Abstract
Phosphodiesterase 10A (PDE10A) plays a key role in the regulation of brain striatal signaling. A PET tracer for PDE10A may serve as a tool to evaluate PDE10A expression in vivo in central nervous system disorders with striatal pathology. Here, we further characterized the binding properties of a previously reported radioligand we developed for PDE10A, [11C]TZ1964B, in rodents and nonhuman primates (NHPs). The tritiated counterpart [3H]TZ1964B was used for in vitro binding characterizations in rat striatum homogenates and in vitro autoradiographic studies in rat brain slices. The carbon-11 labeled [11C]TZ1964B was utilized in the ex vivo autoradiography studies for the brain of rats and microPET imaging studies for the brain of NHPs. MicroPET scans of [11C]TZ1964B in NHPs were conducted at baseline, as well as with using a selective PDE10A inhibitor MP-10 for either pretreatment or displacement. The in vivo regional target occupancy (Occ) was obtained by pretreating with different doses of MP-10 (0.05 - 2.00 mg/kg). Both in vitro binding assays and in vitro autoradiographic studies revealed a nanomolar binding affinity of [3H]TZ1964B to the rat striatum. The striatal binding of [3H]TZ1964B and [11C]TZ1964B was either displaced or blocked by MP-10 in rats and NHPs. Autoradiography and microPET imaging confirmed that the specific binding of the radioligand was found in the striatum but not in the cerebellum. Blocking studies also confirmed the suitability of the cerebellum as an appropriate reference region. The binding potentials (BPND) of [11C]TZ1964B in the NHP striatum that were calculated using either the Logan reference model (LoganREF, 3.96 ± 0.17) or the simplified reference tissue model (SRTM, 4.64 ± 0.47), with the cerebellum as the reference region, was high and had good reproducibility. The occupancy studies indicated a MP-10 dose of 0.31 ± 0.09 mg/kg (LoganREF) / 0.45 ± 0.17 mg/kg (SRTM) occupies 50% striatal PDE10A binding sites. Studies in rats and NHPs demonstrated radiolabelled TZ1964B has a high binding affinity and good specificity for PDE10A, as well as favorable in vivo pharmacokinetic properties and binding profiles. Our data suggests that [11C]TZ1964B is a promising radioligand for in vivo imaging PDE10A in the brain of living subject.
Keywords: PDE10A, radioligand, microPET, C-11, H-3, brain imaging, binding assay, autoradiography
1. Introduction
Phosphodiesterase 10A (PDE10A) is expressed primarily in the medium spiny neurons (MSNs) of the striatum, which is a major input site into basal ganglia of the mammalian brain (Soderling et al., 1999; Strick, 2010). Nigrostriatal terminals release dopamine that engages dopaminergic receptors on MSNs. These receptor interactions lead to changes in cyclic nucleotides mediated by PDE10A. Dysfunction of nigrostriatal transmission and MSN function contributes to multiple neurologic and psychiatric illnesses and may provide novel targets for therapeutic interventions (Nishi et al., 2011). Thus, PDE10A may serve as a reliable biomarker for disease progression or a potential target for therapeutic interventions in various central nervous system (CNS) diseases, such as Parkinson disease, schizophrenia, Huntington disease, and addiction (Celen et al., 2013). Moreover, molecular imaging of PDE10A by PET provides a potential in vivo biomarker of striatal PDE10A function.
Over the past 10 years, tremendous efforts have been made to develop specific PET tracers for imaging PDE10A in the brain. Our group firstly reported the synthesis of a PDE10A PET radiotracer, carbon-11 radiolabeled papaverine (PDE10A inhibition IC50 = 36 nM) in 2010 (Tu et al., 2010). Due to the low retention of the tracer ([11C]papaverine) in rat and monkey striatum, the more potent and specific PDE10A inhibitor MP-10 (IC50 = 0.18 nM) was selected for radiolabelling. However, a radiolabeled metabolite capable of penetrating the blood–brain-barrier limits the clinical utility of [11C]MP-10 for PET quantification of PDE10A (Plisson et al., 2011; Tu et al., 2011). Recently our group reported a series of pyrazole group-containing analogues with a methoxy group on the quinoline fragment, which displayed high potency and selectivity for PDE10A (Li et al., 2013). Among these compounds, TZ1964B has high PDE10A binding potency with an IC50 value of 0.40 ± 0.02 nM and high selectivity for PDE10A versus PDE3A/3B and PDE4A/4B with IC50 > 1500 nM (Li et al., 2013). Biodistribution in normal rats and MP-10 pretreatment in rats revealed that [11C]TZ1964B, named as [11C]1 previously, had high striatal accumulation and good binding specificity for PDE10A (Fan et al., 2014). MicroPET studies in nonhuman primates (NHP) consistently showed good tracer retention in the striatum with rapid clearance from non-target brain regions, and a stable metabolism profile that only one hydrophilic radiometabolite was detected (Fan et al., 2014).
In the present study, we further evaluated the in vitro binding properties of the ligand using its tritiated counterpart [3H]TZ1964B, and characterized the in vivo tracer kinetics and binding characteristics of [11C]TZ1964B in the brains of NHPs, to determine whether this radioligand could permit quantification of PDE10A availability.
2. Materials and methods
2.1 Radioligand preparation
The tritiated compound was custom synthesized by American Radiolabeled Chemicals, Inc. (St. Louis, MO). The carbon-11 labeled compound [11C]TZ1964B was successfully synthesized through O-methylation of the corresponding precursor, following our previous report (Fan et al., 2014).
2.2 Compound preparation
Reagents and standard compounds for in vitro binding assays were purchased from Sigma (St. Louis, MO) and Tocris Biosciences (R&D Systems, Minneapolis, MN). Novel compounds were synthesized in-house. Test compounds (Table 1) were dissolved in N,N-dimethylformamide (DMF) or ethanol to create a stock solution; the desired concentration for in vitro assays was subsequently obtained by further dilution in the Tris-HCl assay buffer (50 mM Tris/HCl, 8.30 mM MgCl2, 1 mM EDTA, pH 7.5). In the blocking and displacement studies of microPET scans, the solution of MP-10 (0.50 mg/mL) was achieved with 10% PEG300/30% cyclodextrin solution/60% water.
Table 1.
Summary of the PDE10A binding affinity of a series compounds obtained from competitive binding study with [3H]TZ1964B using P20 fractions of rat striatum homogenates
| Drugs | Selectivity | Ki, nM |
|---|---|---|
| TZ19106B | PDE10A | 0.015 ± 0.002 |
| MP-10 | PDE10A | 0.20 ± 0.06 |
| TZ1982T | PDE10A | 0.42 ± 0.10 |
| TZ1914B | PDE10A | 36.89 ± 4.51 |
| 9c | PDE10A | 251.40 ± 44.67 |
| 9a | PDE10A | >1000 |
| Papaverine | PDE10A | >1000 |
| Vinpocetine | PDE1 | >1000 |
| Pentazocine | Sigma 1 | >1000 |
| ISO-1 | Sigma2 | >1000 |
| Ditolylguanidine (DTG) | Sigma 1/2 | >1000 |
| WAY-100135 | 5-HT1A | >1000 |
| Haloperidol | D2 | >1000 |
| Eticlopride | D2 | >1000 |
| Vesamicol | VAChT | >1000 |
2.3 Experimental animals
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals under protocols approved by Washington University's Animal Studies Committee. Mature, male Sprague–Dawley (SD) rats (Charles River Laboratories, Inc., Wilmington, MA) were used for in vitro binding assays and in vitro autoradiography. Tail vein injections were performed under light inhalation anesthesia (1-2% isoflurane/oxygen) and euthanasia was done under surgical plane anesthesia. Two male adult cynomolgus monkeys, weighing on average 4-6 kg, served as subjects for microPET studies.
2.4 In vitro binding assay in rat brain homogenates
2.4.1 Brain homogenate preparation
Subcellular fraction was carried out as described by Xie et al. (Xie et al., 2006). Rats were euthanized, and bilateral striatum were dissected and homogenized. Tissue homogenization was carried out in cold (4 °C) homogenization buffer (0.32 M sucrose, 4 mM HEPES-NaOH, 1 mM EDTA, pH 7.4) by vigorous vortexing. The homogenate was then centrifuged for 10 min at 800×g to yield the pellet (P0) and the supernatant (S0). P0 was washed in five volumes of lysis buffer, centrifuged again to yield P1 and S1. S0 and S1 were pooled and centrifuged for 15 min at 9000×g yielding S2 and P2. The supernatant S2 was further spun at 100,000 × g for 60 min using the Beckman Ti45 rotor to yield the high speed membrane fraction P20. P20 was re-suspended into the Tris-HCl assay buffer. Aliquots were stored at −80 °C until use. The protein concentration of the suspension was determined using the DC protein assay (Bio-Rad, Hercules, CA).
2.4.2 Radioligand binding assay
The kinetic analysis of [3H]TZ1964B (~2 nM) binding to rat striatum homogenates P20 (~80 μg /ml) was carried out to measure the association (Kon) and dissociation (Koff) rates. Association and dissociation curves were obtained by recording the amount of the radioligand bound specifically as a function of time at a constant concentration of both the radioligand and the enzyme. Dissociation experiments were carried out by adding 10 μM of MP-10 to the reaction mixture after equilibrium was achieved and the specific binding of the radioligand as a function of time was determined. The values of Kon and Koff were determined by nonlinear regression analysis using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
For the saturation binding assay, rat striatum homogenates P20 were diluted and incubated for 60 min with [3H]TZ1964B in a total volume of 150 μL at 25 °C in 96-well polypropylene plates (Fisher Scientific, Pittsburgh, PA). Each well contained 20 μg protein while the concentrations of the radioligand ranged from 0.20 nM to 10.00 nM. Reactions were terminated by the addition of 100 μL of Tris-HCl buffer at 4 °C, then samples were harvested and filtered rapidly using a 96-well glass fiber filtration plate (Millipore, Billerica, MA) presoaked with 100 μL Tris-HCl assay buffer for 1 hour. Each filter was washed with 5 × 200 μL Tris-HCl buffer then transferred to a scintillation vial with 2 mL of scintillation fluid and counted on a Wallac 1450 MicroBeta TriLux liquid scintillation counter (Perkin Elmer, Boston, MA). The equilibrium dissociation constant (Kd) and maximum number of binding sites (Bmax) were determined by nonlinear regression analysis of one-site saturation binding model using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
For competitive binding assays, rat striatum homogenates P20 (4 μg protein) were incubated with [3H]TZ1964B and each test compound in a total volume of 150 μL in 96 well plates for 60 min at 25 °C. The final concentration of the radioligand in each assay was 2 nM. Concentrations of test compounds ranging from 0.03 nM to 10 μM were added to provide inhibition curves. After incubation, samples were washed 5 times, bound radioactivity counted and analyzed by nonlinear regression using one-site competitive binding model using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
In all the binding assays, the nonspecific binding of the radioligand was obtained in the presence of 10 μM of MP-10. Counts were normalized to mg protein in the sample. Three independent experiments were performed, the results are reported as mean ± standard deviation.
2.5 In vitro autoradiography in rat brain slices
Rats were euthanized, and the whole brain was immediately removed, then were snap frozen over dry ice. Horizontal sections (20 - 30 μm) were cut with a Microm cryostat (Microm HM 550; Microm International, Walldorf, Germany) and mounted on Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA). All the sections were stored in −20 °C for no more than 1 month. The in vitro autoradiography was performed as previously reported (Xu et al., 2010). The sections were thawed and dried for 15 min at room temperature, and pre-incubated for 15 min in Tris-HCl buffer. The brain slices were then incubated for 60 min with [3H]TZ1964B in different concentrations (0.38 - 12 nM) at room temperature. Nonspecific binding was determined from samples that contained additional 10 μM of MP-10. To explore the regional distribution of PDE10A in the brain, sagittal rat brain sections, including striatum, frontal cortex, hippocampus, thalamus, globus pallidus, nucleus accumbens, substantia nigra, and cerebellum, were prepared and incubated with 3 nM [3H]TZ1964B in the presence or absence of 10 μM of MP-10. Following the incubation, all sections received two rinses of 5 min each in ice-cold assay buffers and one quick rinse in ice-cold water. The sections were air-dried overnight and then exposed to the screen (Hyperfilm MP; Amersham-GE Healthcare, Piscataway, NJ) in an imaging cassette (Fuji Photo Film Co., Tokyo, Japan) for 4 weeks at room temperature. The film was then developed with a Kodak X-OMAT 2000A processor (Kodak, Tokyo, Japan). The optical density of images were analyzed by Image J (NIH, Bethesda, MD). The equilibrium dissociation constant Kd of ligand binding were calculated by nonlinear regression analysis of one-site saturation binding model using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA).
2.6 Ex vivo autoradiography in the rat brain
Rats (n = 2) were anesthetized with 2-3% isoflurane/oxygen, and were injected with [11C]TZ1964B (~ 74 MBq) via the tail vein. At 30 min post injection, rats were euthanized and the whole brains were dissected. Intact brains were flash frozen in dry ice, pre-cooled in isopentane and stored on dry ice. Horizontal sections (100 μm) were cut with a Microm cryostat and mounted on superfrost plus glass slides (Fisher Scientific, Pittsburgh, PA). Frozen slides were instantly exposed the film in an imaging cassette BAS- TR2025 (Fuji Photo Film, Tokyo, Japan) for 12 h in −20° C at dark. The distribution of radioactivity was visualized by a Fuji Bio-Imaging Analyzer FLA-7000 (Fuji Photo Film, Tokyo, Japan). Photo-stimulated luminescence (PSL) from the striatum and cerebellum were quantified using Multi Gauge v3.0 software (Fuji Photo Film, Tokyo, Japan). Data were background-corrected and expressed as photo-stimulated luminescence signals per square millimeter (PSL/mm2).
2.7 In vivo microPET studies in NHPs
2.7.1 Subjects
PET imaging was carried out on two adult male cynomolgus monkeys. Subject A underwent 4 baseline scans, a series of scans for blocking studies using various doses of MP-10 (0.05, 0.10, 0.25, 0.50, 1.00, 1.50, and 2.00 mg/kg), and a scan for the displacement study (MP-10, dose of 2.00 mg/kg). Subject B underwent two baseline scans. The data represented the results of 6 baseline and 7 blocking experiments. Fasted animals were immobilized with ketamine (10 mg/kg, intramuscular) and anesthetized with 1.5–2.5% isoflurane via an endotracheal tube. A water-soluble ophthalmic ointment was placed in the eyes. Vital signs were monitored every 5 min, and the temperature was kept constant at ~37 °C with heated water blankets. An intravenous perfusion line permitted hydration and injection of the radiotracer.
2.7.2 PET data acquisition
A MicroPET Focus 220 scanner (Concorde/CTI/Siemens Microsystems, Knoxville, TN) was used for the imaging studies of [11C]TZ1964B in the brains of nonhuman primates. A 10 min transmission scan was performed to check the positioning of the brain within the scanner; this was followed by a 45 min transmission scan for attenuation correction. Subsequently, a 120 min dynamic (3× 1-min frames, 4× 2-min frames, 3× 3-min frames, and 20× 5-min frames) PET scan were acquired after intravenous injection of 185 - 259 MBq of [11C]TZ1964B. Blocking experiments were conducted using escalating doses (0.05, 0.10, 0.25, 0.50, 1.00, 1.50 and 2.00 mg/kg) of MP-10 administered intravenously 5 min prior to the tracer injection. Displacement studies were conducted in subject A by administering MP-10 intravenously at dose of 2.00 mg/kg, 20 min post tracer injection.
2.7.3 PET images processing
Emission scans were corrected using individual attenuation, random correction and model-based scatter correction and reconstructed using filtered back projection as described previously (Miller et al., 1989). PET image reconstructed resolution was < 2.00 mm full width half maximum for all 3 dimensions at the center of the field of view. The monkey's magnetization-prepared rapid gradient echo (MP-RAGE) MR image and the summed PET images were co-registered using Automated Image Registration (AIR) (Woods et al., 1993). For quality control of this co-registration process, the co-registered PET and MRI were superimposed using Analyze 10.0 (AnalyzeDirect, Overland Park, KS) and any misalignments could be detected by misposition of brain edges on both images. For quantitative analyses, three-dimensional regions of interest (ROI) for cerebellum (583 voxels, the voxel size is 1.858 mm × 1.858 mm × 0.796 mm = 2.75 μL), striatum (695 voxels), frontal cortex (263 voxels), occipital cortex (254 voxels), temporal cortex (335 voxels), white matter (257 voxels) and hippocampus (68 voxels) were identified on the MRI and then transformed into baseline PET space using the co-registration transformation matrix. The ROIs once identified on a baseline scan were kept fixed for all subsequent studies on the same animal. These ROIs then were used to extract time activity curves from the dynamic PET images. Radioactivity measures were standardized to the body weight and the dose of radioactivity injected to yield standardized uptake value (SUV). To minimize noise signal in the presentation time tissue-activity curve (TAC) data have been smoothed by “Adjacent Averaging” using the OriginLab 9.1 program (OriginLab Corporation, Northampton, MA), while tracer kinetics analysis was based on original data without smoothing. All image analysis was carried out by investigators blinded to the blocking pretreatment.
2.7.4 Reference based tracer kinetic analysis
Quantification of specific uptake was compared with two tracer kinetic methods: Logan Reference (LoganREF) model (Innis et al., 2007; Logan et al., 1996; Logan et al., 1990) (Figure S1) and simplified reference tissue model (SRTM) (Gunn et al., 1997; Innis et al., 2007; Lammertsma and Hume, 1996) (Figure S2). Using the LoganREF model, the volume of distribution ratio (DVR) was estimated in each ROI using a MATLAB (Mathworks Inc., Natick, MA) implementation of these tracer kinetic methods as previously published (Logan et al., 1990). This enabled calculation of the binding potential (non-displaceable): BPND = DVR-1, which is proportional to the ratio Bmax/Kd for each region of interest with fixed k2’ value 0.10 min−1 (Van Laere et al., 2013b) and CROI values collected from the PET scans. BPND and R1, k2 values were also derived from the application of SRTM model. The model fitting was processed as previously published (Lammertsma and Hume, 1996; Watabe et al., 1996) with CR (reference region tracer radioactive concentration) and CT (target region tracer radioactive concentration) values derived from microPET collections. Percentage test–retest variability (TRV) was calculated as the mean percentage between the absolute difference between test and retest and average test and retest values, 100×| BPND-test – BPND-retest |/(( BPND-test + BPND-retest)/2). The coefficient of variation (CV = 100× standard deviation/mean BPND) is a measure of dispersion and often referred to as the relative standard deviation.
The regional target occupancy (Occ) following pretreatment with MP-10 was calculated directly as the relative change in striatal BPND using both LoganREF and SRTM; and it refers to the percentage of the enzyme PDE10A binding sites bound by the drug molecule (MP-10). The occupancy values were averaged across the striatal regions (putamen and caudate) to estimate the occupancy percentage. In addition, the occupancy values at baseline and after MP-10 pre-treatment were also analyzed using the occupancy plot (Suhara et al., 2003) to provide alternative estimates of the reduction of the specific signal.
3. Results
3.1 Radiochemical synthesis
The chemical structures of [3H]TZ1964B and [11C]TZ1964B are shown in Figure 1. The specific activity of [3H]TZ1964B was 3145 MBq/μmol. [11C] TZ1964B was obtained in high specific activity > 318.2 GBq/μmol (decay corrected to end of bombardment), with radiochemical yield 20 - 30% (decay corrected to end of bombardment,) and radiochemical purity >99%.
Figure 1.
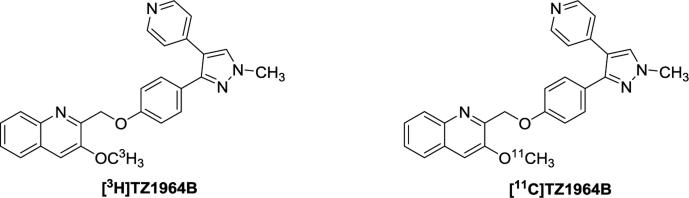
Chemical Structures of [3H]TZ1964B and [11C]TZ1964B
3.2 In vitro binding assays in rat brain homogenates
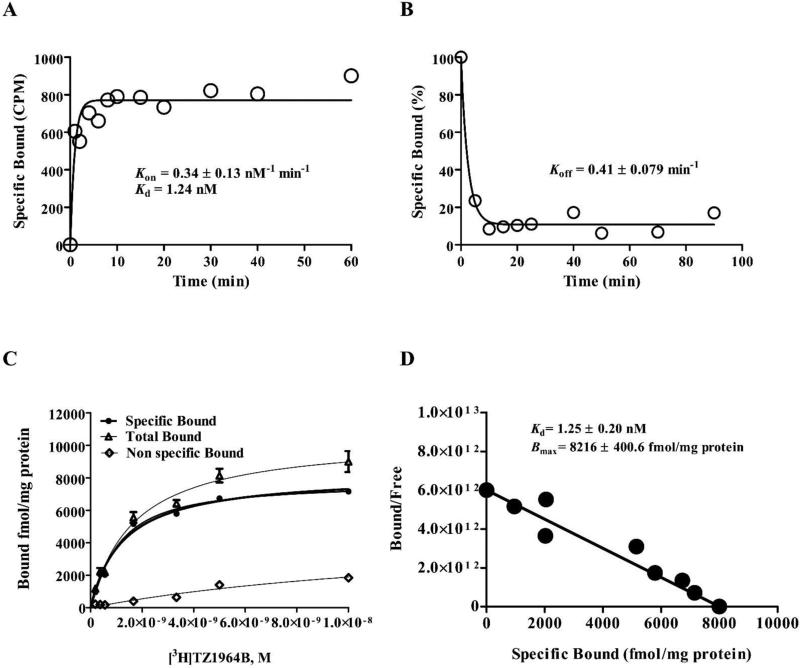
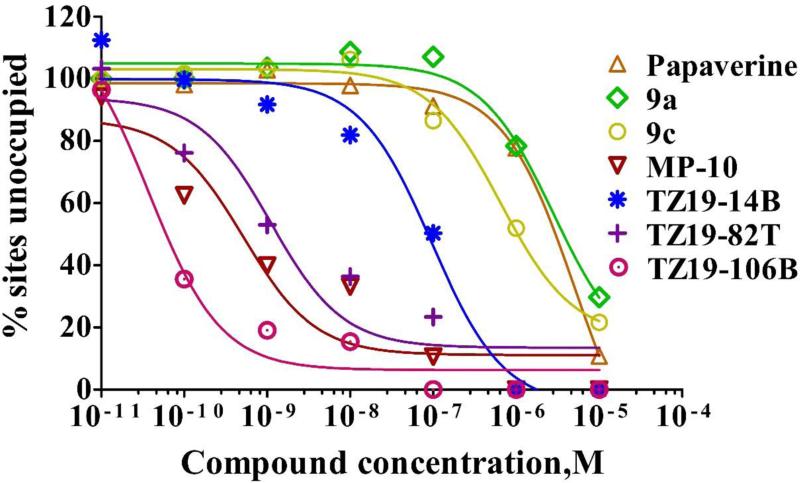
As an initial step, a kinetic binding characteristic of [3H]TZ1964B binding to the PDE10A in rat striatum homogenates P20 was investigated. Both the association and dissociation time courses were shown in Figure 2A&B. After about 10 min at room temperature, > 95% of max specific [3H]TZ1964B binding was achieved (Kon = 0.33 ± 0.13 nM−1 ·min−1). The dissociation rate constant Koff of [3H]TZ1964B binding was 0.41 ± 0.079 min−1. Direct saturation binding studies were carried out using [3H]TZ1964B with rat striatum homogenates P20. A single site binding was observed with Kd = 1.25 ± 0.20 nM and Bmax = 8216 ± 400.60 fmol/mg protein (Figure 2C&D). A series of σ1 receptor, σ2 receptor, vesicular monoamine transporter 2 (VMAT2), vesicular acetylcholine transporter (VAChT), dopaminergic, serotonergic, or PDE1 specific ligands, and known PDE10A inhibitors were tested to characterize the pharmacological profile of [3H]TZ1964B (Chemical structures shown in Figure S3). The Ki values for inhibiting [3H]TZ1964B binding to PDE10A were obtained by competitive binding assays (Table 1). Specific PDE10A inhibitors displaced [3H]TZ1964B binding in rat striatum with variable Ki values ranging from 0.015 nM to 251.4 nM (Table 1, Figure 3). No inhibition of the [3H]TZ1964B binding to PDE10A was observed in the competitive binding studies using PDE1, σ1 receptor, σ2 receptor, VAChT, dopaminergic, and serotonergic ligands.
Figure 2.
Representative kinetic and saturation binding analysis of [3H]TZ1964B to PDE10A in P20 fractions of rat striatum homogenates. (A, association experiment; B, dissociation experiment; C, saturation binding curve; D, Scatchard analysis.)
Figure 3.
Representative competitive binding analysis of the binding of [3H]TZ1964B to PDE10A in P20 fractions of rat striatum homogenates.
3.3 In vitro autoradiography in rat brain slices
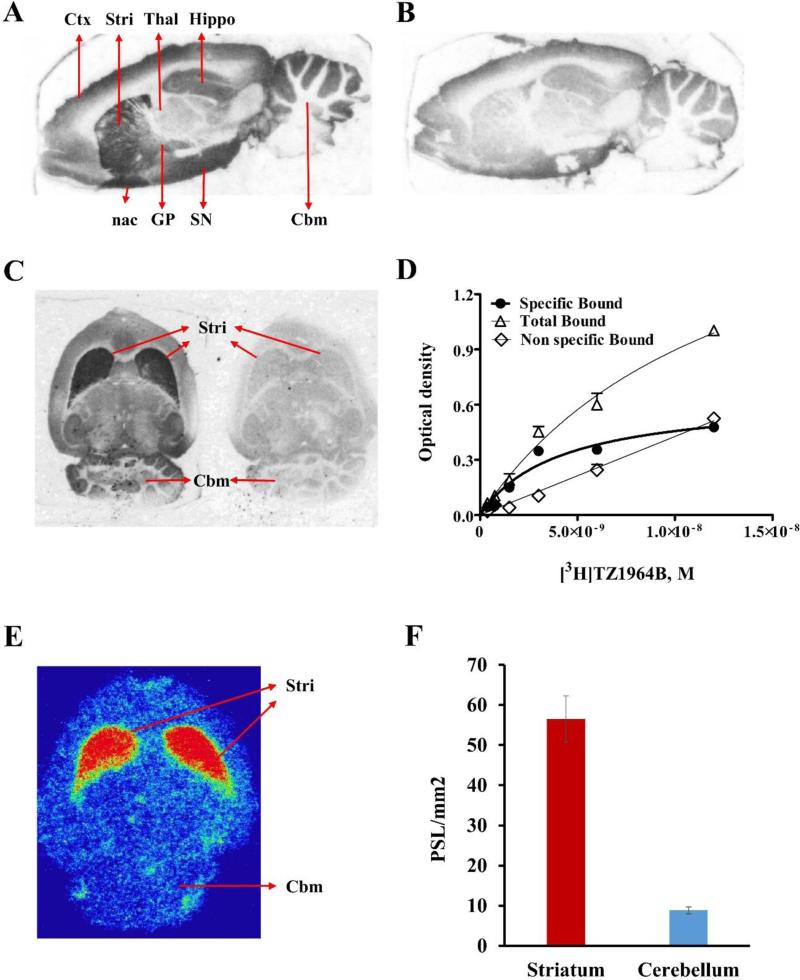
To confirm the selectivity of [3H]TZ1964B in different brain regions, in vitro autoradiography studies were performed using rat brain slices. As shown in Figure 4A&B, [3H]TZ1964B clearly accumulated in the striatum. The addition of 10 μM of MP-10 dramatically reduced the binding of [3H]TZ1964B in the striatum. High radiotracer uptake also was found in globus pallidus, nucleus accumbens, and substantia nigra. Thalamus, cerebellum and frontal cortex showed trace to low level of [3H]TZ1964B accumulation.
Figure 4.
In vitro and/or ex vivo binding specificity and brain distribution of [3H]TZ1964B and [11C]TZ1964B in rat brain. Representative images of in vitro autoradiography of [3H]TZ1964B in rat brain showed high radiotracer uptake in striatum, globus pallidus, nucleus accumbens, and substantia nigra, which could be blocked by MP-10 (A, sagittal section; B, sagittal section with MP-10 blocking; C, horizontal section with and without MP-10 blocking; D. Saturation binding curve obtained from striatal uptake of [3H]TZ1964B). Representative images of ex vivo autoradiography of [11C]TZ1964B in rat brain (E, horizontal section; F, Bar graph showing binding intensity.) confirmed the high radiotracer uptake in the striatum, but not in the cerebellum. PSL/mm2, photo-stimulated luminescence signals per square millimeter.
The specific uptake of [3H]TZ1964B in rat striatum was 5.50-7.50-fold higher than that in cerebellum (Figure 4C, the ratio varied due to different concentrations of [3H]TZ1964B). The saturation binding curve was shown in Figure 4D. [3H]TZ1964B bound to the striatum in rat brain slices with a Kd value of 4.10 ± 1.74 nM.
3.4 Ex vivo autoradiography in the rat brain
Ex vivo autoradiography using [11C]TZ1964B confirmed that the radiotracer clearly labelled the PDE10A sitesin rat striatum with high selectivity, whereas rare accumulation was observed in the cerebellum (Figure 4E & F). The uptake of [11C]TZ1964B in rat striatum was ~6-fold higher than that in the cerebellum.
3.5 In vivo microPET studies in NHPs
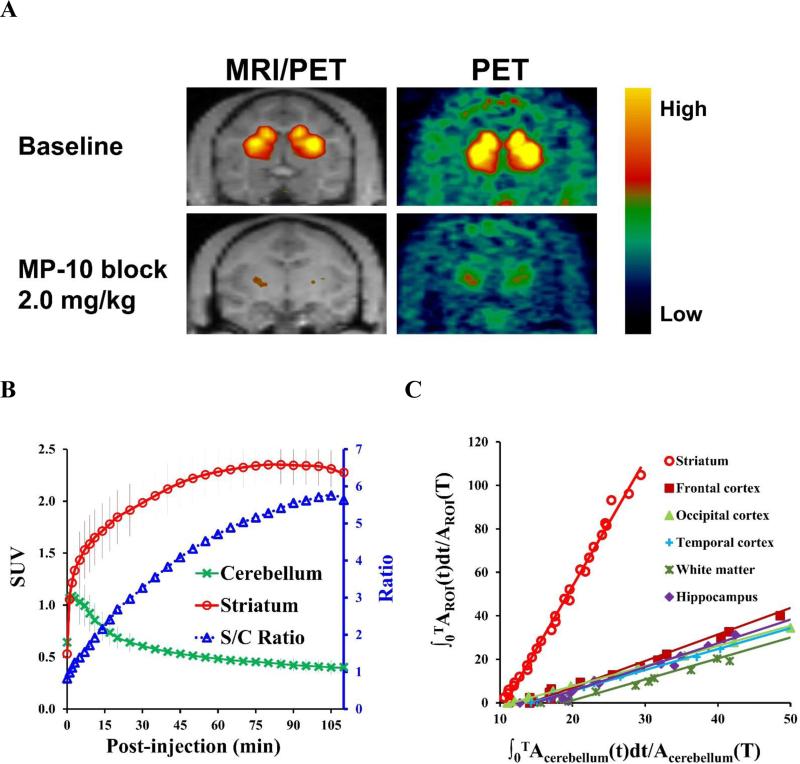
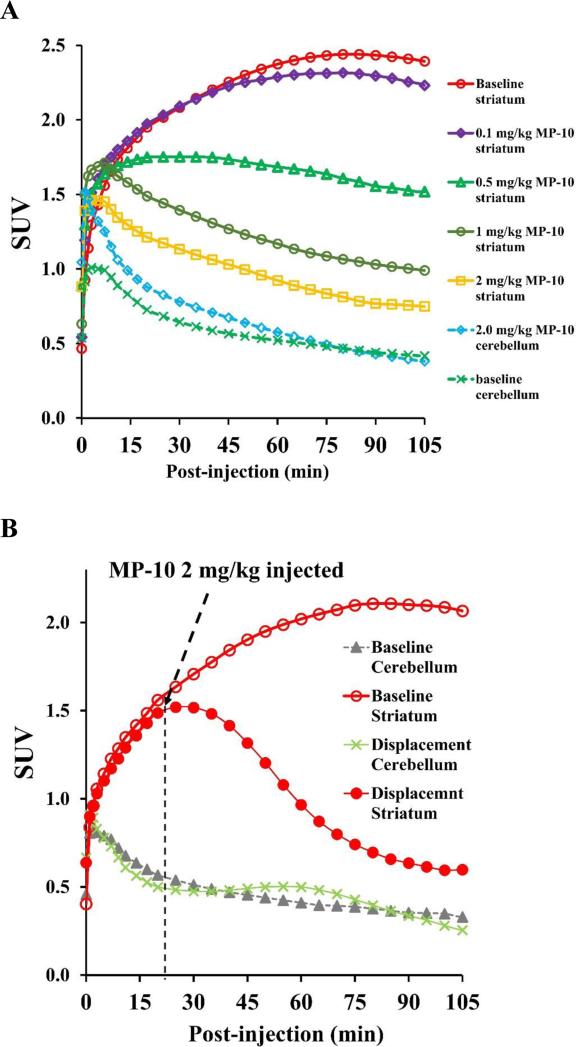
The summed PET images from 0 to 120 min, showing the regional distribution of [11C]TZ1964B at baseline, revealed [11C]TZ1964B retention in striatum (Figure 5A). The selective PDE10A inhibitor MP-10, at a dose of 2.0 mg/kg, reduced significantly the striatal uptake of [11C]TZ1964B, which confirmed the target specificity of [11C]TZ1964B for PDE10A. The average SUV curve (6 baseline scans in 2 NHPs, Figure 5B) showed consistently high accumulation of the radiotracer in the striatum, and fast wash-out in the cerebellum. The striatum to cerebellum ratio was ~5.50 at 90 min post-injection. Using the cerebellum as the reference region, the graphical analysis by LoganREF model showed the uptake of [11C]TZ1964B in the striatum is much higher than other brain regions, including cortex, hippocampus and white matter (Figure 5C).
Figure 5.
In vivo binding specificity and brain distribution of [11C]TZ1964B in non-human primates (NHPs). Representative PET and the co-registered MRI/PET images of [11C]TZ1964B in NHPs (A, summed radioactivity from 0 to 120 min) and averaged SUV curves of striatum and cerebellum (n = 6, B) indicated the tracer consistently accumulated in the NHP striatum, which could be blocked by MP-10 pretreatment. The average striatum to cerebellum (target to reference) ratio near steady state was ~5.50. Graphical analysis by LoganREF model also revealed the striatum has much higher radiotracer uptake than other brain regions (C).
For a 120-min acquisition time of baseline scans, the BPND calculated with the LoganREF model for the striatum was 3.96 ± 0.17 (n = 6, Table 2), using the cerebellum as the reference region. The BPND value using the SRTM for the striatum was 4.64 ± 0.47 (n = 6, Table 2). The examination of test-retest reproducibility and variability was presented in Table 3. The first two scans of subject A and B were used. The average percent changes between test and retest BPND values in the striatum were 4.80% for LoganREF model and 13.80% for SRTM. The test-retest variability (TRV) were 4.62% for LoganREF model and 13.10% for SRTM.
Table 2.
Kinetics parameters of [11C]TZ1964B PET brain imaging in NHPs at baseline
| Regions | Kinetics parameters |
|||
|---|---|---|---|---|
| LoganREF | SRTM | |||
| BPND | BPND | R1 | k2 (min−1) | |
| Striatum | 3.96 ± 0.17 | 4.64 ± 0.47 | 1.06 ± 0.16 | 0.11 ± 0.02 |
| Cerebellum | Reference | |||
(n= 6 scans)
Table 3.
Test-retest variability (TRV) and coefficient of variation (CV) of [11C]TZ1964B PET brain imaging in NHPs at baseline
| Methods | BPstriatum-test (mean ± SD) | CV (%) | BPstriatum-retest (mean ± SD) | CV (%) | Mean change (|%|) | TRV (% ± SD) |
|---|---|---|---|---|---|---|
| LoganREF | 3.93 ± 0.0025 | 0.063 | 4.12 ± 0.063 | 1.52 | 4.80 | 4.62±1.59 |
| SRTM | 4.34 ± 0.23 | 2.27 | 4.94 ± 0.10 | 2.07 | 13.80 | 13.10±3.19 |
(n = 2 subjects, first 2 scans of each subject were included)
As shown in Figure 6A, MP-10 dose-dependently blocked the [11C]TZ1964B uptake in the striatum. When pretreating with MP-10 at dose of 0.25 mg/kg, the striatal BPND was reduced by 26.69% and 17.76%, calculated by LoganREF and SRTM, respectively (Figure 6A, Table 4). Pretreatment using MP-10 at dose of 2.00 mg/kg decreased the striatal BPND by 89.29% (LoganREF) and 85.87% (SRTM) (Figure 6A, Table 4). MP-10 (2.00 mg/kg) administration 20 min post injection of [11C]TZ1964B displaced radiotracer from striatum (Figure 6B). On the other hand, cerebellar uptake was not affected by either pretreatment (blocking) or post-treatment (displacement) studies, validating the use of cerebellum as a suitable reference region.
Figure 6.
Blocking and displacement study of [11C]TZ1964B in NHPs. SUV curves showing the striatal uptake, but not the cerebellar uptake, of [11C]TZ1964B was dose-dependently decreased by MP-10 pretreatment (A). The binding of [11C]TZ1964B in NHP striatum was also reduced by MP-10 displacement (B), indicating a reversible tracer binding property.
Table 4.
Kinetic parameters of [11C]TZ1964B PET brain imaging in NHPs following pharmacological pretreatment
| Doses of MP-10 (mg/kg) | Kinetic parameters (Striatum) | |||||
|---|---|---|---|---|---|---|
| LoganREF | SRTM | |||||
| BPND | Occ% | BPND | R1 | k2 (min−1) | Occ % | |
| 2.00 | 0.4231 | 89.23 | 0.6558 | 1.0209 | 0.04245 | 86.65 |
| 1.50 | 0.4234 | 89.17 | 0.5732 | 0.8844 | 0.08179 | 80.00 |
| 1.00 | 0.8980 | 77.14 | 0.9026 | 0.9932 | 0.05868 | 81.63 |
| 0.50 | 0.8214 | 79.09 | 1.3084 | 0.7716 | 0.08542 | 73.37 |
| 0.25 | 2.8957 | 26.26 | 3.8161 | 1.0628 | 0.08540 | 22.32 |
| 0.10 | 3.7678 | 4.06 | 4.0338 | 1.1128 | 0.10847 | 17.79 |
| 0.05 | 3.7307 | 5.01 | 4.0253 | 0.9354 | 0.09479 | 18.07 |
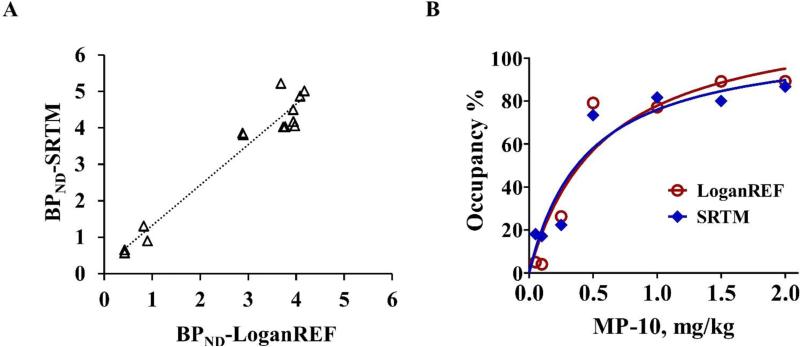
The data acquired from NHPs pretreated with different doses of MP-10 was used to determine the target occupancy after administration of different doses of MP-10 and to estimate the in vivo affinity. The BPND values (both from LoganREF and SRTM) for occupancy studies are shown in Table 4. The reduction of BPND corresponds with increasing dose of pretreatment with MP-10. MP-10 pretreatment doses of 0.05, 0.10, 0.25, 0.50, 1.00, 1.50 and 2.00 mg/kg produced occupancy levels of 5.01%, 4.06%, 26.26%, 79.09%, 77.14%, 89.17% and 89.23% determined by LoganREF (BPND) or 18.07%, 17.19%, 22.32%, 73.37%, 81.63%, 80.00% and 86.65% determined by SRTM (BPND). Scatter plot of BPND in the striatum with SRTM versus BPND LoganREF modeling showed good correlation (r2 = 0.94), including data from both baseline and different doses of MP-10 pretreatments (Figure 7A). The fitted ED50 is 0.31 ± 0.09 mg/kg (LoganREF) and 0.45 ± 0.17 mg/kg (SRTM) (Figure 7B).
Figure 7.
MP-10 dose and PDE10A occupancy relationship. A. Scatter plot of BPND in the striatum with SRTM versus BPND LoganREF modeling showed good correlation. B. Target occupancy curves using LoganREF (red) and SRTM (blue) respectively. The fitted ED50 is 0.31 ± 0.09 mg/kg (LoganREF) and 0.45 ± 0.17 mg/kg (SRTM).
4. Discussion
Recently, several potent radiotracers targeting PDE10A have been developed and validated in vivo by different groups (Barret et al., 2014; Barret, 2012; Celen et al., 2010; Celen et al., 2013; Harada et al., 2015; Hwang et al., 2014; Kehler et al., 2014; Van Laere et al., 2013a; Van Laere et al., 2013b), including another radiolabeled MP-10 analogue, [18F]JNJ42259152. However, as found with MP-10, the possible influence of a brain-penetrating radioactive metabolite of [18F]JNJ42259152 was reported (Celen et al., 2013; Van Laere et al., 2013b). To overcome the common radiolabeled metabolite problem, as reported, we radiosynthesized and preliminarily evaluated a series of new pyrazole group-containing analogues, with a methoxy group on the quinoline fragment (Li et al., 2013). TZ1964B displayed a favorable metabolic stability and was selected as the most promising candidate of PET ligands for PDE10A (Fan et al., 2014). Thus in the present study, [11C]TZ1964B and its radiolabeled counterpart [3H]TZ1964B were further investigated both in vitro and in vivo.
Saturation binding assays in rat striatum homogenates revealed that [3H]TZ1964B has a nanomolar Kd value (1.25 nM), which was consistent with the value obtained from kinetic binding experiments (Koff/ Kon = 1.24 nM). Moreover, obtained from kinetic binding studies, both of the association rate (Kon) and the dissociation rate (Koff) were fast, which indicated that the tracer [3H]TZ1964B binds to the PDE10A binding site quickly, and that the binding is reversible (Hulme and Trevethick, 2010). A similar Kd value (4.10 ± 1.74 nM) in rat striatum was also observed in autoradiographic studies. The slight difference between homogenate binding assays and the brain slice autoradiography has been previously reported (Harada et al., 2015), which might be attributed to the purification of the target protein in the former study.
In competitive binding assays, [3H]TZ1964B has little affinity for PDE1, σ1, σ2, VAChT, dopaminergic, or serotonergic receptors/proteins. Only PDE10A compounds reduced specific binding, confirming the specificity of [3H]TZ1964B binding. Interestingly, among those PDE10A compounds used in competitive binding assay, papavarine and its analogues (9a and 9c) had comparably very large Ki values (>100 nM), while MP-10 and other 3 analogues (TZ1914B, TZ1982T, TZ19106B) had nanomalar Ki values. The different competitive binding patterns of these two categories of PDA10A compounds could be explained by their different binding sites to PDE10A enzyme. According to the crystal structures, MP-10 binds to the “selectivity pocket” of PDE10A that is in proximity to the catalytic site of the enzyme, while papavarine and its analogues only binds to the hydrophobic clamp and exo-binding region (Chappie et al., 2012). Furthermore, the chemical structure of MP-10 analogue TZ1914B shows that the fluorine group in the quinoline fragment locates at the fourth position, instead of at the third position as shown in other 2 MP-10 analogues. The group at the fourth position is close to the M-loop of PDE10A (Chappie et al., 2012), and may be contributed to a comparably low competitive binding affinity of TZ1914B.
Besides nanomolar binding affinity, a high target-to-reference ratio of the radiotracer uptake is required for PET imaging. Therefore, selecting a proper reference region is critical for both in vitro and in vivo validation of novel PET radiotracers. Although the PDE10A distribution in the brain is mainly restricted to the striatum, other brain regions including cortex, thalamus and cerebellum show trace to low levels of PDE10A expression (Seeger et al., 2003). The cerebellum has been used as the reference tissue for the assessment of regional binding potentials for PDE10A radiotracers (Barret, 2012; Kehler et al.,2014; Lin et al., 2015). The frontal cortex and the thalamus were also used as the reference region in human or NHP PET studies (Hwang et al., 2014; Van Laere et al., 2013b). To validate a suitable brain region as the reference for our new tracer, in vitro distribution of [3H]TZ1964B in the brain was examined by in vitro autoradiography. The cerebellum and frontal cortex showed similar low levels of radiotracer uptake, with an even lower level in the thalamus. However, there was no difference in the specific binding in the above three regions. Moreover, our initial microPET imaging of [11C]TZ1964B in NHP showed consistently low tracer accumulation in both the cerebellum and the thalamus, with a slightly high uptake in the frontal cortex.
Accordingly, both the cerebellum and the thalamus may serve as the candidate of reference regions for [11C]TZ1964B PET imaging. In the current study, the cerebellum was chosen for further characterization of radiolabelled TZ1964B both in vitro and in vivo, as the striatum-to-cerebellum ratio is a widely used index for the specificity of PDE10A tracers, permitting comparisons with others radiotracers. The lack of alteration of cerebellar uptake of [3H]TZ1964B and [11C]TZ1964B by blocking or pretreatment with high dose of MP-10 further confirms the suitability of this area as an appropriate reference region for these analyses. The specific uptake of [3H]TZ1964B and [11C]TZ1964B in the rat striatum was 5.50-7.50 fold higher than that in the cerebellum (the reference region), found by in vitro and ex vivo autoradiography, respectively. The striatum-to-cerebellum ratio approximated that from a previous biodistribution study (the ratio was 6.00 at 60 min post-injection) (Fan et al., 2014). Nevertheless, the use of cerebellum as the reference region needs to be confirmed further by arterial blood input analysis in the future.
The in vitro data justified investigation of characterization of the in vivo binding properties of [11C]TZ1964B in NHPs. Baseline scans demonstrated that [11C]TZ1964B consistently and selectively accumulated in the NHP striatum. The average striatum to cerebellum (target to reference) ratio after steady state (~5.50) was close to that observed in in vitro and ex vivo autoradiographic studies. We applied the LoganREF model and the SRTM to determine the binding potential BPND values with the cerebellum as the reference region. The average striatal BPND value was 3.96 (by LoganREF model), and 4.64 (by SRTM). The BPND values using these two models correlated well, although LoganREF model gave lower values, as previously reported in PET imaging analysis of several C-11 labeled radiotracers (Yamasaki et al., 2014; Zanderigo et al., 2013). Moreover, the reproducibility and reliability of the striatal BPND values, calculated by both LoganREF model and SRTM, was evidenced by the low test-retest variability and the low coefficient of variance. The good correlation and the high reliability of these two modelling methods indicated both LoganREF model and SRTM could be applied for PET data analysis of [11C]TZ1964B scans in the brain.
The in vivo binding specificity of [11C]TZ1964B was investigated by pretreatment with different doses of MP-10. The regional tissue time-activity curves revealed that the uptake of [11C]TZ1964B in the striatum, but not in the cerebellum, was reduced following MP-10 pretreatment. In the displacement study, following the MP-10 administration, the uptake of [11C]TZ1964B in the striatum was reduced to the similar level of the cerebellum uptake at the end of PET scan. Along with the in vitro binding assay data, these data demonstrate that[11C]TZ1964B binds specifically to PDE10A, and that the binding is reversible. Furthermore, intravenous injection of MP-10 occupied PDE10A specific striatal binding sites in NHPs. The MP-10 dose of 0.31 ± 0.09 mg/kg (LoganREF) / 0.45 ± 0.17 mg/kg (SRTM) produced about 50% PDE10A occupancy in the striatum. These results provided an example of estimating PDE10A occupancy, and generated key information on the pharmacokinetic-pharmacodynamics relationship of MP-10 (Borroni et al., 2013). The estimated in vivo binding affinity for MP-10 agreed well with the ED50 value measured by [11C]MP-10 (0.30 mg/kg) (Plisson et al., 2011), which further supports the suitability of [11C]TZ1964B in quantifying the in vivo binding sites for PDE10A and evaluating the target occupancy of new-synthesized PDE10A inhibitors.
In conclusion, our results indicate that [11C]TZ1964B has a nanomolar affinity and specificity to PDE10A, has high selectivity for the targeted brain region (striatum), and possesses good characteristics for the in vivo quantification of the PDE10A levels. Furthermore, [11C]TZ1964B has great potential for evaluation of target occupancy of drugs targeting PDE10A. Therefore, [11C]TZ1964B could serve as a suitable candidate for in vivo detecting PDE10A levels in human brain.
Supplementary Material
Highlights.
[3H]TZ1964B has a high potency (Kd = 1.25 nM) and high target selectivity (~6-fold)
[11C]TZ1964B has a high binding potential (3.96-4.64) and high binding specificity in the brain of nonhuman primate
[11C]TZ1964B showed reversible binding property in the monkey striatum
[11C]TZ1964B is a promising PET imaging tracer for assessing PDE10A in the brain
Acknowledgements
This work was supported by the National Institute of Mental Health (NIMH) of the National Institutes of Health (No. MH092797) and National Institute of Neurological Disorders and Stroke (NINDS, No. NS075527, NS061025). The Cyclotron Facility of Washington University provided the [11C]methyl iodide for radiolabeling studies. We would like to thank Emily William, Christina Zukas, Darryl Craig and John Hood for the technique support in NHP microPET studies, Dr. Tom Ellenberger for providing the high-speed centrifuge, Lynne Jones and Adam Rosenberg for manuscript revision, and Jinbin Xu for his technical assistance in the binding assay studies.
Abbreviations
- AIR
automated Image Registration
- BPND
binding potential (non-displaceable)
- CNS
central nervous system
- CR
reference region tracer radioactivity concentration
- CT
target region tracer radioactivity concentration
- DMF
N,N-dimethylformamide
- DVR
volume of distribution ratio
- LoganREF
Logan Reference
- MP-RAGE
magnetization-prepared rapid gradient echo
- MSN
medium spiny neuron
- NHPs
nonhuman primates
- Occ
target occupancy
- PDE10A
phosphodiesterase 10A
- PSL
photo-stimulated luminescence
- ROI
region of interest
- SD
Sprague-Dawley
- SRTM
simplified reference tissue model
- SUV
standardized uptake value
- TRV
percentage test–retest variability
- VAChT
vesicular acetylcholine transporter
- VMAT2
vesicular monoamine transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors do not have any conflict of interest.
References
- Barret O, Thomae D, Tavares A, Alagille D, Papin C, Waterhouse R, McCarthy T, Jennings D, Marek K, Russell D, Seibyl J, Tamagnan G. In Vivo Assessment and Dosimetry of 2 Novel PDE10A PET Radiotracers in Humans: 18F-MNI-659 and 18F-MNI-654. J Nucl Med. 2014;55:1297–1304. doi: 10.2967/jnumed.113.122895. [DOI] [PubMed] [Google Scholar]
- Barret OT, D., Alagille D, Lee H, Papin C, Baldwin R, Jennings D, Marek K, Seibyl J, Tamagnan G. First in vivo assessment of two PDE10 tracers [18F]MNI654 and [18F]MNI659. J. Nucl. Med. 2012;53:361. [Google Scholar]
- Borroni E, Zhou Y, Ostrowitzki S, Alberati D, Kumar A, Hainzl D, Hartung T, Hilton J, Dannals RF, Wong DF. Pre-clinical characterization of [C-11]R05013853 as a novel radiotracer for imaging of the glycine transporter type 1 by positron emission tomography. Neuroimage. 2013;75:291–300. doi: 10.1016/j.neuroimage.2011.11.090. [DOI] [PubMed] [Google Scholar]
- Celen S, Koole M, De Angelis M, Sannen I, Chitneni SK, Alcazar J, Dedeurwaerdere S, Moechars D, Schmidt M, Verbruggen A, Langlois X, Van Laere K, Andres JI, Bormans G. Preclinical Evaluation of F-18-JNJ41510417 as a Radioligand for PET Imaging of Phosphodiesterase-10A in the Brain. Journal of Nuclear Medicine. 2010;51:1584–1591. doi: 10.2967/jnumed.110.077040. [DOI] [PubMed] [Google Scholar]
- Celen S, Koole M, Ooms M, De Angelis M, Sannen I, Cornelis J, Alcazar J, Schmidt M, Verbruggen A, Langlois X, Van Laere K, Andres JI, Bormans G. Preclinical evaluation of [18F]JNJ42259152 as a PET tracer for PDE10A. Neuroimage. 2013;82:13–22. doi: 10.1016/j.neuroimage.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Chappie TA, Helal CJ, Hou XJ. Current Landscape of Phosphodiesterase 10A (PDE10A) Inhibition. Journal of Medicinal Chemistry. 2012;55:7299–7331. doi: 10.1021/jm3004976. [DOI] [PubMed] [Google Scholar]
- Fan J, Zhang X, Li J, Jin H, Padakanti PK, Jones LA, Flores HP, Su Y, Perlmutter JS, Tu Z. Radiosyntheses and in vivo evaluation of carbon-11 PET tracers for PDE10A in the brain of rodent and nonhuman primate. Bioorg Med Chem. 2014;22:2648–2654. doi: 10.1016/j.bmc.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Harada A, Suzuki K, Miura S, Hasui T, Kamiguchi N, Ishii T, Taniguchi T, Kuroita T, Takano A, Stepanov V, Halldin C, Kimura H. Characterization of the binding properties of T-773 as a PET radioligand for phosphodiesterase 10A. Nucl Med Biol. 2015;42:146–154. doi: 10.1016/j.nucmedbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. British Journal of Pharmacology. 2010;161:1219–1237. doi: 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DR, Hu E, Rumfelt S, Easwaramoorthy B, Castrillon J, Davis C, Allen JR, Chen H, Treanor J, Abi-Dargham A, Slifstein M. Initial characterization of a PDE10A selective positron emission tomography tracer [11C]AMG 7980 in non-human primates. Nucl Med Biol. 2014;41:343–349. doi: 10.1016/j.nucmedbio.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Giedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Lida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kehler J, Kilburn JP, Estrada S, Christensen SR, Wall A, Thibblin A, Lubberink M, Bundgaard C, Brennum LT, Steiniger-Brach B, Christoffersen CT, Timmermann S, Kreilgaard M, Antoni G, Bang-Andersen B, Nielsen J. Discovery and Development of C-11-Lu AE92686 as a Radioligand for PET Imaging of Phosphodiesterase10A in the Brain. Journal of Nuclear Medicine. 2014;55:1513–1518. doi: 10.2967/jnumed.114.140178. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Li JF, Jin HJ, Zhou HY, Rothfuss J, Tu ZD. Synthesis and in vitro biological evaluation of pyrazole group-containing analogues for PDE10A. Medchemcomm. 2013;4:443–449. doi: 10.1039/C2MD20239E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SF, Labaree D, Chen MK, Holden D, Gallezot JD, Kapinos M, Teng JK, Najafzadeh S, Plisson C, Rabiner EA, Gunn RN, Carson RE, Huang Y. Further evaluation of [11C]MP-10 as a radiotracer for phosphodiesterase 10A: PET imaging study in rhesus monkeys and brain tissue metabolite analysis. Synapse. 2015;69:86–95. doi: 10.1002/syn.21792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of Cerebral Blood Flow and Metabolism. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Miller TR, Wallis JW, Wilson AD. Interactive reconstruction in single-photon tomography. Eur J Nucl Med. 1989;15:189–193. doi: 10.1007/BF00253792. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat. 2011;5:43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plisson C, Salinas C, Weinzimmer D, Labaree D, Lin SF, Ding YS, Jakobsen S, Smith PW, Eiji K, Carson RE, Gunn RN, Rabiner EA. Radiosynthesis and in vivo evaluation of [11C]MP-10 as a positron emission tomography radioligand for phosphodiesterase 10A. Nucl Med Biol. 2011;38:875–884. doi: 10.1016/j.nucmedbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–126. doi: 10.1016/s0006-8993(03)02754-9. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci U S A. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick CAS, C. J., Menniti FS. PDE10A: a striatum-enriched, dual-substrate phosphodiesterase. In: Joseph ABS, H. F., Miles DH, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. CRC Press; 2010. pp. 237–254. [Google Scholar]
- Suhara T, Takano A, Sudo Y, Ichimiya T, Inoue M, Yasuno F, Ikoma Y, Okubo Y. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Archives of General Psychiatry. 2003;60:386–391. doi: 10.1001/archpsyc.60.4.386. [DOI] [PubMed] [Google Scholar]
- Tu Z, Fan J, Li S, Jones LA, Cui J, Padakanti PK, Xu J, Zeng D, Shoghi KI, Perlmutter JS, Mach RH. Radiosynthesis and in vivo evaluation of [11C]MP-10 as a PET probe for imaging PDE10A in rodent and non-human primate brain. Bioorg Med Chem. 2011;19:1666–1673. doi: 10.1016/j.bmc.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Xu J, Jones LA, Li S, Mach RH. Carbon-11 labeled papaverine as a PET tracer for imaging PDE10A: radiosynthesis, in vitro and in vivo evaluation. Nucl Med Biol. 2010;37:509–516. doi: 10.1016/j.nucmedbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laere K, Ahmad RU, Hudyana H, Celen S, Dubois K, Schmidt ME, Bormans G, Koole M. Human biodistribution and dosimetry of 18F-JNJ42259152, a radioligand for phosphodiesterase 10A imaging. Eur J Nucl Med Mol Imaging. 2013a;40:254–261. doi: 10.1007/s00259-012-2270-1. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Ahmad RU, Hudyana H, Dubois K, Schmidt ME, Celen S, Bormans G, Koole M. Quantification of 18F-JNJ-42259152, a novel phosphodiesterase 10A PET tracer: kinetic modeling and test-retest study in human brain. J Nucl Med. 2013b;54:1285–1293. doi: 10.2967/jnumed.112.118679. [DOI] [PubMed] [Google Scholar]
- Watabe H, Itoh M, Cunningham V, Lammertsma AA, Bloomfield P, Mejia M, Fujiwara T, Jones AKP, Jones T, Nakamura T. Noninvasive quantification of rCBF using positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 1996;16:311–319. doi: 10.1097/00004647-199603000-00017. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, Menniti FS. Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience. 2006;139:597–607. doi: 10.1016/j.neuroscience.2005.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D(3) and D(2) receptor density ratio in the caudate-putamen. Synapse. 2010;64:449–459. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Maeda J, Fujinaga M, Nagai Y, Hatori A, Yui J, Xie L, Nengaki N, Zhang MR. PET brain kinetics studies of (11)C-ITMM and (11)C-ITDM,radioprobes for metabotropic glutamate receptor type 1, in a nonhuman primate. Am J Nucl Med Mol Imaging. 2014;4:260–269. [PMC free article] [PubMed] [Google Scholar]
- Zanderigo F, Ogden RT, Parsey RV. Reference region approaches in PET: a comparative study on multiple radioligands. J Cereb Blood Flow Metab. 2013;33:888–897. doi: 10.1038/jcbfm.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.