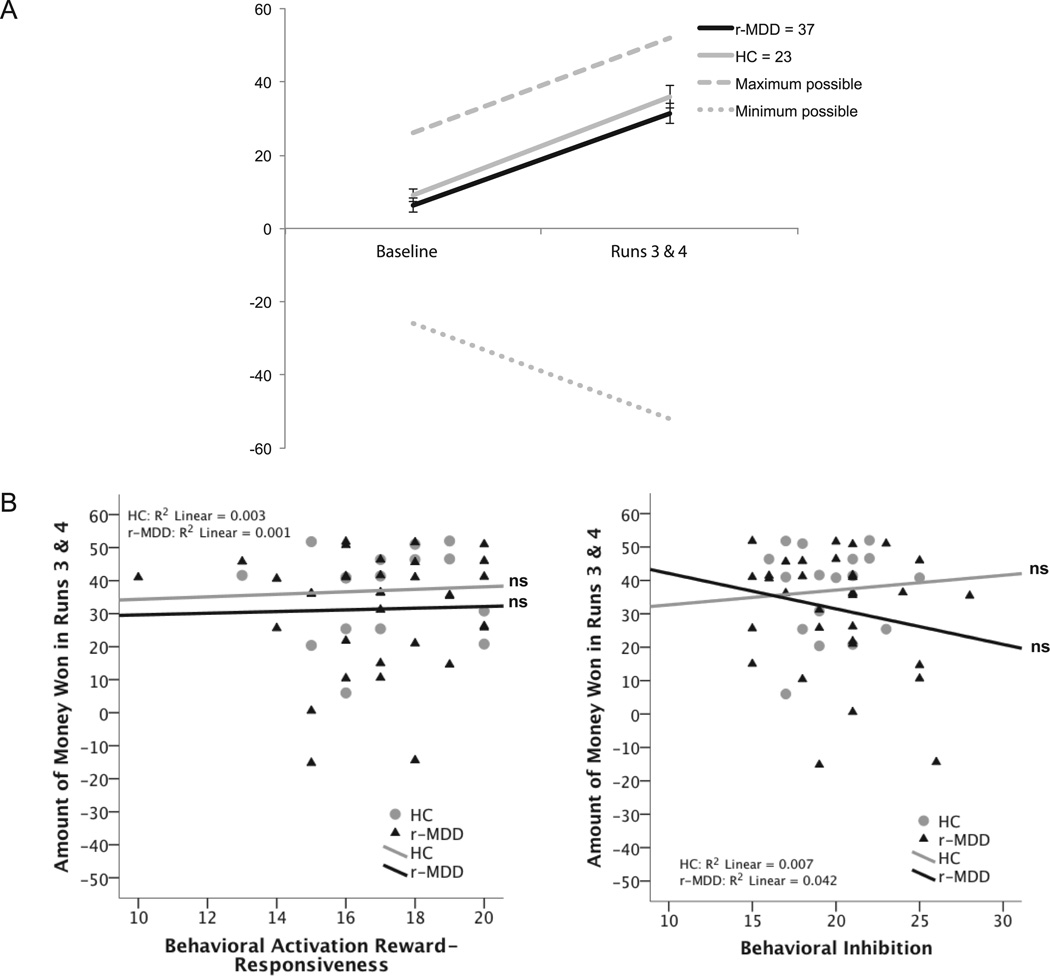
A repeated measures ANOVA revealed that r-MDD participants won equivalent amounts of money to HC participants overall, F(1, 58) = 1.83, p > 0.05, supporting our first hypothesis of equal performance before titration. There was an effect of time, such that both groups won more money as the task went on, F(1, 58) = 119.70, p < 0.001 (see Figure 2). There was no diagnosis-by-task interaction (F(1, 58) = 0.142, p > 0.05), thus failing to support our second hypothesis that HCs would show better adaptation to titration relative to the r-MDD group.
Fig 2.
Panel A: Amount of money won in both groups during baseline and in titrated runs 3 and 4 of the mMIDT. Error bars represent standard error of the mean. Panel B: Scatterplots illustrating amount of money won in runs 3 and 4 in relation to BA Reward-Responsiveness and BI in r-MDD and HC groups.
Hierarchical multiple linear regressions were used to test the third hypothesis, that BAS-RR and BIS would predict AMW. In a between-groups model and an r-MDD group only model, BIS and BAS scores did not predict AMW in the baseline or titrated mMIDT, failing to support our hypothesis. Table 4 displays these results, with the intercept, diagnosis, and BIS/BAS beta coefficients coming from reduced models with non-significant covariates removed.
Table 4.
Predicting Amount of Money Won in Runs 3 & 4 in Study 2
| Both Groups | r-MDD Only | |||||
|---|---|---|---|---|---|---|
| Predictor | Stand. β | t | p | Stand. β | t | p |
| Intercept | - | 4.75 | <0.001 | - | 9.73 | <0.001 |
| Diagnostic Group | −0.15 | −.1.03 | 0.31 | - | - | - |
| BAS-RR | 0.10 | 0.60 | 0.55 | 0.22 | 0.90 | 0.37 |
| BIS | −0.19 | −1.09 | 0.28 | −0.33 | −1.40 | 0.17 |
| BAS-D | 0.06 | 0.38 | 0.71 | −0.03 | −0.15 | 0.89 |
| Interactions | ||||||
| BAS-RR*rMDD | −0.03 | −0.08 | 0.94 | - | - | - |
| BIS*rMDD | 0.05 | 0.15 | 0.88 | - | - | - |
| BAS-D*rMDD | 0.18 | 0.51 | 0.61 | - | - | - |
| HAM-D | - | - | - | 0.36 | 1.22 | 0.24 |
| HAM-A | - | - | - | −0.12 | −0.39 | 0.70 |
| No. of Depressive Episodes | - | - | - | −0.04 | −0.22 | 0.83 |
8. General Discussion
This is the only study to our knowledge to evaluate the behavioral MIDT performance of a currently depressed sample with an indirect comparison to a remitted depressed sample. We found that the r-MDD group performed as well as HCs on the titrated mMIDT, whereas the a-MDD did not. This suggests that depressive symptoms significantly interfered with performance on the task, while remission from MDD eliminated group differences on task accuracy. However, there may be alternative explanations for the current findings. For instance, the r-MDD group may have performed better than the a-MDD group because the r-MDD participants were younger. In fact, the HC group in Study 2 performed significantly better on runs 3 and 4 than the HCs in Study 1. Younger participants may have been quicker to learn computer tasks and game contingencies than slightly older adults. They may have also been more responsive to changes in task parameters. Moreover, reward-seeking deficits in the a-MDD group could have been exacerbated by the scanner environment, which was not present in Study 2. Furthermore, the young age range of the r-MDD could mean that each individual has had fewer episodes of MDD than the older MDD participants. In other words, the a-MDD group could have been at a disadvantage due to scar effects of multiple major depressive episodes.
A key finding of Study 2 was that BIS and BAS scores of the r-MDD participants did not differ from HCs and were unrelated to the amount of money won during the task. The BIS/BAS scale may be measure of affective state in addition to trait affect, thus failing to predict reward-seeking behavior in a euthymic group. In other words, impaired reward-seeking may be a state effect of MDD and not a trait or risk factor. Negative mood state could have also affected the range of BIS/BAS scores. These results contrast previous research that has found disrupted reward-processing in remitted MDD (Dichter et al., 2012; Pechtel et al., 2013), as compared to healthy controls. We may have failed to find similar results due to the titration procedure, which could have helped r-MDD participants perform similarly to the HCs.
Alternatively, it is possible that our data in Study 2 reflect a restricted range of scores on the BIS and BAS scales, especially BAS Reward-Responsiveness. While BAS-RR scores were equivalent between HCs in both studies, the a-MDD group’s BAS-RR was significantly lower and more distributed than the r-MDD group’s. This restricted range of BAS-RR for r-MDD in Study 2 could have attenuated our ability to see effects of BAS-RR on AMW. BIS scores were significantly lower in the Study 1 HCs than in Study 2 but were significantly higher in the a-MDD group than r-MDD, further suggesting that the range of scores in Study 2 may have been restricted.
Overall, our major finding was that MDD individuals perform worse on a behavioral measure of reward-seeking than healthy individuals, and that r-MDD individuals perform equally well as controls. This finding has important clinical implications in that it suggests that the group differences on a reward-seeking task may be due to state effects of illness, such as interfering cognitive deficits such as poor task concentration and enhanced concentration on negative self-focused thoughts (Gotlib and Joormann, 2010). Although frustration tolerance, fatigue, and rumination were not directly assessed, these factors could have confounded or negatively influenced performance in the a-MDD group. Fatigue and ruminative thinking are common symptoms of MDD and could have been exacerbated by the 24-minute task that frequently gave participants rumination-enhancing negative feedback. Additionally, participants with low frustration tolerance may have experienced feelings of discouragement and hopelessness, which could interfere with their performance, deepen their frustration, and continue in such a cycle. The influence of BA on reward-seeking behavior suggests that assessing and remediating anhedonic symptoms in MDD may be essential to symptom improvement and recovery of normal reward learning and pursuit processes, possibly through behavioral activation (Dichter et al., 2010). MDD patients with reward-processing abnormalities may benefit from specific interventions such as cognitive restructuring to increase the salience of rewards.
There are several limitations to the present studies. First, the sample sizes were relatively small. In addition, some of the individuals who completed the mMIDT did not complete BA and BI measures. Second, the age range in Study 2 is narrower than in Study 2, limiting generalizability and the validity of comparison between studies. Future studies should better control for age differences across groups. Third, the a-MDD group’s performance may have been negatively influenced by the scanner environment, as even the Study 1 HCs won less money in runs 3 and 4 than Study 2 HCs.
Further research should examine the functional neural correlates of this phenomenon. Many studies have found that when anticipating reward, HCs show increased activation in the nucleus accumbens and medial caudate, whereas MDD individuals show deactivation in the nucleus accumbens and increased activation in the dorsal anterior cingulate cortex (dACC) (Knutson, et al., 2001; Knutson et al., 2008; McCabe et al., 2009; Pizzagalli et al., 2009b). When anticipating non-reward or punishment, HCs show increased activation in the medial caudate (Knutson et al., 2001) and dACC (Knutson et al., 2008). The basal ganglia may also play a role in consummatory reward processes (Pizzagalli et al., 2009b). Delineating functional differences between MDD and healthy individuals could provide a way to predict first onset or risk of relapse.
Highlights.
Despite the use of a response titration procedure that theoretically eliminates individual and group differences in task, depressed individuals won significantly less money on a reward learning task than healthy controls.
The depressed group’s performance was predicted by reward-responsiveness scores, a measure of the behavioral activation system’s functioning.
Remitted depressed participants performed as well as controls on the reward learning task.
The remitted depressed group’s performance was not predicted by trait affect measures.
Reward learning deficits were only observed during active states of depression.
Acknowledgments
Funding: Research supported by the National Alliance for Research on Schizophrenia and Depression Young Investigator Award (SAL, DTH), KL2 Career Development Award (CDA) (RR 024987, SAL), The National Institute of Mental Health (K23 MH 074459, SAL; R01 MH081911, SAL; T32MH067631, to NAC; and R01 MH050030, JKZ), and Phil Jenkins Research Fund (JKZ). The funders had no role in study design, data collection, analysis, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
Contributors: All authors have approved the final manuscript.
Sophie R. DelDonno: Selection and performing of statistical analyses, interpretation of analyses, drafting and editing manuscript
Anne L. Weldon: Literature search, instrument construction, collection and preparation of data, editing manuscript
Natania A. Crane: Interpretation of statistical analyses, editing manuscript
Alessandra M. Passarotti: Editing manuscript
Patrick J. Pruitt: Conceptualizing and refining research ideas, editing manuscript
Laura B. Gabriel: Collection and preparation of data
Wendy Yau: Collection and preparation of data
Kortni K. Meyers: Collection and preparation of data
David T. Hsu: Conceptualizing research ideas, creating research design, instrument selection, editing manuscript
Stephen F. Taylor: Conceptualizing research ideas, instrument selection, editing manuscript
Mary M. Heitzeg: Conceptualizing research ideas
Ellen Herbener: Refining research ideas
Stewart A. Shankman: Refining research ideas
Brian J. Mickey: Conceptualizing research ideas, creating research design, instrument selection, editing manuscript
Jon-Kar Zubieta: Conceptualizing research ideas, creating research design, instrument selection, editing manuscript
Scott A. Langenecker: Conceptualizing research ideas, creating research design, instrument selection and construction, selection of statistical analyses, interpretation of analyses, editing manuscript
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fifth ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. TRENDS in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. The Journal of Nervous and Mental Disease. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Buyukdura JS, McClintock SM, Croarkin PE. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychological Assessment. 2004;16:244. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. Journal of Affective Disorders. 2010;126:236–244. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: Findings from the Global Burden of Disease Study 2010. PLOS Medicine. 2013;10:1–12. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Park DC. fMRI environment can impair memory performance in young and elderly adults. Brain Research. 2006;1099:133–140. doi: 10.1016/j.brainres.2006.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. Journal of Abnormal Psychology. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14:711–724. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: relationship to subclinical measures of depression. Emotion. 2007;7:68. doi: 10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder: An epidemiological study. Journal of Psychopathology and Behavioral Assessment. 2003;25:25–36. [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips MJ. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, Blumberg HP. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Research: Neuroimaging. 2012;202:30–37. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: Convergent validity and test-retest reliability of the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2007;29:842–853. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- Lin K, Xu G, Lu W, Ouyang H, Dang Y, Lorenzo-Seva U, Lee TM. Neuropsychological performance in melancholic, atypical and undifferentiated major depression during depressed and remitted states: a prospective longitudinal study. Journal of Affective Disorders. 2014;168:184–191. doi: 10.1016/j.jad.2014.06.032. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. Major depressive disorder among adults. NIH Health & Education Mental Health Information; 2013. Retrieved November 23, 2013, from http://www.nimh.nih.gov/statistics/1mdd_adult.shtml. [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Siegle GJ. Rumination and depression: Advances in theory and research. Cognitive Therapy and Research. 2003;27:243–245. [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. Journal of Psychiatric Research. 2013;47:1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research. 2009a;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Holmes A, Dillon D, Goetz E, Birk J, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009b;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Sackeim HA. Psychomotor symptoms of depression. American Journal of Psychiatry. 1997;154:4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectrums. 2008;13:561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Taubitz LE, Pedersen WS, Larson CL. BAS reward responsiveness: A unique predictor of positive psychological functioning. Personality and Individual Differences. 2015;80:107–112. doi: 10.1016/j.paid.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue Pegboard: Norms and studies of reliability and validity. Journal of Applied Psychology. 1948;32:234. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III Wechsler Adult Intelligence Scale - Third Edition administration and scoring manual. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Company; 1997. [Google Scholar]