Abstract Abstract
During the course of a project aiming at the reconstruction of the colonization of the South Pacific islands by tateid gastropods based on molecular data we discovered five new species on New Caledonia belonging to the genera Hemistomia and Leiorhagium, respectively. We describe these species based on morphological, anatomical and genetic data. All five species are morphologically cryptic as they closely resemble or are even indistinguishable from known species stressing the importance of a comprehensive taxonomic approach integrating several methods. As a consequence of their small and fragmented geographic ranges and the rapidly progressing anthropogenic land cover changes on New Caledonia, all five species qualify as critically endangered according to the criteria of the IUCN.
Keywords: Conservation, cryptic species, endemic, integrative taxonomy, IUCN, New Caledonia, South Pacific, spring snails, Tateidae
Introduction
New Caledonia is famous for being a biodiversity hotspot harboring a high number of endemic species (Myers et al. 2000) including a radiation of small freshwater gastropods belonging to the family Tateidae. This radiation is probably of Oligocene origin and comprises more than 50 species in seven genera (Haase and Bouchet 1998, Zielske and Haase 2015). Many of these species are extreme narrow-range endemics known from only few or single sites (Haase and Bouchet 1998), a pattern typical for Truncatelloidea in freshwaters worldwide (e.g. Giusti and Pezzoli 1980, Radoman 1983, Haase 1996, 2008, Ponder and Colgan 2002, Liu and Hershler 2005, Hershler et al. 2011, Delicado and Ramos 2012). In the frame of a project aiming at the reconstruction of the colonization of the South Pacific islands by tateids based on molecular data (Zielske and Haase 2014a, b, 2015, Zielske, Ponder and Haase in preparation) we visited New Caledonia in May 2012 in order to collect suitable material for sequencing. During this expedition we found five new species of the genera Hemistomia Crosse, 1872 and Leiorhagium Haase & Bouchet, 1998, respectively (Figs 1, 2), which we describe herein based on morphological, anatomical and genetic data. All five species qualify as morphologically cryptic as they closely resemble or are even indistinguishable from known species (see Pfenninger and Schwenk 2007). The discovery of new cryptic species was predicted by Haase and Bouchet (1998), whose revision was based solely on morphology and anatomy. In general, cryptic species are common among different spring snail families of Truncatelloidea (e.g., Liu et al. 2003; Haase et al. 2007; Delicado and Ramos 2012; Collado et al. 2013).
Figure 1.
Localities of new species and samples used for morphometric comparisons. Inset shows position of New Caledonia in the Southwest Pacific. Arrows indicate type localities of species represented by more than one sample (see also Table 1).
Figure 2.
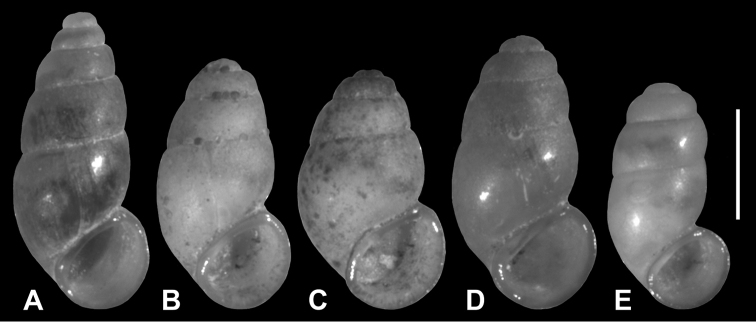
Holotypes. A Hemistomia andreae sp. n. B Leiorhagium adioincola sp. n. C Leiorhagium aremuum sp. n. D Leiorhagium clandestinum sp. n. E Leiorhagium neteae sp. n. Scale bar = 1 mm.
Material and methods
Snails were fixed in 70% ethanol in the field, transferred to propylene glycol for shipping by courier, and returned to ethanol, this time 96%, after arrival in our lab. For measurements, up to 20 snails per sample were photographed under a Zeiss SteREO Discovery.V20 dissecting microscope with a Zeiss Axio Cam MR3. Five dimensions – shell height, shell width, aperture height, aperture width, body whorl width – were measured using the program AxioVision 40 V4.8. (Zeiss) and whorls counted to the nearest eighth (Kerney and Cameron 1979). Up to six shells were dissolved in diluted hydrochloric acid for dissections. Anatomies were photographed as well. These digital images served as template for drawings made on a graphical tablet. For scanning electron microscopy up to three shells, radulae and opercula were cleaned in 5% sodium hypochlorite. The cephalopodia of up to two males were dried using hexamethyldisilazane (Nation 1983). After sputter coating with gold objects were investigated in a Zeiss EVO LS10 Scanning Microscope.
Morphometric analyses of shell measurements including canonical variates analyses (CVA) maximizing the differentiation of groups in multivariate space, (MANOVA), assignment tests, and Hotelling’s T2-tests were conducted in PAST 2.12 (Hammer et al. 2001). Sequential Bonferroni-correction was applied to multiple tests. These analyses also included samples of known, similar species the new ones could be mistaken for (Table 1). The selection of species used in comparisons was based on the phylogenetic analysis.
Table 1.
Locality data of all samples and GenBank accession numbers of specimens represented in phylogeny (see also Fig. 1). The last three species represent the outgroup. Specimens are only distinguished in two cases where more than 1 sequence per sample was used. For museum catalog numbers of NeCa-sample voucher material see Zielske and Haase (2015). Paratypes of species described by Haase and Bouchet (1998) used in morphometric comparisons are accompanied by catalog numbers from the museum in Paris, because these have been assigned only recently.
| Species, sample | Locality | Latitutde, longitude | COI | 16S | IT2 |
|---|---|---|---|---|---|
|
Hemistomia
andreae, NeCa 12_1 Hemistomia andreae, NeCa 12_2 |
Bouloupari, Ouaméni valley | 21°49'46.9"S; 165°56'42.9"E |
KJ490851 KJ490852 |
KJ490767 --- |
KJ490691 --- |
|
Hemistomia
cockerelli, paratypes MNHN IM-2012-2694 |
Bouloupari, Ouaméni, prop. Debels | 21°49'12.0"S; 166°56'36.0"E | |||
| Hemistomia cockerelli, NeCa 11 | Bouloupari, Ouitchambo | 21°48'16.8"S; 166°00'00.8"E | |||
| Hemistomia cockerelli, NeCa 17 | Moindou, road toward barrage | 21°39'52.8"S; 165°43"10.3"E | KJ490857 | KJ490772 | KJ490696 |
| Hemistomia cockerelli, NeCa 21A | Farino, Sentier de la Cascade et des Sources | 21°38'11.9"S; 165°46'36.6"E | KJ490863 | --- | KJ490702 |
| Hemistomia cockerelli, NeCa 36 | Sarraméa, track to “Trou d’Eau” | 21°38'22.1"S; 165°51'37.5"E | |||
| Hemistomia cockerelli, NeCa 54 | Hienghène, Tendo | 20°42'54.7"S; 164°49'20.8"E | |||
| Hemistomia eclima, NeCa 19 | Moindou, road toward barrage | 21°39'58.4"S; 165°43'08.2"E | KJ490858 | KJ490773 | KJ490697 |
| Hemistomia fabrorum, NeCa 1 | Dumbéa, Koé, prop. Oesterlin | 22°08'59.0"S; 166°29'10.6"E | KJ490829 | KJ490749 | KJ490670 |
| Hemistomia fabrorum, NeCa 25B | Sarraméa, road side of RPN 5 | 21°34'15.7"S; 165°49'41.2"E | KJ490867 | KJ490781 | KJ490704 |
| Hemistomia minor, NeCa 30 | Moindou, road side SW Katrikoin | 21°34'21.6"S; 165°41'02.5"E | KJ490872 | KJ490786 | KJ490709 |
| Hemistomia nyo, NeCa 35 | Bourail, Oua Oué | 21°36'50.3"S; 165°35'31.5"E | KJ490880 | KJ490791 | KJ490716 |
| Hemistomia oxychila, NeCa 43A | Poya, road side between Nétéa and Goipin | 21°16'06.0"S; 165°14'32.0"E | KJ490893 | KJ490804 | KJ490726 |
| Hemistomia rusticorum, NeCa 6A | Bouloupari, road side N Nassirah | 21°48'08.0"S; 166°04'14.6"E | KJ490836 | KJ490755 | KJ490677 |
| Hemistomia winstonefi, NeCa 3B | Mont Dore, Rue des Roseaux, prop. Solier | 22°15'42.4"S; 166°34'08.7"E | KJ490834 | KJ490753 | KJ490675 |
| Leiorhagium adioincola, NeCa 43B | Poya, side of road to Goipin | 21°16'06.0"S; 165°14'32.0"E | KJ490895 | KJ490806 | KJ490728 |
| Leiorhagium adioincola, NeCa 49 | Poya, stream into Grotte d‘Adio | 21°15'24.4"S; 165°14'46.4"E | KJ490901 | KJ490812 | KJ490734 |
|
Leiorhagium
ajie, paratypes MNHN IM-2012-2688 |
Houailou, Néoua | 21°24'00.0"S; 165°38'54.0"E | |||
|
Leiorhagium
aremuum, NeCa 33_1 Leiorhagium aremuum, NeCa 33_2 |
Moindou, Aremu valley | 21°35'04.8"S; 165°39'07.5"E |
KJ490878 KJ490879 |
KJ490789 KJ490790 |
KJ490714 KJ490715 |
| Leiorhagium clandestinum, NeCa 30B | Moindou, road side SW Katrikoin | 21°34'21.6"S; 165°41'02.5"E | KJ490874 | --- | KJ490711 |
|
Leiorhagium
douii, paratypes MNHN IM-2012-2681 |
Poya, Grotte d’Adio | 21°15'30.0"S; 165°14'30.0"E | |||
| Leiorhagium inplicatum, NeCa 9B | Bouloupari, road side of RP 4 | 21°44'30.9"S; 166°05'57.9"E | KJ490845 | KJ490762 | KJ490685 |
|
Leiorhagium
kavuneva, paratypes MNHN IM-2012-2690 |
Sarraméa, prop. Bonnard | 21°39'00.0"S; 165°50'48.0"E | |||
| Leiorhagium kavuneva, NeCa 15B | Bouloupari, Oua Tom | 21°47'24.4"S; 165°54'51.2"E | KJ490855 | KJ490770 | KJ490694 |
| Leiorhagium kavuneva, NeCa 27 | Kouaoua, road side N Koh | 21°30'52.2"S; 165°48'05.0"E | KJ490869 | KJ490783 | KJ490706 |
| Leiorhagium kavuneva, NeCa 29 | Kouaoua, road side N Koh | 21°32'02.6"S; 165°49'27.2"E | |||
|
Leiorhagium
monachum, paratypes MNHN IM-2012-2679 |
Poya, Mt. Krapé | 21°23'12.0"S; 165°14'30.0"E | |||
|
Leiorhagium
montfaouense, paratypes MNHN IM-2012-2684 |
Poya, Montfaoué | 21°16'48.0"S; 165°17'42.0"E | |||
| Leiorhagium neteae, NeCa 44B | Poya, beginning of road into Vallée d’Adio | 21°14'47.9"S; 165°15'45.0"E | KJ490897 | KJ490808 | KJ490730 |
| Leiorhagium orokau, NeCa 42 | Poya, near Nétéa | 21°16'32.2"S; 165°12'17.6"E | KJ490891 | KJ490802 | KJ490724 |
| Leiorhagium orokau, NeCa 57 | Hienghène, Tendo | 20°42'43.9"S; 164°47'47.5"E | KJ490912 | KJ490823 | KJ490744 |
| Crosseana crosseana, NeCa 51 | Koumac, seepage in N of town | 20°32'32.2"S; 164°18'33.0"E | KJ490904 | KJ490815 | KJ490737 |
| Crosseana melanosoma, NeCa 50 | Voh, Boyen, overflow of reservoir | 20°49'13.6"S; 164°36'56.4"E | KJ490902 | KJ490813 | KJ490735 |
| Kanakyella gentilsiana, NeCa 58 | Hienghène, Tendo | 20°42'22.4"S; 164°47'20.0"E | KJ490914 | KJ490825 | KJ490746 |
Phylogenetic analyses were based on a selection of sequences generated by Zielske and Haase (2015), who analyzed fragments of the mitochondrial genes (COI) and 16S rRNA as well as the nuclear (ITS2). For lab protocols see Zielske and Haase (2014a, 2015). We restricted the analysis to 3 specimens per species at most and used Kanakyella gentilsiana, Crosseana crosseana, and Crosseana melanosoma as outgroups (Table 1). The alignment of 16S rRNA and ITS2 was generated using secondary structure information using RNAsalsa 0.8.1 (Stocsits et al. 2009) (for details see Zielske and Haase 2015) and checked for ambiguous and randomly similar sites in Aliscore 2.0 (Misof and Misof 2009). We defined seven partitions. PartitionFinder 1.1.0 (Lanfear et al. 2012) identified the following scheme and substitution models as optimal among all possible combinations of separate and merged partitions: COI 1st positions (TrNef+I), COI 2nd positions (F81), COI 3rd positions (TVM+I+Γ), 16S rRNA loops (TrN+ Γ), ITS2 loops (TrNef+I+Γ), joint stems of 16S rRNA and ITS2 (K80+I). With these settings, tree reconstructions were conducted in a (ML) framework using GARLI 2.01 (Zwickl 2006) with 500 replicates. Robustness was assessed by bootstrapping with 200 replicates.
Type and non-type material is deposited at the (MNHN) and at the (NHMW).
Results
Systematic descriptions
Diagnoses and descriptions of Hemistomia and Leiorhagium and data used in our comparisons with the new species were provided by Haase and Bouchet (1998). Locality data include site number, district capital, site, coordinates, and date of collection. Shell measurements are given in Table 2 and not repeated in the descriptions.
Table 2.
Morphometry. Measurements in mm. Shell measures: AH; AW; BWW; SH; SW; W; statistics: CV; max; min; SD. First line of new species contains measurements of holotypes. Note that the holotype was only in case of Leiorhagium clandestinum included in the descriptive statistics. Numbers of whorls were only counted in the new species as this parameter was not used in the statistical analyses.
| New species | |||||||
| SH | SW | AH | AW | BWW | SH/SW | W | |
| Hemistomia andreae sp. n. (N=20) | 2.70 | 1.25 | 0.90 | 0.87 | 1.08 | 2.17 | 5.4 |
| min | 2.40 | 1.10 | 0.80 | 0.75 | 0.97 | 2.00 | 4.50 |
| max | 2.78 | 1.28 | 0.93 | 0.91 | 1.08 | 2.35 | 5.50 |
| mean | 2.60 | 1.18 | 0.85 | 0.82 | 1.02 | 2.20 | 5.14 |
| median | 2.60 | 1.17 | 0.85 | 0.82 | 1.01 | 2.23 | 5.25 |
| SD | 0.11 | 0.05 | 0.04 | 0.04 | 0.03 | 0.11 | 0.28 |
| CV | 4.40 | 3.93 | 4.23 | 4.54 | 2.77 | 4.94 | 5.49 |
| Leiorhagium adioincola sp. n. NeCa 49 (N=20) | 2.29 | 1.24 | 0.88 | 0.87 | 1.09 | 1.84 | 4.50 |
| min | 2.10 | 1.16 | 0.83 | 0.83 | 1.04 | 1.71 | 4.13 |
| max | 2.42 | 1.31 | 0.96 | 0.96 | 1.15 | 1.90 | 4.75 |
| mean | 2.25 | 1.25 | 0.88 | 0.89 | 1.10 | 1.80 | 4.36 |
| median | 2.24 | 1.24 | 0.88 | 0.89 | 1.10 | 1.80 | 4.25 |
| SD | 0.09 | 0.04 | 0.04 | 0.03 | 0.03 | 0.05 | 0.18 |
| CV | 4.21 | 2.96 | 4.15 | 3.92 | 2.94 | 2.91 | 4.24 |
| Leiorhagium aremuum sp. n. (N=20) | 2.19 | 1.35 | 0.97 | 0.91 | 1.16 | 1.62 | 4.25 |
| min | 2.03 | 1.29 | 0.87 | 0.86 | 1.10 | 1.53 | 3.75 |
| max | 2.43 | 1.46 | 1.03 | 1.00 | 1.25 | 1.69 | 4.25 |
| mean | 2.19 | 1.35 | 0.94 | 0.92 | 1.16 | 1.62 | 4.03 |
| median | 2.15 | 1.35 | 0.93 | 0.92 | 1.17 | 1.62 | 4.00 |
| SD | 0.11 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.15 |
| CV | 4.92 | 4.06 | 4.76 | 4.54 | 3.77 | 2.71 | 3.78 |
| Leiorhagium clandestinum sp. n. (N=4) | 2.49 | 1.32 | 0.94 | 0.95 | 1.16 | 1.91 | 4.50 |
| min | 2.23 | 1.26 | 0.89 | 0.88 | 1.07 | 1.77 | 4.25 |
| max | 2.49 | 1.32 | 0.94 | 0.95 | 1.16 | 1.91 | 4.50 |
| mean | 2.38 | 1.28 | 0.91 | 0.92 | 1.10 | 1.86 | 4.41 |
| median | 2.41 | 1.27 | 0.90 | 0.93 | 1.09 | 1.89 | 4.44 |
| SD | 0.11 | 0.03 | 0.02 | 0.03 | 0.04 | 0.06 | 0.12 |
| CV | 4.83 | 2.30 | 2.68 | 3.44 | 4.00 | 3.52 | 2.89 |
| Leiorhagium neteae n. sp. (N=18) | 2.07 | 1.12 | 0.75 | 0.77 | 0.91 | 1.84 | 4.50 |
| min | 1.85 | 0.97 | 0.65 | 0.70 | 0.82 | 1.76 | 4.25 |
| max | 2.23 | 1.17 | 0.79 | 0.80 | 0.95 | 2.01 | 5.00 |
| mean | 2.05 | 1.09 | 0.73 | 0.75 | 0.88 | 1.88 | 4.46 |
| median | 2.04 | 1.10 | 0.73 | 0.75 | 0.87 | 1.88 | 4.38 |
| SD | 0.12 | 0.05 | 0.03 | 0.03 | 0.03 | 0.07 | 0.19 |
| CV | 6.05 | 4.82 | 4.71 | 4.69 | 3.99 | 3.72 | 4.25 |
| Material for comparisons | |||||||
| SH | SW | AH | AW | BWW | SH/SW | ||
| Hemistomia cockerelli Types (N=20) | |||||||
| min | 2.58 | 1.18 | 0.88 | 0.83 | 1.03 | 2.05 | |
| max | 3.21 | 1.39 | 1.03 | 0.97 | 1.16 | 2.40 | |
| mean | 2.79 | 1.27 | 0.94 | 0.91 | 1.09 | 2.19 | |
| median | 2.74 | 1.25 | 0.93 | 0.90 | 1.09 | 2.18 | |
| SD | 0.17 | 0.06 | 0.04 | 0.04 | 0.05 | 0.09 | |
| CV | 6.20 | 4.91 | 4.31 | 4.52 | 4.23 | 4.36 | |
| Hemistomia cockerelli NeCa11 (N=20) | |||||||
| min | 2.20 | 1.06 | 0.77 | 0.73 | 0.94 | 1.93 | |
| max | 2.48 | 1.25 | 0.87 | 0.91 | 1.04 | 2.28 | |
| mean | 2.33 | 1.13 | 0.81 | 0.81 | 0.97 | 2.06 | |
| median | 2.32 | 1.12 | 0.80 | 0.80 | 0.96 | 2.03 | |
| SD | 0.08 | 0.04 | 0.03 | 0.04 | 0.02 | 0.10 | |
| CV | 3.49 | 3.70 | 3.36 | 4.60 | 2.36 | 4.75 | |
| Hemistomia cockerelli NeCa17 (N=20) | |||||||
| min | 2.35 | 1.16 | 0.83 | 0.83 | 1.04 | 1.96 | |
| max | 2.62 | 1.28 | 0.92 | 0.93 | 1.14 | 2.19 | |
| mean | 2.50 | 1.21 | 0.87 | 0.87 | 1.08 | 2.07 | |
| median | 2.51 | 1.21 | 0.87 | 0.88 | 1.08 | 2.07 | |
| SD | 0.07 | 0.04 | 0.03 | 0.03 | 0.02 | 0.07 | |
| CV | 2.90 | 3.20 | 3.16 | 3.25 | 2.32 | 3.43 | |
| Hemistomia cockerelli NeCa21A (N=8) | |||||||
| min | 2.26 | 1.09 | 0.74 | 0.77 | 0.96 | 2.03 | |
| max | 2.74 | 1.23 | 0.89 | 0.87 | 1.08 | 2.38 | |
| mean | 2.49 | 1.17 | 0.84 | 0.83 | 1.03 | 2.12 | |
| median | 2.49 | 1.17 | 0.85 | 0.83 | 1.05 | 2.09 | |
| SD | 0.14 | 0.05 | 0.04 | 0.03 | 0.04 | 0.11 | |
| CV | 5.87 | 4.33 | 5.41 | 3.89 | 3.66 | 5.50 | |
| Hemistomia cockerelli NeCa36 (N=13) | |||||||
| min | 2.32 | 1.14 | 0.79 | 0.82 | 1.03 | 1.97 | |
| max | 2.64 | 1.23 | 0.91 | 0.91 | 1.12 | 2.14 | |
| mean | 2.43 | 1.18 | 0.85 | 0.85 | 1.06 | 2.05 | |
| median | 2.42 | 1.19 | 0.86 | 0.85 | 1.06 | 2.04 | |
| SD | 0.10 | 0.03 | 0.03 | 0.03 | 0.03 | 0.05 | |
| CV | 4.25 | 2.42 | 3.79 | 3.22 | 2.64 | 2.64 | |
| Hemistomia cockerelli NeCa54 (N=20) | |||||||
| min | 2.28 | 1.16 | 0.78 | 0.82 | 1.04 | 1.86 | |
| max | 2.63 | 1.31 | 0.96 | 0.93 | 1.14 | 2.14 | |
| mean | 2.47 | 1.23 | 0.87 | 0.88 | 1.09 | 2.00 | |
| median | 2.47 | 1.23 | 0.86 | 0.87 | 1.10 | 2.02 | |
| SD | 0.10 | 0.04 | 0.04 | 0.03 | 0.03 | 0.07 | |
| CV | 4.18 | 3.08 | 4.18 | 3.28 | 2.62 | 3.62 | |
| Hemistomia nyo NeCa35 (N=7) | |||||||
| min | 2.43 | 1.25 | 0.88 | 0.89 | 1.09 | 1.93 | |
| max | 2.75 | 1.34 | 0.96 | 0.96 | 1.15 | 2.08 | |
| mean | 2.62 | 1.30 | 0.92 | 0.92 | 1.12 | 2.01 | |
| median | 2.69 | 1.30 | 0.91 | 0.91 | 1.11 | 2.03 | |
| SD | 0.12 | 0.04 | 0.03 | 0.03 | 0.02 | 0.06 | |
| CV | 4.80 | 2.84 | 3.30 | 3.10 | 2.00 | 3.00 | |
| Leiorhagium ajie Types (N=6) | |||||||
| min | 2.35 | 1.31 | 0.93 | 0.94 | 1.12 | 1.61 | |
| max | 2.74 | 1.62 | 1.10 | 1.06 | 1.34 | 1.80 | |
| mean | 2.50 | 1.46 | 1.01 | 1.00 | 1.25 | 1.72 | |
| median | 2.43 | 1.46 | 1.01 | 1.00 | 1.27 | 1.70 | |
| SD | 0.16 | 0.12 | 0.07 | 0.05 | 0.08 | 0.07 | |
| CV | 6.50 | 8.31 | 6.95 | 4.88 | 6.32 | 4.12 | |
| Leiorhagium douii Types (N=20) | |||||||
| min | 1.87 | 0.98 | 0.68 | 0.68 | 0.86 | 1.84 | |
| max | 2.50 | 1.16 | 0.84 | 0.79 | 0.97 | 2.16 | |
| mean | 2.06 | 1.05 | 0.73 | 0.71 | 0.91 | 1.96 | |
| median | 2.02 | 1.06 | 0.72 | 0.71 | 0.91 | 1.95 | |
| SD | 0.14 | 0.04 | 0.04 | 0.02 | 0.03 | 0.08 | |
| CV | 7.04 | 4.02 | 5.11 | 3.51 | 3.21 | 4.23 | |
| Leiorhagium kavuneva Types (N=20) | |||||||
| min | 2.17 | 1.17 | 0.78 | 0.82 | 1.02 | 1.77 | |
| max | 2.42 | 1.32 | 0.94 | 0.93 | 1.13 | 1.93 | |
| mean | 2.31 | 1.26 | 0.88 | 0.88 | 1.07 | 1.84 | |
| median | 2.33 | 1.25 | 0.89 | 0.88 | 1.07 | 1.85 | |
| SD | 0.07 | 0.04 | 0.04 | 0.03 | 0.03 | 0.05 | |
| CV | 3.24 | 3.16 | 4.52 | 3.15 | 2.87 | 2.58 | |
| Leiorhagium kavuneva NeCa15B (N=20) | |||||||
| min | 2.20 | 1.21 | 0.84 | 0.88 | 1.07 | 1.76 | |
| max | 2.46 | 1.31 | 0.94 | 0.98 | 1.20 | 1.97 | |
| mean | 2.34 | 1.27 | 0.90 | 0.92 | 1.12 | 1.84 | |
| median | 2.35 | 1.28 | 0.91 | 0.92 | 1.12 | 1.83 | |
| SD | 0.07 | 0.03 | 0.03 | 0.03 | 0.03 | 0.06 | |
| CV | 3.14 | 2.30 | 3.31 | 3.00 | 2.54 | 3.14 | |
| Leiorhagium kavuneva NeCa29 (N=20) | |||||||
| min | 2.17 | 1.20 | 0.85 | 0.85 | 1.06 | 1.76 | |
| max | 2.54 | 1.36 | 1.00 | 0.99 | 1.17 | 1.97 | |
| mean | 2.35 | 1.28 | 0.91 | 0.93 | 1.12 | 1.83 | |
| median | 2.34 | 1.27 | 0.90 | 0.93 | 1.13 | 1.82 | |
| SD | 0.10 | 0.04 | 0.04 | 0.04 | 0.03 | 0.05 | |
| CV | 4.27 | 3.12 | 4.50 | 3.93 | 2.91 | 2.62 | |
| Leiorhagium monachum Types (N=3) | |||||||
| min | 2.07 | 1.04 | 0.72 | 0.69 | 0.88 | 1.88 | |
| max | 2.18 | 1.10 | 0.82 | 0.78 | 0.97 | 2.00 | |
| mean | 2.11 | 1.08 | 0.76 | 0.74 | 0.92 | 1.96 | |
| median | 2.07 | 1.09 | 0.76 | 0.75 | 0.92 | 1.99 | |
| SD | 0.07 | 0.03 | 0.05 | 0.05 | 0.05 | 0.07 | |
| CV | 3.36 | 3.19 | 7.25 | 6.89 | 5.51 | 3.67 | |
| Leiorhagium montfaouense Types (N=10) | |||||||
| min | 1.80 | 1.03 | 0.68 | 0.64 | 0.83 | 1.76 | |
| max | 2.30 | 1.16 | 0.81 | 0.77 | 0.99 | 1.99 | |
| mean | 2.01 | 1.08 | 0.73 | 0.70 | 0.90 | 1.87 | |
| median | 2.02 | 1.05 | 0.72 | 0.68 | 0.89 | 1.86 | |
| SD | 0.15 | 0.05 | 0.05 | 0.04 | 0.06 | 0.09 | |
| CV | 7.73 | 4.89 | 6.40 | 6.29 | 6.87 | 4.79 | |
Genus. Hemistomia
Crosse, 1872
Type species.
Hemistomia caledonica Crosse, 1872
Hemistomia andreae sp. n.
http://zoobank.org/1C80E381-43F7-43EB-9853-425C5C6B925E
Type material.
Holotype MNHN IM 2000-27858; paratypes MNHN IM 2000-27859 (> 50), NHMW 110181 (10).
Type locality.
NeCa 12, Bouloupari: Ouaméni-valley, small stream on W-side of road in secondary forest, 21°49'46.9"S, 165°56'42.9"E, 22 May 2012.
Etymology.
The new species is dedicated to the senior author’s daughter on the occasion of her ‘quinceañera’, the 15th birthday.
Diagnosis.
Hemistomia andreae sp. n. is very similar to Hemistomia cockerelli and Hemistomia nyo. It differs from both in a clearer separation of the opercular pegs and a much more delicate penis. The protoconch of the new species has more whorls than Hemistomia nyo and the palatal denticle is further behind the outer lip.
Shell.
Conical, 2.2 times higher than wide, 4.5-5.5 whorls, without colour, transparent; protoconch faintly pitted with 1-1.25 whorls; palatal denticle large, elongate, c. 1/3 whorl behind outer lip; with columellar fold in the body whorl; aperture slightly higher than wide (Figs 2A, 3A,B, 4A,B).
Figure 3.
Shells (all paratypes). A, B Hemistomia andreae sp. n. C, D Leiorhagium adioincola sp. n. E, F Leiorhagium aremuum sp. n. G Leiorhagium clandestinum sp. n. H, I Leiorhagium neteae sp. n.
Figure 4.

Protoconchs (left) and close-up views of apical microstructure (right). A, B Hemistomia andreae sp. n. C, D Leiorhagium adioincola sp. n. E, F Leiorhagium aremuum sp. n. G, H Leiorhagium clandestinum I, J Leiorhagium neteae sp. n. Scale bars 50 µm (A, C, E, G, I), 10 µm (B, D, F, H, J).
Operculum.
Elongate-ellipsoidal, paucisprial, nucleus submarginal, orange, one large and one small non-calcareous white peg, well separated from each other (N=5) (Fig. 5A,B).
Figure 5.
Operculum. A, B Hemistomia andreae sp. n. C, D Leiorhagium adioincola sp. n. E, F Leiorhagium aremuum sp. n. G, H Leiorhagium neteae sp. n.
External features.
Epidermis without pigment, eyes black.
Mantle cavity.
Ctenidium with 24–26 (2 males) or 25–28 (3 females) filaments; osphradium kidney-shaped, behind middle of ctenidium.
Digestive system.
Radula formula (N=3) (Fig. 6A): R (rhachis or central tooth): 3 1 3/2 2, L (lateral tooth): 3 1 5, M1 (inner marginal tooth): 21–25, M2 (outer marginal tooth): 27–32; stomach without caecum; rectum close to pallial oviduct in females and to prostate in males.
Figure 6.
Radula. A Hemistomia andreae sp. n. B Leiorhagium adioincola sp. n. C Leiorhagium aremuum sp. n. D Leiorhagium neteae sp. n. Arrows indicate membranous junction of flank and face of lateral teeth typical for most Pacific tateid genera (partly dissolved in A and D).
Female genitalia.
Ovary without lobes, proximal end c. 1.25 whorls below apex, comprising 0.25–0.5 whorls, eventually reaching stomach; anterior capsule gland yellow-orange, posterior capsule gland opaque-white, albumen gland milky-white; proximal loop of renal oviduct upright comprising 180°, distal loop short; bursa copulatrix pear-shaped, reaching only slightly behind albumen gland; bursal duct long, entering anterior; seminal receptacle on ventral edge of and as long as bursa (N=3) (Fig. 7A).
Figure 7.
Female genitalia. A Hemistomia andreae sp. n. B Leiorhagium adioincola sp. n. C Leiorhagium aremuum sp. n. D Leiorhagium neteae sp. n. ac anterior capsule gland, ag albumen gland, bc bursa copulatrix, bd bursal duct, go genital opening, od oviduct, pc posterior capsule gland, rs receptaculum seminis, vc vestibular capsule gland, ve ventral channel.
Male genitalia.
Proximal end of lobate testis 1–1.25 whorls below apex, comprising 0.75 whorls, covering proximal end of stomach; vesicula seminalis arising from anterior third of testis; penis fairly delicate with blunt end (N=2) (Fig. 8A,B).
Figure 8.
Penis. A, B Hemistomia andreae sp. n. C Leiorhagium adioincola sp. n. D Leiorhagium aremuum sp. n. E Leiorhagium neteae sp. n. Scale bars = 100 µm.
Remarks.
This is Hemistomia sp. n. 1 of Zielske and Haase (2015). Both Hemistomia andreae sp. n. and Hemistomia cockerelli do have the columellar fold in the body whorl assumed to be unique in Hemistomia nyo by Haase and Bouchet (1998). Hemistomia andreae sp. n. is only known from the type locality.
Genus. Leiorhagium
Haase & Bouchet, 1998
Type species.
Leiorhagium orokau Haase & Bouchet, 1998
Leiorhagium adioincola sp. n.
http://zoobank.org/CCC4F863-76C3-44C2-A4AA-CE9DE0B726AB
Type material.
Holotype MNHN IM 2000-27860; paratypes MNHN IM 2000-27861 (29), NHMW 110182 (5).
Type locality.
NeCa 49, Poya: Massif d’Adio, stream flowing into Grotte d’Adio, open secondary forest, 21°15'24.4"S, 165°14'46.4"E, 29 May 2012.
Other material.
NeCa 43, Poya: small stream on W-side of road between Nétéa and Goipin, on forest edge, 21°16'06.0"S, 165°14'32.0"E, 28 May 2012, MNHN-IM-2012-36075 (23), NHMW 110183 (10).
Etymology.
Adioincola is composed of the name of the area of Adio and the Latin noun incola meaning inhabitant, and thus refers to the type locality of the new species.
Diagnosis.
Leiorhagium adioincola sp. n. is very similar to Leiorhagium kavuneva and Leiorhagium clandestinum sp. n. The former pair differs in penial shape, slender vs. basally broad with long terminal filament. Leiorhagium adioincola sp. n. tends to have fewer radular denticles than Leiorhagium kavuneva. Genetically, these species differed on average at 9.65% of the positions of COI. Due to the lack of anatomical data, both new species can only be distinguished genetically. Their COI sequences differed on average by 9.5% (p-distance).
Shell.
Pupiform, 1.8 times higher than wide, 4.125-4.75 whorls, without colour, transparent; protoconch faintly pitted with c. 1 whorl; palatal denticle a small droplet 1/8 whorl behind outer lip; aperture as high as wide (Figs 2B, 3C, D, 4C, D).
Operculum.
Elongate-ellipsoidal, paucisprial, nucleus submarginal, orange, usually two non-calcareous white pegs, eventually accompanied by a small third one (N=3) (Fig. 5C,D).
External features.
Epidermis without pigment, eyes black.
Mantle cavity.
Ctenidium with 18-19 (3 males) or 21–24 (2 females) filaments; osphradium kidney-shaped, behind middle of ctenidium.
Digestive system.
Radula formula (N=3) (Fig. 6B): R: 4 1 4/2 2, L: 4-5 1 6, M1: 22-27, M2: 21-29; stomach without caecum; rectum close to pallial oviduct in females and to prostate in males.
Female genitalia.
Ovary without lobes, proximal end 1.25 whorls below apex, comprising 0.25-0.5 whorls, eventually reaching stomach; anterior capsule gland yellow-orange, posterior capsule gland opaque-white, albumen gland milky-white; proximal loop of renal oviduct bent forward, distal loop short; bursa copulatrix almost cubical, reaching behind albumen gland; bursal duct long, entering anterior; no seminal receptacle (N=2) (Fig. 7B).
Male genitalia.
Proximal end of lobate testis 1.25–1.5 whorls below apex, comprising 0.5-0.75 whorls, covering proximal end of stomach; vesicula seminalis arising from anterior half of testis; penis slender, terminal end occasionally forming short filament (N=3) (Fig. 8C).
Remarks.
This is Leiorhagium sp. n. 4 of Zielske and Haase (2015). Leiorhagium adioincola sp. n. occurs in the area between the villages of Nétéa and Goipin including the Massif d’Adio.
Leiorhagium aremuum sp. n.
http://zoobank.org/3B015791-A03B-48BB-8C1D-1A829588B5E2
Type material.
Holotype MNHN IM 2000-27862; paratypes MNHN IM 2000-27863 (28), NHMW 110184 (10).
Type locality.
NeCa 33, Moindou: spring-fed stream close to road in Aremu valley, under shrub, 21°35'04.8"S, 165°39'07.5"E, 26 May 2012.
Etymology.
The new species is named after the Aremu valley, where it has been discovered.
Diagnosis.
Leiorhagium aremuum sp. n. is most similar to Leiorhagium ajie, which is, however, larger and slightly more slender, lacks the palatal denticle, and has a more massive penis. The prolonged capsule gland is unique among New Caledonian tateids. The COI sequences had a p-distance of 9.4%.
Shell.
Broadly pupiform, 1.62 times higher than wide, 3.75-4.25 whorls, without colour, transparent; protoconch faintly pitted with 0.75-0.9 whorls; palatal denticle a small droplet 1/8 whorl behind outer lip; aperture practically as high as wide (Figs 2C, 3E,F, 4E,F).
Operculum.
Elongate-ellipsoidal, paucisprial, nucleus submarginal, orange, two non-calcareous white pegs, eventually accompanied by a small third one (N=4) (Fig. 5E, F).
External features.
Epidermis without pigment, eyes black.
Mantle cavity.
Ctenidium with 15-16 (2 males) or 19-20 (2 females) filaments; osphradium elongate, slightly behind middle of ctenidium.
Digestive system.
Radula formula (N=3) (Fig. 6C): R: 4-5 1 4-5/2-3 2-3, L: 4-5 1 4-6, M1: 26-31, M2: 20-32; stomach without caecum; rectum close to pallial oviduct in females, with short loop left of prostate in males.
Female genitalia.
Ovary without lobes, proximal end 1.25-1.75 whorls below apex, comprising 0.25-0.5 whorls, reaching stomach; capsule gland with long and slender, opaque-white vestibulum, anterior capsule gland yellow-orange, toward posterior capsule gland covered with brown spots, posterior capsule gland opaque-white with a central milky section, albumen gland milky-white; proximal loop of renal oviduct bent forward, distal loop long; bursa copulatrix higher than long, reaching behind albumen gland; bursal duct long, entering anterior; no seminal receptacle (N=3) (Fig. 7C).
Male genitalia.
Proximal end of lobate testis 1 whorl below apex, comprising c. 0.75 whorls, covering proximal end of stomach; vesicula seminalis arising from distal third of testis; penis very long and slender (N=2) (Fig. 8D).
Remarks.
This is Leiorhagium sp. n. 3 of Zielske and Haase (2015). Leiorhagium aremuum sp. n. is only known from the type locality.
Leiorhagium clandestinum sp. n.
http://zoobank.org/723A9EA1-CBFC-486A-AA37-69728E99AC3A
Type material.
Holotype MNHN IM 2000-27865; paratypes MNHN IM 2000-27866 (3).
Type locality.
NeCa 30, Moindou: spring along road SW of Katrikoin, under shrub, 21°34'21.6"S, 165°41'02.5"E, 26 May 2012.
Etymology.
The Latin adjective clandestinus means clandestine and refers to the new species’ external identity with Leiorhagium kavuneva.
Diagnosis.
Leiorhagium clandestinum sp. n. is most similar to Leiorhagium adioincola sp. n. and Leiorhagium kavuneva. For the distinction from Leiorhagium adioincola sp. n. see above. Due to the lack of anatomical data, Leiorhagium clandestinum sp. n. and Leiorhagium kavuneva can only be distinguished based on 7.6% average sequence divergence of COI (p-distance).
Shell.
Pupiform, 1.86 times higher than wide, 4.25-5 whorls, without colour, transparent; protoconch very faintly pitted with c. 1 whorl; palatal denticle a small droplet 1/8 whorl behind outer lip; aperture as high as wide (Figs 2D, 3G, 4G, H).
External features.
Epidermis without pigment, eyes black.
Remarks.
This is Leiorhagium sp. n. 2 of Zielske and Haase (2015). Leiorhagium clandestinum sp. n. is only known from the type locality.
Leiorhagium neteae sp. n.
http://zoobank.org/7B81AF32-3FDA-49C7-A316-D84B1A5ED324
Type material.
Holotype MNHN IM 2000-27867; paratypes MNHN IM 2000-27868 (20).
Type locality.
NeCa 44, Poya: stream at side of small road branching off road between Nétéa and Goipin toward the Vallée d’Adio, under shrub close to overgrown garden, 21°14'47.9"S, 165°15'45.0"E, 28 May 2012.
Etymology.
The new species is named after the village of Nétéa, which is closely proximal to our collecting locality.
Diagnosis.
Leiorhagium neteae sp. n. is very similar to Leiorhagium douii and Leiorhagium montfaouense. In Leiorhagium neteae sp. n. the palatal denticle is slightly larger and 1/8 whorl further behind the outher lip. The operculum has only a single denticle compared to 2-3 in Leiorhagium douii and Leiorhagium montfaouense. The distal loop of the renal oviduct of the new species forms a 270° loop counter-clockwise, whereas in the other two species this part of the oviduct is bent 180° clockwise. The penis of Leiorhagium neteae sp. n. is long and slender in contrast to the other species, where it has a broad base and a very long filament.
Shell.
Elongate-pupiform, 1.88 times higher than wide, 4.25–5 whorls, without colour, transparent; protoconch faintly pitted with c. 1 whorl; palatal denticle an elongate droplet c. 1/4 whorl behind outer lip; aperture slightly wider than high (Figs 2E, 3H, I, 4I, J).
Operculum.
Elongate-ellipsoidal, paucisprial, nucleus submarginal, orange, one non-calcareous white peg (N=4) (Fig. 5G, H).
External features.
Epidermis without pigment, eyes black.
Mantle cavity.
Ctenidium with 15 (1 male) or 19-22 (5 females) filaments; osphradium short-elongate, behind middle of ctenidium.
Digestive system.
Radula formula (N=4) (Fig. 6D): R: 4 1 4/2-3 2-3, L: 4-5 1 5, M1: 20-25, M2: 24-27; stomach without caecum; rectum close to pallial oviduct in females, with short loop left of prostate in male.
Female genitalia.
Ovary without lobes, proximal end 1.25-1.5 whorls below apex, comprising 0.25-0.5 whorls, not reaching stomach; anterior capsule gland yellow-orange, posterior capsule gland opaque-white, albumen gland milky-white; proximal loop of renal oviduct bent forward, distal loop short; bursa copulatrix globular, reaching slightly behind albumen gland; bursal duct long, entering anterior; no seminal receptacle (Fig. 7D).
Male genitalia.
Proximal end of lobate testis 1 whorl below apex, comprising slightly more than 0.5 whorls, covering proximal end of stomach; vesicula seminalis arising approximately in middle of testis; penis very long and slender (N=1) (Fig. 8E).
Remarks.
This is Leiorhagium sp. n. 5 of Zielske and Haase (2015). Leiorhagium neteae sp. n. is only known from the type locality.
Morphometry
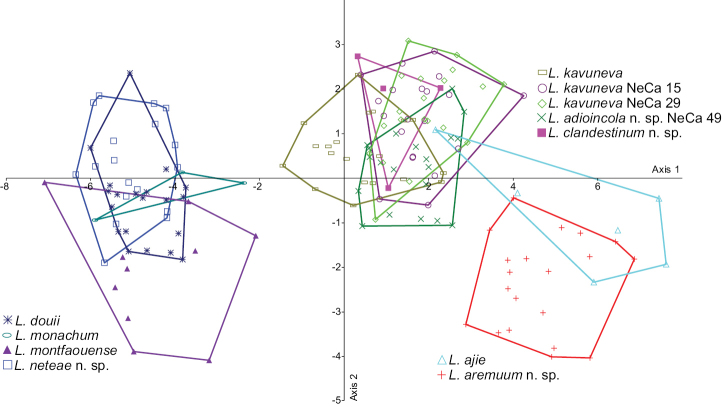
The CVA plot (Fig. 9) comparing species of Hemistomia shows the high variability of Hemistomia cockerelli. The associated MANOVA was highly significant (Wilk’s λ = 0.062, DF1 = 35, DF2 = 490.4, F = 13.16, p = < 0.001). Many pairwise comparisons of populations were significant as well (Table 3). Hemistomia nyo and Hemistomia andreae sp. n. fell within the variation of Hemistomia cockerelli. According to the CVA, they were not more different from each other than from populations of Hemistomia cockerelli. Assignment and jacknifed assignment tests allocated 80 (62.5%) and 67 (52.3%) of a total of 128 shells to their original sample indicating the considerable overlap of shapes.
Figure 9.
CVA plot for Hemistomia. Samples without numbers are paratypes.
Table 3.
Pairwise morphometric comparisons of Hemistomia samples. Hotelling’s T2 tests, based on five shell measures; significance assessed after sequential Bonferroni correction; sample sizes are given in Table 2. *; NS.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 Hemistomia andreae | |||||||
| 2 Hemistomia cockerelli Types | * | ||||||
| 3 Hemistomia cockerelli NeCa11 | * | * | |||||
| 4 Hemistomia cockerelli NeCa17 | * | * | * | ||||
| 5 Hemistomia cockerelli NeCa21 | NS | * | * | NS | |||
| 6 Hemistomia cockerelli NeCa36 | * | * | * | NS | NS | ||
| 7 Hemistomia cockerelli NeCa54 | * | * | * | NS | * | NS | |
| 8 Hemistomia nyo NeCa35 | * | * | * | NS | NS | * | NS |
The CVA (Fig. 10) for Leiorhagium revealed species clusters with Leiorhagium adioincola sp. n. and Leiorhagium clandestinum sp. n. overlapping with Leiorhagium kavuneva and Leiorhagium neteae sp. n. largely grouping with Leiorhagium douii and Leiorhagium monachum. The MANOVA was again highly significant (Wilk’s λ = 0.009, DF1 = 50, DF2 = 669.2, F = 23.56, p = < 0.001), as were most pairwise comparisons (Table 4). Note that comparisons involving Leiorhagium clandestinum sp. n. or Leiorhagium monachum were less meaningful because of the small sample sizes. Assignment and jacknifed assignment tests performed similar as for Hemistomia with only 103 (64.0%) and 88 (54.7%) correctly allocated shells of a total of 161.
Figure 10.
CVA plot for Leiorhagium. Samples without numbers are paratypes.
Table 4.
Pairwise morphometric comparisons of Leiorhagium samples. Hotelling’s T2 tests, based on five shell measures; significance assessed after sequential Bonferroni correction; sample sizes are given in Table 2. *; NS.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Leiorhagium adioincola NeCa49 | ||||||||||
| 2 Leiorhagium aremuum | * | |||||||||
| 3 Leiorhagium clandestinum | NS | * | ||||||||
| 4 Leiorhagium neteae | * | * | * | |||||||
| 5 Leiorhagium ajie Types | * | * | NS | * | ||||||
| 6 Leiorhagium douii Types | * | * | * | * | * | |||||
| 7 Leiorhagium kavuneva Types | * | * | NS | * | * | * | ||||
| 8 Leiorhagium kavuneva NeCa15B | NS | * | NS | * | * | * | * | |||
| 9 Leiorhagium kavuneva NeCa29 | NS | * | NS | * | * | * | * | NS | ||
| 10 Leiorhagium monachum Types | * | * | NS | * | NS | NS | * | * | * | |
| 11 Leiorhagium montfaouense Types | * | * | * | * | * | NS | * | * | * | NS |
Phylogenetic analysis
In the phylogenetic analysis (Fig. 11), Hemistomia and Leiorhagium were sister groups, both with 100% bootstrap support. Within Leiorhagium, the elongate-pupiform species Leiorhagium orokau, Leiorhagium inplicatum and Leiorhagium neteae sp. n. were paraphyletic with respect to the more conical-pupiform species, which received a bootstrap support of 91%. Otherwise, relationships among species of Leiorhagium were not well supported. All four new species were (phylo)genetically well distinct as indicated by the branch lengths expressing genetic distances. Within Hemistomia, the picture was very similar with well differentiated species but otherwise little resolution. Average pairwise uncorrected genetic distances based on the COI-fragment were ≥ 7.4% and are summarized in Table 5.
Figure 11.
Maximum likelihood phylogram showing bootstrap support when > 50%. Outgroup pruned from tree; new species highlighted by bold face type.
Table 5.
Average pairwise uncorrected (p) distances between selected species based on the COI-fragment (in %).
| 1 | 2 | |||
| 1 Hemistomia andreae | ||||
| 2 Hemistomia cockerelli | 8.6 | |||
| 3 Hemistomia nyo | 8.8 | 9.5 | ||
| 1 | 2 | 3 | 4 | |
| 1 Leiorhagium adioincola | ||||
| 2 Leiorhagium ajie | 9.3 | |||
| 3 Leiorhagium aremuum | 10.6 | 9.4 | ||
| 4 Leiorhagium clandestinum | 9.5 | 7.8 | 7.4 | |
| 5 Leiorhagium kavuneva | 9.7 | 8.1 | 8.5 | 7.6 |
Discussion
Our phylogenetic analyses based on DNA sequence data confirmed the suspicion of Haase and Bouchet (1998) that additional cryptic species in this snail fauna will be identified once molecular methods are applied emphasizing the huge morphological variability of certain nominal species. Recent accounts on tateid gastropods from Vanuatu and Fiji (Zielske and Haase 2014a, b) have revealed extensive radiations of morphologically very similar species. However, in contrast to the New Caledonian taxa, the radiations on those archipelagos are comparatively young (Zielske and Haase 2015). Four of the five species described here are hardly distinguishable from known taxa based on measurements despite being genetically well differentiated with even uncorrected distances (see Fregin et al. 2012) of at least 7.4% to their next similar congeners. Whether this means that morphologically similar species occupy similar niches is impossible to tell at this stage because the relationship of shell morphology to habitat has not been investigated among truncatelloidean gastropods except for a few accounts on Potamopyrgus antipodarum (Haase 2003, Holomuzki and Biggs 2006, Kistner and Dybdahl 2013). Although ranges overlap or are contiguous, sibling species have not (yet) been encountered in sympatry, i.e. in the same spring or stream.
The new species provide an additional truncatelloid example stressing the importance of an integrative taxonomic approach combining morphological, anatomical and genetic methods (e.g. Haase et al. 2007, Delicado and Ramos 2012). Given the mosaic nature of evolution of these small gastropods with morphologically as well as genetically cryptic species (e.g., Haase et al. 2007, Haase 2008, Zielske et al. 2011, Delicado and Ramos 2012, Liu et al. 2013), we do not adhere to a fairly strict scheme of species identification as advocated elaborately e.g. by Schlick-Steiner et al. (2010). Instead we advocate the approach of Padial and de la Riva (2010) who have a more natural vision of the evolutionary processes potentially involved in speciation. For instance, they acknowledge that the congruence of different character sets, pivotal for taxonomic decisions for Schlick-Steiner et al. (2010), may be plesiomorphic.
Genetic differentiation was an important indicator of species status. Pairwise p-distances > 7.4% are far above any threshold suggested by advocates of barcoding (e.g., Hebert et al. 2003, 2004; Ratnasingham and Hebert 2007). However, again we do not adhere to a strict scheme as there may be no mitochondrial differentiation between good species as well as considerable variation within species of spring snails (e.g. Haase 2008; Zielske et al. 2014a; see also Fregin et al. 2012). That genetic differentiation does reflect species status for the new taxa is also indicated by the comparison of their branch lengths to branch lengths among morphologically well defined species in our phylogenetic analysis.
While conducting our morphometric analyses we appreciated that the measuring methods applied for the material described previously (Haase and Bouchet 1998) and for this account are incompatible. Obviously, using an ocular micrometer fitted to a dissecting microscope produced inaccurate data, although the measurements were quite consistent judging from the fairly low coefficients of variation, which were of a similar order of magnitude as those computed for the present data. Therefore, we had to re-measure the old samples used in our comparisons.
Another methodological problem almost expectedly occurred in the field. All collections made for our previous monograph (Haase and Bouchet 1998) were geo-referenced from maps. This proved to be fairly inaccurate when we tried to relocate sites in 2012 guided by GPS. Additional difficulties arose from recent road development and land-use changes. Many villages are now accessible on much broader roads than 20 years ago. Construction has obviously destroyed small road-side springs and seepages and changed the course of streams. Other sites were destroyed by extensive fires affecting entire valleys or hills. Crosseana melanosoma, in our analysis part of the outgroup, used to be common when first collected in 1992. Now we found only a few specimens. It remains to be seen whether there are other (extant) populations in the unexplored hinterland of Boyen. In contrast, Hemistomia yalayu, collected in a few seepages on Col d’Amoss in the far Northeast in 1989, is now probably extinct. The entire area has lost its primary vegetation. Today, the fire resistant niaouli (Melaleuca quinquenervia) and shrubland are dominating and streams harbor a very depauperate fauna.
Four of the five new species were found in single sites and the fifth was found at only two sites. Considering the vulnerability of small habitats like springs and the rapid anthropogenic development and changes on New Caledonia just outlined immediately raises concern regarding the chances of long-term survival of these species (see also Haase et al. 2010). Most sites we surveyed were rather easily accessible, close to roads, so that one can assume that there are other populations deeper in the forests or forest remnants. Nevertheless, given that the area of occupancy of each species is certainly less than 10 km2, that ranges of spring snails are almost naturally severely fragmented, and the rapidly progressing change of land cover, areas of occupancy as well as habitat, hence the numbers of populations will decline. Therefore, all five species and probably the majority of New Caledonian tateids qualify as critically endangered according to the criteria (CE, B2,a,II-IV) of the International Union for Conservation of Nature (IUCN 2012).
Supplementary Material
Acknowledgements
This publication would not have been possible without the assistance of numerous local field guides and landowners who provided access to their properties. We thank Christel Meibauer for assistance in the molecular lab. Rabea Schlüter is acknowledged for support at the SEM. We are grateful to the authorities of New Caledonia for issuing the relevant permits. Christine Pöllabauer has been a generous host in Noumea during our field trip. The manuscript benefited from suggestions of two anonymous reviewers and Robert Hershler. Financial support was received from the German Research Foundation (HA 4752/2-1).
Citation
Haase M, Zielske S (2015) Five new cryptic freshwater gastropod species from New Caledonia (Caenogastropoda, Truncatelloidea, Tateidae). ZooKeys 523: 63–87. doi: 10.3897/zookeys.523.6066
References
- Collado G, Valladares M, Mendez M. (2013) Hidden diversity in spring snails from the Andean Altiplano, the second highest plateau on Earth, and the Atacama Desert, the driest place in the world. Zoological Studies 52: . doi: 10.1186/1810-522X-52-50 [Google Scholar]
- Delicado D, Ramos MA. (2012) Morphological and molecular evidence for cryptic species of springsnails [genus Pseudamnicola (Corosella)] (Mollusca, Caenogastropoda, Hydrobiidae). Zookeys 190: 55–79. doi: 10.3897/zookeys.190.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregin S, Haase M, Olson U, Alström P. (2012) Pitfalls in comparisons of genetic distances: A case study of the avian family Acrocephalidae. Molecular Phylogenetics and Evolution 62: 319–328. doi: 10.1016/j.ympev.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Giusti F, Pezzoli E. (1980) Gasteropodi, 2 (Gastropoda: Prosobranchia: Hydrobioidea, Pyrguloidea). Collana Progetto Finalizzato ‘Promozione Quality dell’Ambiente’, Consiglio Nazionale delle Ricerche AQ/ 1147: 1–67. [Google Scholar]
- Haase M. (1996) The radiation of spring snails of the genus Belgrandiella in Austria (Mollusca: Caenogastropoda: Hydrobiidae). Hydrobiologia 319: 119–129. doi: 10.1007/BF00016880 [Google Scholar]
- Haase M. (2003) Clinal variation in shell morphology of the freshwater gastropod Potamopyrgus antipodarum along two hillcountry streams in New Zealand. Journal of the Royal Society of New Zealand 33: 549–560. doi: 10.1080/03014223.2003.9517743 [Google Scholar]
- Haase M. (2008) The radiation of hydrobiid gastropods in New Zealand: a revision including the description of new species based on morphology and mtDNA sequence information. Systematics and Biodiversity 6: 99–159. doi: 10.1017/S1477200007002630 [Google Scholar]
- Haase M, Bouchet P. (1998) Radiation of crenobiontic gastropods on an ancient continental island: the Hemistomia-clade in New Caledonia (Gastropoda: Hydrobiidae). Hydrobiologia 367: 43–129. doi: 10.1023/A:1003219931171 [Google Scholar]
- Haase M, Fontaine B, Gargominy O. (2010) Rissooidean freshwater gastropods from the Vanuatu archipelago. Hydrobiologia 637: 53–71. doi: 10.1007/s10750-009-9985-4 [Google Scholar]
- Haase M, Wilke T, Mildner P. (2007) Identifying species of Bythinella (Caenogastropoda: Rissooidea): A plea for an integrative approach. Zootaxa 1563: 1–16. [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD. (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: . [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, DeWaard JR. (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society London B Biological. Sciences 270: 313–321. doi: 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. (2004) Identification of birds through DNA barcodes. PLoS Biology 2: . doi: 10.1371/journal.pbio.0020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershler R, Liu H-P, Landye JJ. (2011) New species and records of springsnails (Caenogastropoda: Cochliopidae: Tryonia) from the Chihuahuan Desert (Mexico and United States), an imperiled biodiversity hotspot. Zootaxa 3001: 1–32. [Google Scholar]
- Holomuzki JR, Biggs BJF. (2006) Habitat-specific variation and performance trade-offs in shell armature of New Zealand Mudsnails. Ecology 87: 1038–1047. doi: 10.1890/0012-9658(2006)87[1038:HVAPTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- IUCN (2012) IUCN Red List categories and criteria. Version 3.1. Second edition http://www.iucnredlist.org [accessed 2nd of September 2014]
- Kerney MP, Cameron RAD. (1979) A Field Guide to the Land Snails of Britain and North-west Europe. Collins Publishers, London. [Google Scholar]
- Kistner EJ, Dybdahl MF. (2013) Adaptive responses and invasion: the role of plasticity and evolution in snail shell morphology. Ecology and Evolution 3: 424–436. doi: 10.1002/ece3.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. doi: 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Liu H-P, Hershler R. (2005) Molecular systematic and radiation of western North American nymphophiline gastropods. Molecular Phylogenetics and Evolution 34: 284–298. doi: 10.1016/j.ympev.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Liu H-P, Hershler R, Clift K. (2003) Mitochondrial DNA sequences reveal extensive cryptic diversity within a western American springsnail. Molecular Ecology 12: 2771–2782. doi: 10.1046/j.1365-294X.2003.01949.x [DOI] [PubMed] [Google Scholar]
- Liu H-P, Hershler R, Lang B, Davies J. (2013) Molecular evidence for cryptic species in a narrowly endemic western North American springsnail (Pyrgulopsis gilae). Conservation Genetics 14: 917–923. doi: 10.1007/s10592-013-0483-x [Google Scholar]
- Misof B, Misof K. (2009) A Monte Carlo approach successfully identifies randomnes in multiple sequence alignments: a more objective means of data exclusion. Systematic Biology 58: 1–14. doi: 10.1093/sysbio/syp006 [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. doi: 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Padial JM, de la Riva I. (2010) A response to recent proposals for integrative taxonomy. Biological Journal of the Linnean Society 101: 747–756. doi: 10.1111/j.1095-8312.2010.01528.x [Google Scholar]
- Pfenninger M, Schwenk K. (2007) Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evolutionary Biology 7: . doi: 10.1186/1471-2148-7-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder WF, Colgan DJ. (2002) What makes a narrow-range taxon? Insights from Australian freshwater snails. Invertebrate Systematics 16: 571–582. doi: 10.1071/IT01043 [Google Scholar]
- Radoman P. (1983) Hydrobioidea a superfamily of Prosobranchia (Gastropoda), I. Sistematics (sic!). Serbian Academy of Sciences and Arts, Monographs, 547, Department of Sciences, 57, Belgrade, 256 pp. [Google Scholar]
- Ratnasingham S, Hebert PDN. (2007) BOLD: the barcoding of life data system (www.barcodinglife.org). Molecular Ecology Notes 7: 355–364. doi: 10.1111/j.1471-8286.2007.01678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick-Steiner BC, Steiner FM, Seifert B, Stauffer C, Christian E, Crozier RH. (2010) Integrative taxonomy: a multisource approach to exploring biodiversity. Annual Review of Entomology 55: 421–438. doi: 10.1146/annurev-ento-112408-085432 [DOI] [PubMed] [Google Scholar]
- Stocsits RR, Letsch H, Hertel J, Misof B, Stadler PF. (2009) Accurate and efficient reconstruction of deep phylogenies from structured RNAs. Nucleic Acids Research 37: 6184–6193. doi: 10.1093/nar/gkp600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielske S, Glaubrecht M, Haase M. (2011) Origin and radiation of rissooidean gastropods (Caenogastropoda) in ancient lakes of Sulawesi. Zoologica Scripta 40: 221–237. doi: 10.1111/j.1463-6409.2010.00469.x [Google Scholar]
- Zielske S, Haase M. (2014a) When snails inform about geology: Pliocene emergence of islands of Vanuatu indicated by a radiation of truncatelloidean freshwater gastropods (Caenogastropoda: Tateidae). Journal of Zoological Systematics and Evolutionary Research 52: 217–236. doi: 10.1111/jzs.12053 [Google Scholar]
- Zielske S, Haase M. (2014b) New insights into tateid gastropods and their radiation on Fiji based on anatomical and molecular methods (Caenogastropoda: Truncatelloidea). Zoological Journal of the Linnean Society 172: 71–102. doi: 10.1111/zoj.12153 [Google Scholar]
- Zielske S, Haase M. (2015) Molecular phylogeny and a modified approach of character-based barcoding refining the taxonomy of New Caledonian freshwater gastropods (Caenogastropoda, Truncatelloidea, Tateidae). Molecular Phylogenetics and Evolution 89: 171–181. doi: 10.1016/j.ympev.2015.04.020 [DOI] [PubMed] [Google Scholar]
- Zwickl DJ. (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD Dissertation, University of Texas, Austin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.