Abstract
Diffuse infiltration across brain tissue is a hallmark of glioblastoma and the main cause of unsuccessful total resection that leads to tumor reappearance. A subpopulation termed glioblastoma stem cells (GSCs) has been directly related to aggressive invasion; nonetheless, their migratory characteristics and regulation by the microenvironment are still unknown. In this study, we developed a composite matrix of hyaluronan (HA) structurally supported by a collagen-oligomer fibril network to simulate the brain tumor extracellular matrix (ECM) composition. Matrigel-coated microfibers were embedded within the matrix to create a tunable dual niche microenvironment that resembles the vascular network of the brain. This model was compared with the most commonly used in vitro three-dimensional (3D) culture formats, Matrigel and collagen type-I monomer matrices, to study how the mechanical and compositional properties of the ECM alter the migration characteristics of GSC neurospheres. The migration mode, distance, velocity, and morphology of the GSCs were monitored over a 72-h period. The cells altered their migration mode depending on the matrix composition, showing migration by expansive growth in Matrigel matrices, multicellular extension along rigid interfaces (as Matrigel glass and coated microfibers), and mesenchymal single-cell migration in collagen matrices. Velocity and distance of migration within each composition varied according to matrix mechanical properties. In the dual niche system, the presence of HA reduced velocity and number of migratory cells; however, cells that came in contact with the pseudovessels exhibited collective migration by an extensive strand and reached higher velocities than cells migrating individually across the 3D matrix. Our results show that GSCs adopt varied migration mechanisms to invade multiple ECM microenvironments, and the migration characteristics exhibited are highly influenced by the matrix physical properties. Moreover, GSC neurospheres exhibit concomitant single and collective migration as a function of the microenvironment topography to reach the most productive migration strategy.
Introduction
Glioblastoma (GBM) is a highly invasive and lethal brain cancer with an estimated 5-year survival of less than 5%.1 Low prognosis is primarily due to resistance to chemotherapy and radiotherapy coupled with diffuse infiltration into healthy brain parenchyma.2,3 GBM resistance, rapid growth, and propagation have been linked to the presence of a subpopulation of tumor cells with stem cell-like features termed as glioblastoma stem cells (GSCs).4–9 Given the aggressiveness of GSCs, it has been hypothesized that these cells drive the invasion into healthy brain tissue and contribute to regrowth of a new heterogeneous tumor.
It is now well established that tumor cell invasion and maintenance of tumor stem cells are processes regulated by the microenvironment and involve specific interactions with the extracellular matrix (ECM).10–12 The brain ECM has a distinct composition relative to other tissues and organs. It represents a low stiffness and loosely connected network comprised mainly of hyaluronan (HA).13,14 In cases such as glioma development, tumor cells actively remodel their microenvironment by depositing their own ECM, including type-I collagen as a component of the tumor tissue, surrounding environment,15 and GSC niche.16 Interestingly, GBM can successfully invade any part of the brain, yet, unlike other cancers, rarely metastasizes. During invasion, migratory GBM cells preferentially use existing tracks such as myelinated axons in white matter and the basement membrane (BM) surrounding blood vessels, as has been shown by histopathological examination.17 Such invasion pattern suggests the existence of productive infiltration mechanisms mediated by the brain-specific microenvironment that foster tumor expansion.13,18
Despite the well-known characteristics of brain microenvironment and glioma invasion routes, there is still a lack of understanding about the mechanistic migration processes exhibited by GBM cells and specifically by GSCs.19 A critical barrier in the cancer field is that most of the invasion and migration studies are conducted using two-dimensional (2D) substrates that fail to recapitulate the dimensionality, composition, and physical properties of brain tissue.20 Increasingly, direct passage of human tumor cells within xenograft mouse models has been preferred to maintain the in vivo characteristics of cancer cells and the tumor microenvironment; however, processes such as GSC migration are still difficult to monitor and systematically interrogate due to the complexity of these systems and the limited number of GSCs present in the tumor.21 Consequently, three-dimensional (3D) in vitro models that mimic multiple features of the tumor microenvironment and allow the study of important cancer cell subpopulations, such as GSCs, are required to compliment in vivo models and histopathological analysis.
Although previous studies have provided useful insight about phenotypic and invasive characteristics of GBM when cultured in 3D matrices, such as collagen monomers (e.g., telocollagen and atelocollagen),22–24 chemically functionalized HA,25,26 chitosan–alginate,27 and poly(ethylene-glycol) (PEG)-HA,28 these matrices incorporate chemical groups naturally absent in vivo and/or do not represent the composition, structure, or topographical characteristics of the tumor environment. In this study, we recreated the main features of the GBM microenvironment by developing a tunable 3D matrix with a similar composition to glioma ECM with incorporated topographical tracks, which simulate the brain vasculature, to study over time the migratory behavior of GSC neurospheres and cell line GBAM1 (CD133+) (Supplementary Methods, Supplementary Figs. S1A, B, and S2; Supplementary Data are available online at www.liebertpub.com/tea). The composite matrix consisted of a HA network structurally supported by a customizable collagen-oligomer fibril matrix embedded with BM-coated microfibers to provide alternative migratory paths as occurs in vivo.
In addition, for comparison, we generated reconstituted matrices from the most common 3D cell culture substrates, type-I collagen monomers and Matrigel. Matrigel represents a commercial EHS mouse sarcoma extract that mainly comprised laminin and type-IV collagen. This tumor extract, which forms a BM-like hydrogel when warmed to 37°C, has traditionally been used for tumor invasion studies.29 In addition, since solubilized type-I collagen formulations differ significantly in their molecular composition and fibril–matrix formation capacity, we prepared matrices with diverse structural and mechanical properties using commercial monomeric collagen (BD Biosciences, San Jose, CA), as well as atelocollagen and oligomeric collagen extracted from pig skin. We performed 3D culture of GSC (GBAM1) neurospheres in the different matrices to monitor and compare overtime how fundamental migratory behavior, such as migration mode, velocity, maximum distance, and morphology exhibited by GSC neurospheres is regulated by the compositional and physical properties of the different matrices. To the best of our knowledge, this is the first study that evaluates the migration of GSC neurospheres in multiple types of ECMs, including a specifically designed matrix that resembles the main features of the glioma microenvironment.
Materials and Methods
Cell liquid culture
GBM cells, isolated from human surgical tumor specimens sorted by FACS for CD133 expression (GBAM1), were kindly provided by Dr. Phillip Tofilon and the Moffitt Cancer Center. GSC GBAM1 cells (CD133+ >98% from passages 17–23) met all criteria for a stem-like population, including neurosphere formation in stem cell media, expression of the stem-like markers Sox2, Notch, and Nestin, and capability to differentiate into glial cells.30 Cells were maintained in stem liquid media DMEM/F12 (Life Technologies, Carlsbad, CA) supplemented with B27 without vitamin A (Life Technologies) and growth factors, EGF and bFGF (50 ng/mL each; Peprotech, Rocky hill, NJ), at 37°C in an atmosphere of 5% CO2. Cells were propagated in T25 or T75 flasks and fed with complete media every other day. Neurospheres were disaggregated with TrypLE (Life Technologies) and passaged every 7 days.
Synthesis of collagen and collagen-HA matrices
Three-dimensional collagen matrices were generated using rat tail type-I collagen monomers from BD Biosciences, pig skin type-I collagen oligomer,31 and pig skin type-I atelocollagen. All collagens were adjusted to the desired concentration and polymerized by neutralization with 10 × phosphate-buffered saline (PBS) (1 × PBS has a 0.17 M total ionic strength) and 0.1 N sodium hydroxide to reach pH 7.4. During and after neutralization, all reagents were maintained at 4°C and the neutralized collagen solutions were pipetted at volumes of 100 μL in 96 multiwell plates and polymerized at 37°C during 30 min. To generate the composed oligomer-HA matrices, sodium hyaluronate of molecular weight between 351 and 600 KDa (Lifecore Biomedical, Chaska, MN) was dissolved in 10 × PBS and added during neutralization of the oligomer to attain final concentrations of 2 mg/mL oligomer and 2, 5, and 10 mg/mL HA. To mimic the topography generated by the blood vessels in the brain parenchyma, pseudovessels were recreated using sterile rods of PDS II-polydioxanone of diameter 100–150 μm (Ethicon, Blue Ash, OH) coated with Matrigel by immersion. The coated rods were incubated at 37°C for 30 min and immersed in the oligomer-HA matrix before polymerization. To corroborate the presence of Matrigel on the rod surface, sample rods were incubated with anti-Collagen type IV eFluor® 660 antibody (eBiosciences, Santa Clara, CA) and imaged with confocal microscopy (Supplementary Fig. S3). For the control experiments, noncoated rods were used.
Analysis of mechanical properties
Rheological properties of the matrices were measured by oscillatory shear in a stress-controlled AR2000 rheometer (TA Instruments, New Castle, DE) using a parallel plate geometry (40 mm diameter). Each sample (1 mL) was polymerized at 37°C on the rheometer during 30 min, the geometry was set at 725 μm gap distance, and humidity was maintained by a solvent trap as previously described.31 The viscoelastic properties, shear storage modulus (G′), and shear loss modulus (G′′) were determined by a strain–stress sweep from 0.01% to 0.4% at 1 Hz (this range was chosen from predetermined linear viscoelastic response regions). All measurements were conducted on at least three independent samples.
Cell culture in 3D matrices
GBAM1 cells were cultured in complete liquid media for 4 days. Neurospheres were collected using a 100 μm cell strainer (BD Biosciences) and recovered from the strainer by washing with PBS. Before, polymerization neurospheres were suspended in each of the matrices (Matrigel from BD Biosciences, collagen and collagen-HA) to achieve a density of 1–2 neurospheres per 100 μL of polymeric suspension. Volumes of 100 μL of cell–matrix suspension were platted into 96-well plates. Subsequently, the matrices were polymerized at 37°C for 30 min followed by addition of complete liquid media. Cells were cultured at 37°C in an atmosphere of 5% CO2.
Immunofluorescence staining
For N-cadherin detection in GBAM1 cultured in 3D matrices, matrices with embedded neurospheres were fixed with 4% glutaraldehyde, permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO), blocked with 5% bovine serum albumin (Life Technologies), and incubated with the rabbit polyclonal N-cadherin antibody (Santa Cruz Biotech, Santa Cruz, CA) (dilution 1:40) at 4°C for 12 h. The matrix was washed and incubated with IgG anti-rabbit Alexa-488 (Life Technologies) (dilution 1:200) at 4°C for 6 h. Nuclei were counterstained with Hoechst 33342 (Life Technologies) during 1 h.
Laser scanning confocal and light microscopy
Migration of GBAM1 cells within the 3D matrices was quantified using bright field and phase contrast images collected at 1, 24, 48, and 72 h after polymerization of the matrices. All images were collected using an AmScope (10MP) camera on a CKX41 Olympus inverted microscope. Confocal images for detection of N-cadherin and Hoechst 33342 were taken with a Nikon A1R-MP confocal microscope equipped with objectives Apo 40X (1.25 NA) and Plan Fluor 40X-oil (1.3 NA). Confocal reflectance microscopy (CRM) was performed to analyze the structure of the collagen-HA matrices in their hydrated state. The images were obtained from three independent samples for each matrix at random positions using the objective Plan Fluor 20X (0.75 NA) in the reflectance mode.
Analysis of morphology and migration
Bright field and phase contrast images were analyzed using the software ImageJ (NIH, Bethesda, MD). Migration distance was quantified as the distance from the initial perimeter of the neurosphere to the edge of the most external protrusion or migratory cell.23,32 Average migration velocity was calculated by dividing the distance recorded by the time interval chosen (24, 48, or 72 h).
Statistical analysis
Quantitative data were compared by the t-test (α = 0.05), Mann–Whitney, or ANOVA using the statistical software package SAS 9.3 (SAS Institute, Cary, NC).
Results
Modulation of GSC migration by varying the concentration of Matrigel matrices
Invasion of cancer cells has been traditionally studied in Matrigel matrices due to its similar composition to the BM, an in vivo type of ECM rapidly infiltrated by tumor cells during tumor invasion.12,29
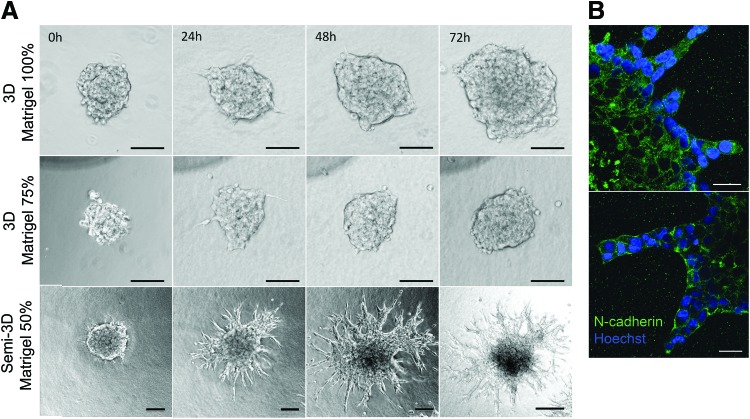
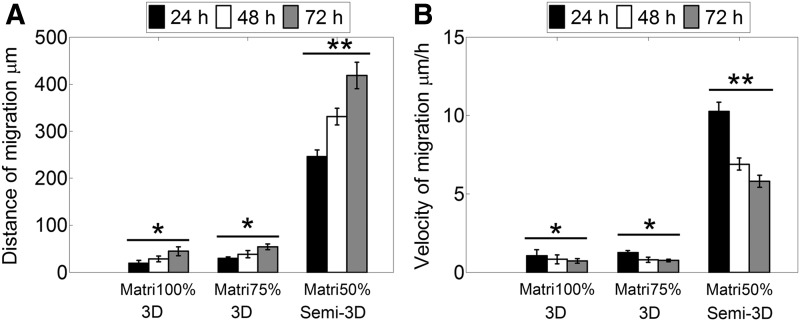
To investigate time-dependent migration of GSCs within matrices of similar composition to BM, neurospheres were seeded within Matrigel (100%), Matrigel (75%)-DMEM/F12 (25% v/v), and Matrigel (50%)-DMEM/F12 (50% v/v) to monitor their migratory behavior over 72 h. During the first hours of culture, the neurospheres developed thin pseudopodia-like extensions that were more abundant and larger in Matrigel 50% compared to other Matrigel matrices. Subsequently, the extensions exhibited in Matrigel 75% and 100% disappeared, and cells continued expanding as a functional aggregate with spherical morphology resembling multicellular migration by expansive growth (Fig. 1A).33 Contrastingly, the initial pseudopodia developed in Matrigel 50% extensions were gradually replaced by large and wide protrusions with elongated tips that grew radially and remained in contact with the neurosphere. Three-dimensional reconstruction from confocal microscopy images of neurospheres cultured in Matrigel 50% showed that the tips of the migratory strands were located on the same focal plane (Supplementary Fig. S4) suggesting that, different to the other 3D matrices, Matrigel 50% allowed the generation of focal contacts with the plate surface generating a semi-3D (or 2.5D) substrate.34 It is possible that at this Matrigel concentration, the material shear storage modulus became low enough (Table 1) for the neurospheres to settle and interact with the rigid surface of the 2D culture plate. To corroborate that multiple cells in contact formed the extensions, we performed immunostaining to detect expression of N-cadherin. The results suggest maintenance of cell–cell contacts in both, the cells forming the neurosphere and cells migrating as multicellular extensions (Fig. 1B). In comparison, GSCs in the 3D Matrigel expanded into significantly smaller distances and with significantly slower velocities compared to the GSCs that were able to contact the rigid culture plate (Fig. 2A, B).
FIG. 1.
(A) Glioblastoma stem cell (GSC) neurospheres present different migration characteristics when seeded within Matrigel matrices of different concentrations. Neurospheres exhibit migration by expansive growth when cultured in three-dimensional (3D) matrices of Matrigel 100% and 75%, while Matrigel 50% exhibits multicellular strand migration due to low compliance of the matrix (semi-3D substrate). Scale bars 100 μm. Migration was determined as the distance from the initial perimeter of the neurosphere to the edge of the most external strand or migratory cell. (B) GSCs cultured in Matrigel 50% develop contacts with rigid surfaces of the plate (semi-3D substrate), giving onset to wide, stable, and long protrusions that comprised one or more cells with intact and stable cell–cell contacts. Neurospheres after 72h of culture were fixed and stained with Hoechst 33342 and N-cadherin-Alexa 488. Staining for nuclei was incomplete due to the high density of the neurosphere. Scale bars 25 μm. Color images available online at www.liebertpub.com/tea
Table 1.
Viscoelastic Properties of the Different Matrices Used for Three-Dimensional Migration Studies of Glioblastoma Stem Cell (n = 3, Mean ± SEM)
| Matrix | Shear storage modulus G′ (Pa) | Matrix | Shear storage modulus G′ (Pa) |
|---|---|---|---|
| Matrigel 100% | 56.5 ± 3.1 | BD-Col 3 mg/mL | 24.5 ± 0.3 |
| Matrigel 75% | 35.6 ± 0.8 | BD-Col 1.5 mg/mL | 6.5 ± 1.9 |
| Matrigel 50% | 17.9 ± 0.3 | Atelocollagen 4 mg/mL | 39 ± 2.1 |
| Oligomer 3 mg/mL | 885 ± 92.3 | Atelocollagen 2 mg/mL | 4 ± 1.3 |
| Oligomer 2 mg/mL | 397 ± 32.7 | HA:2 mg/mL-Col:2 mg/mL | 126.2 ± 14.6 |
| Oligomer 1.5 mg/mL | 225 ± 14.1 | HA:5 mg/mL-Col:2 mg/mL | 107.9 ± 19.6 |
| Oligomer 1 mg/mL | 90 ± 5.2 | HA:10 mg/mL-Col:2 mg/mL | 36.1 ± 0.6 |
HA, hyaluronan.
FIG. 2.
(A) Distance of migration reached by GSCs increases progressively in all the matrices. Cells cultured in Matrigel 50% that exhibit long protrusions due to contact with rigid surfaces (semi-3D substrate) present the highest distance. (B) Velocity of migration of GSC at varying times in different Matrigel matrices. The maximum velocity was reached in Matrigel 50% at 24 h and decreased gradually afterward. Values marked as * are statistically different from values marked as **p-value < 0.05. n = 9 (Matrigel 100%, 50%), n = 6 (Matrigel 75%), error bars indicate ± SEM.
Type-I collagen 3D matrices support single-cell migration of GSCs regardless of collagen concentration or formulation
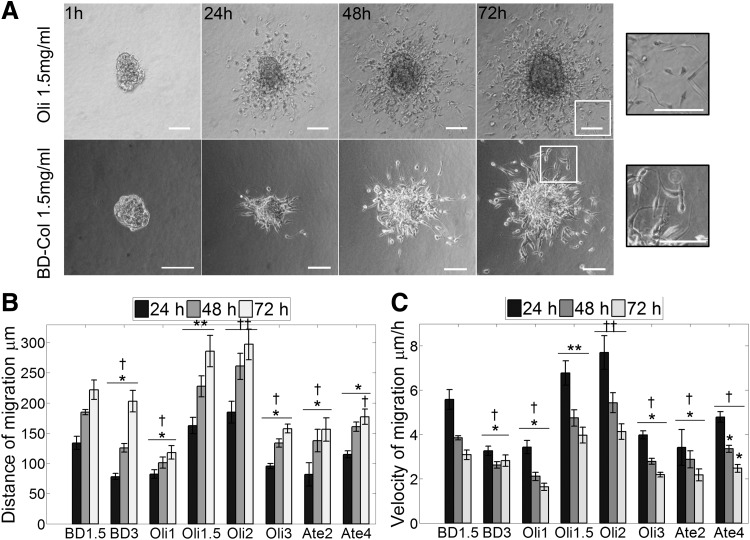
Fibrillar collagens are ubiquitous components of the ECM in the majority of tissues and organs and have been demonstrated to be present within glioma tissues and their surrounding ECM during tumor progression.35 To further explore the morphology and migration characteristic of GSCs, type-I collagen matrices with diverse characteristics were generated using type-I oligomers (Oli) isolated through acid solubilization of pig skin, type-I atelocollagen from pepsin-treated pig skin (Atelo), and BD Biosciences type-I collagen from rat tail (BD). Migration of GSCs in all collagen matrices occurred by single-cell migration (Fig. 3A and Supplementary Fig. S5) consistent with the migration mode presented by other glioma cells in commercial collagen.22,23 Different concentration of collagen and absence of telopeptides (atelocollagen) or collagen source (commercial rat tail or pig skin) did not modulate the migration mode exhibited. The neurospheres cultured in collagen developed thin pseudopodia-like extensions with subsequent detachment of single cells from the neurosphere (Fig. 3A). Migration occurred in all directions with cells presenting a spindle-shape morphology resembling mesenchymal single-cell migration.
FIG. 3.
GSCs exhibit elongated single-cell movement in collagen-I, BD1.5 (BD collagen 1.5 mg/mL), Oli (oligomer), and Ate (atelocollagen). (A) GSC neurospheres embedded in multiple types of collagen type-I matrices present a single migration mode. Scale bars 100 μm. (B) Cells cultured in acid-solubilized matrices of low collagen concentration (BD-1.5 and Oli-1.5, Oli-2 mg/mL) presented the highest distance of migration. (C) Maximum velocity was reached during the first 24 h of culture and decreased subsequently. Similar to B, the highest velocity was reached in BD-1.5 mg/mL and Oli-1.5, 2 mg/mL. Values marked as * are statistically different from **p-value < 0.05, † are statistically different from ††p-value < 0.05. n = 9 (oligomer), n > 4 (BD collagen and atelocollagen), error bars indicate ± SEM.
Collagen formulation and concentration influence migration distance and velocity of GSCs
Parameters such as collagen source, isolation method, and polymerization reaction conditions dictate the mechanical and physical properties of self-assembled collagen matrices31 and are expected to affect migration distance and velocity. While previous studies have explored glioma migration in collagen, only standard commercial monomeric collagen has been used to generate 3D matrices.23,24,32 Unlike conventional monomer matrices, which represent entanglements of long fibrils, oligomers induce interfibril associations to yield branched fibril networks. As such, oligomer matrices present higher stiffness compared to monomer matrices prepared at the same collagen concentration (fibril density). In this study, collagen matrices were prepared at concentrations ranging from 1 to 4 mg/mL, and GSC migration distance and velocity were measured as a function of concentration, formulation, and matrix stiffness.
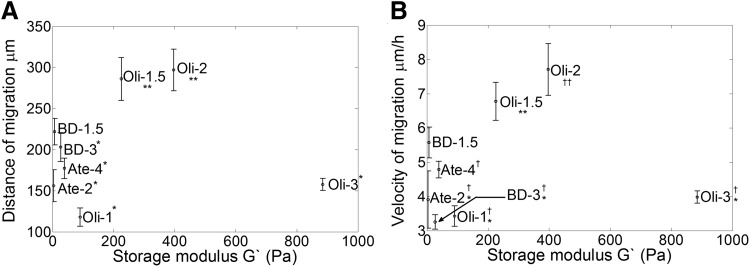
Variation of collagen concentration affected the distance and migration velocity of GSCs (Fig. 3B, C). The relationship between concentration and velocity was specific for each of the collagen formulations. Atelocollagen matrices, prepared from collagen molecules in which telopeptide regions have been enzymatically eliminated, showed short migration distances and low matrix stiffness values (Fig. 4A, B and Table 1) along with low velocities that slightly increased with concentration (Fig. 3C). BD collagen matrices also exhibited low stiffness and induced slightly higher although nonstatistically different levels of invasion compared to atelocollagen. However, the velocity decreased moderately as the collagen concentration increased (Fig. 3C). In contrast to other formulations, oligomer presented the highest stiffness at the same fibril density (Fig. 4A, B and Table 1) and a broad range of migration distances as a function of collagen concentration. The velocity and distance increased with oligomer concentration until reaching a maximum in 2 mg/mL matrices; subsequently, velocity and distance decreased for matrices of higher concentration (3 mg/mL) (Fig. 3B, C). Interestingly, matrices such as BD collagen and atelocollagen that represented the low end of the matrix stiffness spectrum (4–39 Pa) supported low migration distances and velocities similar to those obtained in matrices. Oli-3 that represented the highest matrix stiffness (885 Pa) evaluated and exhibited higher cell–matrix contacts but reduced porosity. Analysis of oligomer matrices encompassing a wider range of stiffness values (90–885 Pa) suggested an optimum range where the maximum migration is reached (Fig. 4A, B). GSCs cultured within matrices Oli-1.5 and Oli-2 (225–397 Pa) of similar stiffness values to brain tissue (100–600 Pa)36 supported the greatest migration.
FIG. 4.
Optimal migration distance and velocity of GSCs in collagen-I occur at stiffness levels in the range of normal brain. (A) Migration distance (at 72 h) and (B) migration velocity (at 24 h) are modulated by matrix stiffness (G′). Values marked as * are statistically different from **, † are statistically different from ††, p-value < 0.05. Oligomer matrices with G′ of 225 Pa (oligomer 1.5 mg/mL) and 397 Pa (oligomer 2.0 mg/mL) supported the greatest migration distance and velocity compared to other collagen matrices. Error bars indicate ± SEM.
Presence of HA in composite matrices reduces GSC migration
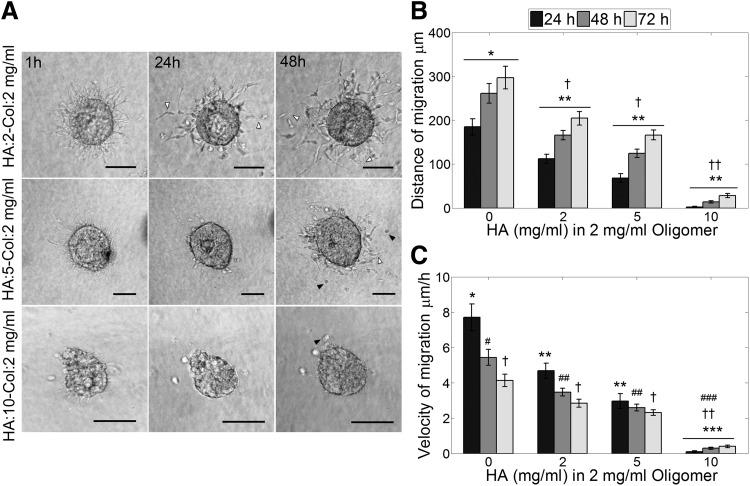
To recreate some essential features of glioma ECM composition and study the migratory behavior of GSCs, composite matrices of HA and type-I collagen (Col) were generated with 0, 2, 5, and 10 mg/mL of HA and a constant oligomer concentration of 2 mg/mL. GSC neurospheres cultured in Col-HA matrices exhibited early pseudopodia extension followed by single-cell migration similar to what was observed in collagen-only matrices. Nevertheless, increasing HA concentration proportionally reduced migration as well as the frequency of cell detachment from the neurospheres (Fig. 5A, B). Cells cultured in oligomer 2 mg/mL exhibited the greatest migration distance, while cells in HA:2-Col:5 mg/mL and HA:2-Col:5 mg/mL presented a significant reduction of migration compared to only oligomer matrices. The most drastic reduction was observed in HA:10-Col:2 mg/mL, where very few cells detached from the neurosphere and migrated considerable short distances. Cells that left the neurosphere were able to increase the migration velocity over time, different to what was observed for the other matrices (Fig. 5C). In addition, presence of HA induced morphological changes of the migratory cells. Cells switched from an elongated morphology observed in only oligomer and HA:2-Col:2 mg/mL matrices to a mixed population of elongated and rounded cells in HA:5-Col:2 mg/mL matrices (Fig. 5A) and to a mostly rounded morphology in HA:10-Col:2 mg/mL matrices.
FIG. 5.
(A) GSCs present single-cell migration in composite oligomer–hyaluronan (HA) matrices similar to only oligomer matrices. Increasing concentration of HA decreases the number of migrating cells and alters the morphology of the migratory cells from elongated to spherical shaped. White arrowheads point out elongated migrating cells and black arrowheads point out rounded migrating cells. Scale bars 100 μm. (B) Distance of migration is reduced with increasing presence of HA. All matrices present statistically different distance at all time points, except HA:2-Col:2 and HA:5-Col:2 mg/mL. (C) Velocity decreased with time as observed with other matrix types, except HA:10-Col:2 mg/mL matrix where the velocity increased with time. Values marked as * are statistically different from **, ** are statistically different from *** p-value < 0.05, † are statistically different from †† p-value < 0.05, #, ##, and ### are statistically different, p-value < 0.05. n = 9, error bars indicate ± SEM.
Addition of HA modulates fibril microstructure and mechanical properties of oligomer matrices
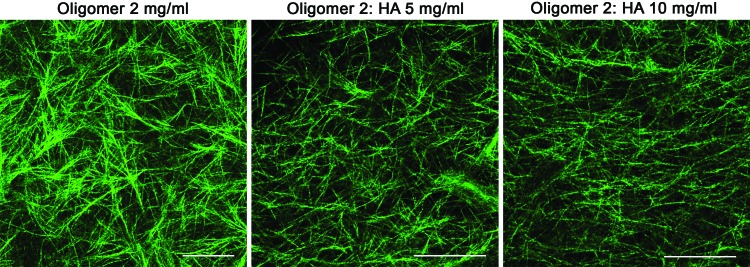
To further analyze how the incorporation of HA altered the mechanical properties of the matrices and the migratory behavior of GSCs, we performed viscoelastic shear tests of the composite matrices. Increasing HA concentration reduced the storage and loss moduli, while slightly decreasing the phase angle (δ) of the matrix (Supplementary Table S1). The greater values of the storage modulus compared to the loss modulus indicate that the matrix dynamic response is mainly controlled by the elastic (solid) phase. Structural changes of the matrix caused by HA were examined by CRM and suggest that HA incorporation does not affect fibril density, as was expected for matrices of the same collagen concentration, but disrupts the formation of interfibril associations, reducing the fibril branching (Fig. 6). Since collagen fibril branching is correlated with matrix stiffness,37 the reduction of fibril associations caused by HA might have contributed to stiffness reduction.
FIG. 6.
Confocal reflectance microscopy of oligomer matrices with varied concentrations of HA. HA presence altered the collagen interfibril associations. Scale bars 50 μm. Color images available online at www.liebertpub.com/tea
GSCs exhibit multiple types of migration
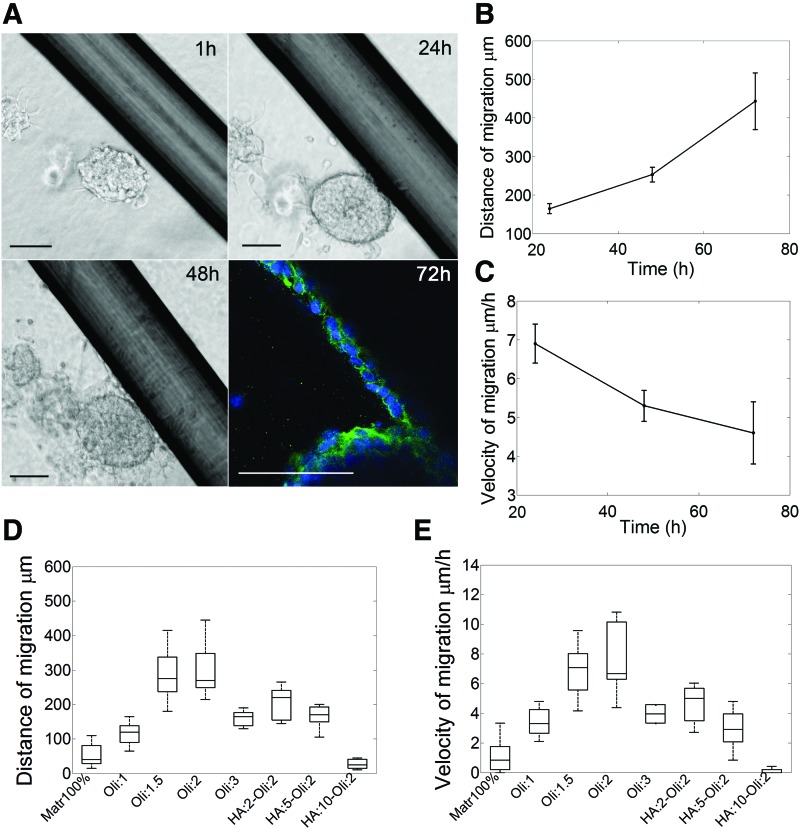
We next asked what would be the overall migratory response of GSCs in a 3D in vitro microenvironment that mimics the composition and topography of the glioma environment in a single 3D construct. To address this question, we cultured GSC neurospheres in a composite matrix of HA:10-Col:2 mg/mL with embedded Matrigel-coated microfibers to imitate the structural tracts formed by the blood vessels that offer the cells diverse migratory paths. We observed that neurospheres located close to the fibers migrated toward the fiber and developed strand collective migration using the topographical cues as physical support (Fig. 7A). Cells migrating along the rods maintained cell–cell contacts during movement, as was identified by the expression of N-cadherin, and did not separate from the neurospheres, whereas cells facing the collagen-HA matrix directly (no rods nearby) detached from the neurosphere and exhibited single-cell migration, as was observed previously in collagen-only and collagen-HA matrices. A similar behavior was presented when noncoated rods were used (Supplementary Fig. S6), indicating that, regardless of the presence of Matrigel coating, the cells used the microfibers to migrate collectively.
FIG. 7.
(A) GSC neurospheres exhibited multiple migration modes in the composite matrix as the single-cell migration mode across the 3D matrix and collective strand migration along the topographical cues present in the matrix. Staining for nuclei was incomplete due to the density of the neurosphere. Scale bars 100 μm. (B) Distance of migration along the rods increases with time and present values comparable to Matrigel 50%, n = 4, error bars indicate ± SEM. (C) Velocity of migration along the rods slowly decreases with time presenting values comparable with other types of matrices such as oligomer 2 mg/mL, n = 4, error bars indicate ± SEM. (D) Comparison of GSC maximum distance of migration in different types of matrices at 72 h, n = 9. (E) Comparison of GSC velocity of migration in different types of matrices at 24 h, n = 9. Color images available online at www.liebertpub.com/tea
The velocity and maximum distance for collective migration along the rods (Fig. 7B, C) were comparable with the values obtained for single-cell migration in oligomer 2 mg/mL matrices and are greater than the values obtained for migration in oligomer-HA matrices. The results indicate that multicellular strand migration exhibited during durotaxis, such as along the structural cues in composite matrices and in the semi-3D (Matrigel 50%) matrix, is the most productive and fastest migration mode (Fig. 7D, E), as it favors the generation of combined motility force and maintains important paracrine signaling.10
Discussion
Migration of GSCs has been linked to tumor reappearance; nevertheless, the migratory characteristics of this cell population in diverse types of ECMs have not been previously studied in detail. In this study, we used multiple 3D formats commonly used for tumor invasion studies, such as Matrigel and type-I collagen, as well as a novel composite in vitro model that mimics the ECM composition, physical properties, and topography of the glioma microenvironment. Our results indicate that GSCs exhibit varied velocities and migration modes such as collective migration, expansive growth, single-cell migration, and combined collective–single migration as a function of the composition and physical characteristics of the 3D microenvironment.
In 3D matrices of Matrigel, the neurospheres adopted a rounded morphology resembling expansive growth. Such morphology can be associated with the structural characteristics of the matrix. Matrigel presents a different cross-linking pattern as well as a larger pore size than native BM,12,38 and does not confine or resist neurosphere expansion. As a consequence, cells tend to exhibit characteristics related to proteolysis-independent migration, such as amoeboid morphology for single cells39 or expansive growth,40 consistent with the morphology observed in Matrigel 75% and 100%. Our results also suggest that reduction of matrix stiffness as in Matrigel 50% generates a semi-3D (or 2.5D) substrate, where close contact between the neurospheres and the rigid surface of the culture plate allows the formation of focal contacts and stable multicellular strands supporting greater migration distances.
The drastic change of GSC morphology to strictly elongated, mesenchymal-like single migration in type-I collagen corroborates the relevance of the ECM properties on the regulation of cancer cell migration. Fibrillar collagen, although nonpresent in normal brain ECM, has been found in glioma tissues, surrounding ECM,15 and as a component of the GSC niche,16 suggesting that glioma cells deposit it to support tumor maintenance. While previous studies have explored the migration of glioma cells in collagen matrices using established cell lines and commercial collagen,23,24,32 we focused on studying the invasiveness of GSCs due to their role on tumor migration and regrowth. In addition, we generated matrices with oligomer collagen that, as recent reports showed, offers a number of advantages over conventional monomeric collagens, including hierarchical collagen fibril assembly that recapitulates that observed in vivo, presence of tissue-specific intermolecular cross-links, short polymerization time, and customization over a broad range of relevant physicochemical features.31 GSC neurospheres exhibited single-cell detachment and mesenchymal migration in all the types of collagen matrices studied, consistent with the migration mode presented by other glioma cells lines in 3D collagen matrices.22,23 The velocity and distance of migration were modulated by the concentration of the collagen as was expected due to changes in the fibril density and pore size of the matrix.11,31 In atelocollagen matrices where no crosslinks are present, increasing concentration derived in higher migration due to higher generation of cell-matrix contacts and proteolysis-independent migration. In covalently cross-linked matrices such as BD collagen and oligomer, increasing collagen concentration reduces the pore size and the available space for cell movement; hence, migration relies on the ability of the cell to degrade and remodel the ECM, or to deform its body until reaching an appropriate size to move through the pores.41 Similarly, reduction of the collagen concentration and consequent increase of the pore size can also reduce migration due to the lack of structural support for the development of cell–matrix contacts and generation of traction force to propel movement. These differences in migration due to the collagen matrix concentration are similar to observations by other authors23 and support the existence of an optimum collagen concentration range where the fibril density and pore size support the maximum cell migration.42–44
HA enrichment of the ECM and overexpression of CD44 receptors have been associated with glioma successful invasion of brain parenchyma.45–47 Interestingly, when increasing quantities of HA were incorporated to collagen matrices to resemble glioma microenvironment, the number of migratory cells and migration distance were reduced. In addition, the cell morphology shifted to a more rounded appearance. Previous studies of collagen-HA 3D matrices24,25,48 have related increasing HA concentrations with matrix stiffness and subsequent reduction of cell migration with adoption of rounded morphology. Similarly, in PEG-HA matrices, increased stiffness has been derived in changes of cell morphology.28 Nevertheless, our results showed that incorporation of noncross-linking HA decreases matrix stiffness (Supplementary Table S1), but also induces adoption of cell-rounded morphology. As other studies of acid-solubilized collagen matrices have shown, HA incorporation increases interstitial fluid movement resistance without affecting fibril density.49 Consistently, Col-HA matrices presented a similar fibril density upon HA addition; however, the interfibrillar associations were disrupted. Therefore, we suggest that the stiffness reduction is linked to reduction fibrillar branching caused by HA during matrix polymerization,37 and the adoption of cell-rounded appearance is mainly due to increased resistance of the interstitial fluid caused by HA hydration that opposes an additional barrier for cell deformation and movement.
It is accepted that GBM presents a rapid infiltration pattern with preference for myelinated axons and blood vessels.14 Recently, it was shown using in vivo xenograft models that GBM cells present directional and efficient migration (greater net distance and velocity) along blood vessels, while migration through the parenchyma occurs by expression of multiple pseudopodia with constant changes in direction.50 Certain GBM cell lines also presented a chain-like morphology when extending from the tumor to the blood vessels.51 In line with such findings, our results, obtained using an in vitro composite matrix, suggest that GSCs detect the asymmetric rigidity gradient presented in the environment and develop different migration modes according to the mechanical properties of the structures to invade. Migration across the collagen-HA matrix, which poses a low-stiffness environment, triggers cell detachment and single-cell migration with constant changes of direction; however, when rigid structures (as the microrods) are presented to the neurospheres, the cells develop collective strand migration along the directional tracts. Such a migration mode by multicellular strands was present along rods, regardless of the percentage of Matrigel coverage, on the rod surface. Hence, we suggest that the rigidity of the topographical clue coupled with the proximity of the neurosphere to the clue induce durotaxis and formation of strand collective migration, and that this type of collective migration is likely to be present not only along blood vessels but also along myelinated axons.52,53
In this study, we describe a new composite tunable matrix that resembles important features of the glioma microenvironment such as ECM composition, mechanical properties, and preexisting structural cues to dissect mechanistic understanding regarding the migratory behavior of GSCs in 3D environments. Furthermore, we analyzed multiple matrices of varied mechanical properties using Matrigel and diverse types of fibrillar collagen to assess how the migratory characteristics of GSCs are affected by the physical properties of the matrix. Our results indicate that GSC neurospheres are able to exhibit multiple velocities and migration modes such as collective migration (expansive growth and strand)54 and single-cell migration (mesenchymal) as a function of the mechanical and compositional properties of the matrices. In a composite collagen-HA matrix, the migration of GSCs was reduced by the presence of HA; nonetheless, cells adopted a productive and fast migration as a collective strand using the preexisting topographical cues presented as migratory paths. Taken together, the results suggest that GSC migration is not limited to a unique migration mode as is usually observed in in vitro studies, but is able to exhibit concomitantly multiple migration modes (collective and single) as a response to the heterogeneity of the environment. The recreation of additional characteristics of cancer environments such multiple cell coculture and functional vascular networks in controllable 3D-models is a powerful tool to study cancer development and progression. Additional efforts in this area will contribute to elucidate fundamental mechanisms such as how cell sensing of the microenvironment composition and mechanical characteristics induce the adoption of different migration mechanisms.
Supplementary Material
Acknowledgments
The authors would like to thank Rucha Joshi for the valuable assistance with the preparation of the collagen matrices and rheological testing, Dr. Phillip Tofilon and the Moffitt Cancer Center for providing the GSCs GBAM1, and Aaron Taylor for assistance with confocal microscopy. This study was partially supported by the Purdue University Office of Vice President for Research Incentive Grant Program (S.L.V.H.) and the IUPUI Signature Center Initiative for the Cure of Glioblastoma (J.L.R.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Dolecek T.A., Propp J.M., Stroup N.E., and Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14 Suppl 5, v1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakada M., Nakada S., Demuth T., Tran N.L., Hoelzinger D.B., and Berens M.E. Molecular targets of glioma invasion. Cell Mol Life Sci 64, 458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima F.R.S., Kahn S.A., Soletti R.C., Biasoli D., Alves T., da Fonseca A.C.C., et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 1826, 338, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Schonberg D.L., Lubelski D., Miller T.E., and Rich J.N. Brain tumor stem cells: molecular characteristics and their impact on therapy. Mol Aspects Med 39, 82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magee J.A., Piskounova E., and Morrison S.J. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21, 283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Ontanon P., Orgaz J.L., Aldaz B., Elosequi-Artola A., Martino J., Berciano M., et al. Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells 31, 1075, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Cheng L., Wu Q., Guryanova O.A., Huang Z., Huang Q., Rich J.N., et al. Elevated invasive potential of glioblastoma stem cells. Biochem Biophys Res Commun 406, 643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persano L., Rampazzo E., Basso G., and Viola G. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 85, 612, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Lathia J.D., Heddleston J.M., Venere M., and Rich J.N. Deadly teamwork: neural cancer stem cells and the tumor microenvironment. Cell Stem Cell 8, 482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedl P., and Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Carey S.P., Kraning-Rush C.M., Williams R.M., and Reinhart-King C.A. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 33, 4157, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willis A.L., Sabeh F., Li X.-Y., and Weiss S.J. Extracellular matrix determinants and the regulation of cancer cell invasion stratagems. J Microsc 251, 250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellail A.C., Hunter S.B., Brat D.J., Tan C., and Van Meir E.G. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol 36, 1046, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gritsenko P.G., Ilina O., and Friedl P. Interstitial guidance of cancer invasion. J Pathol 226, 185, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Huijbers I.J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One 5, e9808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motegi H., Kamoshima Y., Terasaka S., Kobayashi H., and Houkin K. Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology 34, 378, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Giese A., Bjerkvig R., Berens M.E., and Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21, 1624, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mourad P.D., Farrell L., Stamps L.D., Chicoine M.R., and Silbergeld D.L. Why are systemic glioblastoma metastases rare? Systemic and cerebral growth of mouse glioblastoma. Surg Neurol 63, 511, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Vehlow A., and Cordes N. Invasion as target for therapy of glioblastoma multiforme. Biochim Biophys Acta 1836, 236, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Baker B.M., and Chen C.S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125(Pt 13), 3015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoma C.R., Zimmermann M., Agarkova I., Kelm J.M., and Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69, 29, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Kaufman L.J., Brangwynne C.P., Kasza K.E., Filippidi E., Gordon V.D., Deisboeck T.S., et al. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J 89, 635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Motte S., and Kaufman L.J. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials 31, 5678, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Rao S.S., Dejesus J., Short A.R., Otero J.J., Sarkar A., and Winter J.O. Glioblastoma behaviors in three-dimensional collagen-hyaluronan composite hydrogels. ACS Appl Mater Interfaces 5, 9276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananthanarayanan B., Kim Y., and Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 32, 7913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedron S., Becka E., and Harley B.A.C. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34, 7408, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Kievit F.M., Florczyk S.J., Leung M.C., Veiseh O., Park J.O., Disis M.L., et al. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 31, 5903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C., Tong X., and Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol Pharm 11, 2115, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Benton G., Kleinman H.K., George J., and Arnaoutova I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer 128, 1751, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., et al. Identification of a cancer stem cell in human brain tumors identification of a cancer stem cell in human brain tumors. Cancer Res 63, 5821, 2003 [PubMed] [Google Scholar]
- 31.Kreger S.T., Bell B.J., Bailey J., Stites E., Kuske J., Waisner B., et al. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 93, 690, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman L.J., Brangwynne C.P., Kasza K.E., Filippidi E., Gordon V.D., Deisboeck T.S., et al. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J 89, 635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedl P., Locker J., Sahai E., and Segall J.E. Classifying collective cancer cell invasion. Nat Cell Biol 14, 777, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Rao S.S., Bentil S., DeJesus J., Larison J., Hissong A., Dupaix R., et al. Inherent interfacial mechanical gradients in 3D hydrogels influence tumor cell behaviors. PLoS One 7, e35852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne L.S., and Huang P. The pathobiology of collagens in glioma. Mol Cancer Res 11, 1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levental I., Georges P.C., and Janmey P.A. Soft biological materials and their impact on cell function. Soft Matter 3, 299, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Bailey J.L., Critser P.J., Whittington C., Kuske J.L., Yoder M.C., and Voytik-Harbin S.L. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 95, 77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe R.G., and Weiss S.J. Breaching the basement membrane: who, when and how? Trends Cell Biol 18, 560, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Poincloux R., Collin O., Lizárraga F., Romao M., Debray M., Piel M., et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci U S A 108, 1943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen-Ngoc K.-V., Cheung K.J., Brenot A., Shamir E.R., Gray R.S., Hines W.C., et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci U S A 109, E2595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf K., Te Lindert M., Krause M., Alexander S., Te Riet J., Willis A.L., et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201, 1069, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engler A., Bacakova L., Newman C., Hategan A., Griffin M., and Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J 86, 617, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyton S.R., and Putnam A.J. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 204, 198, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Pathak A., and Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A 109, 10334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delpech B., Maingonnat C., Girard N., Chauzy C., Maunoury R., Olivier A., et al. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur J Cancer 29A, 1012, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Park J.B., Kwak H., and Lee S. Role of hyaluronan in glioma invasion. Cell Adh Migr 2, 202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourguignon L.Y.W. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol 18, 251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Sun C., Wilhelm M.E., Fox L.J., Zhu J., and Kaufman L.J. Influence of chondroitin sulfate and hyaluronic acid on structure, mechanical properties, and glioma invasion of collagen I gels. Biomaterials 32, 7932, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreger S.T., and Voytik-Harbin S.L. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol 28, 336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirata E., Yukinaga H., Kamioka Y., Arakawa Y., Miyamoto S., Okada T., et al. In vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J Cell Sci 125(Pt 4), 858, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Watkins S., Robel S., Kimbrough I.F., Robert S.M., Ellis-Davies G., and Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5, 4196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrie R.J., Doyle A.D., and Yamada K.M. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 10, 538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson J., Nowicki M.O., Lee C.H., Chiocca E.A., Viapiano M.S., Lawler S.E., et al. Quantitative analysis of complex glioma cell migration on electrospun polycaprolactone using time-lapse microscopy. Tissue Eng Part C Methods 15, 531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedl P., Locker J., Sahai E., and Segall J.E. Classifying collective cancer cell invasion. Nat Cell Biol 14, 777, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.