Abstract
Acinetobacter baumannii is one of the most important antibiotic-resistant nosocomial bacteria. We investigated changes in the clinical and molecular epidemiology of A. baumannii over a 10-year period. We compared the data from 2 prospective multicenter cohort studies in Spain, one performed in 2000 (183 patients) and one in 2010 (246 patients), which included consecutive patients infected or colonized by A. baumannii. Molecular typing was performed by repetitive extragenic palindromic polymerase chain reaction (REP-PCR), pulsed-field gel electrophoresis (PFGE), and multilocus sequence typing (MLST).
The incidence density of A. baumannii colonization or infection increased significantly from 0.14 in 2000 to 0.52 in 2010 in medical services (p < 0.001). The number of non-nosocomial health care-associated cases increased from 1.2% to 14.2%, respectively (p < 0.001). Previous exposure to carbapenems increased in 2010 (16.9% in 2000 vs 27.3% in 2010, p = 0.03). The drugs most frequently used for definitive treatment of patients with infections were carbapenems in 2000 (45%) and colistin in 2010 (50.3%). There was molecular-typing evidence of an increase in the frequency of A. baumannii acquisition in non-intensive care unit wards in 2010 (7.6% in 2000 vs 19.2% in 2010, p = 0.01). By MSLT, the ST2 clonal group predominated and increased in 2010. This epidemic clonal group was more frequently resistant to imipenem and was associated with an increased risk of sepsis, although not with severe sepsis or mortality.
Some significant changes were noted in the epidemiology of A. baumannii, which is increasingly affecting patients admitted to conventional wards and is also the cause of non-nosocomial health care-associated infections. Epidemic clones seem to combine antimicrobial resistance and the ability to spread, while maintaining their clinical virulence.
INTRODUCTION
Acinetobacter baumannii has become one of the most significant antibiotic-resistant bacteria causing nosocomial infections worldwide.3,7 From an epidemiologic point of view, its most remarkable features as a nosocomial pathogen are its ability to survive in the hospital environment and to develop resistance to multiple antimicrobial agents. Although typical clonal outbreaks associated with a common environmental reservoir have often been described,34 the situation in many hospitals is much more complex. In these situations, the epidemiology of A. baumannii may be difficult to decipher because sporadic and epidemic clones, as well as different reservoirs (including environmental ones and colonized patients), may coexist, challenging the efficacy of infection control measures.1,24 Until now, the intensive care units (ICUs) have been regarded as the epicenters of the epidemiology of A. baumannii7,34; nevertheless, spreading to conventional wards does occur,21 and in some areas, long-term care facilities have recently been shown to play an important role.22,29 Better knowledge of the current situation and of the clinical and molecular epidemiology of A. baumannii among hospitals will be helpful for designing useful infection control strategies for different centers.
From a clinical point of view, A. baumannii is usually considered to be a pathogen with limited virulence, although invasive infections are associated with increased morbidity and mortality in predisposed patients.11,18 This is probably related to the very limited therapeutic alternatives active against this pathogen, a situation which worsened with the rapid spread of extremely drug-resistant isolates.15 However, most of the available information applies to specific types of infection or to patients admitted to certain wards, so that the overall clinical importance and features of health care-associated A. baumannii infection is not as well described.23 Recent changes in the epidemiology make it necessary to reappraise the clinical features and impact of A. baumannii infections in the various populations affected.
In 2000, we performed a multicenter study in Spain to investigate the epidemiology of A. baumannii and the clinical features of infections caused by this pathogen.4,13,25 We repeated the study in 2010, with the main objective of updating the information and analyzing the changes occurred during this 10-year interval. We specifically compared epidemiologic determinants (including rates, type of wards affected, acquisition types, and predisposing factors), clinical features (such as sources and severity of infections, therapy, outcomes, and microbiologic determinants (antimicrobial susceptibility and clonality) between the 2 periods. As a secondary objective, we investigated the epidemiologic and clinical implications of specific clonal groups of A. baumannii.
METHODS
Study Design, Sites, and Participants
Two prospective multicenter, hospital-based cohort studies were performed, using the same methodology, which included all new patients infected or colonized by A. baumannii. The first (“2000 cohort”) was carried out from November 1 to November 30, 2000, in 27 acute care hospitals in Spain (patients from a specialized center for paraplegic patients were excluded from this analysis); 14 of these were teaching hospitals and 21 had ≥500 beds. Data from this cohort have been published previously.3,13,25 The second (“2010 cohort”) study was undertaken in 38 Spanish acute care hospitals between February 1 and March 31, 2010; 23 were teaching hospitals and had ≥500 beds. Twenty-two centers participated in both studies.
Patients from whom A. baumannii was isolated from any clinical sample were prospectively included in the cohorts, as detected daily by reviewing the microbiology reports in the participating centers. Active surveillance samples (such as samples performed to detect colonization for infection control purposes) were not considered. Patients were excluded if colonized or infected by A. baumannii during the previous year. All patients were followed for 30 days after isolation of the A. baumannii.
The study was approved by the Ethics Committee of the Hospital Universitario Virgen Macarena. The need to obtain informed consent was waived due to the observational nature of the study.
Variables and Definitions
The following data were collected: age, sex, type of acquisition, ward of admission, chronic underlying conditions, severity of the underlying condition according to the McCabe classification,20 invasive procedures, antibiotic use in the previous 2 months, infection or colonization and type of infection, according to CDC criteria,16 hospital stay before and after isolation of A. baumannii, therapy, and 30-day all-cause mortality. It should be noted that isolating the pathogen in a clinical sample together with the presence of clinical signs of infection were not sufficient to regard the patient as infected by A. baumannii; other sources of infection had to have been ruled out, and quantitative or semi-quantitative culture results were taken into account for urine samples, tracheal aspirates, bronchial brushes, bronchoalveolar lavages and catheter tips. When it was not possible to identify whether the status of the patient was infected or colonized, the patient was considered to be only colonized. Severity of systemic inflammatory response was classified as sepsis, severe sepsis or shock, using standard definitions.19 Acquisition was classified as nosocomial or community, according to conventional CDC definitions16; community acquisition was considered health care associated if any of the following was demonstrated: hospital admission during the previous year; and/or attention at a day hospital, specialized care home, or dialysis in the previous 3 months. Hospital wards were classified as ICUs, medical wards or surgical wards. Medical treatments were chosen by physicians in charge of the patients.
Microbiologic Studies
All isolates, presumptively identified as Acinetobacter species at each center using conventional phenotypic and/or biochemical methods, were sent to the reference laboratories for definitive identification with ARDRA analysis,31 and, for 2010 isolates, also with MALDI-TOF (Microflex LT; Bruker Daltonics GmbH, Leipzig, Germany). Only patients whose isolates were definitively identified as A. baumannii were included. At the reference laboratory, microdilution susceptibility testing was performed, according to the recommendations of the Clinical and Laboratory Standards Institute.5 For sulbactam, tigecycline, and rifampin, isolates with an MIC of ≤8, 1, and 4 mg/L, respectively, were considered as susceptible.12 Pooled susceptibility data for all A. baumannii isolates (including repeat isolates from the same patients) have been reported previously12; here we included only the first isolate obtained from each patient.
Clonal relationships between the first isolates obtained from each patient were determined by repetitive extragenic palindromic polymerase chain reaction (REP-PCR), in conditions reported previously.32 Representative strains of each REP-PCR profile at each hospital were also studied by pulsed-field gel electrophoresis (PFGE),28,30 using FPQuest II software version 4.5 (Bio-Rad) to analyze the band patterns, and by multilocus sequence typing (MLST), following the protocol developed by the Pasteur Institute (Paris, France). Isolates of the same REP-PCR and PFGE types were assigned the sequence type (ST) found for the representative isolate studied by MSLT.
Statistical Analysis
Overall, the data are analyzed in terms of the whole set of hospitals (WSH) and of hospitals that participated in both studies (HPBS). The incidence density rates are provided as number of new cases per 1000 patient-days, and compared by Poisson regression modeling. Categorical variables are described as percentages, and continuous variables as medians and interquartile ranges. Proportions were compared using the chi-square test or the Fisher exact test as appropriate, and continuous variables using the Mann-Whitney U test. To control for confounders, multivariate analysis was performed using logistic regression. Variables with a univariate p value < 0.1 were included, and selected using a stepwise backward procedure.
RESULTS
Incidence Rates
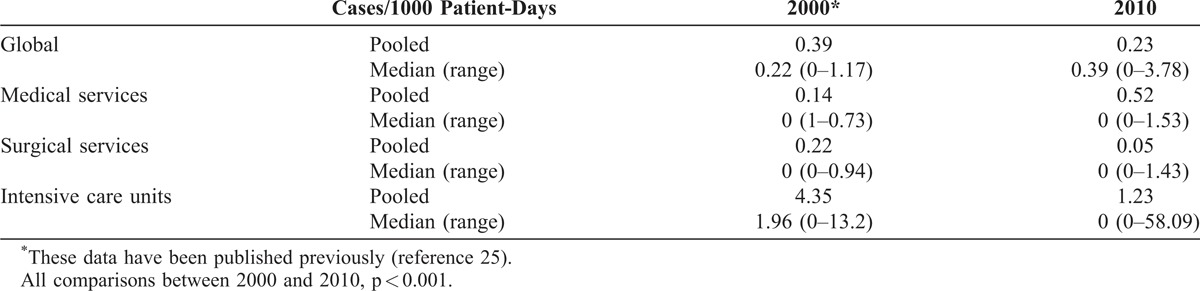
Overall, 246 patients with colonization or infection due to A. baumannii were detected in 30 of the 38 hospitals included in the 2010 cohort; in the 2000 cohort, 183 patients were detected in 25 of 27 acute care hospitals taking part. The incidence density rates of A. baumannii colonization/infection in 2000 and 2010 across the WSH are shown in Table 1. Overall, the incidence density rate decreased, particularly in surgical services and intensive care units, while it significantly increased in medical services. For HPBS, the rate decreased significantly in 8 hospitals but increased in 1. As regards changes in specific types of ward, the results were similar to those described for the WSH.
TABLE 1.
Incidence Density Rate of Colonization/Infection Due to Acinetobacter baumannii in the Whole Set of Hospitals
Predisposing Factors, 2010 vs 2000
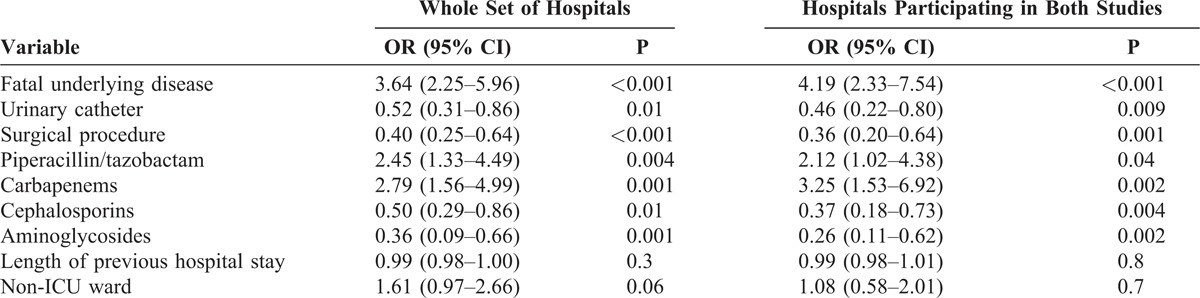
To investigate potential changes in the epidemiology of A. baumannii, we compared the frequency of exposure to predisposing conditions of patients in the 2000 and 2010 cohorts. The data are shown in Table 2 for both the WSH and the HPBS. The proportion of patients admitted to the ICU decreased in 2010, while that of patients admitted to medical wards increased; cases of non-nosocomial acquisition increased, as did patients suffering from fatal underlying diseases. Overall, invasive procedures were less frequent in 2010 patients, consistent with fewer ICU admissions. Finally, there was reduced previous exposure to cephalosporins and aminoglycosides, and increased exposure to piperacillin/tazobactam, carbapenems, and fluoroquinolones. The results of multivariate analysis by logistic regression are shown in Table 3.
TABLE 2.
Epidemiologic and Predisposing Features of Patients Colonized or Infected Due to A. baumannii in Spanish Acute Care Hospitals in 2000 and 2010∗

TABLE 3.
Multivariate Analysis of Demographic and Predisposing Factors of Patients Colonized or Infected Due to A. baumannii in 2010 Compared to 2000
Clinical Features, Outcome, and Treatment, 2010 vs 2000
In the WSH, the numbers (percentage) of patients considered to have an A. baumannii infection, rather than colonization, were 102 (55.7%) and 151 (61.4%), in 2000 and 2010, respectively (p = 0.2); the percentages in the HPBS were 55.2% and 63.6%, respectively (p = 0.1). Types of infection are shown in Table 4. Overall, the percentage of patients with intraabdominal infections increased in 2010, while meningitis/ventriculitis decreased. In patients with pneumonia in 2000 and 2010, 27 (71.1%) and 37 (63.8%) respectively, had ventilator-associated pneumonia (p = 0.4).
TABLE 4.
Types of Infection, Treatment, and Outcome Among Patients With A. baumannii, in 2000 and 2010∗
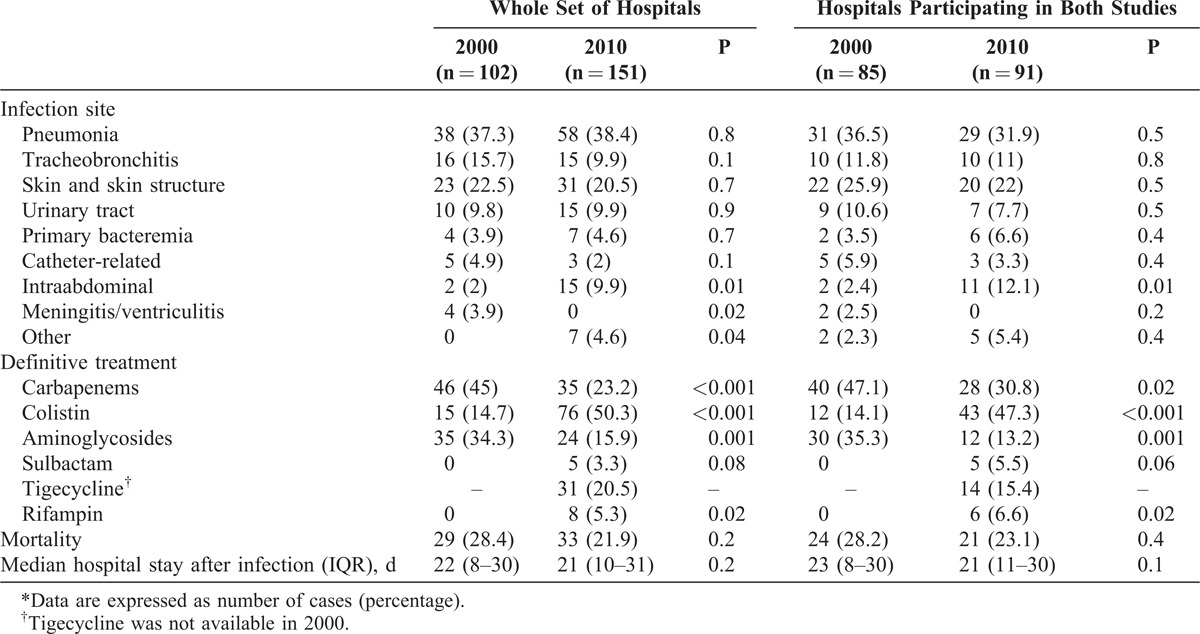
The most frequently used drugs in 2000 for the definitive treatment of patients with A. baumannii infections were carbapenems and, in 2010, colistin (see Table 4). A significant increase in the percentage of patients treated with colistin and rifampin was noted in 2010, while the use of carbapenems and aminoglycosides was reduced. Tigecycline was used to treat a significant proportion of 2010 episodes, but was not used for purposes of comparison, since it was unavailable in 2000. Among HPBS patients, and after controlling for site of infection and severity of systemic inflammatory response, the probability of being treated with carbapenems or aminoglycosides in 2010 was lower (odds ratio [OR], 0.45; 95% [CI], 0.21–0.93; p = 0.03, and OR, 0.17; 95% CI, 0.07–0.44; p < 0001, respectively), and higher for colistin (OR, 10.12; 95% CI, 4.01–25.49; p < 0.001).
There were no differences between the 2 periods in terms of crude mortality or length of hospital stay after infection (see Table 4).
A. baumannii Infections According to Ward of Admission
To outline the clinical features of A. baumannii infections, we used pooled data from the 2000 and 2010 studies for the WSH. A. baumannii was more frequently associated with infection among ICU patients (135/211, 63.9%) compared to patients admitted to medical (56/106, 52.8%) or surgical wards (54/102, 52.9%) (p < 0.05 for both comparisons). The most frequent sites of infection in ICU patients were pneumonia (56%), tracheobronchitis (15%), and intraabdominal infections (7%), and among patients admitted to medical and surgical wards the most frequent sites were skin and skin structure infections (27% and 50%, respectively), urinary tract infections (23% and 17%), and pneumonia (23% and 11%). Crude mortality was higher in ICU patients than among medical or surgical patients (28% vs. 17% and 9%; p < 0.05). Of patients admitted to medical and surgical wards, 38% and 29%, respectively, had previously been admitted to the ICU.
Acquisition was considered as non-nosocomial but health care associated in 38 patients (35 in 2010, 3 in 2000; see Table 2); all these patients had chronic underlying conditions and previous specialized health care contact; 31 had been previously hospitalized, 10 were residents of nursing homes, 6 were receiving hemodialysis, and 2 attended a day-hospital (several had more than 1 of these expositions). Twenty-three (60.5%) were considered to be infected, and types of infections were pneumonia (6 patients), tracheobronchitis2, soft tissue infections (9, mostly secondary to chronic ulcers), urinary tract infections2, and others4.
Molecular Epidemiology and Susceptibility of A. baumannii Isolates
Using a highly discriminatory molecular typing technique such as REP-PCR, a wide clonality was evident. Concerning the isolates from the WSH, 68 REP-PCR different profiles were in 2010, and 40 profiles (16.2%) were made up of just 1 isolate. This was very similar to the situation in the acute care hospitals in 2000 (62 profiles among 183 isolates, 41 of them made up of just 1 isolate). The isolates obtained from 28 of the 38 patients (73.6%) with non-nosocomial acquisition showed a close clonal relationship to at least 1 other isolate obtained from a nosocomial case. Three REP-PCR profiles were found in more than 1 hospital: in 2000, R66 was found in 3 hospitals; in 2010, R11 was found in 2 and R31 in 5 hospitals; these results were confirmed by PFGE. These 3 REP-PCR/PFGE profiles belonged to ST2 by MSLT (see below).
Ten and twelve hospitals in 2000 and 2010, respectively, had ≥5 cases with the same REP-PCR profile; 1 profile was predominant in all of them, making up a median of 70% of isolates from the same center (range, 43%-100%). The percentage of non-ICU patients who harbored 1 of the epidemic isolates but had not previously been admitted to an ICU (thereby suggesting that A. baumannii had been acquired in a conventional ward) was 7.6% (8/104) in 2000, and 19.2% (30/156) in 2010 (p = 0.01).
Using a technique more appropriate for evaluating long-term evolution of clonality such as MSLT, 47 different STs (22 in 2000, 29 in 2010) were identified in the WSH. Predominant STs were ST2 (227 isolates [52.9%] from 25 hospitals), ST79 (46 isolates [10.7%] from 9 hospitals), ST181 (34 isolates [7.9%] from 2 hospitals), and ST179 (13 isolates [3%] from 3 hospitals). Only STs 2, 79, and 181 were found in both 2000 and 2010.
Regarding the isolates from the HPBS, the evolution of the predominant STs was as follows. The proportion of ST2 isolates increased from 42.9% in 2000 to 59% in 2010 (p = 0.01); these were found in 5 hospitals in both 2000 and 2010, in 3 hospitals in 2000 only, and in 4 in 2010 only. There was no apparent regional clustering. ST79 caused a similar percentage of cases in both 2000 and 2010 (9.1% vs. 8.4%; p = 0.9). It was isolated at 3 hospitals in the same region in 2000 and had persisted in 2 of these in 2010, when it was also found in 2 other centers located at a considerable distance from the others. ST181 was isolated in just 1 hospital in 2000 and at a different hospital in a city at some distance in 2010. Finally, ST179 was found in 2 hospitals in 2000, and in none in 2010.
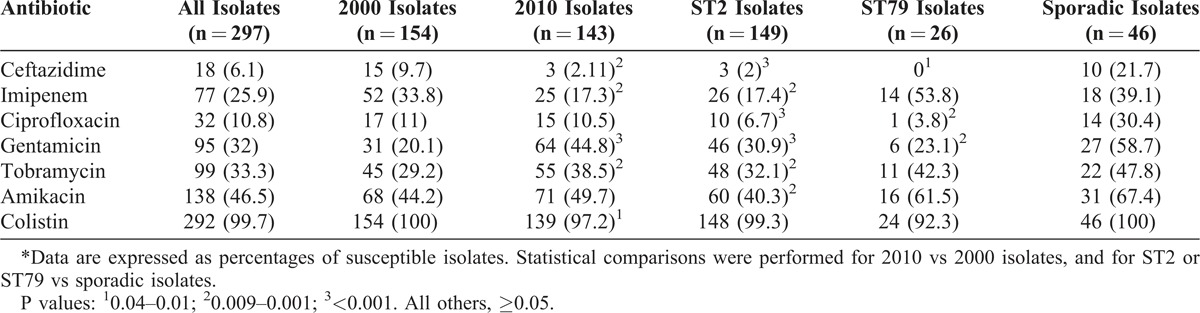
The susceptibility data of all isolates by study period, and of ST2, ST79, and sporadic isolates (for example, each causing 3 cases or less) in the HPBS are shown in Table 5. Overall, A. baumannii isolates were less frequently susceptible to ceftazidime, imipenem, and colistin and more frequently susceptible to gentamicin and tobramycin in 2010 compared to 2000; ST2 and ST79 were less frequently susceptible to ceftazidime, ciprofloxacin, and tobramycin, compared to sporadic isolates, with ST2 also being less susceptible to imipenem, tobramycin, and amikacin. ST2 and ST79 isolates were more frequently susceptible to imipenem in 2000 compared to 2010 (25.8% vs. 10.8%, p = 0.01 for ST2; and 78.6% vs. 20.5%, p = 0.006 for ST79), with the opposite being true with gentamicin (19.7% vs. 39.8%, p = 0.008 for ST2; and 7.1% vs. 41.7%, p = 0.06 for ST79). Differences among other antibiotics were not significant.
TABLE 5.
Susceptibility Data for A. baumannii Isolates From Hospitals Taking Part in Both Studies (2000 and 2010)∗
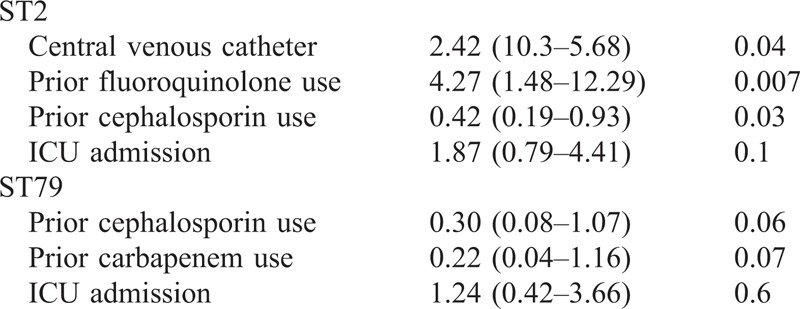
The epidemiologic and clinical features of patients harboring ST2 and ST79 isolates were compared with those of patients with sporadic sequence types. Univariate comparisons are shown in Table 6. The multivariate analysis of factors predisposing to acquisition of ST2 or ST79 is shown in Table 7. As regards outcome, sepsis and severe sepsis/septic shock were more frequent among patients with ST2 isolates; there were no differences in mortality (see Table 6). After adjusting for age, ICU admission, site of infection, and severity of underlying condition, ST2 isolates remained associated with an increased risk of sepsis (OR, 4.13; 95% CI, 1.28–13.36; p = 0.01), but not with severe sepsis/septic shock (OR, 2.06; 95% CI, 0.64–6.64; p = 0.2) or mortality (OR, 1.49; 95% CI, 0.48–4.64; p = 0.4); ST79 was not associated with sepsis (OR, 1.66; 95% CI, 0.33–8.37; p = 0.5), severe sepsis/septic shock (OR, 2.17; 95% CI, 0.42–12.13; p = 0.3), or mortality (OR, 0.33; 95% CI, 0.02–4.22; p = 0.3).
TABLE 6.
Exposure to Predisposing Factors and Clinical Features of Patients With A. baumannii Isolates Belonging to ST2, ST79, or Sporadic Clones in Hospitals Participating In Both Studies∗
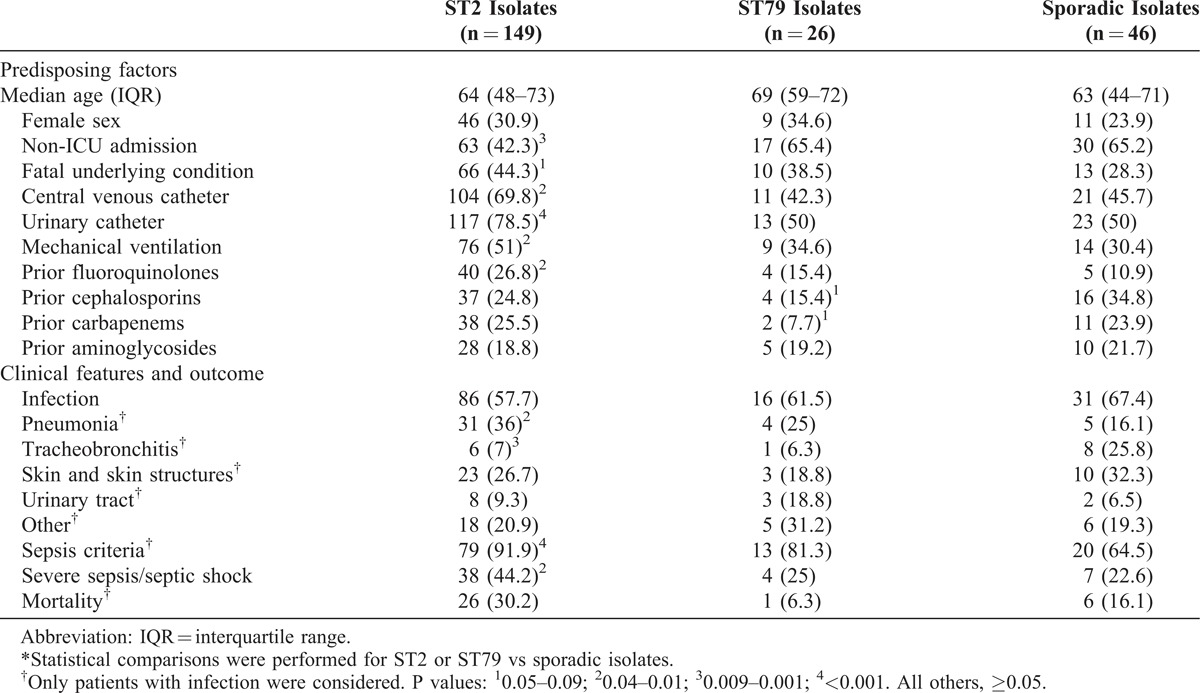
TABLE 7.
Multivariate Analysis of Risk Factors for Acquisition of A. baumannii ST2 or ST79
DISCUSSION
The current study provides comprehensive information about the general clinical and molecular epidemiology of A. baumannii, its clinical features, and the changes that occurred over a 10-year interval in a wide sample of Spanish hospitals.
Of note, the incidence density rate of A. baumannii decreased; this was specifically associated with lower rates in the ICUs, which could be linked to more active infection control in this kind of unit. Nonetheless, incidence density increased in medical services, which, together with the molecular typing data, indicates that, in many hospitals, this organism is now spreading within medical wards. Molecular typing showed that some clones are spreading from patients who acquired the organism in the ICU and were then transferred to a medical ward, and also that epidemic clones were found exclusively in medical or surgical wards in more hospitals than was expected, suggesting that these wards are now primary reservoirs for them. The influx and spread of A. baumannii clones from the ICU to conventional wards has been shown previously in specific centers, and was suspected in a study including 3 hospitals in Israel where molecular typing was not performed21; to our knowledge, the current study is the first to use molecular typing techniques across a wide sample of centers in order to demonstrate the magnitude of the influx. The spread of epidemic clones to conventional wards represents a significant challenge for infection control activities, since the reservoirs in these wards may be diverse, including environmental surfaces and colonized patients; in situations of this complexity, only a comprehensive “bundle” approach to interventions maintained over long periods of time may be successful.2,26 It is worth mentioning that, according to a recent survey, infection control measures against A. baumannii are not applied uniformly, particularly in non-ICU wards.14 Physicians attending patients in medical and surgical wards should be aware that skin and skin structure and urinary tract infections are the most frequent infections caused by A. baumannii in those wards, rather than pneumonia, which is the infection most frequently found in ICU patients.
Another aspect of interest is the significant increase in non-nosocomial cases. Both clinical epidemiology and molecular data suggest that the organism was acquired during previous contact with the health care system. Significantly, some of these patients were residents in long-term care centers. Recently, in specific areas, nursing homes and post-acute care facilities have been seen to play an important role in the epidemiology of A. baumannii,22,29 which increases the complexity of controlling it when this kind of center is involved.6
With regard to predisposing factors, we found some important changes in previous exposure to antibiotics. In 2010, more patients had previously received piperacillin/tazobactam or carbapenems and fewer had received cephalosporins or aminoglycosides; this could be due to changes in the epidemiology of resistance among gram-negative bacilli, and particularly the spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae.27 Also, some of these changes in antimicrobial exposure were consistent with the fact that 2010 isolates were more frequently resistant to carbapenems and less frequently to aminoglycosides, as previously reported.12
Significant changes in the treatment of A. baumannii infections were noted as a result of the increase in the rate of carbapenem resistance (which is probably associated with the spread of ST2); colistin has replaced carbapenems as the most frequently used drug. Tigecycline is frequently used now, usually in combination, which may explain the reduced use of aminoglycosides. In addition, rifampin or sulbactam was used to treat some patients in 2010. Whether combination therapy is more effective is a controversial issue; a recent randomized controlled trial found that colistin plus rifampicin was not superior to colistin alone, although the rate of eradication was higher.10 It is noted that the dose used in that trial (2 MU bid) was lower than the dose recommended now (3 MU bid after a loading dose). These data indicate an urgent need for new studies and new drugs.
A wide clonal diversity was found when studied by REP-PCR, a highly discriminatory technique, which was useful to evaluate transmission within specific areas in each period. However, MLST, a technique used to investigate the long-term evolutionary relationships of organisms, showed a single predominant clonal group, ST2, which included >50% of the isolates. These results were very similar to those obtained in a previous study of 729 epidemic strains received by the Spanish reference laboratory for the molecular investigation of outbreaks (Centro Nacional de Microbiología, Instituto de Salud Carlos III) between 1997 and 2007.33 ST2 corresponds to the clone known as international clone II, the most prevalent in other countries of southern Europe.8,9 These results underline the importance of using highly discriminatory techniques, such as REP-PCR or PFGE, for short-term investigations of the local epidemiology of A. baumannii, while MLST provides a comprehensive view of the epidemiology in wider scenarios. Our results suggest that ST2, associated with increasing carbapenem resistance, is spreading further, and, in fact, the overall increase in carbapenem resistance seems to be related to the development of resistance in strains belonging to the dominant clonal groups, ST2 and ST79. Of note, although more studies would be needed, exposure to fluoroquinolones seems to be associated with an increased risk of harboring an ST2 isolate. The prudent use of fluoroquinolones may therefore be regarded as an extra measure for avoiding the further spread of ST2.
Finally, ST2 was associated with an increased risk of sepsis (although not of severe sepsis or septic shock). Because it is difficult to control for all potential confounders, we cannot assign ST2 greater clinical virulence. As this clonal group is associated with increased antimicrobial resistance, it suggests that the further acquisition of resistance genes gives ST2 a survival advantage, and at least it did not reduce the clinical virulence, probably due to the genomic plasticity of A. baumannii.17
The current study has some limitations. First, although both 2000 and 2010 studies included a large number of hospitals, they are not representative of Spanish hospitals as a whole, and not all centers took part in both studies. Second, since the study periods were of limited duration, the number of cases from some hospitals was low; also, the studies were performed during different months so that seasonality may have had some influence. Finally, it was sometimes difficult to decide whether the patients were colonized or infected, despite the careful application of clinical and microbiologic criteria. The strengths of our study include the fact that it was prospective and multicenter in nature, reference techniques were used to identify A. baumannii, and clinical data were integrated with molecular typing.
In conclusion, some important changes were noted in the epidemiology of A. baumannii. First, a reduction in the ICU rates or an increase in the acquisition in conventional wards (particularly in medical units) and in non-nosocomial, health care-associated infections was evident. Second, some changes in the predisposing features of the patients were noted, with a decrease in the exposure to some invasive procedures and an increase in previous use of carbapenems, which is probably related to the fact that carbapenem-resistance also significantly increased. And third, colistin has replaced carbapenems as the cornerstone of therapy, but resistance to colistin is increasing. Finally, a successful clonal group was shown to be responsible for about half of infections, and it seems to combine antimicrobial resistance and the ability to spread, while maintaining its clinical virulence.
ACKNOWLEDGMENTS
The authors thank the Platform for Genotyping of Pathogens and Public Health (Institut Pasteur, Paris, France) for coding MLST alleles and profiles as available at www.pasteur.fr/mlst. The authors are grateful for the support of the Spanish Group for Nosocomial Infections (GEIH) and the Spanish Group for Antimicrobial Mechanisms of Action and Resistance (GEMARA) from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) for their support in the development of the project.
APPENDIX. PARTICIPANTS OF THE GEIH/GEMARA/REIPI-Ab2010 GROUP
Other participants from the GEIH/GEMARA/REIPI-Ab2010 group are as follows: Hospital SAS La Línea (Antonio Sánchez Porto, Luis Vallejo), Complejo hospitalario de Ourense (Begoña Fernández Pérez, José Carlos Villar Chao), Hospital Gregorio Marañón (Belén Padilla Ortega, Emilia Cercenado Mansilla), Hospital Virgen del Rocío (JA Márquez Vácaro, A Gutiérrez-Pizarraya), Hospital de Navarra (José Javier García Irure), Hospital Costa del Sol-Marbella (Alfonso del Arco Jiménez, Javier de la Torre Lima), Hospital General de Valencia (Concepción Gimeno Cardona, Vicente Abril), Consorci Hospitalari de Vic (Joseph Vilaró Pujals, Marian Navarro Aguirre), Policlínica Guipúzkoa (José Antonio Jiménez Alfaro, Carlos Reviejo Jaca), Hospital Puerta del Mar (Pilar Marín Casanova, Francisca Guerreo), Complejo Hospitalario de Soria (Teresa Nebreda Mayoral, María José Fernández Calavia), Hospital Universitario de Alicante (Esperanza Merino de Lucas, Alfredo Zorraquino), Hospital Infanta Cristina (Eugenio Garduño Eseverri, Luis López Sánchez), Hospital Universitario Central de Asturias (Ana Fleites Gutiérrez, Azucena Rodríguez Guardado), Hospital Donostia (José María García-Arenzana Anguera), Complejo Hospitalario Torrecárdenas (Serafín López Palmero, Manuel Rodríguez Maresca), Complejo Hospitalario Xeral-Calde Lugo (Fernando García Garrote, José Varela Otero), Hospital Universitario Reina Sofía de Córdoba (Elisa Vidal Verdú, Fernando Rodríguez López), Hospital Universitario de Santiago Compostela (Fernanda Pardo Sánchez, E. Ferrer Vizoso), Hospital Sant Pau (Mercé Gurgui, Roser Pericas), Hospital Galdakao-Usansolo (Pedro María Olaechea Astigarraga, Rafael Ayarza Igartua), Hospital Son Dureta (María Dolores Maciá Romero, Enrique Ruiz de Gopegui Bordes), Hospital Puerta de Hierro (María Isabel Sánchez Romero), Hospital Juan Grande (Jesús García Mata, María José Goyanes), Hospital San Cecilio (José Hernández Quero, Trinidad Escobar Lara), Hospital Sant Joan de Reus (Frederic Ballester Bastardie, Simona Iftimie), Hospital de Motril (María Isabel Galán Navarro, María Luz Cádiz Gurrea), Hospital San Agustín de Linares (Carmen Amores Antequera, Montserrat Gómez), Hospital de Granollers (Carmina Martí Salas, Jordi Cuquet Peragosa), Hospital de Segovia (Susana Hernando Real, Pablo A. Carrero González), Complejo Hospitalario de Pontevedra (María Angeles Pallarés González, Sergio Rodríguez Fernández), Hospital de Bellvitge (Miquel Pujol Rojo, Fe Tubau), Hospital Virgen de la Victoria de Málaga (Enrique Nuño Alvarez, María Ortega Torres), Hospital Doctor Moliner (Salvador Giner Almaraz, Elena Hortelano), Hospital 12 de Octubre (Fernando Chaves Sánchez, Ana García Reyne), Hospital del Mar (Juan Pablo Horcajada Gallego, Concha Segura), Hospital San Agustín de Avilés (Gema Sierra Dorado, Raquel Yano Escudero), Complejo Hospitalario Materno Insular de Gran Canaria (María Elena Dorta Hung, Cristóbal del Rosario).
Footnotes
Abbreviations: CI = confidence interval, HPBS = hospitals participating in both studies, ICU = intensive care unit, MLST = multilocus sequence typing, OR = odds ratio, PCR = polymerase chain reaction, PFGE = pulsed-field gel electrophoresis, REP-PCR = repetitive extragenic palindromic PCR, ST = sequence type, WSH = whole set of hospitals.
MV and MEC contributed equally to this study.
Financial support and conflicts of interest: The study was funded by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, cofinanced by European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008), and FIS (PI 10/00056 and PI 11/02046). LMM has been a consultant for Pfizer; has served as speaker for Merck, Pfizer, Janssen-Cilag, and Astra-Zeneca; and has received research support from Merck, Wyeth, Janssen-Cilag, and Astra-Zeneca. JGM has served as speaker for Astellas, Merck, and Pfizer. AP has been a consultant for Merck and Pfizer; has served as speaker for Wyeth, Astra-Zeneca, Merck, and Pfizer; and has received research support from Merck, Pfizer, and Wyeth. JRB has been a consultant for Wyeth, Merck, and Pfizer; has served as speaker for Wyeth, Merck, Pfizer, Astra-Zeneca, and GlaxoSmithKline; and has received research support from Merck and Wyeth.
Contributor Information
Collaborators: the GEIH/GEMARA/REIPI-Ab20101 Group
References
- 1.Abbo A, Navon-Venezia S, Hammer-Muntz O, et al. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005;11:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. Thammasat University Pandrug-Resistant Acinetobacter baumannii Control Group. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008;47:760–767. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, et al. ESKAPE Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Cisneros JM, Rodriguez-Bano J, Fernandez-Cuenca F, et al. Risk factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain. A nationwide study. Clin Microbiol Infect. 2005;11:874–879. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and CLSI. Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing–20th Informational Supplement. Approved Standard M100-S20. Wayne, PA:NCCLS; 2010. [Google Scholar]
- 6.de Medina T, Carmeli Y. The pivotal role of long-term care facilities in the epidemiology of Acinetobacter baumannii: another brick in the wall. Clin Infect Dis. 2010;50:1617–1618. [DOI] [PubMed] [Google Scholar]
- 7.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nature Rev Microbiol. 2007;5:939–951. [DOI] [PubMed] [Google Scholar]
- 8.Di Popolo A, Giannouli M, Triasi M, et al. Molecular epidemiological investigation of multi-drug resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin Microbiol Infect. 2011;17:197–201. [DOI] [PubMed] [Google Scholar]
- 9.Drancourt L, Passet V, Nemec A, et al. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57:349–358. [DOI] [PubMed] [Google Scholar]
- 11.Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez Cuenca F, Tomas Carmona M, Caballero Moyano F, et al. Actividad de 18 agentes antimicrobianos frente a aislados clinicos de Acinetobacter baumannii: segundo estudio nacional multicentrico (proyecto GEIH-REIPI-Ab 2010). Enferm Infecc Microbiol Clin. 2013;31:4–9. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez Cuenca F, Pascual A, Ribera A, et al. Diversidad clonal y sensibilidad a los antimicrobianos de Acinetobacter baumannii aislados en hospitales Espanoles. Estudio multicentrico nacional: proyecto GEIH-Ab 2000. Enferm Infecc Microbiol Clin. 2004;22:267–271. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Ortega L, Arch O, Perez-Canosa C, et al. Control measures for Acinetobacter baumannii: a survey from Spanish hospitals. Enferm Infecc Microbiol Clin. 2011;29:36–38. [DOI] [PubMed] [Google Scholar]
- 15.Higgins PG, Dammhayn C, Hackel M, et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. [DOI] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 17.Imperi F, Antunes LC, Blom J, et al. The genomics of Acinetobacter baumannii: insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life. 2011;63:1068–1074. [DOI] [PubMed] [Google Scholar]
- 18.Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11:30–38. [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 20.McCabe WR, Jackson GG. Gram-negative bacteremia I. Etiology and ecology. Arch Intern Med. 1962;110:847–855. [Google Scholar]
- 21.Paul M, Weinberger M, Siegman-Igra Y, et al. Acinetobacter baumannii: emergence and spread in Israeli hospitals 1997-2002. J Hosp Infect. 2005;60:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65:1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez F, Ponce-Terashima R, Adams MD, et al. Are we closing in on an “elusive enemy”? The current status if our battle with Acinetobacter baumannii. Virulence. 2011;2:86–90. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Bano J, Bonomo RA. Multidrug-resistant Acinetobacter baumanni: “eyes wide shut”? Enferm Infecc Microbiol Clin. 2008;26:185–186. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, et al. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25:819–824. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide endemic multidrug-resistant (MDR) Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Bano J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther. 2008;6:671–683. [DOI] [PubMed] [Google Scholar]
- 28.Seifert H, Dolzani L, Bressan R, et al. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J Clin Microbiol. 2005;43:4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sengstock DM, Thyagarajan R, Apalara J, et al. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010;50:1611–1616. [DOI] [PubMed] [Google Scholar]
- 30.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaneechoutte M, Dijkshoorn L, Tjernberg I, et al. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1995;33:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villalon P, Valdezate S, Medina-Pascual MJ, et al. Clonal diversity of nosocomial epidemic Acinetobacter baumannii isolated in Spain. J Clin Microbiol. 2011;49:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villegas MV. Acinetobacter outbreaks 1977-2000. Infect Control Hosp Epidemiol. 2003;24:284–295. [DOI] [PubMed] [Google Scholar]


