Abstract
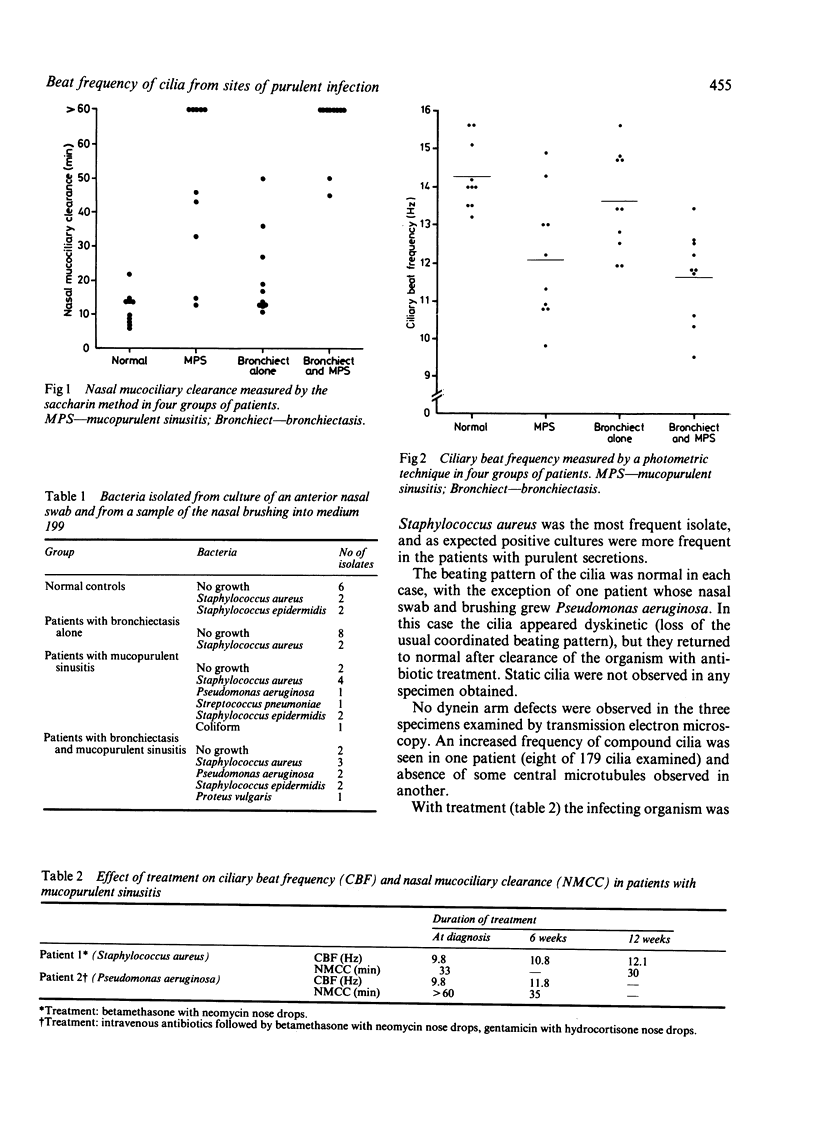
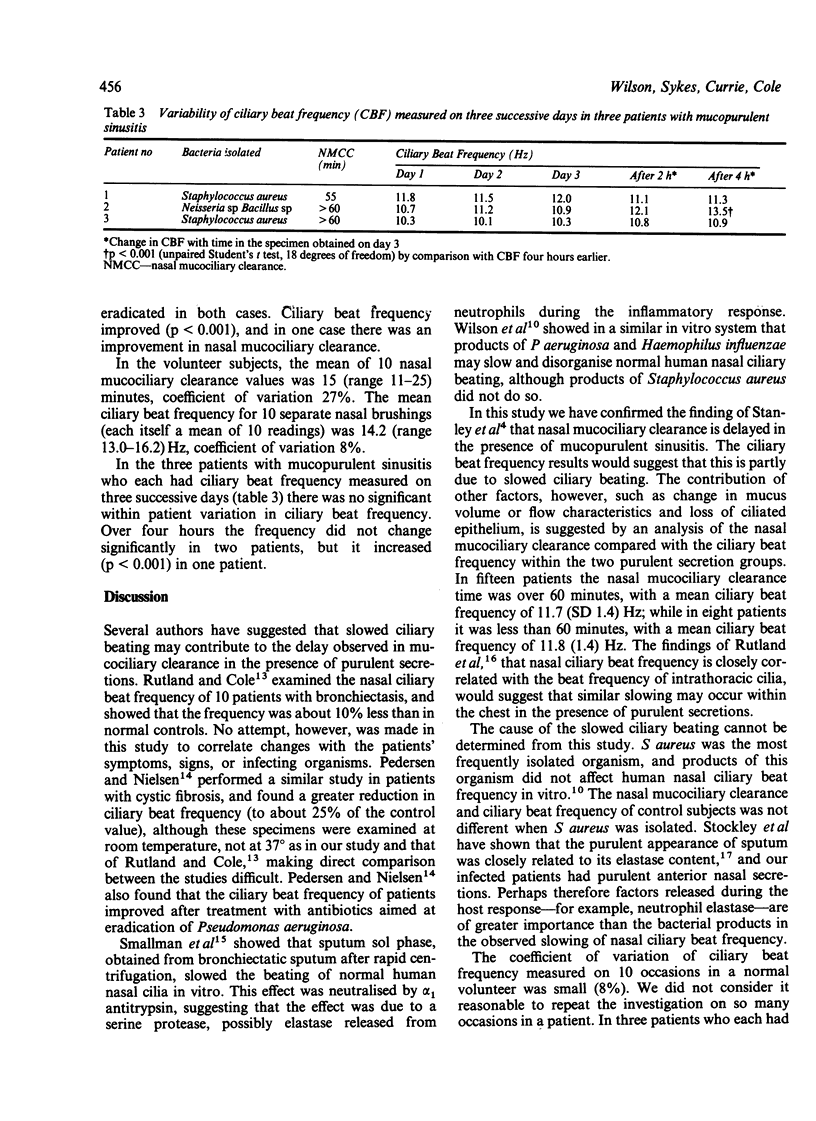
Mucociliary clearance depends on the interaction between cilia and mucus; it is delayed in the presence of purulent secretions. Nasal mucociliary clearance was examined by the saccharin method and nasal ciliary beat frequency by a photometric technique. Four groups were studied: normal controls, patients with bronchiectasis without nasal symptoms, patients with chronic mucopurulent sinusitis alone, and patients with chronic mucopurulent sinusitis and bronchiectasis. Nasal mucociliary clearance was prolonged in infected patients. Cilia obtained from the site of purulent secretions were found to beat more slowly in vitro (mucopurulent sinusitis 12.1 Hz, mucopurulent sinusitis and bronchiectasis 11.6 Hz), than those obtained from normal controls (14.3 Hz) and from patients with bronchiectasis alone (13.6 Hz). The cause of the ciliary slowing seemed most likely to be the release of host factors during the inflammatory response, rather than the particular organism isolated. Ciliary slowing may contribute to the observed delay of mucociliary clearance in conditions in which purulent secretions are present.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürgi H. Fibre systems in sputum. Bull Physiopathol Respir (Nancy) 1973 Mar-Apr;9(2):191–198. [PubMed] [Google Scholar]
- Dulfano M. J., Adler K. B. Physical properties of sputum. VII. Rheologic properties and mucociliary transport. Am Rev Respir Dis. 1975 Sep;112(3):341–347. doi: 10.1164/arrd.1975.112.3.341. [DOI] [PubMed] [Google Scholar]
- Kollberg H., Mossberg B., Afzelius B. A., Philipson K., Camner P. Cystic fibrosis compared with the immotile-cilia syndrome. A study of mucociliary clearance, ciliary ultrastructure, clinical picture and ventilatory function. Scand J Respir Dis. 1978;59(6):297–306. [PubMed] [Google Scholar]
- Lourenço R. V., Loddenkemper R., Carton R. W. Patterns of distribution and clearance of aerosols in patients with bronchiectasis. Am Rev Respir Dis. 1972 Dec;106(6):857–866. doi: 10.1164/arrd.1972.106.6.857. [DOI] [PubMed] [Google Scholar]
- Reimer A., von Mecklenburg C., Toremalm N. G. The mucociliary activity of the upper respiratory tract. III. A functional and morphological study on human and animal material with special reference to maxillary sinus diseases. Acta Otolaryngol Suppl. 1978;356:1–20. [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981 Sep;36(9):654–658. doi: 10.1136/thx.36.9.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Rutland J., Griffin W. M., Cole P. J. Human ciliary beat frequency in epithelium from intrathoracic and extrathoracic airways. Am Rev Respir Dis. 1982 Jan;125(1):100–105. doi: 10.1164/arrd.1982.125.1.100. [DOI] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. J., Griffin W. M., Wilson R., Greenstone M. A., Mackay I. S., Cole P. J. Effect of betamethasone and betamethasone with neomycin nasal drops on human nasal mucociliary clearance and ciliary beat frequency. Thorax. 1985 Aug;40(8):607–612. doi: 10.1136/thx.40.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. J., Wilson R., Greenstone M. A., Mackay I. S., Cole P. J. Abnormal nasal mucociliary clearance in patients with rhinitis and its relationship to concomitant chest disease. Br J Dis Chest. 1985 Jan;79(1):77–82. doi: 10.1016/0007-0971(85)90010-5. [DOI] [PubMed] [Google Scholar]
- Stanley P., MacWilliam L., Greenstone M., Mackay I., Cole P. Efficacy of a saccharin test for screening to detect abnormal mucociliary clearance. Br J Dis Chest. 1984 Jan;78(1):62–65. [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M., Starkie C. M. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984 Jun;39(6):408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Wanner A., Hirsch J., Farrell P. M. Tracheal mucociliary transport in patients with cystic fibrosis and its stimulation by terbutaline. Am Rev Respir Dis. 1975 Jun;111(6):733–738. doi: 10.1164/arrd.1975.111.6.733. [DOI] [PubMed] [Google Scholar]


