Abstract
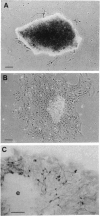
Subventricular zone (SVZ) cells proliferate spontaneously in vivo in the telencephalon of adult mammals. Several studies suggest that SVZ cells do not differentiate after mitosis into neurons or glia but die. In the present work, we show that SVZ cells labeled in the brains of adult mice with [3H]thymidine differentiate directly into neurons and glia in explant cultures. In vitro labeling with [3H]thymidine shows that 98% of the neurons that differentiate from the SVZ explants are derived from precursor cells that underwent their last division in vivo. This report identifies the SVZ cells as neuronal precursors in an adult mammalian brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Das G. D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965 Jun;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Kirn J. R., Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990 Sep 21;249(4975):1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988 Sep 22;335(6188):353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M., Nottebohm F. Proliferation "hot spots" in adult avian ventricular zone reveal radial cell division. Neuron. 1990 Jul;5(1):101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. Neuron production in the hippocampus and olfactory bulb of the adult rat brain: addition or replacement? Ann N Y Acad Sci. 1985;457:163–172. doi: 10.1111/j.1749-6632.1985.tb20804.x. [DOI] [PubMed] [Google Scholar]
- Bayer S. A., Yackel J. W., Puri P. S. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982 May 21;216(4548):890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F., Jolly R. D. The subependymal plate and associated ependyma in the dog. An ultrastructural study. J Neurocytol. 1972 Jul;1(1):69–84. doi: 10.1007/BF01098647. [DOI] [PubMed] [Google Scholar]
- Goldman S. A. Neuronal development and migration in explant cultures of the adult canary forebrain. J Neurosci. 1990 Sep;10(9):2931–2939. doi: 10.1523/JNEUROSCI.10-09-02931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday A. L., Cepko C. L. Generation and migration of cells in the developing striatum. Neuron. 1992 Jul;9(1):15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977 Sep 9;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- McDermott K. W., Lantos P. L. Distribution and fine structural analysis of undifferentiated cells in the primate subependymal layer. J Anat. 1991 Oct;178:45–63. [PMC free article] [PubMed] [Google Scholar]
- Morshead C. M., van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992 Jan;12(1):249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. A., Nottebohm F. N. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984 Sep 7;225(4666):1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Privat A., Leblond C. P. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol. 1972 Nov;146(3):277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985 Mar 1;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards L. J., Kilpatrick T. J., Bartlett P. F. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]