Abstract
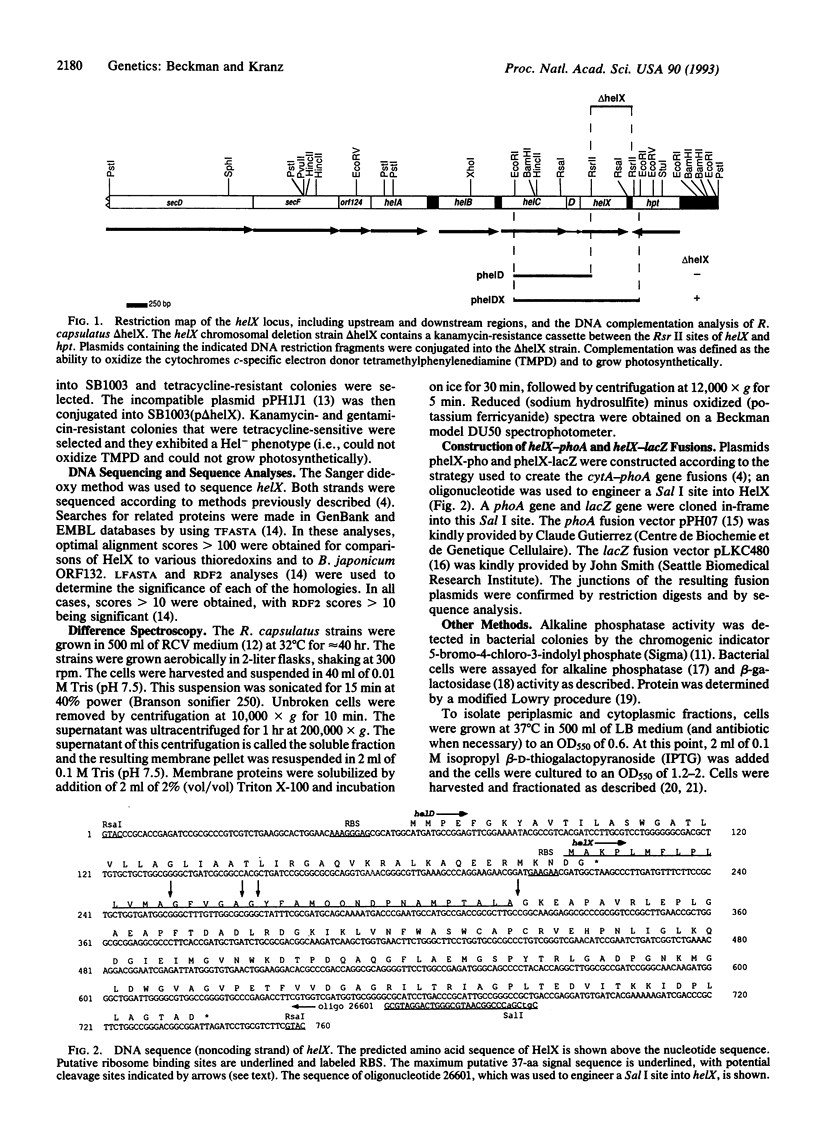
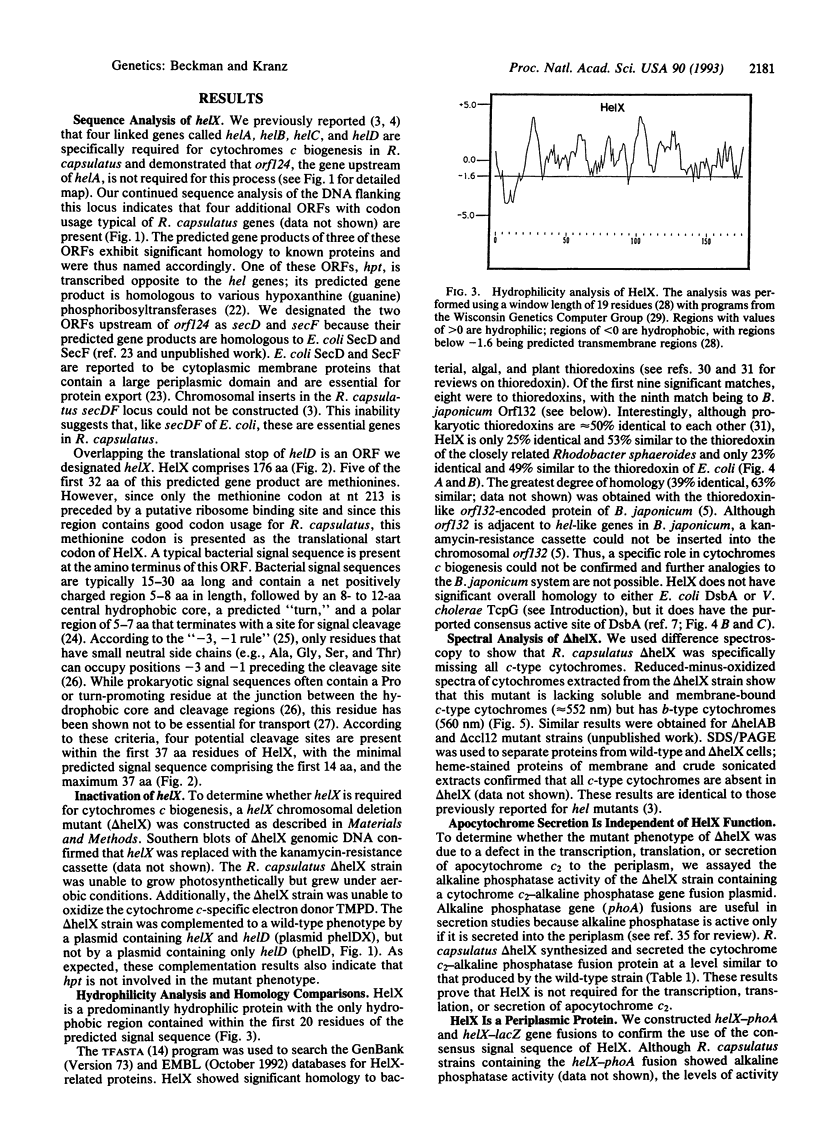
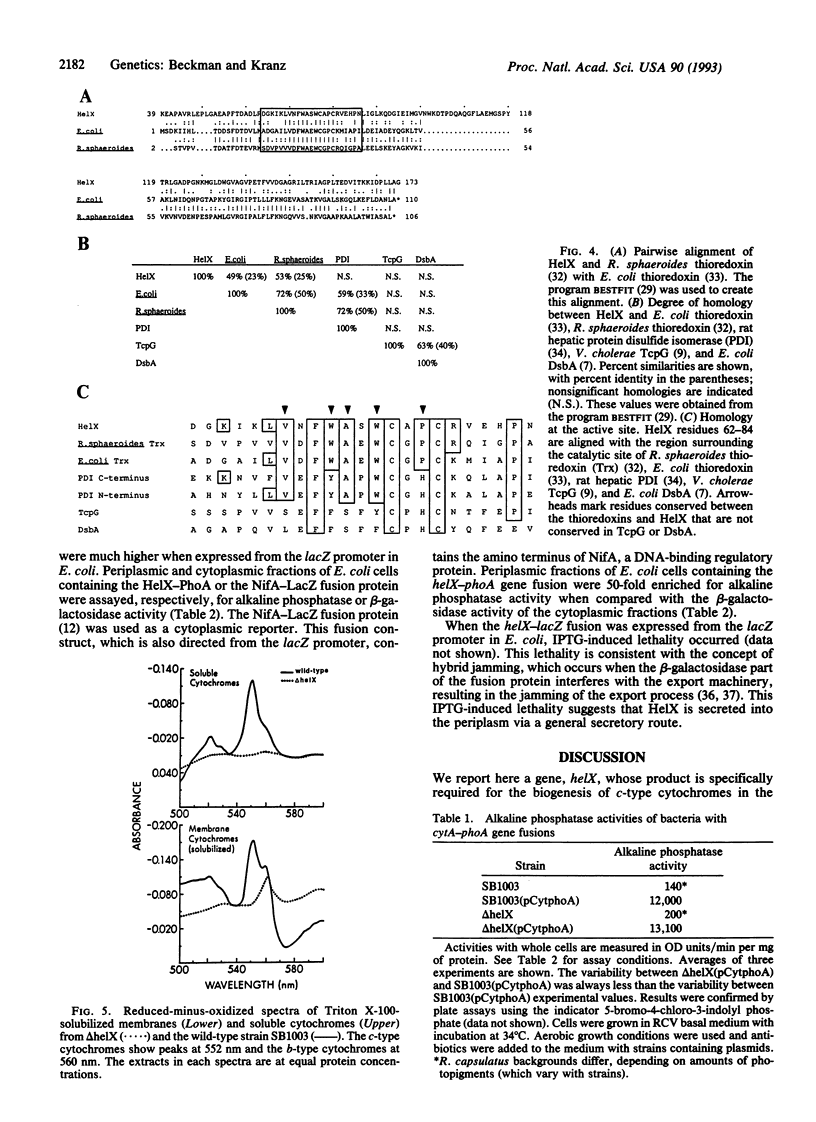
Rhodobacter capsulatus is a Gram-negative photosynthetic bacterium that requires c-type cytochromes for photosynthetic electron transport. Our studies demonstrate that the gene helX is required for the biogenesis of c-type cytochromes in R. capsulatus. A helX chromosomal deletion mutant cannot grow photosynthetically, due to a deficiency of all c-type cytochromes. The predicted amino acid sequence of the helX gene product (176 residues) is related to that of thioredoxin and shares active-site homology with protein disulfide isomerase. Cytochrome c2-alkaline phosphatase gene fusions are used to show that HelX is not required for the transcription, translation, or secretion of apocytochrome c2. HelX-alkaline phosphatase and HelX-beta-galactosidase gene fusions are used to demonstrate that HelX is a periplasmic protein, which is consistent with the presence of a typical signal sequence in HelX. Based on these results, we propose HelX functions as a periplasmic disulfide oxidoreductase that is essential for cytochromes c biogenesis. This role is in accordance with the observation that both heme and the cysteines of apocytochromes c (Cys-Xaa-Yaa-Cys-His) must be in the reduced state for covalent linkage between the two moieties to occur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Beckman D. L., Kranz R. G. A bacterial homolog to HPRT. Biochim Biophys Acta. 1991 Dec 2;1129(1):112–114. doi: 10.1016/0167-4781(91)90223-9. [DOI] [PubMed] [Google Scholar]
- Beckman D. L., Trawick D. R., Kranz R. G. Bacterial cytochromes c biogenesis. Genes Dev. 1992 Feb;6(2):268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Biel S. W., Biel A. J. Isolation of a Rhodobacter capsulatus mutant that lacks c-type cytochromes and excretes porphyrins. J Bacteriol. 1990 Mar;172(3):1321–1326. doi: 10.1128/jb.172.3.1321-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Eklund H., Gleason F. K., Holmgren A. Structural and functional relations among thioredoxins of different species. Proteins. 1991;11(1):13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- Enosawa S., Ohashi A. A simple and rapid assay for heme attachment to apocytochrome c. Anal Biochem. 1987 Jan;160(1):211–216. doi: 10.1016/0003-2697(87)90632-4. [DOI] [PubMed] [Google Scholar]
- Foster-Hartnett D., Kranz R. G. Analysis of the promoters and upstream sequences of nifA1 and nifA2 in Rhodobacter capsulatus; activation requires ntrC but not rpoN. Mol Microbiol. 1992 Apr;6(8):1049–1060. doi: 10.1111/j.1365-2958.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- Gardel C., Johnson K., Jacq A., Beckwith J. The secD locus of E.coli codes for two membrane proteins required for protein export. EMBO J. 1990 Oct;9(10):3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D. H., Neupert W. Biogenesis of mitochondrial c-type cytochromes. J Bioenerg Biomembr. 1990 Dec;22(6):753–768. doi: 10.1007/BF00786929. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Devedjian J. C. A plasmid facilitating in vitro construction of phoA gene fusions in Escherichia coli. Nucleic Acids Res. 1989 May 25;17(10):3999–3999. doi: 10.1093/nar/17.10.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. R., Beringer J. E. A physical map of pPH1JI and pJB4JI. Plasmid. 1984 Sep;12(2):139–141. doi: 10.1016/0147-619x(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Kamitani S., Akiyama Y., Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992 Jan;11(1):57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Characterization of nif regulatory genes in Rhodopseudomonas capsulata using lac gene fusions. Gene. 1985;40(2-3):203–215. doi: 10.1016/0378-1119(85)90043-5. [DOI] [PubMed] [Google Scholar]
- Kranz R. G. Isolation of mutants and genes involved in cytochromes c biosynthesis in Rhodobacter capsulatus. J Bacteriol. 1989 Jan;171(1):456–464. doi: 10.1128/jb.171.1.456-464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laforet G. A., Kendall D. A. Functional limits of conformation, hydrophobicity, and steric constraints in prokaryotic signal peptide cleavage regions. Wild type transport by a simple polymeric signal sequence. J Biol Chem. 1991 Jan 15;266(2):1326–1334. [PubMed] [Google Scholar]
- Lee C., Li P., Inouye H., Brickman E. R., Beckwith J. Genetic studies on the inability of beta-galactosidase to be translocated across the Escherichia coli cytoplasmic membrane. J Bacteriol. 1989 Sep;171(9):4609–4616. doi: 10.1128/jb.171.9.4609-4616.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D. W., Stuart R. A., Neupert W. Biogenesis of cytochrome c1. Role of cytochrome c1 heme lyase and of the two proteolytic processing steps during import into mitochondria. J Biol Chem. 1989 Jun 15;264(17):10156–10168. [PubMed] [Google Scholar]
- Oda K., Yamato K., Ohta E., Nakamura Y., Takemura M., Nozato N., Akashi K., Kanegae T., Ogura Y., Kohchi T. Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. A primitive form of plant mitochondrial genome. J Mol Biol. 1992 Jan 5;223(1):1–7. doi: 10.1016/0022-2836(92)90708-r. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek J. A., Taylor R. K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pille S., Chuat J. C., Breton A. M., Clément-Métral J. D., Galibert F. Cloning, nucleotide sequence, and expression of the Rhodobacter sphaeroides Y thioredoxin gene. J Bacteriol. 1990 Mar;172(3):1556–1561. doi: 10.1128/jb.172.3.1556-1561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. C., Daldal F. Physiological electron donors to the photochemical reaction center of Rhodobacter capsulatus. Biochim Biophys Acta. 1987 Dec 17;894(3):370–378. doi: 10.1016/0005-2728(87)90115-0. [DOI] [PubMed] [Google Scholar]
- Ramseier T. M., Winteler H. V., Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991 Apr 25;266(12):7793–7803. [PubMed] [Google Scholar]
- Tai S. P., Kaplan S. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J Bacteriol. 1985 Oct;164(1):181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. lacZY gene fusion cassettes with KanR resistance. Nucleic Acids Res. 1988 Apr 25;16(8):3587–3587. doi: 10.1093/nar/16.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]


