Abstract
Hematopoietic stem cell transplantation (HSCT) recipients lacking HLA-matched related donors have increased graft-vs.-host disease (GVHD) and non-relapse mortality (NRM). Bortezomib added to reduced-intensity conditioning can offer benefit in T-replete HLA-mismatched HSCT, and may also benefit myeloablative conditioning (MAC) transplants. We conducted a phase II trial of short-course bortezomib plus standard tacrolimus/methotrexate after busulfan/fludarabine MAC in 34 patients with predominantly myeloid malignancies. Fourteen (41%) received 8/8 HLA-matched unrelated donor (MUD) and 20 (59%) received 7/8 HLA-mismatched related/unrelated donor (MMRD/MMUD) peripheral blood stem cell grafts. Median age was 49 years (range, 21–60) and median follow-up was 25 months (range, 11–36). The regimen was well tolerated. No dose-modifications were required. Neutrophil and platelet engraftment occurred at a median of 14 (range, 10–33) and 17 (range, 10–54) days respectively. Median 30-day donor chimerism was 99% (range, 90–100). 100-day grade II-IV and III-IV acute GVHD incidence was 32% and 12% respectively. One-year chronic GVHD incidence was 50%. Two-year cumulative incidence of both NRM and relapse was 16%. Two-year progression-free and overall survival was 70% and 71% respectively. Outcomes were comparable to an 8/8 MUD MAC cohort (n=45). Immune reconstitution was robust. Bortezomib-based MAC HSCT is well tolerated, with HLA-mismatched outcomes comparable to 8/8 MUD MAC HSCT, and is suitable for randomized evaluation.
Keywords: HLA-mismatch, proteasome-inhibitor, allogeneic, myeloablative, transplantation, t-cell replete
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is potentially curative in advanced or aggressive hematologic malignancies. While a sibling donor matched at HLA-A, -B, -C, and – DRB1 is optimal, only ~30% of patients who may benefit from HSCT have such a donor available. 1 The likelihood of finding a matched unrelated donor (MUD) varies between racial and ethnic groups. Accepting a 7/8 HLA-match increases the likelihood of identifying an adult donor for all; from the highest likelihood group (whites of European descent (75%→97%)), to the lowest likelihood group (blacks of South or Central American descent (16%→66%)),1 but at the expense of worse outcomes. In myeloablative conditioning (MAC) HSCT, observational studies comparing 7/8 vs. 8/8 HLA-matched unrelated donors document an increased rate of 100-day severe grade III-IV acute GVHD (37% vs. 28%) and 1–2 year NRM (34–45% vs. 22–36%), with worse PFS (38–41% vs. 47–52%) and OS (41–43% vs. 52–54%). 2–4
The proteasome-inhibitor bortezomib can selectively deplete proliferating alloreactive T lymphocytes, reduce Th1 cytokines, and block APC activation.5,6 Bortezomib may also spare regulatory T cells (Treg) that may be relevant in GVHD control.7 Administered early after stem cell infusion, bortezomib can control GVHD in MHC-mismatched mouse HSCT and maintain therapeutic graft-versus-tumor responses,8–10 while avoiding the severe colonic toxicity that delayed or prolonged bortezomib administration can induce in mice.9
In a previous study of T-replete reduced-intensity conditioning (RIC) we showed that a bortezomib-based GVHD prophylaxis regimen (day +1, +4, +7 plus standard-of-care tacrolimus/methotrexate (tac/mtx)) appeared safe and efficacious in HLA-mismatched HSCT, comparable to HLA-matched transplantation. 11,12 Prospective randomized controlled trials of bortezomib-based T-replete RIC HSCT are ongoing at the national level (BMTCTN 1203). We therefore undertook a phase II trial to determine whether bortezomib-based GVHD prophylaxis is also effective with the more cytotoxic conditioning regimen intensity in MAC HSCT using matched unrelated and mismatched donors.
Patients and Methods
This prospective single-arm phase II trial was approved by the institutional review board of the Dana-Farber Cancer Institute/Harvard Cancer Center (DFCI 11-007). Written informed consent was obtained prior to enrollment.
Trial Cohort
participants with various hematologic malignancies aged 18–60 years and lacking a timely 8/8 HLA-matched (-A, -B, -C, -DRB1) related donor (MRD) received an 8/8 HLA-matched unrelated (MUD) or a 1-locus mismatched related or unrelated donor (MMRD, MMUD). Participants with HIV infection, active hepatitis B or C, abnormal renal (serum creatinine >ULN, creatinine clearance <60 ml/min) or pulmonary (FEV1, FVC or DLCO <60%) or hepatic function (serum total bilirubin >ULN, serum ALT or AST >2x ULN), ECOG performance status >2, uncontrolled infections, peripheral neuropathy ≥grade 2 within 21 days prior, or history of seizures were excluded. Enrollment time period was 2011–2012 and dataset was locked May 1, 2014. MAC comprised fludarabine (40 mg/m2 IV) and busulfan (130 mg/m2 IV, without PK dose adjustment) daily on days -7, -6, -5, -4. Target unmanipulated peripheral blood stem cell (PBSC) dose was ≥2×106 CD34+ cells/kg. GVHD prophylaxis comprised tacrolimus (starting day -3, to achieve a target serum level of 5–10 ng/ml); methotrexate (15 mg/m2 IV on days +1, 10 mg/m2 IV on days +3, +6, +11); and bortezomib (1.3 mg/m2 IV on days +1, +4 and +7, in accordance with the standard 72-hour bortezomib dose-interval). Tacrolimus taper commenced day +100, with the goal to be off immune suppression by day +180 in the absence of GVHD.
Standard-of-Care Comparator Cohort
Clinical outcomes were also obtained for all adult hematologic malignancy patients (n=45) undergoing off-protocol 8/8 MUD PBSC MAC HSCT at our center between 2010–2012 with standard-of-care tac/mtx prophylaxis dosed similar to the study cohort (tacrolimus starting day -3; methotrexate on days +1, +3, +6, and ±11). Tacrolimus taper routinely commenced around week 9, with the goal to be off immune suppression by 6 months in the absence of GVHD. HSCT eligibility criteria were similar to those above. MAC comprised cyclophosphamide/TBI. Target PBSC dose was ≥2×106 CD34+ cells/kg. Median follow-up in survivors was 36 months (range, 13–49).
Supportive care
Participants received filgrastim 5 µg/kg daily from day +12 until an absolute neutrophil count (ANC) >1000 cells/µl was attained, and at least 12 months of Pneumocystis jiroveci and HSV/VZV prophylaxis. Anti-fungal prophylaxis was not routine.
Immune reconstitution assays
Immune reconstitution assays: CD4+ T cells were defined as CD3+CD4+; CD4+ naïve cells were defined as CD4+, CD45RO−; CD4+ memory cells were defined as CD4+CD45RO+; CD8+ T cells were defined as CD3+CD8+; CD8+ naïve cells were defined as CD8+CD45RO−CD62L+; CD8+ memory cells were defined as CD8+CD45RO+; CD8+ terminal effector cells were defined as CD8+CD45RO−CD62L−; CD4 regulatory T cells (Treg) were defined as CD3+CD4+CD25med-highCD127low; NK cells as CD56+CD3−; and B cells as CD19+. Fifty μl whole blood (15% EDTA) in 5 ml polystyrene round-bottom reaction tubes was incubated with fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1, BD Biosciences), anti-CD4 APC-H7 (clone RPA-T4, BD Biosciences), anti-CD8 Pacific-Orange (clone RPA-T8, Biolegend), anti-CD25 PE-Cy7 (clone M-A251, BD Biosciences), anti-CD127 PE-Cy5 (clone eBioRDR5, eBioscience), anti-CD62L APC (clone DREG-56, BD Biosciences), CD45RO FITC (clone UCHL1, BD Biosciences) for T cell subsets; anti-CD56 PE (clone B159, BD Biosciences), anti-CD3 V450 (clone UCHT1, BD Biosciences) for NK/NKT cells; anti-CD19 APC (clone HIB19, BD Biosciences) for B cells. RBC lysis with 500 μl 1× BD Pharm Lyse followed. Immune reconstitution flow cytometry analysis utilized FACSCanto II (BD Bioscience) and FACSDiva software (BD Bioscience).
Statistical considerations
Baseline characteristics were reported descriptively. Neutrophil and platelet engraftment was the number of days to absolute neutrophil count (ANC) ≥500 cells/µl and platelet count ≥20,000 cells/µl respectively, in the absence of transfusions. Acute GVHD was graded per the consensus grading system.13 PFS was measured from the date of stem cell infusion to disease relapse/progression or death. Patients alive without disease relapse/progression were censored at the time last seen alive and progression-free. OS was measured from the date of stem cell infusion to death from any cause. Patients alive or lost to follow-up were censored at the time last seen alive. PFS and OS were estimated by the method of Kaplan-Meier. The log-rank test was used for comparisons of Kaplan-Meier curves. Cumulative incidence of GVHD was constructed reflecting time to relapse or death without GVHD as a competing event. Cumulative incidence of non-relapse mortality and relapse with or without death were constructed reflecting time to relapse and time to non-relapse death respectively as competing risks. Difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method.14 Immunologic parameters were analyzed descriptively and compared using the exact Wilcoxon-rank-sum test. All testing was two-sided at the significance level of 0.05 and multiple comparisons were not adjusted. All calculations were done using SAS 9.3 (SAS Institute Inc, Cary, NC), and R version 2.13.2 (the CRAN project).
Results
Study Cohort
Thirty-four participants enrolled in the phase II study. Baseline characteristics including diagnoses and disease risk index (DRI) are presented in Table 1. The majority (n=27, 79%) had myeloid disease. The median participant age was 49 years (range, 21–60). The majority received mismatched grafts. Fourteen participants (41%) received 8/8 HLA-matched unrelated (MUD) grafts, and 20 (59%) received 1-locus HLA-mismatched grafts (18 MMUD, 2 MMRD) (Supplementary Table A). The median follow-up time among survivors is 25 months (range, 11–36).
Table 1.
Baseline characteristics of the bortezomib study cohort
| N | % | |
|---|---|---|
| Median Age in Years (range) | 49 | (21, 60) |
| Age>=50 | 16 | 47.1 |
| Patient Sex | ||
| M | 14 | 41.2 |
| F | 20 | 58.8 |
| Donor Sex | ||
| M | 19 | 55.9 |
| F | 15 | 44.1 |
| Male Patient & Female Donor | 4 | 11.8 |
| HLA typing at -A,-B,-C, -DRB1 | ||
| 8/8 Matched Unrelated (MUD) | 14 | 41.2 |
| 7/8 Unrelated (MMUD) | 18 | 52.9 |
| mismatch locus: | ||
| -A | 8 | |
| -B | 1 | |
| -C | 6 | |
| -DRB1 | 3 | |
| 7/8 Related (MMRD) | 2 | 5.9 |
| mismatch locus : | ||
| -A | 1 | |
| -DRB1 | 1 | |
| Diagnosis | ||
| AML | 17 | 50 |
| CML | 1 | 2.9 |
| MM/PCD | 1 | 2.9 |
| ALL | 2 | 5.9 |
| MDS | 6 | 17.6 |
| MPD | 3 | 8.8 |
| NHL | 4 | 11.8 |
| Graft Source | ||
| PBSC | 34 | 100 |
| GVHD prophylaxis | ||
| Tac/Bort/MTX | 34 | 100 |
| Patient or Donor CMV seropositivity | ||
| Yes | 29 | 85.3 |
| Disease Risk Index | ||
| Low | 1 | 2.94 |
| Intermediate | 24 | 70.59 |
| High | 9 | 26.47 |
| HCT -CI | ||
| 0 | 15 | 44.1 |
| 1–2 | 9 | 26.5 |
| ≥3 | 10 | 29.4 |
One participant died on day 11 prior to engraftment. For the remainder, the median time to neutrophil and platelet engraftment time was 14 days (range, 10–33) and 17 days (range, 10–54) respectively. Total nucleated cell (TNC) donor chimerism by day 30 (D30) was 99% (range, 90–100) and by day 100 (D100) was also 99% (range, 73–100). The regimen was well tolerated. No bortezomib doses were missed or reduced due to toxicity. No SAE attributable to bortezomib (e.g. neuropathy) was documented. Non-hematologic toxicities, with organ dysfunction (hepatic, renal, pulmonary) and metabolic/endocrine abnormalities were anticipated after MAC HSCT. None experienced hepatic VOD.
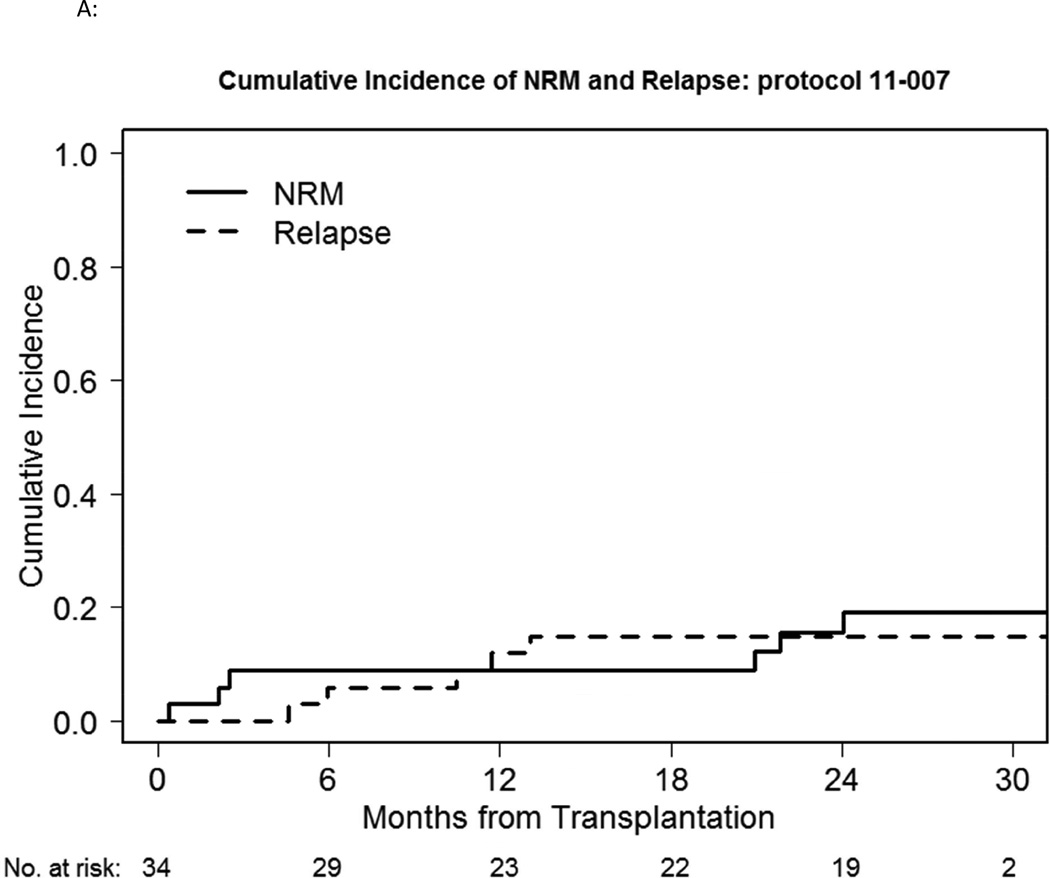
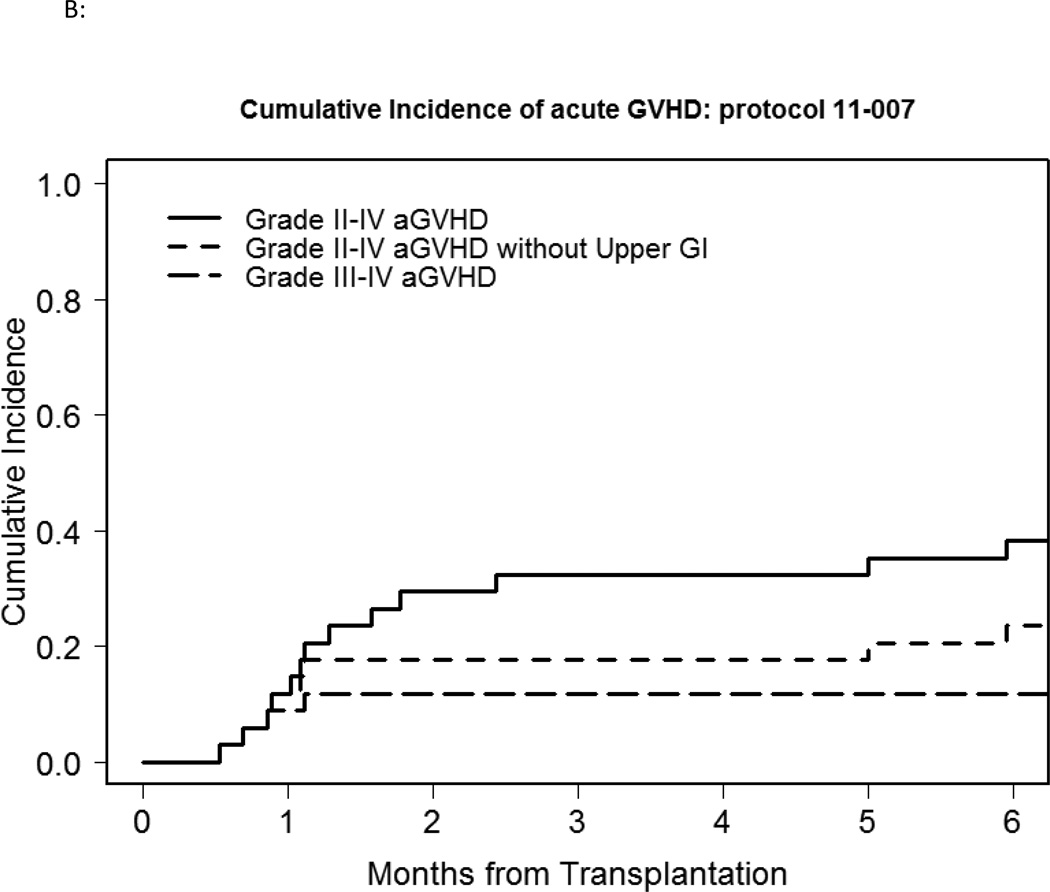
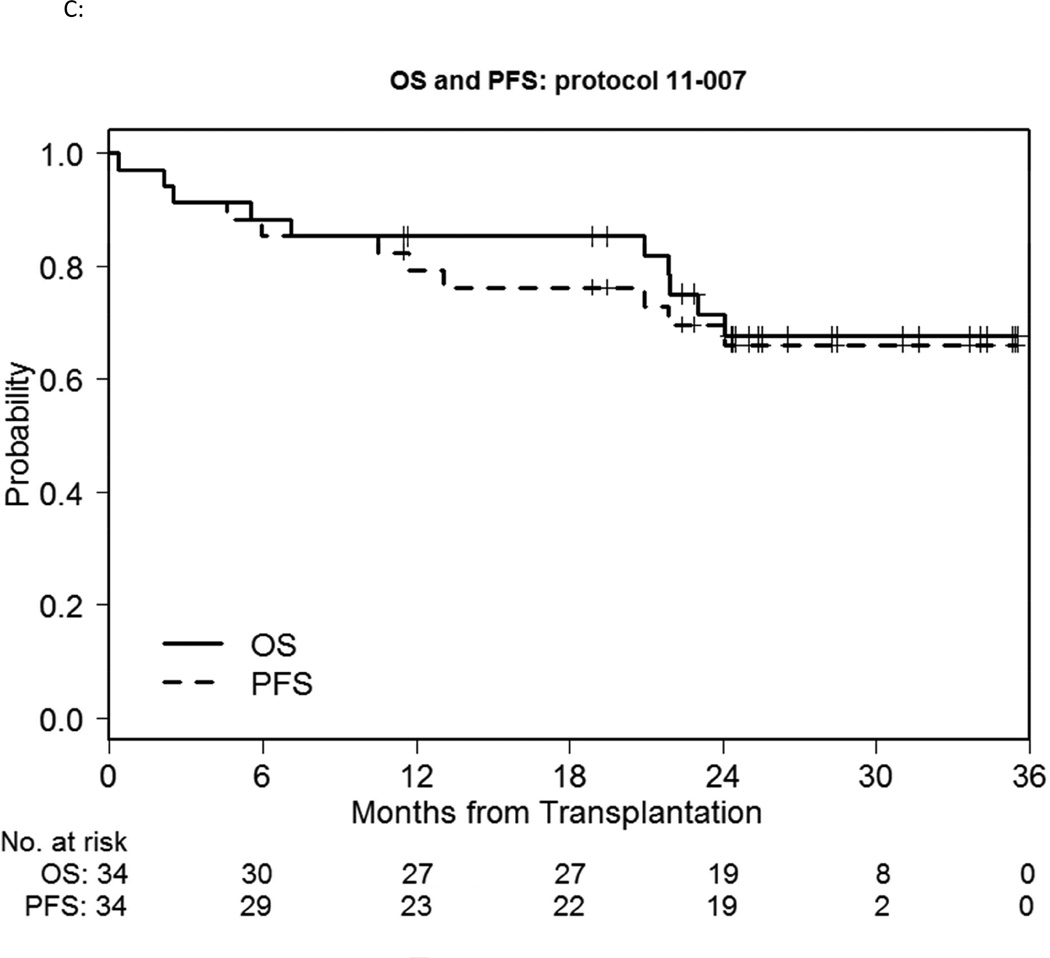
Six participants died without evidence of disease relapse/progression, a 2-year cumulative NRM incidence of 16% (Figure 1A). Three died of infection: one participant each with enterococcus/aspergillus infection; pneumonia/respiratory failure; gram negative rod/gram positive cocci sepsis, all non-attributable to bortezomib. Five participants relapsed, a 2-year cumulative incidence of relapse of 16% (Figure 1A). GVHD incidence was low, with 3 deaths. Grade II-IV acute GVHD occurred in 13 participants, 5 of whom experienced isolated upper GI GVHD (GI stage 1, overall grade II). The median time of grade II-IV acute GVHD onset was 34 days (range, 16–181), with a 100-day and 180-day cumulative incidence of 32% and 38% respectively (Figure 1B). 180-day cumulative incidence of grade II-IV acute GVHD involving skin, liver and/or lower gut was 24%. Four participants developed grade III-IV severe acute GVHD (2 had grade IV acute GVHD), a 180-day cumulative incidence of 12%. Chronic GVHD occurred in 21 patients, with a median time to onset of 241 days (range, 110–807). The 1-year cumulative incidence of cGVHD was 50%. Of these, 18 had extensive chronic GVHD, a 1-year cumulative incidence of 41%. The 2-year PFS and OS was 70% and 71% respectively (Fig 1C).
Figure 1.
A: NRM and relapse incidence of the bortezomib study cohort.
B: Grade II-IV and III-IV acute GVHD incidence of the bortezomib study cohort
C: OS and PFS of the bortezomib study cohort
Comparison with MUD MAC HSCT
Trial outcomes were retrospectively compared to a near contemporaneous standard-of-care MAC cohort (n=45) from 2010–2012, receiving 8/8 MUD with T-replete PBSC grafts and tac/mtx GVHD prophylaxis. The cohorts were similar with regards to diagnoses, patient sex, donor-recipient sex match, CMV serostatus, DRI and HCT-CI scores (Supplementary Table B). In the standard-of-care cohort, 1 patient died prior to neutrophil engraftment and another died prior to platelet engraftment. The median time to neutrophil engraftment was 14 days (range, 11–60), comparable to the bortezomib-based cohort (p=0.44); and the median time to platelet engraftment was 20 days (range, 12–139), possibly delayed compared to the bortezomib-based cohort (p=0.07). Despite older patients (p=0.02) and use of HLA-mismatched grafts (p<0.001) in the bortezomib-based vs. standard-of-care cohort, the cumulative incidence of D180 grade II-IV acute GVHD (38% (95% CI 22%–54%) vs. 56% (95% CI 40%–69%); p=0.044, Supplementary Figure 1) and cumulative incidence of grade III-IV severe acute GVHD (12% (95% CI 4%–25%) vs. 27% (95% CI 15%–40%); p=0.07) appeared possibly lower in the presence of bortezomib. 1-year cumulative incidences of chronic GVHD, and 2-year NRM and relapse were similar. Importantly, 2-year PFS and OS were similar despite use of HLA-mismatched transplantation in the bortezomib-based cohort. Comparing the subset of patients who received bortezomib-based 7/8 MMUD/MMRD vs. 8/8 MUD standard-of-care MAC cohort also yielded similar outcomes (Supplementary Table C)
Comparison of bortezomib-MUD vs. -MMUD/MMRD Cohorts
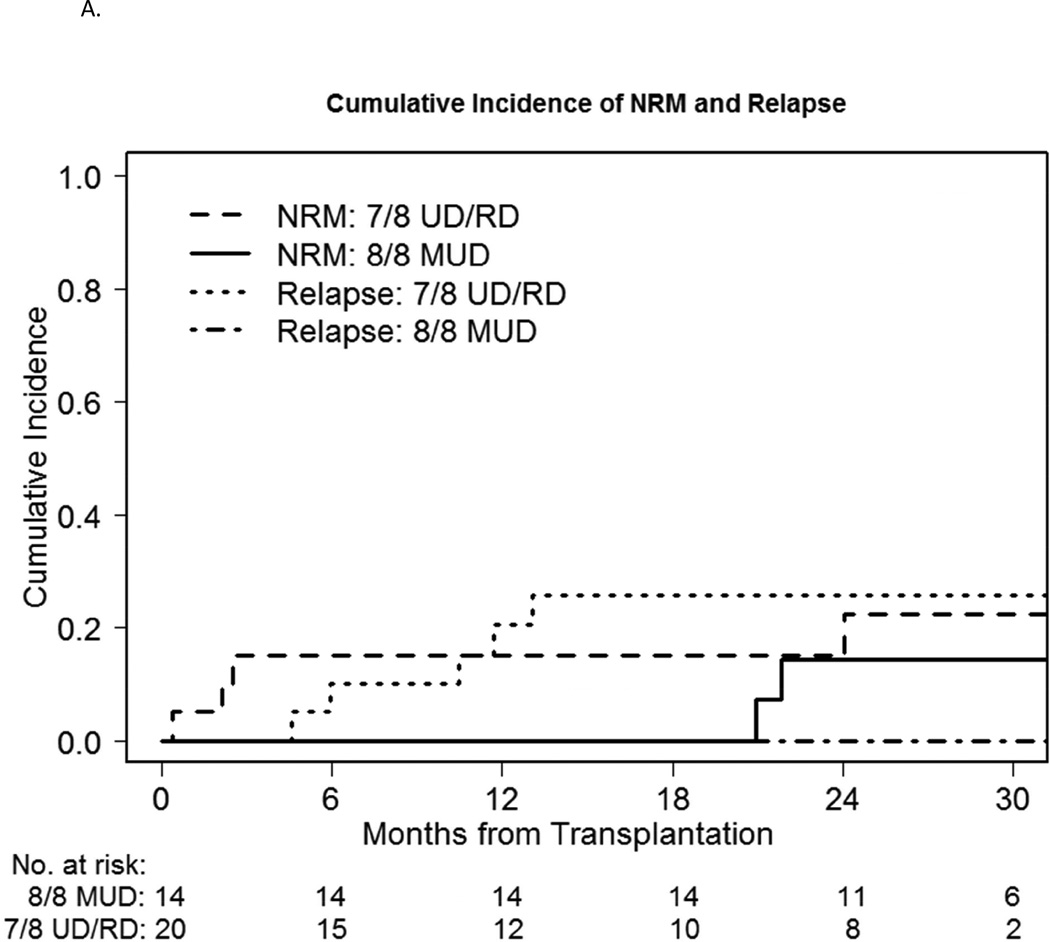
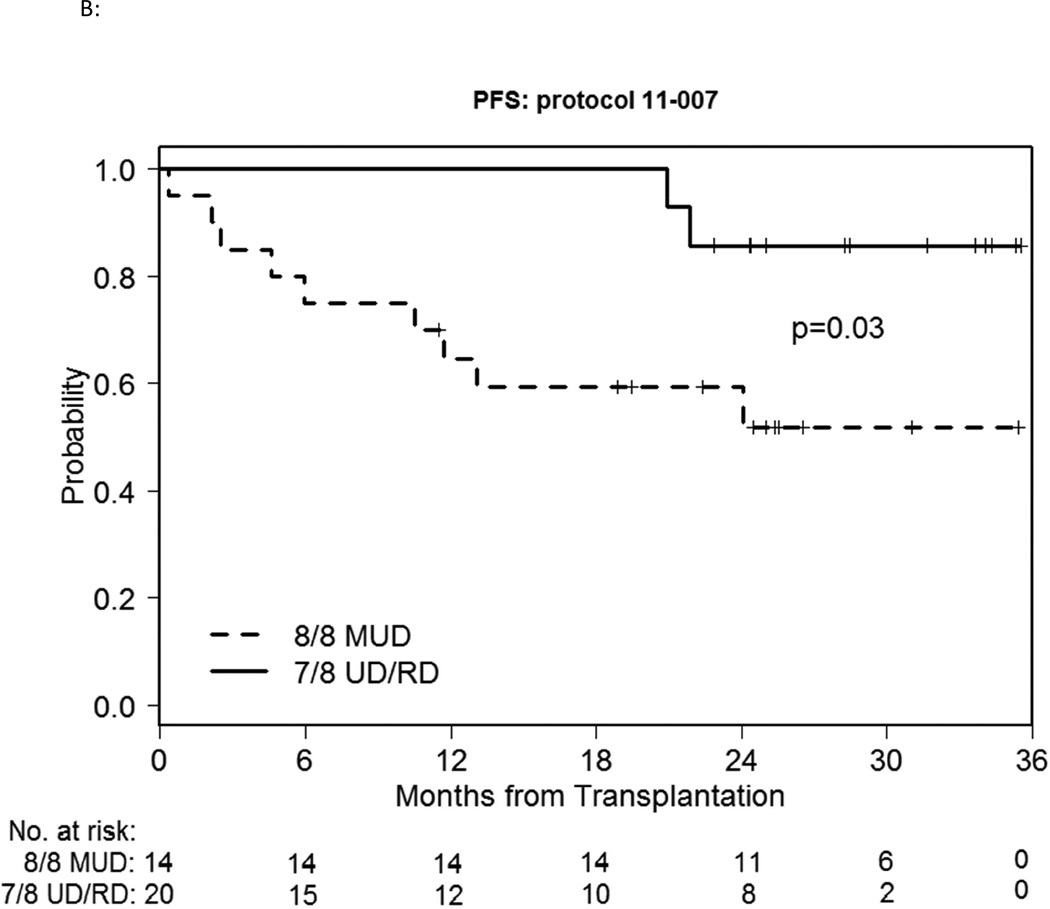
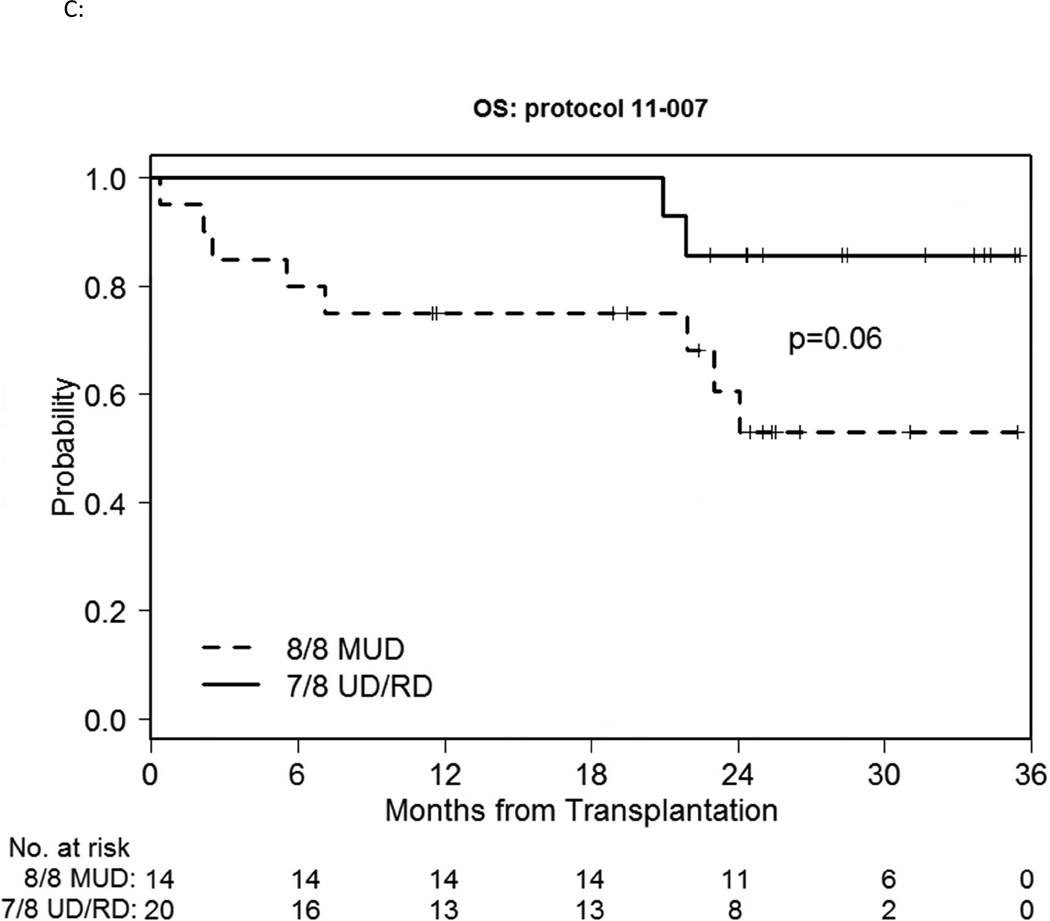
We also compared MUD vs. MMUD/MMRD outcomes within the study cohort. The median time to engraft neutrophils and platelets did not differ meaningfully. Median D30 and D100 TNC chimerism was 98–99% for both groups and time points. 2-year cumulative incidence of NRM was 14% vs.15% respectively (p=0.52) (Figure 2A). 180-day cumulative incidence of GVHD was possibly different, with grade II-IV acute GVHD involving the skin, liver and/or lower gut at 14% vs. 30%, and grade III-IV severe acute GVHD of 7% vs. 15% respectively (Figure 2B). However this difference did not reach statistical significance (p=0.28, 0.48 respectively). 1-year cumulative incidence of cGVHD was 57% vs. 45% (p=0.40) and extensive chronic GVHD was 43% vs. 40% respectively (p=0.74). Overall the HLA-matched patients did better than the mismatched patients, with a 2-year relapse incidence of 0% vs. 26% respectively (p=0.044) (Figure 2A) and a 2-year PFS of 86% vs. 59% respectively (p=0.03) (Figure 2C), with a 2-year OS of 86% vs. 61% respectively (p=0.06) (Figure 2D).
Figure 2.
A: NRM and relapse incidence of the bortezomib-MUD vs. -MMUD/MMRD cohorts
B: PFS of the bortezomib-MUD vs. -MMUD/MMRD cohorts
C: OS of the bortezomib-MUD vs. -MMUD/MMRD cohorts
Immune reconstitution
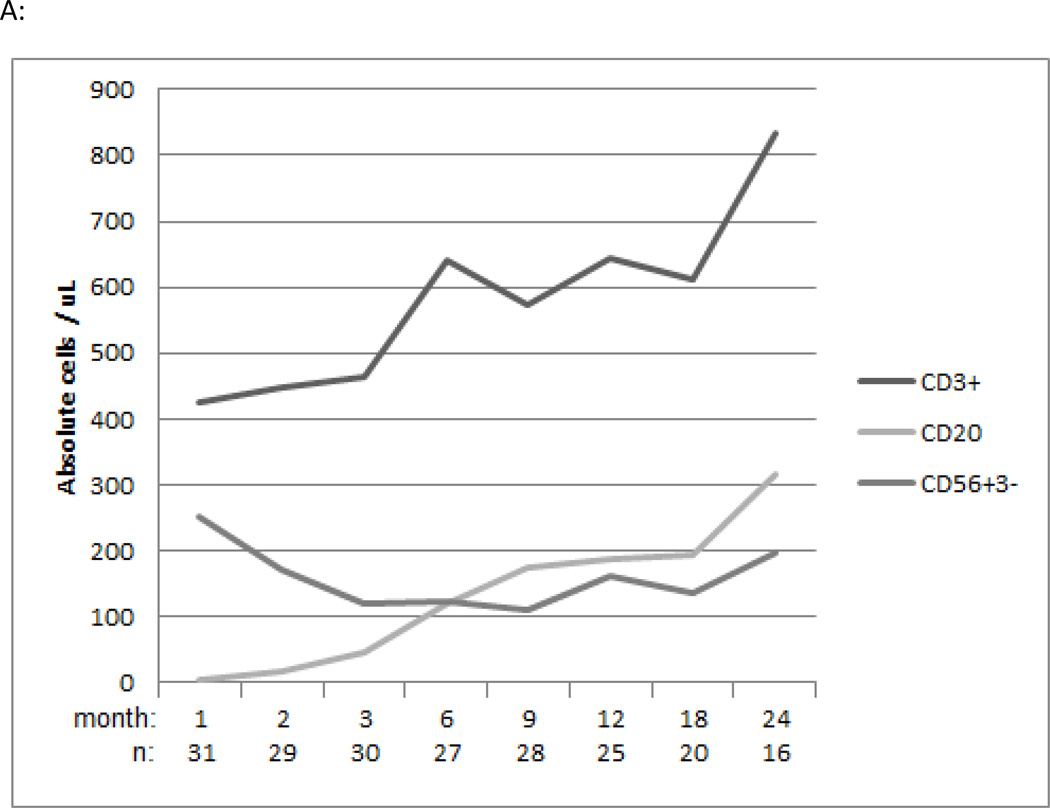
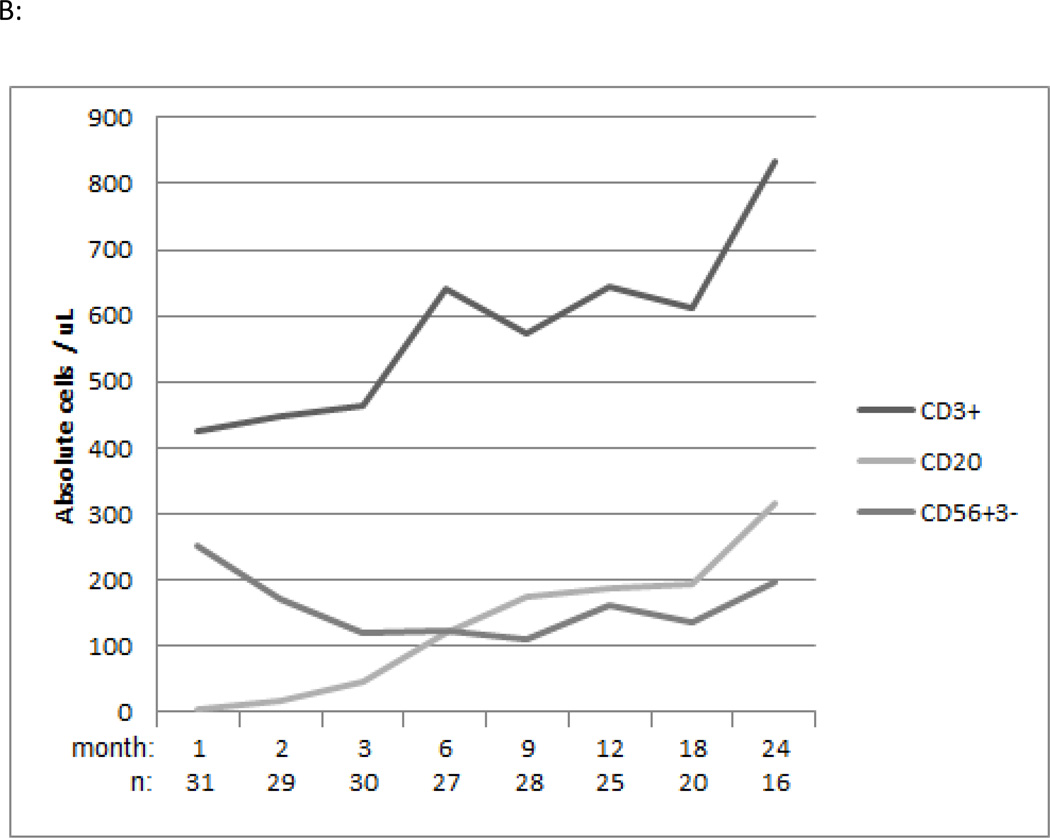
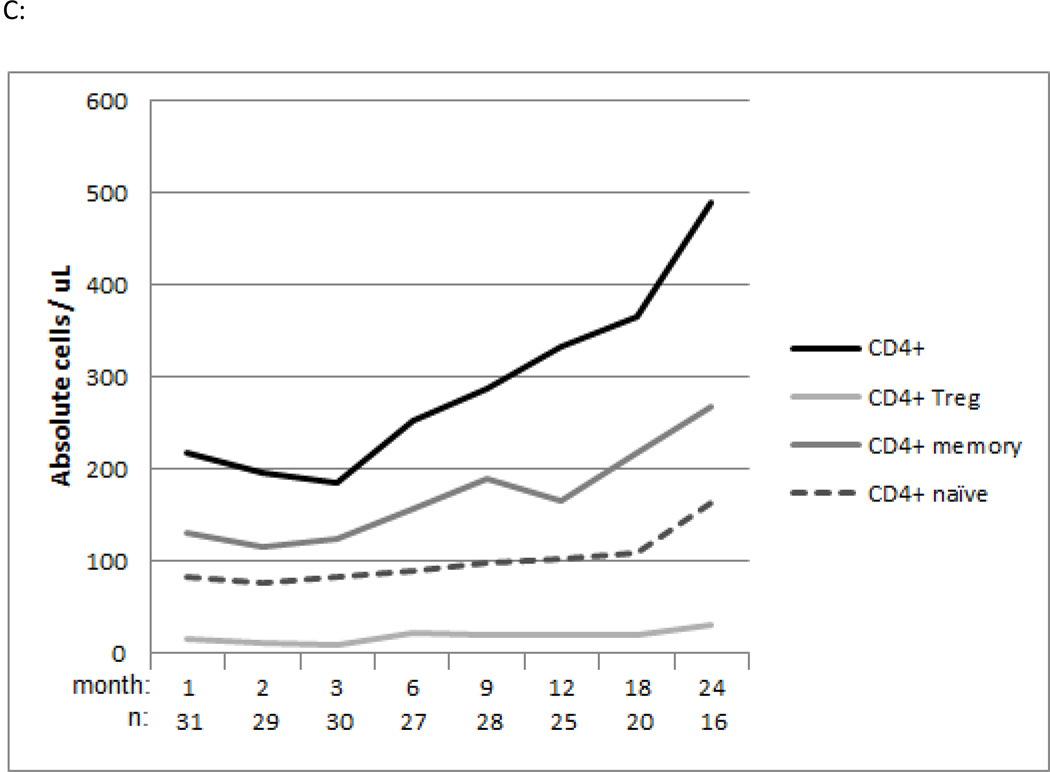
In the bortezomib-based study cohort the median total CD3+ T cell count/μl at 1, 6 and 12 months post-transplantation was 426 (Q1–3, 246–687), 640 (Q1–3, 342–1135), and 643 (Q1–3, 351–1129) respectively (Figure 3A). The median CD20+ B cell count/μl at 1, 6 and 12 months post-transplantation was 5 (Q1–3, 2–11), 120 (Q1–3, 50–304) and 194 (Q1–3, 73–309) respectively Figure 3A). The median CD56+CD3- NK cell count/μl at 1, 6 and 12 months post-transplantation was 252 (Q1–3, 193–371), 122 (Q1–3, 76–183) and 162 (Q1–3, 88–251) respectively (Figure 3A). Regarding T subset reconstitution, the median total CD8+ T cell count/μl at 1, 6 and 12 months post-transplantation was 144 (Q1–3, 63–401), 224 (Q1–3, 113–647) and 266 (Q1–3, 126–461) (Figure 3B). The median total CD4+ T cell count/μl at 1, 6 and 12 months post-transplantation was 218 (Q1–3, 143–340), 253 (Q1–3, 215–376) and 333 (Q1–3, 186–462) respectively (Figure 3C). CD4+Treg and CD4+ and CD8+ naïve and memory T cell reconstitution was also assessed (Figure 3B, C). Additionally, immunologic recovery did not appear impaired in bortezomib-MMUD/MMRD compared with bortezomib-MUD recipients (data not shown).
Figure 3.
Immune reconstitution of the bortezomib cohort
A: Median values of absolute CD3+ T, CD20+ B and CD56+CD3- NK cell counts
B: Median values of absolute CD8+, CD8+ naïve and CD8+ memory cell counts
C: Median values of absolute CD4+, CD4+ naïve, CD4+ memory, and CD4+ Treg cell counts
Discussion
Most adult hematologic malignancy patients who may benefit from HSCT lack an available sibling donor and are usually transplanted from either a matched unrelated donor, or 1-locus HLA-mismatched donors (with umbilical cord blood and haploidentical donors also being considered comparable). For 8/8 MUD, with improvements in DNA based typing and supportive care, survival outcomes are similar to MRD HSCT. 15,16 However MUD HSCT is still associated with increased acute grade II-IV (52% vs. 34%) and III-IV (21% vs.16%) GVHD and NRM (RR 2.76; p<0.01). 17 The use of 1-locus mismatched donors adds risk. A retrospective study of 2825 MAC HSCT recipients with myeloid disease predominantly grafted with bone marrow (BM) from 7/8 vs. 8/8 MUD documented increased severe acute grade III-IV GVHD at 100 days (37% vs. 28%; p<0.001) and higher 1-year NRM (45% vs. 36%; p<0.001), with poorer 1-year DFS (38% vs. 47%; p<0.001) and OS (43% vs. 52%; p<0.001). 2 A more recent retrospective analysis of 1360 adult acute leukemia patients receiving BM or PBSC grafts from 7/8 vs. 8/8 HLA-matched donors also documented increased 2-year NRM (34%–38% vs. 22%–24%) and poorer 2-year DFS for patients in remission at time of MAC HSCT (39%–41% vs. 50–52%). 3 Another retrospective analysis of 2646 adult patients with various hematologic malignancies (including lymphoma, myeloma) receiving predominantly MAC transplantation with PBSC grafts from 7/8 vs. 8/8 HLA-matched donors documented a higher rate of NRM (p<0.01) and poorer OS (p<0.01; 2 year survival of 41% vs. 54% respectively). 4 These studies indicate that regardless of graft source (BM, PBSC), worse outcomes are anticipated in patients lacking 8/8 HLA-matched sibling donors due to increased acute GVHD, NRM, and poorer DFS and OS. Novel regimens to improve outcomes for such patients would represent a major advance.
Bortezomib has immunomodulatory properties relevant to allogeneic HSCT, and based on its encouraging results in HLA-mismatched RIC HSCT, we prospectively evaluated a regimen of short-course bortezomib plus tacrolimus and methotrexate for MAC HSCT recipients lacking 8/8 HLA-matched related donors. Bortezomib, limited to 3 doses early after transplantation (day +1, +4 and +7) appears to have little systemic toxicity. No patient developed toxicities associated with more prolonged bortezomib therapy (e.g. neuropathy, colonic necrosis), and no hepatic VOD was noted despite the lack of PK-targeted Busulfan conditioning. Treatment-related toxicity after bortezomib-based MAC HSCT is in the range previously reported for HLA-matched transplantation.
The bortezomib-based MAC HSCT regimen appears efficacious. The 100-day cumulative incidence of grade II-IV acute GVHD was 32%, and both MUD and MMUD/MMRD survival (2-year OS of 86% and 61% respectively) were substantially better than anticipated compared with registry data. 2–4,17 However, retrospective registry outcomes may not represent an adequate comparator. In order to place our findings in context, we therefore compared the bortezomib-MUD/MMUD/MMRD cohort with a near-contemporaneous MUD MAC HSCT cohort receiving a T-replete PBSC graft and standard-of-care tac/mtx GVHD prophylaxis at our center. The cohort was similar to the bortezomib-based cohort in most parameters including disease risk index and comorbidity scores. It differed with regards to systematic use of Cy/TBI (vs. Bu/Flu) MAC, whose impact, if any, however remains uncertain. While some retrospective and prospective cohort studies indicate a survival benefit of Bu- (primarily Bu/Cy) vs. TBI-based (primarily Cy/TBI) MAC, other analyses fail to document such benefit, and a phase III randomized trial indicates impaired survival of Bu/Flu (vs. Bu/Cy) MAC HSCT, suggesting that use of ablative Bu/Flu conditioning in the bortezomib-based study cohort is unlikely to provide a-priori survival advantage. 18–21 We document that despite the increased patient age and HLA-mismatched donor use in the study cohort, the bortezomib- and standard-of-care MUD MAC HSCT cohorts had similar clinical outcomes of engraftment, NRM, relapse, chronic GVHD and survival, with a possible reduction in acute GVHD with bortezomib use. We also assessed the effect of bortezomib on immunologic reconstitution, which appeared robust in the study cohort.
Compared to both published retrospective analyses and institutional MUD MAC control cohorts with standard GVHD prophylaxis, outcomes of both MUD and 1-locus mismatched MAC HSCT with the addition of bortezomib appear promising. While these phase II results are encouraging, we caution that such retrospective and non-randomized comparisons have inherent limitations, being subject to bias and confounding, even in apparently well matched cohorts such as those described above. However, they are useful in a hypothesis-generating context, hereby providing support for prospective randomized evaluation of bortezomib-based MAC HSCT.
In the myeloablative context, a short-course bortezomib-based regimen is safe, with evidence of efficacy in acute GVHD prophylaxis and with 1-locus mismatched survival comparable to 8/8 MUD HSCT. Bortezomib appears an active agent in MAC HSCT, and is a candidate for prospective randomized evaluation.
Supplementary Material
Highlights.
Phase II trial of bortezomib-based GVHD prophylaxis after myeloablative HSCT (n=34)
Regimen was safe and efficacious for patients lacking HLA-matched sibling donors
Bortezomib-based prophylaxis is suitable for prospective randomized evaluation
Acknowledgements
We thank clinical research nurses Susan Stephenson RN and Mildred Pasek RN. J.K. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. This study was supported in part by Millennium Pharmaceuticals Inc. and Otsuka Pharmaceuticals Inc.; the Jock and Bunny Adams Education and Research Endowment; and by the National Institutes of Health CA183560, CA183559, and P01CA142106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study identifier: NCT01323920 (http://www.ClinicalTrials.gov)
Disclosures: JK: Takeda Pharmaceuticals Advisory Board.
The study was presented in part at the American Society of Hematology Annual Meeting 2013.
Authorship
Research design: JK, HTK, BRB, JR, EPA
Data: JK, PBL, BB, CGR, MJC, PA, CSC, VTH, BG, SN, JR, RJS, JHA, EPA
Statistical analysis: JK, HTK
Manuscript preparation: JK
Manuscript editing and review: JK, HTK, PBL, BB, CGR, MJC, PA, CSC, VTH, BG, SN, JR, BRB, RJS, JHA, EPA
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furst D, Muller C, Vucinic V, et al. High-resolution HLA matching in hematopoietic stem cell transplantation: a retrospective collaborative analysis. Blood. 2013;122:3220–3229. doi: 10.1182/blood-2013-02-482547. [DOI] [PubMed] [Google Scholar]
- 5.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108:551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 6.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Lee JI, Shin JY, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 8.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K, Wilkins DE, Anver MR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–3959. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koreth J, Stevenson K, Kim HT, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. Journal of Clinical Oncology. 2012 doi: 10.1200/JCO.2012.42.0984. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 15.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 16.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 17.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen--a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–709. doi: 10.1200/JCO.2011.40.2362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.