Abstract
Exfoliation syndrome (XFS) is the commonest recognizable cause of open angle glaucoma world-wide. To better understand the etiology of XFS, we conducted a genome-wide association study (GWAS) on 1,484 patients and 1,188 controls from Japan, and followed up the most significant findings on a further 6,901 patients and 20,727 controls from 17 countries across 6 continents. We discovered a significant association between a new locus (CACNA1A rs4926244) and increased susceptibility to XFS (Odds ratio [OR] = 1.16, P = 3.36 × 10−11). Although overwhelming association at the LOXL1 locus was confirmed, the key SNP marker (LOXL1 rs4886776) demonstrated allelic reversal depending on ethnic grouping (In Japanese: ORA-allele= 9.87, P = 2.13 × 10−217; In non-Japanese: ORA-allele= 0.49, P = 2.35 × 10−31). Our findings represent the first genetic locus outside of LOXL1 which surpasses genome-wide significance for XFS, and provides insight into the biology and pathogenesis of the disease.
XFS is a generalized disorder of the extracellular matrix that manifests most conspicuously in the eye. The exfoliation material consists of cross-linked, amyloid-like fibrillar material and glycoproteins. Apart from ocular tissues, this material deposits around blood vessels particularly in association with elastic connective tissue, and could be found in other organs1. The build-up of exfoliation material deposits and pigment in the trabecular meshwork can damage this tissue and impede the drainage of aqueous humor from the eye thus resulting in elevated intraocular pressure and glaucomatous optic neuropathy. Exfoliation glaucoma is the most serious known complication of XFS2.
The first GWAS on XFS was reported in 2007 and successfully identified LOXL1 as a major susceptibility locus for XFS3. Since then, multiple studies have uniformly corroborated the association of genetic variants of LOXL1 with XFS4–21. However, data from these studies showed associated alleles for LOXL1 SNPs frequently undergo allelic reversal depending on ethnic group22. These findings suggest that complex genetic mechanisms are present for XFS pathogenesis, and the possibility that additional susceptibility loci for XFS remain to be identified. We assembled an international, multi-institutional collaborative effort across 6 continents comprising 17 countries to conduct a GWAS discovery and two-staged replication study of XFS (see Methods, Supplementary Table 1, and Supplementary Figure 1). Participating subjects provided written informed consent under the oversight of all local institutional review boards in accordance with the tenets of the Declaration of Helsinki.
For the GWAS discovery stage, we genotyped 717,991 SNP markers on 1,578 Japanese patients with XFS and 1,215 controls using the Illumina OmniExpress microarray. Control subjects were drawn from the same hospital where the XFS patients were first identified. A total of 1,484 cases and 1,188 controls passed quality control (QC) filters for call-rate, relatedness, heterozygosity and ancestry (see Methods for QC details) and were included for downstream association analysis. Multiple markers in strong linkage disequilibrium (LD) at the LOXL1 locus showed strong evidence of association with XFS (Supplementary Figure 2a), with rs4886776 (P = 7.37 × 10−137) serving as the sentinel SNP.
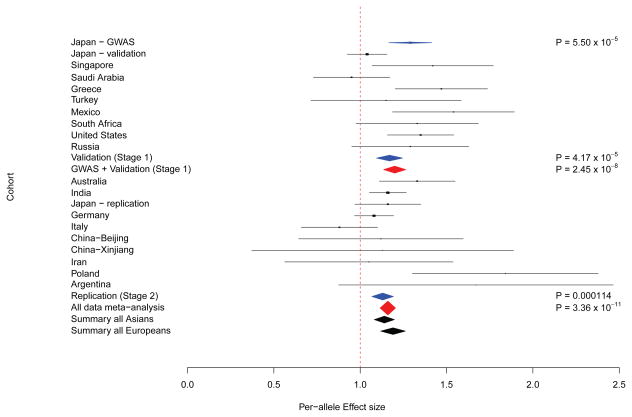
A total of 66 SNPs outside of LOXL1 showed evidence of association with XFS surpassing P < 1 × 10−4 at the GWAS discovery stage. We thus designed validation assays for these 66 SNP markers, together with LOXL1 rs4886776, and genotyped them in a follow up collection of 2,628 XFS cases and 8,947 controls drawn from 9 countries (Stage 1 validation, see Supplementary Table 1). For each SNP examined, we conducted a fixed-effects meta-analysis to summarize the observations across the nine studies. One SNP marker (rs4926244), mapping within the CACNA1A gene that showed association in the GWAS discovery stage at P = 5.50 × 10−5 (ORG-allele = 1.29) was also significant in this validation stage (ORG-allele = 1.17, P = 4.17 × 10−5). Thus for rs4926244, meta-analysis of both the discovery and validation stages revealed genome-wide significant association (ORG-allele = 1.20, P = 2.45 × 10−8) (Figure 1, Supplementary Table 2, and Supplementary Figure 2b). Results for all 67 SNP markers from the GWAS discovery and Stage 1 replication are appended in Supplementary Table 2. We did not observe consistent evidence of association at CNTNAP2, a locus previously reported to associate with XFS from a pooled GWAS study23, as well as other previously reported candidate genes (Supplementary Table 3).
Figure 1.
Forest plot for the associations between CACNA1A rs4926244 and Exfoliation syndrome in discovery and follow up case-control collections. The black lines denote the 95% confidence intervals of the odds ratio for each collection. The diamonds denote summary results for the GWAS, Validation and Replication stages (blue), as well as the GWAS and Validation meta-analysis, and meta-analysis of data from all collections (red). Asian and European ethnic summaries are in black diamonds.
We subjected CACNA1A rs4926244 to further technical scrutiny in a third, independent dataset consisting of 4,273 XFS cases and 11,780 controls drawn from 8 additional countries (Stage 2, replication; see Supplementary Table 1). The association maintained significance, consistent with findings observed in the two previous stages (ORG-allele = 1.13, P = 1.14 × 10−4). Together, the combined discovery and two-stage replication patient collections consisting of 8,385 XFS cases and 21,915 controls provide evidence for association between the minor G allele at rs4926244 and XFS (P = 3.36 × 10−11). These data suggest risk for XFS increases approximately 1.16 fold for each copy of the minor G allele (Figure 1 and Supplementary Table 4). This association appears to be consistent with minimal heterogeneity when stratified for Asian (ORG-allele = 1.14, P = 7.46×10−6), European (ORG-allele=1.19, P=1.90×10−6) or South African (ORG-allele = 1.33, P = 0.11)(Phet = 0.5, I2 = 0%) ethnic groups (Figure 1).
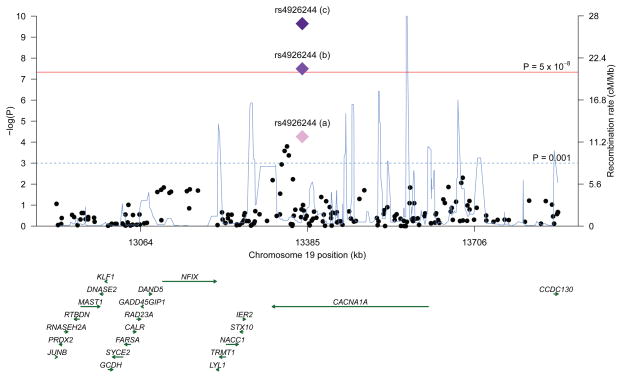
SNP rs4926244 resides within an intronic region near the 3′ end of CACNA1A. It is flanked closely by recombination events (Figure 2) and is confined to its own linkage disequilibrium (LD) block (Supplementary Figure 3). We did not observe association with any genetic marker surpassing the nominal threshold of P < 0.001 outside of this critical region (Figure 2)24. We next performed imputation for unobserved SNPs at the CACNA1A locus on the basis of 1,000 Genomes cosmopolitan project data using the Phase 3 release (June 2014, see Methods) across the GWAS discovery collection. We were able to successfully impute 5602 SNPs across the CACNA1A locus. However, subsequent association analysis utilizing the imputed SNPs did not identify additional genetic associations that surpassed the statistical significance of rs4926244 (Supplementary Figure 4). Notably, the most significant SNPs emerging from the cosmopolitan imputation analysis are intronic and are all moderate-to-highly correlated with rs4926244 (Supplementary Table 5). None of these correlated SNP markers lies in strong motifs for transcription factor binding sites as identified by ENCODE. They also do not tag any common non-synonymous variants in CACNA1A (Supplementary Table 6). Haplotype association analysis assessing SNPs in a 2, 3, and 4 marker sliding window did not reveal evidence of association surpassing that observed for rs4926244 (lowest haplotype P-value = 0.00021; Supplementary Table 7), and we further note that all but one haplotype showing evidence of association exceeding P < 0.0005 in the GWAS dataset contained SNP rs4926244 (Supplementary Table 7). This suggests that rs4926244 is likely driving the common-variant haplotype association results, and that detailed fine-mapping of this locus using deep re-sequencing may be required. Examination of a recently available large-scale eQTL mapping database indicates the risk allele G at rs4926244 is modestly correlated with lower CACNA1A mRNA levels in peripheral blood cells (Z=−3.00, P=0.0027), suggesting that it may influence XFS risk through an effect on CACNA1A expression25. Further work will be needed to evaluate its effect in human ocular tissues.
Figure 2.
Regional association and recombination rate plot for the CACNA1A rs4926244 locus. The left y axis represents –log10 P values for association with exfoliation syndrome and the right y axis represents the recombination rate. The x axis represents base-pair positions along the chromosome (human genome Build 37). The diamonds denote the summary of each experimental stage, a) for the GWAS discovery, b) for the meta-analysis between GWAS discovery and validation stages, and c) for the meta-analysis between GWAS discovery, validation, and replication stages.
Initial analysis of the LOXL1 locus in the GWAS discovery dataset of Japanese descent demonstrated strong association at rs4886776 (ORA-allele = 8.31, P = 7.37 × 10−137). The strength of this association vastly exceeded that of marker rs3825942 (responsible for a p.G153D substitution in exon 1 of LOXL1), which has been the most widely tested and reported SNP association prior to this analysis22. Performing the analysis after conditioning for the allele dosage of rs4886776 extinguished the signal of association for every other genetic marker within the LOXL1 locus. Conversely, conditioning the analysis for allele dosage at rs3825942 still resulted in genome-wide significant association at many of the other LOXL1 SNPs, including rs4886776 (Supplementary Table 8). These data suggest that within the Japanese GWAS discovery set, the observed association at LOXL1 can be attributed to rs4886776 alone. We note that rs4886776 is in high LD with rs1048661 (r2=0.98 in 1000 genomes Asians), a SNP responsible for another non-synonymous substitution (p.R141L) in LOXL1 but was not directly genotyped in our dataset. However, we were able to successfully impute rs1048661 in our GWAS discovery dataset and we confirmed strong association between it and XFS (ORT-allele = 8.13, P = 1.32 × 10−126). SNP rs1048661 has been previously reported to show strong association with XFS in multiple populations, although the risk allele is reversed depending on which ethnic group is being studied11,22. This SNP is also in LD with several other LOXL1 SNPs in potential transcription factor binding sites (Supplementary Table 6)26,27.
Both rs4886776 and rs3825942 are in moderate pair-wise linkage disequilibrium (r2 = 0.23). When we genotyped rs4886776 through the 2,628 XFS cases and 8,947 controls from Stage 1 validation (Supplementary Table 1), we noted very strong evidence of consistent association in the Japanese (ORA-allele = 21.7, P = 1.54 × 10−135), leading to an overwhelmingly significant association in the Japanese cases and controls analyzed (ORA-allele = 9.87, P = 2.13 × 10−217). Strikingly in non-Japanese, the direction of the association was opposite to that seen in the Japanese (ORA-allele = 0.49, P = 2.35 × 10−31)(Supplementary Figure 5). Such a scenario echoes recently reported observations for the reversed effect of rs3825942 on XFS risk in South Africans, and suggests that the genetic mechanism whereby LOXL1 exerts its effect on individual susceptibility to XFS is complex22. We failed to detect any evidence of statistically significant interaction between CACNA1A rs4926244 and the sentinel LOXL1 polymorphisms, suggesting that both loci impact XFS risk via distinct biological pathways.
CACNA1A encodes for the alpha 1A subunit of the type P/Q voltage-dependant calcium channel. Calcium channels are responsible for the transport of calcium ions across cell membranes and play a key role in a cell’s ability to generate and transmit electrical signals. Previous electron microscopy studies on human XFS eyes showed the presence of high calcium concentration in direct association with aggregating XFS fibrils28. In addition it is well known that fibrillin utilizes calcium to form stable aggregates29. Thus, it can be hypothesized that altered function of a calcium channel could lead to alterations of calcium concentrations that may facilitate the formation of XFS aggregates.
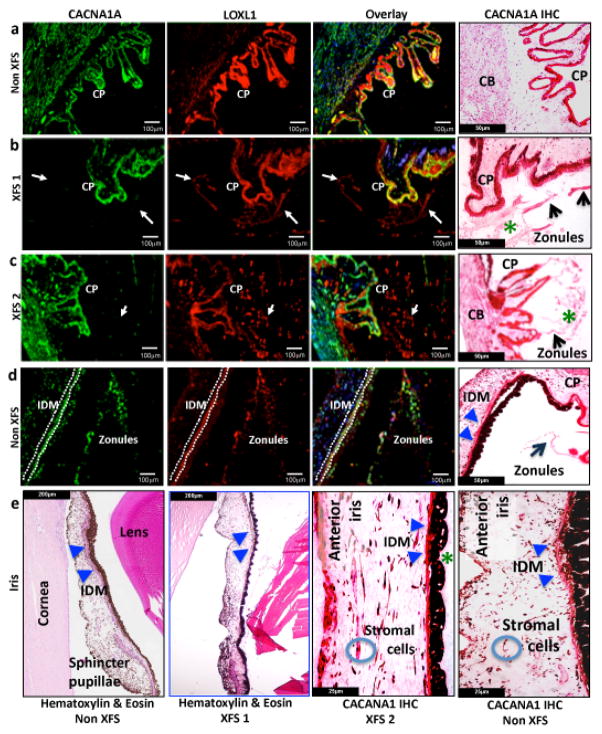
As there is a paucity of information on CACNA1A expression in the eye, we examined the mRNA expression profile and protein expression of CACNA1A from a variety of human ocular tissues and cell lines respectively (Supplementary Figure 6). CACNA1A mRNA expression was detected in all of the ocular tissues we studied with the exception of the optic nerve head (Supplementary Figure 6, panel A). Expression of different CACNA1A isoforms appear to be higher in human ocular tissue-derived cells than in cells of non-ocular origin (Supplementary Figure 6, panel B). Immunofluorescence and immunohistochemistry analysis was also performed on adult human eyes which showed positive immunoreactivity for CACNA1A in multiple human ocular tissues (Figure 3, Supplementary Figure 7 & 8). The distribution of CACNA1A was similar in human ocular tissues of individuals with or without XFS (Figure 3, Supplementary Figures 8 & 9). Positive staining and localization of CACNA1A in the human eye was further corroborated by immunofluorescence microscopy analysis in mouse eyes. (Supplementary Figure 10). In the human eyes, we observed positive CACNA1A immunoreactivity in the ciliary body and iris (Figure 3). Positive staining of CACNA1A was also seen in the anterior lens epithelium but not in the acellular capsule and the cornea (Supplementary Figure 7 & 9). The optic nerve glia and vascular endothelial cells also showed positive immunoreactivity with CACNA1A (Supplementary Figure 9). In the retina, strong diffuse CACNA1A staining was seen in the photoreceptor inner segments (IS), inner nuclear layer (INL) and outer nuclear layer (ONL) and nerve fibre layer (NFL) of non XFS globes in comparison to the XFS globes where focal and patchy immunostaining of the IS, ONL, INL and NFL was observed. Light microscopy comparison of the irides in XFS eyes against non-XFS eyes showed typical XFS findings of exfoliated material on the posterior iris and atrophic iris pigment epithelium, as well as the possible atrophy of the iris dilator muscle in XFS eyes (Figure 3). Double immunofluorescence microscopy for CACNA1A and LOXL1 was also performed in human eyes with XFS and without XFS and showed co-localisation of CACNA1A and LOXL1 only in the epithelium of the ciliary processes. The exfoliated material in XFS eyes showed LOXL1 positive staining with negligible CACNA1A immunoreactivity. The ciliary body and iris smooth musculature had CACNA1A positive immunostaining but were negatively stained for LOXL1 in both XFS and non-XFS eyes (Figure 3). This observation raises the possibility that CACNA1A and LOXL1 contribute to XFS pathology through different mechanisms at different ocular sites.
Figure 3.
CACNA1A and LOXL1 protein expression and light microscopic analysis in XFS and non-XFS control eyes. Immunolocalisation of CACNA1A in human non-XFS and XFS globes show CACNA1A positive immunoreactivity in the smooth musculature of the ciliary body (CB) and pigmented and non-pigmented ciliary process (CP) epithelium with variable staining in the zonules (Panels a to c: CACNA1A immunofluorescence (IF) panel; CACNA1A immunohistochemical (IHC) panel, 40x; zonules, white and black arrows; exfoliated material, green asterix). In contrast, LOXL1 immunoreactivity is present only in the exfoliated material and the CP epithelium, (LOXL1 IF panel; zonules, white arrows). Double immunofluorecence analysis shows colocalisation of CACNA1A and LOXL1 within the non-pigmented and pigmented epithelium of the CP but not in the CB smooth muscles or the zonules (IF overlay panel; zonules, white arrows). Light microscopy comparison of non-XFS and XFS irides show the typical XFS findings of exfoliated material (green asterix) on the posterior iris and atrophic iris pigment epithelium with possible atrophy of the iris dilator muscle (blue arrowheads) in XFS irides. The sphincter pupillae in both non-XFS and XFS shows negligible differences (H&E panels). CACNA1A positive immunoreactivity is also seen in the anterior iris border, iris stromal cells, the iris dilator (blue arrowheads, H&E and IHC panels) and sphincter muscles as well as the iris pigmented epithelium in both XFS and Non-XFS irides (Panel d & e: CACNA1A IF and CACNA1A IHC panels). Stromal cells are highlighted by the blue circles. For CACNA1A, LOXL1, and overlay panels a) through to d), each unit on the scale bar represents 100 μm. For CACNA1A IHC panels a) to d), each unit on the scale bar represents 50 μm. For panel e) hematoxylin & eosin non-XFS as well as hematoxylin & eosin XFS1, each unit on the scale bar represents 200 μm. For panel e) CACNA1A IHC XFS2 as well as CACNA1A IHC non-XFS, each unit on the scale bar represents 25 μm.
In summary, we have identified a susceptibility locus mapping to CACNA1A using a three-staged GWAS study design. Further investigation of this locus is now warranted to uncover the mechanisms via which CACNA1A affects individual susceptibility to XFS.
Online Methods
Patient recruitment
Detailed information on all XFS sample collections can be found in Supplementary note. All patients and controls were enrolled into the study following informed consent and ethical approval from the relevant national and regional institutional review boards for each sample collection. DNA was extracted from patient blood samples using standard, well-described laboratory procedures for genetic analysis.
Genotyping
For the GWAS discovery stage, genome-wide genotyping was performed using the Illumina OmniExpress beadchips, following manufacturer’s instructions (see URLs). For validation (Stage 1), genotyping was performed using the Sequenom MassArray platform (see URLs). For replication (Stage 2), genotyping was performed using Applied Biosystems Taqman probes (see URLs) for India, Germany, Italy, China, Iran, Poland, and Argentina. The Australian replication collection had GWAS genotyping on all cases and controls using Illumina genome-wide arrays, and CACNA1A rs4926244 was directly genotyped from the GWAS arrays. We perform cross-platform concordance checks and verify >99.9% concordance of genotypes for SNP markers of interest reported here (e.g. rs4926244).
Statistical analysis
Stringent quality control filters were used to remove poorly performing samples and SNP markers in both the GWAS discovery and validation phases. The SNPs with call rates of less than 95%, minor allele frequency of less than 1 percent, or showing significant deviation from Hardy-Weinberg Equilibrium (P-value for deviation < 1 × 10−6) were removed from further statistical analysis. Likewise, samples with an overall genotyping success rate of less than 95% were removed from further analysis. The remaining samples were then subjected to biological relationship verification by using the principle of variability in allele sharing. Identity-by-state information was derived using PLINK (see URLs). For those pairs of individuals who showed evidence of cryptic relatedness (possibly either due to duplicated or biologically related samples), we removed the sample with the lower call rate before performing principal component (PC) analysis. PC analysis was undertaken to account for spurious associations resulting from ancestral differences of individual SNPs and PC plots were performed using the R statistical program package (see URLs). For the GWAS discovery stage, all XFS cases had genetically matched controls as visualized spatially on PC analysis (Supplementary Figures 11a and 11b). A total of 581,023 autosomal SNPs passed quality filters for call rate and minor allele frequency (see Methods) and were included for further analysis. Genotypes were contrasted between XFS cases and controls using logistic regression, with adjustments for the top 6 principal components of genetic ancestry to further minimize confounding due to cryptic population stratification. We did not observe any evidence of genomic inflation (λgc = 1; Supplementary Figure 12), suggesting that the association results were not confounded by external artefacts such as genetic mismatch between cases and controls. Crucially, we did not observe any deviation or dispersion of test statistics when patients with XFS without glaucoma were compared to patients with exfoliation glaucoma (Supplementary Figure 13). This suggests that our primary analysis approach in combining all XFS patients into an overall case group is valid.
For the GWAS discovery, validation, and replication stages, analysis of association with XFS disease status was carried out using allele-based score tests (1 degree of freedom), which models additive effects of the minor allele on disease risk. For the GWAS discovery stage, we incorporated the top six principal components of genetic stratification into the logistic regression model while performing the analysis for association to minimize the effect of residual population stratification. As the follow up validation (Stage 1) and replication (Stage 2) phases only tested a limited number of genetic markers, we were unable to adjust for population stratification in these follow up sample collections. However, association tests were performed by site to minimize population stratification and then subsequently combined in meta-analysis as described below. All P-values reported here are two-tailed.
Meta-analysis was conducted using inverse variance weights for each sample collection, which calculates an overall Z-statistic, its corresponding P-value, and accompanying odds ratios for each SNP analyzed. The meta-analysis is performed under the fixed effects model30. Analysis of linkage disequilibrium was performed using the R software package (version 2.9.0) and associated rmeta and RColorBrewer analytical packages.
Genotype Imputation
Fine-scale imputation at CACNA1A was performed using all 1,484 XFS cases and 1,188 controls passing the standard GWAS QC checks. The imputation and phasing of genotypes were carried out using IMPUTE2 (see URLs) with cosmopolitan population haplotypes based on data from 2535 individuals from 26 distinct populations around the world obtained from the 1000 Genomes project Phase 3 (Jun 2014) release for reference panel construction. Imputed genotypes were called with an impute probability threshold of 0.90 with all other genotypes classified as missing. Additional quality control filters were applied to remove SNPs with a call rate of < 99% should the SNP have a minor allele frequency (MAF) below 5% in either cases or controls. For common SNPS with MAF above 5%, the filtering criteria were set at less than 95% call rate.
Power calculations
All statistical power calculations were performed as previously described31. We present these power calculations for each of the following conditions; a) GWAS discovery stage only, b) GWAS discovery plus stage 1 validation, and c) GWAS discovery plus stage 1 validation plus stage 2 replication (Supplementary Table 9).
Expression analysis
RT-PCR in human ocular tissues: Multiple transcript variants encoding different isoforms have been found for CACNA1A. Here we assessed for the presence of either of the two major transcripts known for CACNA1A, which encode 2506 and 2261 amino acid isoforms, CaV2.1 variant-1 and 2 (CaV2.1_V2 and CaV2.1_V1, NCBI Reference Sequences: NM_001127222.1/NP_001120694.1 and NM_001127221.1/NP_001120693.1). All of the known critical regulatory elements of the CaV2.1 C-terminal tail are included in both variants, but the polyQ domain is excluded from the short-tail CaV2.1_V1 isoform32. We utilized 5 pairs of primers spread across and common to both transcripts to assess the presence of either transcript in eye tissues (Supplementary Table 10) CACNA1A transcript expression was assessed by semi quantitative reverse transcription PCR (RT-PCR) using the above mentioned CACNA1A specific primers on total RNA extracted from a variety of ocular tissues (anterior sclera, cornea, iris, trabecular meshwork, lens capsule, retina, choroid, optic nerve head and optic nerve) with TRIzol® Reagent (Invitrogen, Carlsbad, California) in accordance with the manufacturer’s protocol. First-strand cDNA synthesis was performed with SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, California). Semi quantitative RT-PCR was performed according to manufacturer’s protocol, with the SYBR® Green Master Mix (Invitrogen, Carlsbad, California) using the above CACNA1A primers. RT-PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining. The ubiquitously expressed beta-actin (ACTB) gene was amplified using specific primers (Supplementary Table 10) and used as amplification and normalizing control.
Western Blotting
Cell lines obtained from American Type Culture Collection (Manassas, VA, USA) were the human retinal pigment epithelial cell line (APRE19), human cervical adenocarcinoma cell line (Hela S), human breast adenocarcinoma cell line (MCF7), and human embryonic kidney epithelial cell line (HEK 293). Human nonpigmented ciliary epithelial cell line (NPCE) is a kind gift from Prof. Miguel Coca-Prados from Yale School of Medicine. Human Trabecular Meshwork cell line (HTM) was purchased from PromoCell GmbH (Heidelberg, Germany). Cell lysates were obtained by lysing individual cell lines with lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 0.2 mM NaVO4, 10 mM NaF, 0.4 mM EDTA, and 10% glycerol). SDS-PAGE resolved proteins were transferred to Hybond-C Extra nitrocellulose membranes (Amersham Life Science Inc., Arlington Heights, IL, USA). Membranes were blocked by 5% nonfat milk, 0.1% Tween 20 in Tris-buffered saline (20 mM Tris-HCl, pH 7.6, 150 mM NaCl) for 1 h before incubation with CACNA1A (1:1000) from Abcam Inc. (Cambridge, MA, USA), Actin-horseradish peroxidase (HRP) (1:50000) from Santa Cruz Biotechnology (Dallas, TX, USA). Blocking and blotting of antibodies were performed in 10% horse serum (Sigma-Aldrich Corp., St. Louis, MO, USA), 0.1% Tween 20 in phosphate-buffered saline for 1 h. The bound primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (GE Healthcare Biosciences, Pittsburgh, PA, USA), and visualized by Luminata Forte Western HRP substrate (Millipore, Bedford, MA, USA).
Immunofluorescence Confocal Microscopy
Immunofluorescence confocal microscopy was performed on antigen retrieved 4μm paraffin sections. Blocking of tissue sections was performed with blocking buffer (10% FBS, 0.1% PBS-Tween; 1x pen/strep) for 1 hour at RT. CACNA1A (Abcam Inc., Catalog#: Ab81011 from Abcam) and LOXL1 (ABNOVA, Catalog # : H00004016-B01P from Abnova) antibodies were diluted at 1:300 ratio with blocking buffer and incubated overnight at 4°C. Secondary FITC (1:300) or Cy3 (1:300) labeled anti-mouse or anti-rabbit antibodies (Jackson Laboratories, Westgrove, PA, USA) were also diluted in blocking buffer and incubated at RT for 1 hour followed by application of Vectashield with 4′,6-diamidino-2-phenyl-indole (DAPI) (Vector Laboratories, Burlingame, CA, USA). Cover-slips were then used to overlay the sections and stored in the dark at 4°C until viewing with Olympus Fluoview 1000 confocal microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
Immunohistochemistry
A total of 7 archival enucleated globes were retrieved for IHC analysis with CACNA1A (1:50) (Abcam Inc., Cambridge, MA, USA) and LOXL1 (1:50) (ABNOVA, Taipei, Taiwan) antibodies. Three human eye globes from Saudi Arabia and 1 from Denmark with a history of advanced XFS were obtained courtesy of Deepak Edward (KKESH, Riyadh Saudi Arabia) and Steffen Heegaard (University of Copenhagen, Denmark) respectively. Three additional eyes from Singapore (SGH-SNEC Ophthalmic pathology service, SGH Pathology) that were exenterated for other external pathological diagnoses that did not involve the globe were used as non-XFS controls. Immunohistochemistry (IHC) analysis was performed according to our previously published protocol33. In brief, paraffin sections were cut at 4μm and placed on coated slides. Immunohistochemistry was performed using the Leica Bond Polymer Refine detection kit DS9800 (Leica Microsystems GmbH, Wetzlar, Germany). Slides were heated for 20 minutes at 60°C and then loaded onto the Leica Bond III autostainer for immunohistochemical staining. Staining on the autostainer consisted of dewaxing, antigen retrieval using Leica Bond ER2 solution for 20mins at 100°C, antibody incubation for 20minutes at room temperature followed by staining using the Bond polymer refine kit. Polymeric alkaline phosphatase-AP linker antibody conjugate system (Vision Biosystems, Norwell, MA, USA) and counterstaining with haematoxylin were applied for visualization, after which the slides were dehydrated and cover-slipped.
Immunofluorescence confocal microscopy in mice
C57BL/6J and B6(Cg)-Tyrc-2J/J mice were obtained from The Jackson Laboratory production facility. Mice were housed with a 14-hour-light/10-hour-dark cycle, under the same conditions as previously described34. All experiments were performed in compliance with the ARVO statement for use of animals in ophthalmic and vision research and approved by The Jackson Laboratory Animal Care and Use Committee. Adult mice were deeply anesthetized and perfused with 1X phosphate buffered saline (PBS) and eyes were enucleated. Fresh tissue was frozen in optimal cutting temperature (OCT) compound frozen by liquid nitrogen cooled isopentane and stored at −80°C or eyes were fixed overnight in 4% paraformaldehyde. Fixed eyes were cyroprotected in 30% sucrose, rinsed in 1X PBS and frozen in OCT for sectioning. Eyes were cut into 12 μm sections. Primary antibodies used to stain sections included anti-CACNA1A (1:500, Millipore, Billerica, MA, USA), anti-Endomucin (EMCN; 1:500; eBioscience, San Diego, CA, USA), and anti-smooth muscle Actin (ACTA2; 1:500; Abcam, Cambridge, MA, USA). Alexafluor secondary antibodies from Invitrogen were used to label by immunofluorescence. Sections were desiccated, washed in 1X PBS with 0.5% Triton and incubated with primary antibodies overnight. Sections were subsequently washed in 1X PBS and incubated with secondary antibodies for 60 minutes. DAPI was used to stain nuclei. For each experiment, at least four sections from four eyes were assessed. Microscopy was performed on the Axio Imager (Zeiss).
Supplementary Material
Quantile-quantile plot of the discovery stage P-values contrasting patients with exfoliation syndrome without glaucoma (N = 788) against patients presenting with exfoliation syndrome accompanied by glaucoma (N = 594). A total of 102 exfoliation syndrome cases did not have the glaucoma sub-diagnosis.
Manhattan plots for the genome-wide association study on exfoliation syndrome showing
a) Discovery phase (N = 1,484 cases and 1,188 controls) data only and
b) meta-analysis of the discovery and validation (stage 1) phases comprising up to N = 4,112 cases and N = 10,135 controls. CACNA1A rs4926244 was brought forward for replication (stage 2).
Regional association analysis of the CACNA1A locus. The genomic locations of the genes flanking CACNA1A are also shown. The locations for rs4926244, T666M, and R583Q are shown in the context of CACNA1A. P-values on the y axis are truncated at P < 1 × 10−10.
Regional association plot of the CACNA1A locus for SNP markers imputed using cosmopolitan population haplotypes based on data from 2535 individuals from 26 distinct populations around the world obtained from the 1000 Genomes project Phase 3 (Jun 2014) release for reference panel construction. No imputed genetic marker showed evidence of association surpassing the directly genotyped rs4926244.
A total of 17 imputed SNPs at the CACNA1A locus show P < 0.001 in terms of association with XFS. The individual SNP IDs and correlation with rs4926244 are appended in Supplementary Table 5. The dashed vertical lines mark regions of recombination flanking rs4926244 and the 17 SNPs correlated with it.
Forest plot for the associations between LOXL1 rs4886776 and Exfoliation syndrome in the discovery and Stage 1 follow up sample collections. The black lines denote the 95% confidence intervals of the odds ratio for each collection. The blue diamonds denote the 95% confidence interval for summary observations for Japan, non-Japan, and overall meta-analysis.
Regional association analysis of the CACNA1A locus. The genomic organization of CACNA1A is super-imposed. The locations for rs4926244, T666M, and R583Q are shown in the context of CACNA1A. P-values on the y axis are truncated at P < 1 × 10−10.
Sample collections for Exfoliation syndrome cases and controls for the GWAS, validation (Stage 1 follow up), and replication (Stage 2 follow up) phases.
Association results for the 67 SNPs which surpassed P < 1 × 10−4 at the discovery stage and brought forward to the validation stage (see Supplementary table 1 for patient collections in the validation stage).
Association results for previously reported candidate genes for Exfoliation syndrome.
Detailed association analysis between CACNA1A rs4926244 and exfoliation syndrome in all collections.
Distribution of XFS cases (number in red) and controls (number in black) from all participating sites.
Top imputed SNPs (exceeding P < 0.001) from the XFS GWAS using the 1000G Cosmopolitan reference panel (Phase 3 release). The vast majority are highly correlated with the index SNP rs4926244 which is shown here for reference and comparison purposes (pairwise D′ >0.8 for all SNPs, and pairwise r2 > 0.8 for 10 out of 17 imputed SNPs).
RegulomeDB annotation of the top LOXL1 and CACNA1A SNPs (rs4886776 and rs4926244 respectively, marked with an *) and those in LD with them (1000 genomes Asians r2>0.8).
Haplotypes at the CACNA1A locus showing association with XFS surpassing P < 0.0005. The haplotypes were phased and derived from 2 marker, 3 marker, and 4 marker sliding windows across CACNA1A. All haplotypes in this manner are nearly perfectly tagged by the single SNP rs4926244, suggesting that the associations observed from the haplotype analysis could be driven by rs4926244.
Association results between markers at the LOXL1 locus on Chromosome 15 at susceptibility to XFS. Shown here are all SNP markers surpassing 1 × 10−7 on primary association testing. Additional analyses conditioning for key LOXL1 SNPs (rs4886776 and rs3825942) are also appended.
Study power (expressed in %) as a function of allele odds ratio (OR) and minor allele frequency (MAF). Conditions surpassing 80% power are highlighted in yellow. The power calculations are presented for a) the GWAS discovery stage (α < 1 × 10−4), b) the GWAS and validation stage 1 (α < 5 × 10−8), c) the GWAS, validation stage 1, and replication stage 2 (α < 5 × 10−8)
Analysis of CACNA1A expression in human ocular tissues:
Panel A) The CACNA1A specific 250bp RT-PCR product was seen in anterior sclera (AS), cornea (C), lens capsule (LC), iris (I), trabecular meshwork (TM), retina and retinal pigment epithelium (R), choroid (CH) and the optic nerve (ON). RT-PCR product was not observed for the optic nerve head (ONH). The amplification product shown was from a PCR that used CACNA1A primers F4 5′-CAGAGCAAGGCCAAGAAGC-3′ and R4 5′-CTTGTTCCGGACTCCATGTG-3′. The ubiquitously expressed gene, ACTB was used as the normalizing control. A no template sample acted as the negative control (NC) to ensure non-contamination of the RT-PCR reaction mix. M denotes molecular-weight marker.
Panel B) Whole cell lysates from ARPE19, NPCE, HTM, HelaS, MCF7, and HEK293 were analyzed for CACNA1A expression. Two CACNA1A protein bands ~275kDa and ~250kDa were observed (arrows), which likely correspond to the 2506 and 2261 amino acid isoforms, CaV2.1_V2 and CaV2.1_V1 (NCBI Reference Sequences: NP_001120694.1 and NP_001120693.1). Human ocular tissue derived cell lines (ARPE19, NPCE, and HTM( displayed a higher CACNA1A expression levels when compared against the non-ocular derived cell lines (HelaS, MCF7 and HEK293). Amongst the ocular cell lines, ARPE19 and HTM cells expressed higher levels of the larger 275kDa protein while NPCE cell line expressed higher level of the smaller 250kDa protein.
Immuno-fluorescense analysis of CACNA1A distribution within the ocular tissues. Panel A) CACNA1A staining was detected in the corneal epithelium (Epi), cornea endothelium (Endo) and corneal stroma. Panel B) Ocular expression of CACNA1A protein was found to be differently expressed in tissues involved in the aqueous humor outflow pathways with the highest level of immune-reactivity in the ciliary muscle (CM), followed by the iris dilator muscle (IDM), ciliary processes (CP), trabecular meshwork (TM), and Schlemm’s canal (SC). No CACNA1A was detected in the sclera. Panel C) CACNA1A was expressed most abundantly in the iris sphincter muscle (ISM), followed by iris pigmented epithelium (IPE) and iris stroma. Panel D) CACNA1A expression was also observed in the lens epithelium (Lens Epi), but not in the lens fibers. Panel E) Strong immunofluorescence labeling of CACNA1A in the ganglion cell layer (GCL), inner nuclear layer (INL) and outer nuclear layer (ONL) was observed. Ubiquitous labeling of other retinal layers such as nerve fibers layer (NFL), rods and cones layer (RCL) and retinal pigment epithelium (RPE) was also detected.
A to C, XFS human eyes: CACNA1 Immunohistochemistry staining (1:100k Abcam, red chromophobe) of 3 PXF globes. Ciliary body (A&B) and ciliary processes (A to C) show positive staining at low magnification. (20x). Asterix (*) highlights the non-staining Exfoliative material along the zonules and ciliary processes. Zonules show focal staining for CACANA1 (arrow).
D &E, XFS human eyes: CACNA1 immunohistochemistry showing the positive staining of the non pigmented ciliary epithelium (NPCE) and pigmented ciliary epithelium (PCE) and ciliary body smooth muscles. (40x). Asterix (*) highlights the non-staining Exfoliative material.
F & G, Normal human eyes: CACNA1A immunohistochemistry showing the positive staining of the non pigmented ciliary epithelium (NPCE) and pigmented ciliary epithelium (PCE) and ciliary body smooth muscles.
Immunolocalisation of CACNA1 in the lens, cornea, retina and optic nerve (CACANA1 IHC panel, 40x ) in both non-XFS and XFS eyes show positive immunoreactivity in the anterior lens epithelium, corneal epithelial cells, stromal keratocytes and corneal endothelial cells. The optic nerve glia and vascular endothelial cells also show positive CACANA1 staining in both non-XFS and XFS eyes. In non-XFS retina, strong diffuse immunoreactivity is seen in the photoreceptor inner segments (IS), inner nuclear layer (INL) and outer nuclear layer (ONL) and nerve fiber layer (NFL) of non XFS globes in comparison to the XFS globes where focal and patchy immunostaining of the IS, ONL, INL and NFL is observed.
Panel c) CACANA1 positive immunoreactivity is seen in the anterior iris border, iris stromal cells, the iris dilator and sphincter pupillae as well as the pigmented iris epithelium in both XFS and Non-XFS irides (Iris panel: CACANA1 IHC panels). Exfoliated material does not show positive staining with CACANA1 (asterix, CACANA1IHC XFS2 panel) in contrast to its LOXL1 positive immunoreactivity (asterix, LOXL1 IHC XFS1 panel).
Ancestry analysis of the GWAS dataset using principal components. Panel a) shows the ancestry of the XFS cases and controls from Japan relative to the international HapMap panels. Panel b) shows direct genetic ancestral matching between the Japanese XFS cases and controls.
Quantile-quantile plot of the GWAS discovery stage P-values. No genomic inflation was observed.
Acknowledgments
The authors thank the staff and participants of all studies for their important contributions. We thank Khai-Koon Heng, Xiao-Yin Chen, Hui-Meng Soo, Shi-Qi Mok, Andrea Jamuth, Nicole Foxworth and Monika Elbl for technical assistance. This research was funded by the Biomedical Research Council, Agency for Science, Technology and Research, Singapore. J.L.W. acknowledges support from the National Institutes of Health/National Eye Institute grants (NIH/NEI R01 EY020928 and NIH/NEI P30 EY014104). SWMJ acknowledges support from EY11721 and is an Investigator of the Howard Hughes Medical Institute. L.R.P. acknowledges support from a Harvard Medical School Distinguished Ophthalmology Scholar Award and the Harvard Glaucoma Center of Excellence. J.H.F. acknowledges support from the National Institutes of Health/National Eye Institute grants (EY023512 and EY018825). Zhenglin Yang acknowledges support from the National Natural Science Foundation of China (81025006, and 81170883), as well as from the Department of Science and Technology of Sichuan Province, China (2012SZ0219 (Z.Y.) and 2011jtd0020 (Z.Y.). M. Schwab acknowledges support from the Robert Bosch Stiftung, Stuttgart, Germany and The German Cancer Consortium (DKTK), Germany. The Australian case cohort was funded by grants from the Ophthalmic Research Institute of Australia and the National Health and Medical Research Council (NHMRC) project #535044. The Thessaloniki Eye Study was co-funded by the European Union (European Social Fund) and Greek national funds under the Act “Aristia” of the Operational Program “Education and Lifelong Learning” (see Supplementary Note). The Blue Mountains Eye Study (BMES) GWAS and genotyping costs was supported by Australian NHMRC, Canberra Australia (NHMRC project grant IDs 512423, 475604 and 529912), and the Wellcome Trust, UK as part of Wellcome Trust Case Control Consortium 2 (A Viswanathan, P McGuffin, P Mitchell, F Topouzis, P Foster, grant IDs 085475/B/08/Z and 085475/08/Z). K.P.B. is an NHMRC Senior Research Fellow and J.E.C. is an NHMRC Practitioner Fellow. M.A.B. is a NHMRC Principal Research Fellow. A.W.H. is a NHMRC Peter Doherty Fellow.
Footnotes
Author Contributions
T.A., M.O., & C.C.K. conceived the project. M.O., T.M., R.R.A., A.H., S.N., J.E.C., A.W.H., D.A.M., P.M., J.J.W., Y.S.A., J.C.Z., Y.N., T.Z., M.P.,L.J., Y.X.W., S.W., D.P., PG.S., Y.I., R.S.K., M.U., S.Manabe., K.H., S.Kazama., R.I., Y.M., K.Miyata., K.S., T.H., E.C., K.I., S.I., A.Y., M.Y., Y.K., M.A., T.O., T.Sakurai, T.Sugimoto, H.C., K.Y., S.Y.A., E.A.O., S.A.A-O., O.O., L.A-J, S.A.S., Y.Y., C.O., M.R.K., A.N.B., S.Y., E.L.A., E.K-J., U.L., P.C., R.M.R., A.Z., T.C., R.Ramakrishnan., K.N., R.V., P.Z., X.C., D.G-V., S.A.P., R.H., S-L.H., U-C.W-L., C.M., U.S-S., S.Moebus., N.Weisschuh., R.S., A.G., I.L., J.G.C., M.C., Q.Y., V.V., P.Founti., A.C., A.L., E.A., A.L.C., M.R.W., D.J.R., I.M-B., K.Mori, S.Heegaard., W.L.M.A., J.B.J., L.X., J.M.L., F.L., N.Wang., P.Frezzotti., S.Kinoshita, J.H.F., M.I., D.P.E., L.R.P., T.K., J.L.W., F.T., N.Y., & R.Ritch conducted patient recruitment and phenotyping. Z.L., S.U., M.K., K.P.B., M.A.B., J.J.W., Y.G., K.Y.T., L.H., W.Y.M., S.Q.P., B.Z., J.S., N.Z., Z.Y., & S.V. performed genotyping experiments. J.M.H., A.S.Y.C., M.C.L., E.N.V., G.R.H., & S.W.M.J. led and performed immunohistochemistry and immunofluorescence experiments. Z.L., K.P.B., R.A.F., P.L., K.K.A-A., L.A.S., L.H., K.S.S., J.N.F., M.N., F.M., N.G., M.M., S.U., M.K., Y.Y.T., J.H.K., A.E.A.K., S.Herms, Y.L., K.T., B.Z., J.S., N.Z., S.V., Z.Y., G.R.H., A.C.O., F.P., & A.G., performed analysis. E.N.V., T.Y.W., C.Y.C., A.M.H., M.M.N., B.C., E.S., M.S., & A.R. contributed genetic and genotyping data from control populations. The manuscript was drafted by C.C.K., with critical input from T.A., R.R.A., L.R.P., J.L.W., F.P., F.T., M.D., S.W.M.J., R.Ritch, and M.A.H. The manuscript was approved by all authors. C.C.K was responsible for obtaining financial support for this study.
Members of consortia and study groups are listed in the Supplementary Note.
URLs
Illumina, www.illumina.com; Sequenom, www.sequenom.com; Applied Biosystems, http://www.appliedbiosystems.com/; PLINK, http://pngu.mgh.harvard.edu/~purcell/plink/; R statistical program package, www.r-project.org; IMPUTE2, http://mathgen.stats.ox.ac.uk/impute/impute_v2.html
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References for main text
- 1.Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Ritch R, Schlotzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 3.Thorleifsson G, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, et al. Ethnicity-based subgroup meta-analysis of the association of LOXL1 polymorphisms with glaucoma. Mol Vis. 2010;16:167–77. [PMC free article] [PubMed] [Google Scholar]
- 5.Fingert JH, et al. LOXL1 mutations are associated with exfoliation syndrome in patients from the midwestern United States. Am J Ophthalmol. 2007;144:974–975. doi: 10.1016/j.ajo.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi H, Gotoh N, Ueda Y, Nakanishi H, Yoshimura N. Lysyl oxidase-like 1 polymorphisms and exfoliation syndrome in the Japanese population. Am J Ophthalmol. 2008;145:582–585. doi: 10.1016/j.ajo.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Fan BJ, et al. DNA sequence variants in the LOXL1 gene are associated with pseudoexfoliation glaucoma in a U.S. clinic-based population with broad ethnic diversity. BMC Med Genet. 2008;9:5. doi: 10.1186/1471-2350-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, et al. Genetic association of LOXL1 gene variants and exfoliation glaucoma in a Utah cohort. Cell Cycle. 2008;7:521–4. doi: 10.4161/cc.7.4.5388. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt AW, et al. Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet. 2008;17:710–6. doi: 10.1093/hmg/ddm342. [DOI] [PubMed] [Google Scholar]
- 10.Pasutto F, et al. Association of LOXL1 common sequence variants in German and Italian patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1459–63. doi: 10.1167/iovs.07-1449. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki M, et al. Association of LOXL1 gene polymorphisms with pseudoexfoliation in the Japanese. Invest Ophthalmol Vis Sci. 2008;49:3976–80. doi: 10.1167/iovs.08-1805. [DOI] [PubMed] [Google Scholar]
- 12.Fan BJ, et al. LOXL1 promoter haplotypes are associated with exfoliation syndrome in a U.S. Caucasian population. Invest Ophthalmol Vis Sci. 2011;52:2372–8. doi: 10.1167/iovs.10-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf C, et al. Lysyl oxidase-like 1 gene polymorphisms in German patients with normal tension glaucoma, pigmentary glaucoma and exfoliation glaucoma. J Glaucoma. 2010;19:136–41. doi: 10.1097/IJG.0b013e31819f9330. [DOI] [PubMed] [Google Scholar]
- 14.Lemmela S, et al. Association of LOXL1 gene with Finnish exfoliation syndrome patients. J Hum Genet. 2009;54:289–97. doi: 10.1038/jhg.2009.28. [DOI] [PubMed] [Google Scholar]
- 15.Aragon-Martin JA, et al. Evaluation of LOXL1 gene polymorphisms in exfoliation syndrome and exfoliation glaucoma. Mol Vis. 2008;14:533–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, et al. Evaluation of LOXL1 polymorphisms in exfoliation syndrome in a Chinese population. Mol Vis. 2009;15:2349–57. [PMC free article] [PubMed] [Google Scholar]
- 17.Mossbock G, et al. Lysyl oxidase-like protein 1 (LOXL1) gene polymorphisms and exfoliation glaucoma in a Central European population. Mol Vis. 2008;14:857–61. [PMC free article] [PubMed] [Google Scholar]
- 18.Challa P, et al. Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol Vis. 2008;14:146–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Ramprasad VL, et al. Association of non-synonymous single nucleotide polymorphisms in the LOXL1 gene with pseudoexfoliation syndrome in India. Mol Vis. 2008;14:318–22. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano M, et al. Novel common variants and susceptible haplotype for exfoliation glaucoma specific to Asian population. Sci Rep. 2014;4:5340. doi: 10.1038/srep05340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori K, et al. LOXL1 genetic polymorphisms are associated with exfoliation glaucoma in the Japanese population. Mol Vis. 2008;14:1037–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Williams SE, et al. Major LOXL1 risk allele is reversed in exfoliation glaucoma in a black South African population. Mol Vis. 2010;16:705–12. [PMC free article] [PubMed] [Google Scholar]
- 23.Krumbiegel M, et al. Genome-wide association study with DNA pooling identifies variants at CNTNAP2 associated with pseudoexfoliation syndrome. Eur J Hum Genet. 2011;19:186–93. doi: 10.1038/ejhg.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rioux JD, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet. 2001;29:223–8. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- 25.Westra HJ, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–43. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–7. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–4. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlotzer-Schrehardt U, Kortje KH, Erb C. Energy-filtering transmission electron microscopy (EFTEM) in the elemental analysis of pseudoexfoliative material. Curr Eye Res. 2001;22:154–62. doi: 10.1076/ceyr.22.2.154.5522. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt DP, Ono RN, Sakai LY. Calcium stabilizes fibrillin-1 against proteolytic degradation. J Biol Chem. 1997;272:1231–6. doi: 10.1074/jbc.272.2.1231. [DOI] [PubMed] [Google Scholar]
- 30.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–50. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 32.Tsou WL, Soong BW, Paulson HL, Rodriguez-Lebron E. Splice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6. Neurobiol Dis. 2011;43:533–42. doi: 10.1016/j.nbd.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MC, et al. Expression of the Primary Angle Closure Glaucoma (PACG) Susceptibility Gene PLEKHA7 in Endothelial and Epithelial Cell Junctions in the Eye. Invest Ophthalmol Vis Sci. 2014;55:3833–41. doi: 10.1167/iovs.14-14145. [DOI] [PubMed] [Google Scholar]
- 34.Smith RS, et al. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000;9:1021–32. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantile-quantile plot of the discovery stage P-values contrasting patients with exfoliation syndrome without glaucoma (N = 788) against patients presenting with exfoliation syndrome accompanied by glaucoma (N = 594). A total of 102 exfoliation syndrome cases did not have the glaucoma sub-diagnosis.
Manhattan plots for the genome-wide association study on exfoliation syndrome showing
a) Discovery phase (N = 1,484 cases and 1,188 controls) data only and
b) meta-analysis of the discovery and validation (stage 1) phases comprising up to N = 4,112 cases and N = 10,135 controls. CACNA1A rs4926244 was brought forward for replication (stage 2).
Regional association analysis of the CACNA1A locus. The genomic locations of the genes flanking CACNA1A are also shown. The locations for rs4926244, T666M, and R583Q are shown in the context of CACNA1A. P-values on the y axis are truncated at P < 1 × 10−10.
Regional association plot of the CACNA1A locus for SNP markers imputed using cosmopolitan population haplotypes based on data from 2535 individuals from 26 distinct populations around the world obtained from the 1000 Genomes project Phase 3 (Jun 2014) release for reference panel construction. No imputed genetic marker showed evidence of association surpassing the directly genotyped rs4926244.
A total of 17 imputed SNPs at the CACNA1A locus show P < 0.001 in terms of association with XFS. The individual SNP IDs and correlation with rs4926244 are appended in Supplementary Table 5. The dashed vertical lines mark regions of recombination flanking rs4926244 and the 17 SNPs correlated with it.
Forest plot for the associations between LOXL1 rs4886776 and Exfoliation syndrome in the discovery and Stage 1 follow up sample collections. The black lines denote the 95% confidence intervals of the odds ratio for each collection. The blue diamonds denote the 95% confidence interval for summary observations for Japan, non-Japan, and overall meta-analysis.
Regional association analysis of the CACNA1A locus. The genomic organization of CACNA1A is super-imposed. The locations for rs4926244, T666M, and R583Q are shown in the context of CACNA1A. P-values on the y axis are truncated at P < 1 × 10−10.
Sample collections for Exfoliation syndrome cases and controls for the GWAS, validation (Stage 1 follow up), and replication (Stage 2 follow up) phases.
Association results for the 67 SNPs which surpassed P < 1 × 10−4 at the discovery stage and brought forward to the validation stage (see Supplementary table 1 for patient collections in the validation stage).
Association results for previously reported candidate genes for Exfoliation syndrome.
Detailed association analysis between CACNA1A rs4926244 and exfoliation syndrome in all collections.
Distribution of XFS cases (number in red) and controls (number in black) from all participating sites.
Top imputed SNPs (exceeding P < 0.001) from the XFS GWAS using the 1000G Cosmopolitan reference panel (Phase 3 release). The vast majority are highly correlated with the index SNP rs4926244 which is shown here for reference and comparison purposes (pairwise D′ >0.8 for all SNPs, and pairwise r2 > 0.8 for 10 out of 17 imputed SNPs).
RegulomeDB annotation of the top LOXL1 and CACNA1A SNPs (rs4886776 and rs4926244 respectively, marked with an *) and those in LD with them (1000 genomes Asians r2>0.8).
Haplotypes at the CACNA1A locus showing association with XFS surpassing P < 0.0005. The haplotypes were phased and derived from 2 marker, 3 marker, and 4 marker sliding windows across CACNA1A. All haplotypes in this manner are nearly perfectly tagged by the single SNP rs4926244, suggesting that the associations observed from the haplotype analysis could be driven by rs4926244.
Association results between markers at the LOXL1 locus on Chromosome 15 at susceptibility to XFS. Shown here are all SNP markers surpassing 1 × 10−7 on primary association testing. Additional analyses conditioning for key LOXL1 SNPs (rs4886776 and rs3825942) are also appended.
Study power (expressed in %) as a function of allele odds ratio (OR) and minor allele frequency (MAF). Conditions surpassing 80% power are highlighted in yellow. The power calculations are presented for a) the GWAS discovery stage (α < 1 × 10−4), b) the GWAS and validation stage 1 (α < 5 × 10−8), c) the GWAS, validation stage 1, and replication stage 2 (α < 5 × 10−8)
Analysis of CACNA1A expression in human ocular tissues:
Panel A) The CACNA1A specific 250bp RT-PCR product was seen in anterior sclera (AS), cornea (C), lens capsule (LC), iris (I), trabecular meshwork (TM), retina and retinal pigment epithelium (R), choroid (CH) and the optic nerve (ON). RT-PCR product was not observed for the optic nerve head (ONH). The amplification product shown was from a PCR that used CACNA1A primers F4 5′-CAGAGCAAGGCCAAGAAGC-3′ and R4 5′-CTTGTTCCGGACTCCATGTG-3′. The ubiquitously expressed gene, ACTB was used as the normalizing control. A no template sample acted as the negative control (NC) to ensure non-contamination of the RT-PCR reaction mix. M denotes molecular-weight marker.
Panel B) Whole cell lysates from ARPE19, NPCE, HTM, HelaS, MCF7, and HEK293 were analyzed for CACNA1A expression. Two CACNA1A protein bands ~275kDa and ~250kDa were observed (arrows), which likely correspond to the 2506 and 2261 amino acid isoforms, CaV2.1_V2 and CaV2.1_V1 (NCBI Reference Sequences: NP_001120694.1 and NP_001120693.1). Human ocular tissue derived cell lines (ARPE19, NPCE, and HTM( displayed a higher CACNA1A expression levels when compared against the non-ocular derived cell lines (HelaS, MCF7 and HEK293). Amongst the ocular cell lines, ARPE19 and HTM cells expressed higher levels of the larger 275kDa protein while NPCE cell line expressed higher level of the smaller 250kDa protein.
Immuno-fluorescense analysis of CACNA1A distribution within the ocular tissues. Panel A) CACNA1A staining was detected in the corneal epithelium (Epi), cornea endothelium (Endo) and corneal stroma. Panel B) Ocular expression of CACNA1A protein was found to be differently expressed in tissues involved in the aqueous humor outflow pathways with the highest level of immune-reactivity in the ciliary muscle (CM), followed by the iris dilator muscle (IDM), ciliary processes (CP), trabecular meshwork (TM), and Schlemm’s canal (SC). No CACNA1A was detected in the sclera. Panel C) CACNA1A was expressed most abundantly in the iris sphincter muscle (ISM), followed by iris pigmented epithelium (IPE) and iris stroma. Panel D) CACNA1A expression was also observed in the lens epithelium (Lens Epi), but not in the lens fibers. Panel E) Strong immunofluorescence labeling of CACNA1A in the ganglion cell layer (GCL), inner nuclear layer (INL) and outer nuclear layer (ONL) was observed. Ubiquitous labeling of other retinal layers such as nerve fibers layer (NFL), rods and cones layer (RCL) and retinal pigment epithelium (RPE) was also detected.
A to C, XFS human eyes: CACNA1 Immunohistochemistry staining (1:100k Abcam, red chromophobe) of 3 PXF globes. Ciliary body (A&B) and ciliary processes (A to C) show positive staining at low magnification. (20x). Asterix (*) highlights the non-staining Exfoliative material along the zonules and ciliary processes. Zonules show focal staining for CACANA1 (arrow).
D &E, XFS human eyes: CACNA1 immunohistochemistry showing the positive staining of the non pigmented ciliary epithelium (NPCE) and pigmented ciliary epithelium (PCE) and ciliary body smooth muscles. (40x). Asterix (*) highlights the non-staining Exfoliative material.
F & G, Normal human eyes: CACNA1A immunohistochemistry showing the positive staining of the non pigmented ciliary epithelium (NPCE) and pigmented ciliary epithelium (PCE) and ciliary body smooth muscles.
Immunolocalisation of CACNA1 in the lens, cornea, retina and optic nerve (CACANA1 IHC panel, 40x ) in both non-XFS and XFS eyes show positive immunoreactivity in the anterior lens epithelium, corneal epithelial cells, stromal keratocytes and corneal endothelial cells. The optic nerve glia and vascular endothelial cells also show positive CACANA1 staining in both non-XFS and XFS eyes. In non-XFS retina, strong diffuse immunoreactivity is seen in the photoreceptor inner segments (IS), inner nuclear layer (INL) and outer nuclear layer (ONL) and nerve fiber layer (NFL) of non XFS globes in comparison to the XFS globes where focal and patchy immunostaining of the IS, ONL, INL and NFL is observed.
Panel c) CACANA1 positive immunoreactivity is seen in the anterior iris border, iris stromal cells, the iris dilator and sphincter pupillae as well as the pigmented iris epithelium in both XFS and Non-XFS irides (Iris panel: CACANA1 IHC panels). Exfoliated material does not show positive staining with CACANA1 (asterix, CACANA1IHC XFS2 panel) in contrast to its LOXL1 positive immunoreactivity (asterix, LOXL1 IHC XFS1 panel).
Ancestry analysis of the GWAS dataset using principal components. Panel a) shows the ancestry of the XFS cases and controls from Japan relative to the international HapMap panels. Panel b) shows direct genetic ancestral matching between the Japanese XFS cases and controls.
Quantile-quantile plot of the GWAS discovery stage P-values. No genomic inflation was observed.