Abstract
Retinal vascular diseases, including diabetic retinopathy, neovascular age related macular degeneration, and retinal vein occlusion, are leading causes of blindness in the Western world. These diseases share several common disease mechanisms, including vascular endothelial growth factor (VEGF) signaling, hypoxia, and inflammation, which provide opportunities for common therapeutic strategies. Treatment of these diseases using laser therapy, anti-VEGF injections, and/or steroids has significantly improved clinical outcomes. However, these strategies do not address the underlying root causes of pathology, and may have deleterious side effects. Furthermore, many patients continue to progress toward legal blindness despite receiving regular therapy. Nanomedicine, the engineering of therapeutics at the 1-100 nm scale, is a promising approach for improving clinical management of retinal vascular diseases. Nanomedicine-based technologies have the potential to revolutionize the treatment of ophthalmology, through enabling sustained release of drugs over several months, reducing side effects due to specific targeting of dysfunctional cells, and interfacing with currently “undruggable” targets. We will discuss emerging nanomedicine-based applications for treatment of complications associated with retinal vascular diseases, including angiogenesis and inflammation.
Graphical Abstract
1. Introduction
The retina is a light sensitive layer of tissue lining the inner surface of the eye and is responsible for vision. Retinal diseases featuring vascular dysfunction, including diabetic retinopathy (DR), neovascular or “wet” age related macular degeneration (AMD), and retinal vein occlusion (RVO) are among the leading causes of blindness in the world [1]. The key complications which cause vision loss in these diseases include ocular neovascularization (or angiogenesis), as well as macular edema, and current therapies primarily address molecular mechanisms underlying these pathologies [2, 3].
Current treatment strategies for retinal vascular diseases involve laser-based targeting of dysfunctional vessels as well as intraocular injection of drugs, including anti-vascular endothelial growth factor (VEGF) therapies or steroids [1, 3, 4]. However, these treatments are not always effective, and many patients do not recover functional vision following treatment [5]. Furthermore, some treatments, such as laser, are not curative and can ablate healthy retinal tissue along with pathologic cells, resulting in irreversible damage even if disease progression is reduced. Furthermore, conventional therapeutic modalities in ophthalmology have been associated with off-target effects, the need for frequent injections to maintain relevant bioavailability, and lack of accessibility to intracellular targets. Therefore, new technologies are warranted for addressing these challenges toward overall improvement of clinical outcomes in the ophthalmology clinic.
Nanotechnology, the manipulation of matter on the 1-100 nm scale, once centered primarily upon engineering practices in the semiconductor industry, has now evolved into a science of nanomedicine which promises to advance clinical management of a number of diseases with a vascular component, including cancer [6, 7] and cardiovascular disease [8], with emerging applications in ocular diseases [9-12]. Nanomedicine enables new clinical approaches due to the unique properties achievable on the nanoscale, the same scale on which most biological interactions occur. Engineering therapeutics on this scale enables disease-specific targeting of drugs through covalent functionalization of the therapy with bioactive ligands to enable high affinity protein-ligand/drug interactions with specific tissues or cell types [13, 14]. Furthermore, by encapsulating therapies within a targeted nanoparticle, a packet of therapeutics can be delivered specifically to diseased cells to achieve a high therapeutic index with minimal dosing frequency and side effects [15]. Nanoengineering of drugs through their encapsulation can also provide a mechanism for sustained release of drug over periods tunable from days to months, and if the drug is poorly soluble in the body, nanoencapsulation can improve the drug's delivery efficiency [16, 17]. The range of therapeutics amenable to nanoengineering include nucleic acid drugs such as antisense oligonucleotides and small interfering RNA (siRNA), to hydrophobic or hydrophilic small molecules, to proteins such as antibodies [15, 18, 19]. Therefore, nanomedicine is uniquely suited toward improving outcomes in retinal vascular disease. The major challenge toward clinical translation of nanomedicines, however, will be the ability to translate studies in preclinical models toward patients, as the behavior of such diverse materials in the clinical setting has yet to be fully understood, and regulatory guidelines for such studies are still in development.
In this review, an overview of retinal vascular disease pathology and current clinical management strategies will be discussed, followed by an overview of current and emerging approaches harnessing nanomedicine for the improved treatment of the significant disease components and causes of retinal vascular diseases, including angiogenesis, loss of vascular integrity, and inflammation. Integrated into the discussion will be the potential of these technologies for clinical translation.
2. Pathology and Clinical Management of Retinal Vascular Diseases
2.1 Diabetic Retinopathy
Diabetic retinopathy (DR) is a retinal vascular disease which can occur in both Type I and Type II diabetics, and is a leading cause of blindness in the working age population (ages 20-74) [20]. A subset of DR patients develop severe vision threatening complications, which are proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME) [21]. In early DR, retinal capillary degeneration and nonperfusion, microaneurysms, and inflammation is thought to contribute to hypoxia-ischemia and the upregulation of VEGF signaling. The increase in VEGF can lead to increased retinal vascular hyperpermeability and thickness (edema) and/or preretinal neovascularization (PDR) [22].
In this condition, diabetic insults, primarily poor glycemic control, lead to blood vessel damage and inflammation, breakdown of the blood-retinal barrier (BRB), and leakage into the center of the eye [1, 23]. In order to maintain proper exchange of nutrients and oxygen the BRB must be properly maintained. Breakdown of the BRB is a hallmark step in diabetic retinopathy resulting in increased vascular permeability [24, 25]. Furthermore, the damage of blood vessels as indicated by microaneurysms, hemorrhages, capillary closure, and loss in BRB integrity alters retinal oxygen diffusion efficiency, which can lead to ischemia-induced pathologic ocular neovascularization (NV) in a stage of the disease called proliferative diabetic retinopathy (PDR) [25]. Some mediators responsible for maintaining BRB integrity include the occludin and claudin class of endothelial tight junction proteins, both of which mediate direct cell-to-cell attachments, as well as supporting cells called pericytes which wrap around endothelial cells lining blood vessels and may play a number of trophic an structural roles [23, 26]. In the retina, a decrease in tight junction proteins and loss of pericytes have been observed in patient specimens and models of diabetic retinopathy [26].
Patients with advanced stages of DR can also acquire diabetic macular edema (DME); a microvascular complication that results in macular thickening leading to capillary leakage and microaneurysms [27]. Although not every patient with DR will acquire DME (25%), once vascular leakage and angiogenesis occurs the likelihood of complete vision loss greatly increases [28]. Since the number of people with diabetes is expected to be around 420 million by 2030 due to an overall increase in obesity as well as an increase in life's expectancy, it is crucial to find new therapies that target vascular dysfunctions such as the ones found in DR and DME [29]. Relevant targets in treatment of DR and DME include inflammation, angiogenesis, and vascular permeability, and current treatments are intended to target one or more of these disease components.
Since the 1980's, laser photocoagulation has emerged as the standard treatment for PDR and DME, the major conditions contributing to vision loss in patients with DR [30]. One of the main goals of laser treatment is to increase oxygen tension in hypoxic regions of the inner retina where vascular injury has occurred [31]. The laser is used to photocoagulate and destroy pathologic vessels responsible for retinal ischemia, thus normalizing oxygen tension in the retina [32]. Recently, advances in laser technology have led to the use of retinal navigated laser treatments which allow for better image quality, improved laser spot accuracy as well as diminished pain reported by patients [30]. Although laser therapy can slow progression of DR and macular edema secondary to DR, this treatment is not curative and does not always halt the progressive loss in visual acuity, and is a destructive approach as healthy retina in the proximity of the laser targeted region is irreversibly ablated [33]. An emerging treatment paradigm which may be available in the near future to patients with DR/DME are intraocular injection of anti-VEGF therapies, or combination therapies of anti-VEGF drugs with laser treatment. Anti-VEGF targeted therapies, including ranibizumab, bevacizumab, and aflibercept neutralize intraocular VEGF, which has been shown to contribute to key events in pathology such as vascular proliferation and permeability which underlie PDR, DME, neovascular AMD, and macular edema secondary to RVO [1, 2, 34]. However, a significant number of patients have failed to respond to both types of treatment in clinical trials, warranting new therapeutic options for refractory patients [35]. Furthermore, intraocular injection is an invasive procedure which can cause complications such as endophthalmitis and retinal detachment, and concerns have been raised regarding systemic side effects of anti-VEGF antibodies or other targeted anti-VEGF drugs leaking into the circulation from the ocular compartment, potentially causing hypertension and adverse cardiovascular events [36, 37]. Thus, any new therapeutic strategies will need to address these possible limitations observed in clinical practice.
2.2 Neovascular Age-Related Macular Degeneration
Age related macular degeneration (AMD) is an incurable eye disease affecting the elderly population, and is characterized by deterioration of the macula, the central portion of the retina responsible for sharp visual acuity. Over 30 million people worldwide are estimated to have some form of AMD. The majority of AMD patients have the “dry” form of the disease, which is described as the formation of yellow deposits called drusen in the submacular space [38]. Dry AMD can cause some central vision loss attributable to retinal structural alteration due to drusen deposition, but most patients retain working visual acuity [39]. However, dry AMD can progress into neovascular, or “wet” AMD. Approximately 10-15% of AMD patients (approximately 2 million) are estimated to have neovascular, or “wet” age related macular degeneration (AMD), which is the leading cause of vision loss in the elderly, and is poised to be appear in epidemic proportions due to the aging of the baby boomer population [39, 40]. In neovascular AMD, pathologic ocular NV occurs in the subretinal space in a layer of blood vessels called the choroid which nourishes the outer retinal tissue. In this region, inflammation as evidenced by inflammatory biomarker expression and infiltration of cells such as macrophages has been observed [41, 42]. The choroidal neovascularization (CNV) that is characteristic of this disease features aberrant growth of blood vessels from the choroidal space into the retina, and on the order of days, can lead to leakage of fluid into the macula, retinal detachment, scarring, and ultimately blindness [43]. Therefore, diagnosis of CNV in AMD patients requires prompt and effective treatment.
While there is no currently available treatment for dry AMD, effective anti-VEGF therapies have been in use for treatment of CNV and have spared vision in a significant number of neovascular AMD patients [44]. However, a substantial fraction of neovascular AMD patients are non-responders or otherwise refractory to anti-VEGF treatment, and still progress toward legal blindness, perhaps due to acquisition of anti-VEGF resistance (tachyphylaxis) with repeat injections, or other molecular mediators underlying CNV not addressed by therapy [45-47]. For these reasons, additional therapies in conjunction with anti-VEGF or as an alternative to anti-VEGF are needed for more universal effective responses to therapeutic intervention in neovascular AMD.
2.3 Retinal Vein Occlusion
Retinal vein occlusion (RVO) is the second most prevalent retinal vascular disease following DR, affecting over 16 million persons worldwide [48]. The disease, associated with atherosclerosis, diabetes, and lifestyle choices such as smoking, features blockage of retinal blood flow in the central or branch retinal veins leading to leakage of blood and retinal swelling. The resulting compromised blood flow can lead to inflammatory responses as well as ocular NV and macular edema, thus sharing pathology with other retinal vascular diseases [3].
Treatment approaches for RVO are focused on controlling ocular NV and macular edema secondary to the disease, and include laser photocoagulation, anti-VEGF therapy, and/or corticosteroid therapy depending on the stage of disease [49]. Of the third therapeutic strategy, implantation of sustained-release anti-inflammatory dexamethasone devices have been shown to reduce edema and improve visual quality in RVO patients when administered alone, with improvement in outcomes when administered as part of a combinative strategy with laser treatment or anti-VEGF therapy [50]. These therapies have frequently proven to be effective in slowing the progression of RVO, but fail to address the root cause of the disease, which is vascular occlusion. Similar to clinical management of other retinal vascular diseases, RVO therapeutic outcomes would be improved with introduction of new therapies used in conjunction with current techniques.
Nanotechnology-Guided Treatment of Retinal Vascular Disease
3.1 Ocular Neovascularization
A primary pathological target for therapy in retinal vascular diseases such as neovascular (“wet”) AMD and proliferative diabetic retinopathy involves the abnormal growth of blood vessels (“ocular neovascularization”), which can disrupt and damage retinal structure and lead to vision loss. Anti-VEGF treatments, while effective in a significant number of patients, still does not prevent progression to legal blindness in many patients [51]. Furthermore, as previously discussed, treatment with anti-VEGF therapies may have side effects require monthly intraocular injections and may have side effects throughout the body. Nevertheless, these vessel growth-arresting therapies remain a front line strategy in many diseases and nanomedicine has the potential to improve upon the clinical outcomes using this approach.
Potential advantages of nanoengineering of anti-VEGF therapies may include reduced dosing frequency and maintenance of anti-VEGF drugs at a therapeutically-effective dose for a longer period of time at the disease site, using strategies based upon sustained release nanoparticles. For example, Yandrapu et al. developed a technology for encapsulation of nanoparticles in porous microparticles (NPinPMP) for the sustained release of the anti-VEGF antibody bevacizumab [52]. This strategy involves the encapsulation of poly lactic acid (PLA) nanoparticles coated with bevacizumab within porous sustained release poly lactic co-glycolic acid (PLGA) nanoparticles. This drug delivery strategy enabled sustained release of anti-VEGF for several months in rat models, which has important implications for reducing injection frequency in patients. Similarly, polymers consisting of polycaprolactone dimethacrylate (PCM) and poly hydroxyethyl methacrylate (poly HEMA) exhibited sustained release of bevacizumab ranging up to 4 months in rabbit models [53]. These types of polymeric systems offer interesting advantages of being injectable as a liquid through the needle, then gelling (either naturally or via a light-activated stimulus, depending on polymers used) in situ within intraocular compartments, enabling sustained depot release of drug over weeks to months.
Nanotechnologies also enable sustained release and enhanced bioavailability of small molecules relevant to treatment of ocular neovascularization. Novel dipeptide based nanotubes comprised of phenylalanine and α,β-dehydrophenylalanine were shown to be effective vehicles for the sustained intravitreal delivery of pazopanib, a small molecule inhibitor of receptor tyrosine kinase involved in the intracellular signaling of VEGF and a number of other growth factors involved in ocular neovascularization [54]. Nanoparticles based on polylactic acid (PLA) and polyethylene oxide (PEO) were shown to penetrate retina and localize within RPE, and were capable of sustained release of integrin antagonists for therapy of CNV [55]. If these nanomedicine-based approaches can be successfully translated, sustained release of anti-VEGF and other anti-angiogenic therapies in patients at a therapeutically-relevant concentration may improve outcomes while minimizing dosage intervals. Polymers such as PLA, PLGA, and PEO or PEG are already incorporated within several FDA approved drug formulations, so their continued incorporation into drug delivery systems for clinical applications is expected.
As an alternative to sustained delivery of anti-angiogenic therapies in the eye, technologies for targeting gene therapies to specific sites of ocular neovascularization can be utilized. As an example, Luo et al. developed PLGA nanoparticles that were surface functionalized with RGD peptide for the targeted delivery of recombinant Flt23K intraceptor plasmid to inhibit progression of CNV lesions in primate and murine AMD models upon intravenous administration (Figure 1) [56]. RGD motifs bind to integrin receptors which are upregulated in ocular neovascularization [57]. Flt23k intraceptor binds to VEGF and sequesters it in the endoplasmic reticulum for effective inhibition. Long-lasting gene therapies delivered systemically have the potential advantage of eliminating the need for intraocular injections, and also minimizing dosing frequency as the therapeutic duration of gene therapies may be longer compared to small molecules or antibodies. Furthermore, these types of targeting strategies enable confinement of therapy to where it is needed in the retina, which is important given the possibility of off-target effects and toxicity associated with anti-VEGF administration [58, 59].
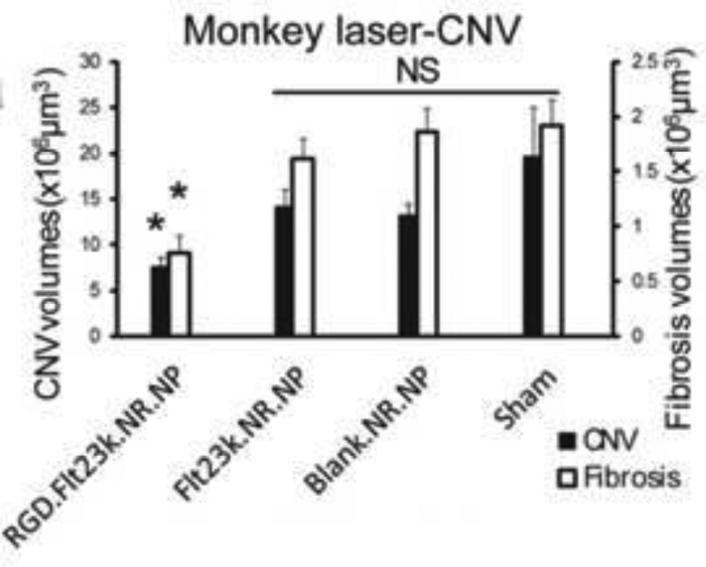
Figure 1.
Targeted therapy of CNV lesions using RGD-targeted nanoparticles. In a monkey model of laser induced CNV, RGD targeted nanoparticles loaded with Flt23k intraceptor plasmid suppressed CNV formation (perlecan staining) and fibrosis (collagen I staining) 4 weeks following a single intravenous injection of nanoparticles. Reproduced with permission from.
Likewise, gene therapies encoding short hairpin RNAs (shRNA) and delivered using nanoparticles are also promising for clinical translation. As a recent example, PLGA nanoparticles encapsulating a plasmid containing VEGF-A shRNA were also shown to be efficacious in regression of murine corneal vascularization [60]. Alternatively, small interfering RNA (siRNA) therapies can be delivered to the retina for relatively transient but potent inhibition of neovascularization. Recently, Zhang et al. used a PEGylated liposome-protamine-hyaluronic acid nanoparticle system originally developed to deliver siRNA targeting the VEGFR1 mRNA to RPE cells and a laser induced murine CNV model [61]. siRNA is efficiently encapsulated by these nanoparticulate formulations, and CNV was inhibited significantly compared to control vehicles and naked siRNA. Synthetic gene therapy vehicles have strong potential for translation; gene therapy vectors such as nanoparticles based on poly-lysine compaction of DNA are well-tolerated by the retina [62]. Furthermore, protamine is a naturally occurring polycation and hyaluronic acid is a polyanionic polysaccharide, and neither polymer exhibits toxicity as demonstrated by preclinical studies. However, extensive clinical studies are warranted to confirm these observations.
3.2 Retinal Hypoxia
While current treatments such as anti-VEGF are used to arrest neovascularization once upon fundus examination, it would be ideal to treat at-risk patients prior to the onset of neovascularization, at a point which the patient can avoid any vision loss. Therefore, biomarkers of early retinal vascular disease identifiable through screening are being sought to improve outcomes. To this end, retinal ischemia and subsequent hypoxia have been associated with susceptibility of the tissue to ocular neovascularization in several retinal vascular diseases, as is manifested by inner retinal or choroidal vascular degeneration at the structural level, and HIF-1α signaling at the molecular level. Hypoxia has been associated with diabetic retinopathy, retinopathy of prematurity, vein occlusion, and age related macular degeneration [63-65]. Ischemia can be detected in the patient through retinal imaging technologies which measure oxygen content of vascular supply and blood flow, and it is also possible to detect retinal cellular hypoxia [66-69]. Therefore, retinal imaging and ophthalmologic examination may offer opportunities for early therapeutic intervention prior to advanced disease.
To complement early detection with timely treatment, nanotechnology based therapies could be useful for targeting hypoxic retinal tissue through several potential strategies, in order to prevent initiation and/or progression of retinal vascular diseases. One method is through sustained delivery of HIF-1 inhibitors. Hypoxia-inducible factor-1 (HIF-1) is known to upregulate VEGF-A, PDGF-BB and other proangiogenic factors [70]. Therefore targeting HIF-1 can be more effective in inhibiting neovascularization than targeting VEGF alone. Towards this application, nanoparticles composed of branched polyethylene glycol and poly(sebacic acid) (PSA-PEG) were synthesized for sustained delivery of a chemotherapeutic HIF-1 antagonist, doxorubicin to the retina [71], with controlled release of doxorubicin observed for over 105 days following intraocular injection in a rabbit model. Furthermore, these nanoparticles enabled sustained inhibition of intraretinal neovascularization for over 35 days in mouse models engineered to express VEGF in photoreceptors. Doxorubicin inhibits HIF-1 by preventing its binding to DNA, but it has poor aqueous solubility, and it precipitates at the retinal surface after intraocular injections [71]. PSA-PEG nanoparticles showed sustained release of drug within the retina in animal models of neovascularization. In rabbits, the drug was present for at least 3 months in the aqueous humor and vitreous after intraocular injection. It was also shown to suppress choroidal and retinal NV and did not cause retinal toxicity. Similarly, PLGA nanoparticles bearing HIF-1 shRNAs were effective in inhibiting CNV formation [72]. Using these formulations, plasmid DNA-mediated expression in the RPE layer of rat models of CNV was observed for over 4 weeks following intravitreal injection, and HIF-1 plasmid shRNA loaded nanoparticles significantly inhibited neovascularization.
A second potentially useful role for nanomedicine in hypoxia targeting involves the site-specific delivery of drugs to hypoxic tissues by means of targeting ligands. For example, hypoxia-induced expression of Annexin A2 or carbonic anhydrase IX can be exploited for targeting therapeutic nanoparticles in vivo [73, 74]. Small molecules such as 2-nitroimidazoles (e.g. pimonidazole) can be covalently coupled to other small molecules to enable their selective nitroreductase-assisted accumulation in hypoxic cells, as demonstrated in cancer studies [75]. Selectivity of drug delivery vehicles toward hypoxic retinal cells would facilitate confinement of therapy and minimization of off-target effects, and possibly lower drug dosing amounts and frequencies.
Additionally, as an adjuvant to laser photocoagulation, which is used to ablate hypoxic retinal tissue to curb neovascularization, nanosensors could be used to “highlight” regions of retinal hypoxia to enable direct visualization of hypoxic tissue and targeting of only hypoxic regions with the argon laser. This could possibly be used to guide prophylactic photocoagulation therapy prior to onset of ocular neovascularization in at-risk patients, and reduce peripheral retinal damage and irreversible vision loss. We have recently developed hypoxia biosensors based on fluorescein which may play a useful role in translating this strategy in the clinic (Figure 2) [69]. These dyes accumulate selectively in hypoxic cells, enabling their in vivo visualization and laser targeting.
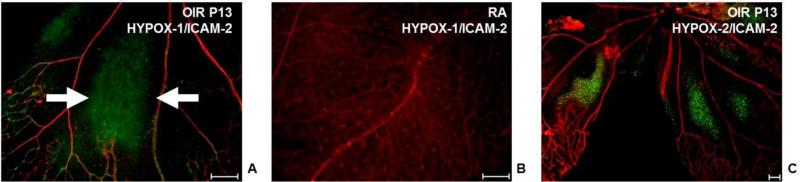
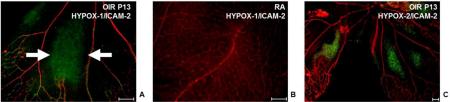
Figure 2.
Detection of retinal hypoxia using fluorescein based molecular imaging probes HYPOX-1 and HYPOX-2. (A-C) Retinal flatmounts from mouse models of oxygen induced retinopathy (OIR, A) or mice reared in room air (B) intravenously injected with the hypoxia sensing probe HYPOX-1 (green) were stained with the endothelial cell marker ICAM-2 (red). (C) Retinal flatmount from OIR mouse intravitreally injected with HYPOX-2 (green) and stained with ICAM-2 (red). Accumulation of HYPOX-1 and HYPOX-2 is observed in the avascular retina. Scale bars = 100 μM. Reproduced with permission from.
3.3 Retinal Inflammation
Along with hypoxia, retinal inflammation is an important early component associated with retinal vascular diseases, and is therefore an important therapeutic target. For example, leukostasis and expression of inflammatory endothelial occur early in the pathogenesis of diabetic retinopathy [23], and dysfunctional macrophages are thought to contribute to AMD pathogenesis [76]. One strategy to curb retinal inflammation involves sustained release of anti-inflammatory small molecules. For example, Nishida et al. developed amphiphilic poly (γ-glutamic acid) nanoparticles containing dexamethasone to suppress phagocytic cells in retinal disorders [77]. These nanoparticles, consisting of a biodegradable, hydrophilic backbone of γ-PGA and a hydrophobic L-phenylalanine (Phe) side chain, feature advantages such as protease resistance which make them promising drug delivery vectors [78-80]. These dexamethasone loaded nanoparticles suppressed TNFα and MCP-1 expression in cultured macrophages upon TNFα stimulation. Furthermore, in an animal model of NMDA-induced excitotoxic retinal damage, microglia activation was significantly suppressed. Other nanoparticle-based delivery methods to combat retinal inflammation rely on gene therapies. For example, nanoparticle-mediated delivery of plasminogen kringle 5 expression plasmids were shown to be not only anti-inflammatory in mouse models of diabetic retinopathy, but were also anti-angiogenic in mouse models of preretinal and subretinal neovascularization [81, 82].
In addition to classical sustained release approaches, nanoparticles that sense and/or quench reactive oxygen species (ROS) associated with inflammation have recently been developed for applications in other fields and may be promising for suppression of inflammatory retinal diseases. For example, micelles encapsulating therapeutics have been developed featuring ROS-degradable polymers on the surface, enabling ROS-activated drug delivery for vascular applications [83-85]. Other types of nanoparticles, such as those based on carbon or rare earth metals (e.g. cerium oxide), are effective scavengers or quenchers of ROS and are thus potent anti-inflammatory agents [86-94]. Recently, cerium oxide nanoparticles (nanoceria) were demonstrated to be potent inhibitors of neovascularization and inflammation induced by oxidative stress (Figure 3) [92]. Furthermore, with a single intravitreal injection, nanoceria exhibited a number of other sustained therapeutic effects, including downregulation of apoptosis and reduction in vascular leakage. Although a single intravitreal injection of nanoceria resulted in retinal retention of nanoparticles for as long as 120 days, no effects on retinal function or viability were observed, collectively suggesting that the long term safety and efficacy of nanoceria in retinal diseases make them very promising candidates for clinical trials in ophthalmology [95].
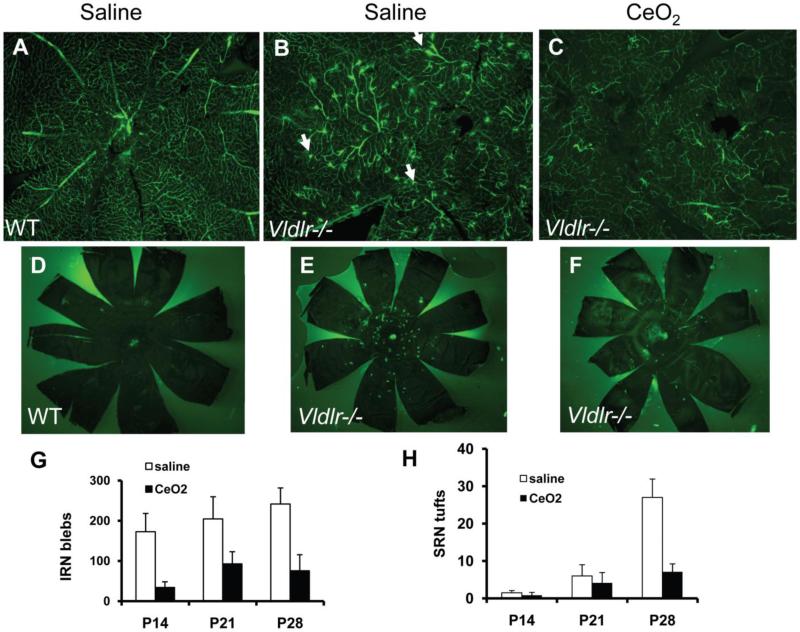
Figure 3.
Inhibition of pathologic vascular lesions in the Vldlr -/- mouse model using cerium oxide nanoparticles (nanoceria). This mouse model exhibits intraretinal (IRN) vascular lesions (B) and subretinal (SRN) vascular lesions (E) which are suppressed by a single injection of nanoceria (G,H). Reproduced with permission from.
An additional approach to targeting inflammation with nanotechnology relies on site-specific targeting of inflammatory biomarkers expressed on dysfunctional endothelial cells, such as cell adhesion molecules (CAMs) and selectins. These proteins promote leukocyteendothelial interactions such as rolling, arrest, and transmigration across endothelial barriers lining blood vessels, and are thus important therapeutic targets for inhibiting retinal inflammation [13, 23]. Nanoparticles loaded with drug and functionalized with anti-ICAM or anti-VCAM antibodies can be used to target inflamed endothelial cells, as well as pericytes, and CAM mediated endocytosis may be useful for traversing the blood-brain or blood-retinal barriers [8, 96, 97]. Therefore, nanomedicines which exploit leukocyte adhesion molecules are promising candidates for site-specific delivery of therapies to sites of retinal inflammation.
Subsets of retinal inflammatory cells may also be targeted by nanoparticles. Kannan and co-workers have developed dendrimer based systems for specifically targeting activated microglia and astrocytes in the brain and retina [98-102]. Furthermore, activated macrophages can be targeted by polymeric mannosylated drug delivery vehicles for applications using cargoes such as siRNAs which modulate the macrophage phenotype to inhibit inflammation [103-105]. Therefore, nanomedicines may play a useful role in immunomodulation of the retina for applications in several retinal vascular diseases.
3. Conclusion
Inspired in part by advances in the application of nanomedicine to the treatment of cardiovascular diseases and cancer, ophthalmic nanotechnology promises to address a number of barriers to successful management of blinding retinal vascular diseases. Advantages of nanomedicine include sustained release of drugs over weeks to months, while maintaining drug concentration at a therapeutically-relevant amount, as well as site-specific targeting of diseased tissue, sparing healthy tissue from off-target effects. Nanomedicines also enable accessibility of therapies to previously undruggable targets, including intracellular molecules, with unprecedented affinity and efficacy. Patients may experience the benefits of nanomedicines in terms of reduced dosing frequency, fewer side effects, reduced treatment time, and most importantly, improved clinical outcomes. While preclinical studies of nanomedicines in the retina are promising, considerable progress must continue to be made in order to successfully translate these technologies to the clinic, in the form of extensive safety and biodistribution studies, followed by proof of concept in non human primates and eventually patients.
Table 1.
Recent preclinical applications of nanotechnology for therapy of retinal vascular disease.
| Technology | Key Findings | References |
|---|---|---|
| Nanoparticles in Porous Microparticles (NPinPMP) | Sustained release of bevacizumab over several months in rodent models | 52 |
| In situ gelling polymeric gels | Sustained release of bevacizumab for at least 60 days in rabbit models | 53 |
| Dipeptide Nanotubes | Improved bioavailability of pazopanib in ocular compartments | 54 |
| Polymeric nanoparticles based on poly-lactic acid (PLA) and polyethylene oxide (PEO) | Improved half-life of peptide-based integrin antagonist for therapy of experimental CNV | 55 |
| RGD- targeted polylactic-co-glycolic acid (PLGA) nanoparticles | Targeted, nonviral gene therapy of experimental CNV using Flt23k intraceptor plasmid DNA cargo | 56 |
| Lipsosome-polymer hybrid nanoparticles | Efficient nanoparticle mediated inhibition of experimental CNV using VEGFR1 siRNA loaded nanoparticles | 61 |
| Poly(sebacic acid)-Polyethylene glycol nanoparticles | Sustained doxorubicin-mediated inhibition of ocular neovascularization (intraretinal NV and CNV), with sustained release of weeks to months | 71 |
| PLGA nanoparticles | Sustained RPE plasmid DNA expression for over 4 weeks and sustained inhibition of CNV in rat models | 72 |
| Poly(gamma-glutamic acid) nanoparticles conjugated with L-Phe | Targeted delivery of dexamethasone to microglia and macrophages, for inhibition of inflammation in models of NMDA-induced toxicity | 77 |
| PLGA nanoparticles | Suppression of vascular leakage, inflammation, and neovascularization via sustained expression of plasmid DNA encoding for plasminogen kringle 5 | 81,82 |
| Cerium oxide nanoparticles (Nanoceria) | Sustained inhibition of pro-inflammatory and pro-angiogenic gene expression in mouse model of AMD, via catalytic antioxidant activity | 92 |
| Polyamidoamine (PAMAM) dendrimers | Targeting of fluocinolone-PAMAM conjugate to retinal microglia for inhibition of retinal degeneration in rat models | 101 |
Acknowledgements
This work was supported by the American Diabetes Association, NIH grants EY023397, T32EY007135, P30EY008126, and Research to Prevent Blindness (Dolly Green Special Scholar Award, Unrestricted Grant to Vanderbilt Eye Institute).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brand CS. Management of retinal vascular diseases: a patient-centric approach. Eye. 2012;26(Suppl 2):S1–16. doi: 10.1038/eye.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Progress in retinal and eye research. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med. 2010;363:2135–2144. doi: 10.1056/NEJMcp1003934. [DOI] [PubMed] [Google Scholar]
- 4.Kim LA, D'Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. The American journal of pathology. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 7.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol Oncol. 2008;26:74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Jayagopal A, Linton MF, Fazio S, Haselton FR. Insights into atherosclerosis using nanotechnology. Current atherosclerosis reports. 2010;12:209–215. doi: 10.1007/s11883-010-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo DH, Lee TG, Kim JH. Nanotechnology and nanotoxicology in retinopathy. Int J Mol Sci. 2011;12:8288–8301. doi: 10.3390/ijms12118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanotechnology in ophthalmology. Can J Ophthalmol. 2010;45:457–476. doi: 10.3129/i10-090. [DOI] [PubMed] [Google Scholar]
- 11.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug discovery today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188. doi: 10.3389/fphar.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickline SA, Neubauer AM, Winter P, Caruthers S, Lanza G. Applications of nanotechnology to atherosclerosis, thrombosis, and vascular biology. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:435–441. doi: 10.1161/01.ATV.0000201069.47550.8b. [DOI] [PubMed] [Google Scholar]
- 14.Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003;107:1092–1095. doi: 10.1161/01.cir.0000059651.17045.77. [DOI] [PubMed] [Google Scholar]
- 15.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayagopal A, Sussman EM, Shastri VP. Functionalized solid lipid nanoparticles for transendothelial delivery. IEEE transactions on nanobioscience. 2008;7:28–34. doi: 10.1109/TNB.2008.2000147. [DOI] [PubMed] [Google Scholar]
- 18.Forrest ML, Kwon GS. Clinical developments in drug delivery nanotechnology. Adv Drug Deliv Rev. 2008;60:861–862. doi: 10.1016/j.addr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Labhasetwar V. Nanotechnology for drug and gene therapy: the importance of understanding molecular mechanisms of delivery. Curr Opin Biotechnol. 2005;16:674–680. doi: 10.1016/j.copbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R, American Diabetes A. Diabetic retinopathy. Diabetes Care. 2003;26(Suppl 1):S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 21.Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF, G. Eye Diseases Prevalence Research, The prevalence of diabetic retinopathy among adults in the United States. Archives of ophthalmology. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 22.Song SJ, Wong TY. Current concepts in diabetic retinopathy. Diabetes & metabolism journal. 2014;38:416–425. doi: 10.4093/dmj.2014.38.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghadam AH. Mechanisms of Blood–Retinal Barrier Breakdown in Diabetic Retinopathy. Ophthalmology Research:Visual Dysfunction in Diabetes. 2012:105–122. [Google Scholar]
- 25.Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 26.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, Cunha-Vaz J, Ambrosio AF. Calcium dobesilate inhibits the alterations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59:2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 28.Antonetti DA, Klein R, Gardner TW, Diabetic retinopathy N. Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 29.Wenick AS, Bressler NM. Diabetic macular edema: current and emerging therapies. Middle East African journal of ophthalmology. 2012;19:4–12. doi: 10.4103/0974-9233.92110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kernt M CR, Cserhati S, Seidensticker F, Liegl RG, Lang J, Haritoglou C, Kampik A, Ulbig MW, Neubauer AS. Pain and accuracy of focal laser treatment for diabetic macular edema using a retinal navigated laser (Navilas®) Clinical Ophthalmology. 2012;6:289–296. doi: 10.2147/OPTH.S27859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79:435–440. doi: 10.1034/j.1600-0420.2001.790502.x. [DOI] [PubMed] [Google Scholar]
- 32.Muqit MM, Sanghvi C, McLauchlan R, Delgado C, Young LB, Charles SJ, Marcellino GR, Stanga PE. Study of clinical applications and safety for Pascal((R)) laser photocoagulation in retinal vascular disorders. Acta Ophthalmol. 2010 doi: 10.1111/j.1755-3768.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- 33.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 34.Kleinman ME, Baffi JZ, Ambati J. The multifactorial nature of retinal vascular disease. Ophthalmologica. 2010;224(Suppl 1):16–24. doi: 10.1159/000315152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kernt M, Cheuteu RE, Cserhati S, Seidensticker F, Liegl RG, Lang J, Haritoglou C, Kampik A, Ulbig MW, Neubauer AS. Pain and accuracy of focal laser treatment for diabetic macular edema using a retinal navigated laser (Navilas) Clin Ophthalmol. 2012;6:289–296. doi: 10.2147/OPTH.S27859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simo R, Hernandez C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia. 2008;51:1574–1580. doi: 10.1007/s00125-008-0989-9. [DOI] [PubMed] [Google Scholar]
- 37.Tezel TH, Kaplan HJ. Are intravitreal anti-VEGF antibodies safe? Ocul Immunol Inflamm. 2007;15:1–2. doi: 10.1080/09273940701246314. [DOI] [PubMed] [Google Scholar]
- 38.Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011;95:14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Progress in retinal and eye research. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarbin MA, Montemagno C, Leary JF, Ritch R. Regenerative nanomedicine and the treatment of degenerative retinal diseases. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2012;4:113–137. doi: 10.1002/wnan.167. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye. 2011;25:127–139. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machalinska A, Kawa MP, Marlicz W, Machalinski B. Complement system activation and endothelial dysfunction in patients with age-related macular degeneration (AMD): possible relationship between AMD and atherosclerosis. Acta Ophthalmol. 2012;90:695–703. doi: 10.1111/j.1755-3768.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- 43.Maguire MG, Alexander J, Fine SL, G Complications of Age-related Macular Degeneration Prevention Trial Research, Characteristics of choroidal neovascularization in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2008;115:1468–1473. 1473, e1461–1462. doi: 10.1016/j.ophtha.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 44.Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144:627–637. doi: 10.1016/j.ajo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 45.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009;29:723–731. doi: 10.1097/IAE.0b013e3181a2c1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forooghian F, Chew EY, Meyerle CB, Cukras C, Wong WT. Investigation of the role of neutralizing antibodies against bevacizumab as mediators of tachyphylaxis. Acta Ophthalmol. 2011;89:e206–207. doi: 10.1111/j.1755-3768.2009.01773.x. [DOI] [PubMed] [Google Scholar]
- 47.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008;115:2199–2205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY, C International Eye Disease, The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319. e311. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–910. doi: 10.1111/j.1445-5994.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 50.Chan A, Leung LS, Blumenkranz MS. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant (Ozurdex) for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Clin Ophthalmol. 2011;5:1043–1049. doi: 10.2147/OPTH.S13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? The British journal of ophthalmology. 2012;96:1–2. doi: 10.1136/bjophthalmol-2011-301236. [DOI] [PubMed] [Google Scholar]
- 52.Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in Porous Microparticles Prepared by Supercritical Infusion and Pressure Quench Technology for Sustained Delivery of Bevacizumab. Molecular pharmaceutics. 2013;10:4676–4686. doi: 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyagi P, Barros M, Stansbury JW, Kompella UB. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Molecular pharmaceutics. 2013;10:2858–2867. doi: 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panda JJ, Yandrapu S, Kadam RS, Chauhan VS, Kompella UB. Self-assembled phenylalanine-alpha,beta-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. Journal of controlled release : official journal of the Controlled Release Society. 2013;172:1151–1160. doi: 10.1016/j.jconrel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Kim H, Csaky KG. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. Journal of controlled release : official journal of the Controlled Release Society. 2010;142:286–293. doi: 10.1016/j.jconrel.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 56.Luo L, Zhang X, Hirano Y, Tyagi P, Barabas P, Uehara H, Miya TR, Singh N, Archer B, Qazi Y, Jackman K, Das SK, Olsen T, Chennamaneni SR, Stagg BC, Ahmed F, Emerson L, Zygmunt K, Whitaker R, Mamalis C, Huang W, Gao G, Srinivas SP, Krizaj D, Baffi J, Ambati J, Kompella UB, Ambati BK. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS nano. 2013;7:3264–3275. doi: 10.1021/nn305958y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proceedings of the National Academy of Sciences. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PloS one. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qazi Y, Stagg B, Singh N, Singh S, Zhang X, Luo L, Simonis J, Kompella UB, Ambati BK. Nanoparticle-mediated delivery of shRNA.VEGF-a plasmids regresses corneal neovascularization. Investigative ophthalmology & visual science. 2012;53:2837–2844. doi: 10.1167/iovs.11-9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu HA, Liu YL, Ma ZZ, Wang JC, Zhang Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Investigative ophthalmology & visual science. 2011;52:4789–4794. doi: 10.1167/iovs.10-5891. [DOI] [PubMed] [Google Scholar]
- 62.Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PloS one. 2009;4:e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD) Ageing Res Rev. 2009;8:349–358. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia inducible factors. Experimental eye research. 2006;83:473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Archives of ophthalmology. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- 66.Kristjansdottir JV, Hardarson SH, Halldorsson GH, Karlsson RA, Eliasdottir TS, Stefansson E. Retinal oximetry with a scanning laser ophthalmoscope. Investigative ophthalmology & visual science. 2014;55:3120–3126. doi: 10.1167/iovs.13-13255. [DOI] [PubMed] [Google Scholar]
- 67.Mezu-Ndubuisi OJ, Teng PY, Wanek J, Blair NP, Chau FY, Reddy NM, Raj JU, Reddy SP, Shahidi M. In vivo retinal vascular oxygen tension imaging and fluorescein angiography in the mouse model of oxygen-induced retinopathy. Investigative ophthalmology & visual science. 2013;54:6968–6972. doi: 10.1167/iovs.13-12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahidi M, Wanek J, Blair NP, Little DM, Wu T. Retinal tissue oxygen tension imaging in the rat. Investigative ophthalmology & visual science. 2010;51:4766–4770. doi: 10.1167/iovs.09-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evans SM, Kim K, Moore CE, Uddin MI, Capozzi ME, Craft JR, Sulikowski GA, Jayagopal A. Molecular probes for imaging of hypoxia in the retina. Bioconjugate chemistry. 2014;25:2030–2037. doi: 10.1021/bc500400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 71.Iwase T, Fu J, Yoshida T, Muramatsu D, Miki A, Hashida N, Lu L, Oveson B, Lima e Silva R, Seidel C, Yang M, Connelly S, Shen J, Han B, Wu M, Semenza GL, Hanes J, Campochiaro PA. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. Journal of controlled release : official journal of the Controlled Release Society. 2013;172:625–633. doi: 10.1016/j.jconrel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang C, Wang YS, Wu H, Zhang ZX, Cai Y, Hou HY, Zhao W, Yang XM, Ma JX. Inhibitory efficacy of hypoxia-inducible factor 1alpha short hairpin RNA plasmid DNA-loaded poly (D, L-lactide-co-glycolide) nanoparticles on choroidal neovascularization in a laser-induced rat model. Gene therapy. 2010;17:338–351. doi: 10.1038/gt.2009.158. [DOI] [PubMed] [Google Scholar]
- 73.Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2010;20:3467–3474. doi: 10.1016/j.bmcl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Valapala M, Thamake SI, Vishwanatha JK. A competitive hexapeptide inhibitor of annexin A2 prevents hypoxia-induced angiogenic events. Journal of cell science. 2011;124:1453–1464. doi: 10.1242/jcs.079236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, Jo DG, Ahn CH, Suh YD, Kim K, Kwon IC, Lee DS, Park JH. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials. 2014;35:1735–1743. doi: 10.1016/j.biomaterials.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 76.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. The Journal of clinical investigation. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryu M, Nakazawa T, Akagi T, Tanaka T, Watanabe R, Yasuda M, Himori N, Maruyama K, Yamashita T, Abe T, Akashi M, Nishida K. Suppression of phagocytic cells in retinal disorders using amphiphilic poly(gamma-glutamic acid) nanoparticles containing dexamethasone. Journal of controlled release : official journal of the Controlled Release Society. 2011;151:65–73. doi: 10.1016/j.jconrel.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 78.Yoshikawa T, Okada N, Oda A, Matsuo K, Matsuo K, Kayamuro H, Ishii Y, Yoshinaga T, Akagi T, Akashi M, Nakagawa S. Nanoparticles built by self-assembly of amphiphilic gamma-PGA can deliver antigens to antigen-presenting cells with high efficiency: a new tumor-vaccine carrier for eliciting effector T cells. Vaccine. 2008;26:1303–1313. doi: 10.1016/j.vaccine.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 79.Ng HI, Fernando GJ, Kendall MA. Induction of potent CD8(+) T cell responses through the delivery of subunit protein vaccines to skin antigen-presenting cells using densely packed microprojection arrays. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:477–484. doi: 10.1016/j.jconrel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Uto T, Akagi T, Akashi M, Baba M. Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccine. J Med Virol. 2008;80:11–19. doi: 10.1002/jmv.21029. [DOI] [PubMed] [Google Scholar]
- 81.Park K, Chen Y, Hu Y, Mayo AS, Kompella UB, Longeras R, Ma JX. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes. 2009;58:1902–1913. doi: 10.2337/db08-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin J, Zhou KK, Park K, Hu Y, Xu X, Zheng Z, Tyagi P, Kompella UB, Ma JX. Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor. Investigative ophthalmology & visual science. 2011;52:6230–6237. doi: 10.1167/iovs.10-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta MK, Martin JR, Werfel TA, Shen T, Page JM, Duvall CL, Cell Protective ABC. Triblock Polymer-Based Thermoresponsive Hydrogels with ROS-Triggered Degradation and Drug Release. Journal of the American Chemical Society. 2014;136:14896–14902. doi: 10.1021/ja507626y. [DOI] [PubMed] [Google Scholar]
- 84.Martin JR, Gupta MK, Page JM, Yu F, Davidson JM, Guelcher SA, Duvall CL. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35:3766–3776. doi: 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta MK, Meyer TA, Nelson CE, Duvall CL. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:591–598. doi: 10.1016/j.jconrel.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ali SS, Hardt JI, Dugan LL. SOD activity of carboxyfullerenes predicts their neuroprotective efficacy: a structure-activity study. Nanomedicine. 2008;4:283–294. doi: 10.1016/j.nano.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiology of aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Ali SS, Hardt JI, Quick KL, Kim-Han JS, Erlanger BF, Huang TT, Epstein CJ, Dugan LL. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free radical biology & medicine. 2004;37:1191–1202. doi: 10.1016/j.freeradbiomed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Wong LL, McGinnis JF. Nanoceria as bona fide catalytic antioxidants in medicine: what we know and what we want to know. Advances in experimental medicine and biology. 2014;801:821–828. doi: 10.1007/978-1-4614-3209-8_103. [DOI] [PubMed] [Google Scholar]
- 90.Cai X, Seal S, McGinnis JF. Sustained inhibition of neovascularization in vldlr-/-mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK NF-kappaB pathway. Biomaterials. 2014;35:249–258. doi: 10.1016/j.biomaterials.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong LL, Hirst SM, Pye QN, Reilly CM, Seal S, McGinnis JF. Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PloS one. 2013;8:e58431. doi: 10.1371/journal.pone.0058431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kyosseva SV, Chen L, Seal S, McGinnis JF. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Experimental eye research. 2013;116:63–74. doi: 10.1016/j.exer.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine. 2013;8:1483–1508. doi: 10.2217/nnm.13.133. [DOI] [PubMed] [Google Scholar]
- 94.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature nanotechnology. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 95.Wong LL, Hirst SM, Pye QN, Reilly CM, Seal S, McGinnis JF. Correction: Catalytic Nanoceria Are Preferentially Retained in the Rat Retina and Are Not Cytotoxic after Intravitreal Injection. PloS one. 2013;8 doi: 10.1371/journal.pone.0058431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu J, Rappaport J, Muro S. Specific binding, uptake, and transport of ICAM-1-targeted nanocarriers across endothelial and subendothelial cell components of the blood-brain barrier. Pharmaceutical research. 2014;31:1855–1866. doi: 10.1007/s11095-013-1289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Voinea M, Manduteanu I, Dragomir E, Capraru M, Simionescu M. Immunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells--a potential tool for specific drug delivery. Pharmaceutical research. 2005;22:1906–1917. doi: 10.1007/s11095-005-7247-3. [DOI] [PubMed] [Google Scholar]
- 98.Xu Q, Kambhampati SP, Kannan RM. Nanotechnology approaches for ocular drug delivery. Middle East African journal of ophthalmology. 2013;20:26–37. doi: 10.4103/0974-9233.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:151–165. doi: 10.1089/jop.2012.0232. [DOI] [PubMed] [Google Scholar]
- 100.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Science translational medicine. 2012;4:130ra146. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iezzi R, Guru BR, Glybina IV, Mishra MK, Kennedy A, Kannan RM. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33:979–988. doi: 10.1016/j.biomaterials.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 102.Dai H, Navath RS, Balakrishnan B, Guru BR, Mishra MK, Romero R, Kannan RM, Kannan S. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine. 2010;5:1317–1329. doi: 10.2217/nnm.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ortega RA, Barham WJ, Kumar B, Tikhomirov O, McFadden ID, Yull FE, Giorgio TD. Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages. Nanoscale. 2014 doi: 10.1039/c4nr03962a. [DOI] [PubMed] [Google Scholar]
- 104.Yu SS, Lau CM, Barham WJ, Onishko HM, Nelson CE, Li H, Smith CA, Yull FE, Duvall CL, Giorgio TD. Macrophage-specific RNA interference targeting via “click”, mannosylated polymeric micelles. Molecular pharmaceutics. 2013;10:975–987. doi: 10.1021/mp300434e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, Dickerson JH, Hubbell JA, Giorgio TD. Size-and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. International journal of nanomedicine. 2012;7:799–813. doi: 10.2147/IJN.S28531. [DOI] [PMC free article] [PubMed] [Google Scholar]