Abstract
Stress is a potential etiology contributor to both post-traumatic stress disorders (PTSD) and major depression. One stress-related neuropeptide that is hypersecreted in these disorders is corticotropin releasing factor (CRF). Dysregulation of CRF has long been linked to the emotion and mood symptoms that characterize PTSD and depression. However, the idea that CRF also mediates the cognitive disruptions observed in patients with these disorders has received less attention. Here we review literature indicating that CRF can alter cognitive functions. Detailed are anatomical studies revealing that CRF is poised to modulate regions required for learning and memory. We also describe preclinical behavioral studies that demonstrate CRF’s ability to alter fear conditioning, impair memory consolidation, and alter a number of executive functions, including attention and cognitive flexibility. The implications of these findings for the etiology and treatment of the cognitive impairments observed in stress-related psychiatric disorders are described.
Keywords: corticotropin releasing hormone, stress, fear conditioning, declarative memory, executive function, attention, working memory, post-traumatic stress disorder (PTSD), major depression, sex difference
Introduction
Some of the most common psychiatric disorders are post-traumatic stress disorder (PTSD) and major depression, which have a lifetime prevalence of 5.7% and 14.4%, respectively (Kessler et al., 2012). The defining symptoms of these disorders are different, such that PTSD is characterized by the re-experiencing of a traumatic event, avoidance, and hyperarousal, while depression is characterized by a persistent low mood often accompanied by feelings of hopelessness, helplessness, and anhedonia (American Psychiatric Association, 2013). Despite differences in their diagnostic criteria, PTSD and depression share several features. For example, patients with these disorders suffer from cognitive deficits, reporting impairments in learning, memory, and attention (for review see, Aupperle et al., 2012; Marazziti et al., 2010; Milad et al., 2006; Samuelson, 2011). These deficits impact daily function, thereby compounding the disruptions in affect caused by these disorders. Another shared feature is stress, and, in fact, PTSD and depression are sometimes referred to as stress-related disorders. PTSD, by definition, is precipitated by a traumatic event (Breslau, 2009; Shabsigh and Rowland, 2007). Stress also is associated with the onset and severity of depression (Kendler et al., 1995; Melchior et al., 2007; Newman and Bland, 1994). Moreover, patients with these disorders have alterations in stress circuitry (Hamilton et al., 2008; Karl et al., 2006; Kitayama et al., 2005), as well as dysregulated stress hormones and stress-related neuropeptides (Deuschle et al., 1997; Elzinga et al., 2003; Holsboer, 2001; Nemeroff et al., 1984; Yehuda et al., 2005). Given the common features of PTSD and depression, it is likely that these disorders share some etiological factors.
One stress-related neuropeptide that is linked to both PTSD and major depression is corticotropin releasing factor (CRF; e.g., Gold and Chrousos, 2002; Kasckow et al., 2001; Nemeroff and Vale, 2005). CRF acts at the level of the pituitary to initiate the hypothalamic pituitary adrenal (HPA) axis response, as well as centrally to modulate brain regions that regulate behavioral responses to stress (Bale and Vale, 2004; Owens and Nemeroff, 1991; Vale et al., 1981). Although typically CRF release facilitates appropriate stress coping, its hypersecretion is thought to be maladaptive (Holsboer and Ising, 2008; Kasckow et al., 2001; Nemeroff, 1996). In fact, some patients with PTSD and depression have elevated levels of CRF in their cerebrospinal fluid, which positively correlates with symptom severity (Baker et al., 2005; Baker et al., 1999; Banki et al., 1992; Bremner et al., 1997; Nemeroff et al., 1984; Sautter et al., 2003). Moreover, in postmortem tissue of depressed patients, high levels of CRF and altered CRF receptor expression indicative of protracted CRF dysregulated are observed (Austin et al., 2003; Bissette et al., 2003; Raadsheer et al., 1994; Wang et al., 2008). Single nucleotide polymorphisms (SNPs) in the CRF1 receptor gene also have been reported in patients with PTSD and depression (Amstadter et al., 2011; Liu et al., 2006; Polanczyk et al., 2009; Wasserman et al., 2008). Collectively, these studies suggest that alterations in the CRF system could contribute to the symptoms of these stress-related disorders.
To more directly link CRF hypersecretion to disordered behavior, researchers have turned to non-human animal models where causality can more easily be tested. The focus of much of this work has been to identify how CRF and the activation of CRF receptors alter anxiety and endocrine responses to stress. These preclinical studies have shown, for example, that CRF overexpression leads to an anxious phenotype (Stenzel-Poore et al., 1994; van Gaalen et al., 2002). Studies on the two CRF receptors, CRF1 and CRF2, have revealed that they can differentially modulate stress-related behavior. Specifically, CRF1 receptor activation initiates the HPA axis response and leads to anxiogenic behavior (Bale and Vale, 2004; Contarino et al., 1999; Heinrichs et al., 1997; Smith et al., 1998; Takahashi, 2001; Timpl et al., 1998). In contrast, activation of CRF2 receptors attenuates the HPA axis, and, in some cases, decreases anxiety (Bale et al., 2000; Bale and Vale, 2004; Coste et al., 2000; Coste et al., 2001). The underlying mechanisms of the sometimes opposing actions of CRF1 and CRF2 receptors are unclear. However, in the dorsal raphe, differences in the density of CRF1 and CRF2 receptors on serotonergic versus GABAergic neurons are thought to underlie different functions (Commons and Valentino, 2002). Additionally, distinct trafficking of CRF1 and CRF2 receptors within dorsal raphe neurons has been linked to alterations in stress-coping strategies (Waselus et al., 2009). However, more research is needed to understand the molecular basis for the sometimes opposing effects of CRF1 and CRF2 receptors in other brain regions.
In addition to the effects of CRF on the regulation of anxiety and endocrine responses to stress, an underexplored but intriguing possibility is that CRF also mediates the changes in cognition observed in patients with PTSD and depression. This idea is based on the fact that CRF and its receptors are found in regions critical for learning and memory (Justice et al., 2008; Merchenthaler, 1984; Primus et al., 1997; Van Pett et al., 2000). Moreover, emerging preclinical research suggests that mnemonic processes can be mediated by CRF. Here we review these studies and provide evidence that the cognitive disruptions that impair function in patients with PTSD and depression could result from high levels of CRF.
CRF and Fear Learning
Fear is an emotional response to a threat or perceived threat. In addition to expressing fear, animals can learn about cues that predict threatening situations and remember those cues to promote future survival. A growing body of literature suggests a critical role for CRF in learning about fearful situations, and these findings may be clinically relevant. Although learning about threating situations is adaptive, it becomes maladaptive when traumatic memories are activated inappropriately or persistently, and such responses are linked to the etiology of stress-related psychiatric disorders. PTSD in particular is thought to be caused, at least in part, by dysregulated fear learning (e.g., Blechert et al., 2007; Mahan and Ressler, 2012; Milad et al., 2006; Orr et al., 2000; Pitman, 1989; VanElzakker et al., 2014; Wessa and Flor, 2007). Learning disruptions can occur at the time of the traumatic event when associations between the trauma and various environmental cues become so strong that they later trigger intrusive recollections (Orr et al., 2000; Pitman, 1989). Additionally, patients with PTSD can have difficulty extinguishing responses to cues associated with the trauma (Blechert et al., 2007; Wessa and Flor, 2007). Although abnormal fear learning is most associated with PTSD, depressed patients and even the children of depressed and anxious mothers have disrupted fear learning (Nissen et al., 2010; Waters et al., 2014). Thus, alterations in the mnemonic aspects of fear processing may be a premorbid risk factor for several stress-related psychiatric disorders.
In the laboratory, fear learning is studied utilizing the fear conditioning procedure. The rodent version of this task pairs an initially neutral stimulus, typically a tone, with an aversive unconditioned stimulus (US), typically a footshock. Because the tone proceeds and predicts the footshock, the rodent forms an association between these two stimuli, and the tone becomes a conditioned stimulus (CS). This association is tested 24 hours after the CS–US pairings when the tone is presented in a novel context and freezing (i.e., ceasing all motion as a defensive behavior) during the tone is measured. This freezing response is considered a conditioned response (CR), and the magnitude of freezing is thought to reflect the strength of the CS–US association. This simple procedure has been elegantly utilized to elucidate the circuitry critical for fear learning (e.g., Davis, 1992; Davis and Whalen, 2001; Fanselow and Poulos, 2004; Johansen et al., 2011; LeDoux, 2000; Maren, 2005; Medina et al., 2002; Quirk et al., 1995; Sah and Westbrook, 2008). This circuit consists of sensory regions that process stimuli, areas that regulate the mnemonic aspects of the task, and regions involved in generating the expression of fearful responses. Specifically, the CS and US are first processed by sensory regions, such as the auditory and somatosensory thalamus and cortices. This sensory information then converges on neurons in the lateral nucleus of the amygdala (LA). Through CS–US pairings, synaptic plasticity within the LA region enhances neuronal responses to the CS, indicating that the LA is critical for forming the association. The LA then projects both directly and indirectly (via the basal nucleus and intercalated masses) to the central nucleus of the amygdala (CE). The CE regulates the expression of fear via projections to brain regions involved in autonomic (lateral hypothalamus), endocrine (paraventricular nucleus of the hypothalamus), and defensive (periaqueductal gray) responses.
It is clear from this prior work that fear conditioning requires a network of brain regions. Interestingly, CRF is positioned to modulate many of these areas, including those involved in both non-mnemonic and mnemonic aspects of fear conditioning. For example, CRF receptors are found in thalamic and cortical regions involved in audition and somatosensation (Fig. 1; Primus et al., 1997; Van Pett et al., 2000). Therefore, CRF could directly modulate sensory processing of the CS and US, a possibility, which to our knowledge, has never been tested. CRF and its receptors are also present in regions critical for fear expression, including the lateral hypothalamus, paraventricular nucleus of the hypothalamus, and periaqueductal gray (Fig. 1; Merchenthaler, 1984; Potter et al., 1994; Van Pett et al., 2000). In fact, local infusions of CRF into the periaqueductal gray increases defensive behavior, such as freezing during fear conditioning (Carvalho-Netto et al., 2007; Stiedl et al., 2005). Anatomically, CRF is also positioned to affect amygdala regions involved in the mnemonic aspects of fear conditioning (Fig.1). Both types of CRF receptors (CRF1 and CRF2) are found in the LA and CE regions, although CRF1 receptors are expressed at higher levels than CRF2 receptors (Chalmers et al., 1995; Justice et al., 2008; Van Pett et al., 2000; Weathington and Cooke, 2012). CRF immunoreactivity is found throughout the amygdala, but the CE in particular has a large number of CRF expressing cell bodies (Gray, 1993; Swanson et al., 1983). Interestingly, CRF projections from the CE terminate in fear expressing regions, including the lateral hypothalamus and periaqueductal gray (Gray, 1993; Gray and Magnuson, 1992), indicating that CRF may be a critical neuropeptide that links the amygdala with regions involved in fear expression.
Figure 1.
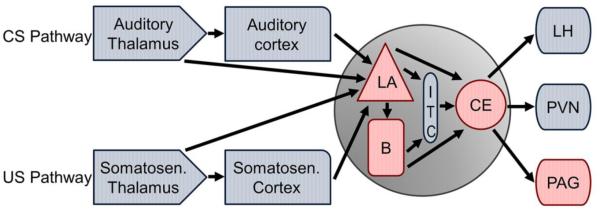
Schematic illustrating CRF receptors in regions critical for fear conditioning. CRF is poised to modulate regions involved in sensory processing, fear learning, and fear expression. Regions with CRF1 receptors are shaded in blue, while those with both CRF1 and CRF2 receptor expression are shaded in red. B: basal nucleus of the amygdala; CE: central nucleus of the amygdala; ITC: intercalated masses; LA: lateral nucleus of the amygdala; LH: lateral hypothalamus; PAG: periaqueductal gray; PVN: paraventricular nucleus of the hypothalamus
There is evidence to suggest that moderate levels of CRF are actually required for appropriate fear learning. Exposure to footshock (the most common US) increases CRF expression in the amygdala of male rats (Yamano et al., 2004). This increase may be critical for fear conditioning because reducing the effects of CRF in both the basolateral amygdala (BLA; which includes the LA) and the CE disrupts the consolidation or stabilization of fear memories in male rats (Hubbard et al., 2007; Pitts and Takahashi, 2011; Pitts et al., 2009). Conversely, in the BLA of male rats, increasing free endogenous CRF concentrations by displacing CRF from its binding protein enhances memory consolidation of fearful events (Roozendaal et al., 2008).
The above studies suggest that CRF acts to enhance consolidation, however manipulations that cause very high CRF levels indicate that CRF can also have the opposite effect on the consolidation of fear memories (Isogawa et al., 2012). Specifically, in male rats, the addition of CRF into the LA by microinfusion immediately before or after training impairs fear conditioning, a time course consistent with CRF inducing an impairment in consolidation (Isogawa et al., 2012). Similarly, fear conditioning is disrupted in CRF overexpressing male mice, although the exact nature of the mnemonic deficit (e.g., disruption in acquisition, consolidation, or retention) is difficult to determine given their persistently high levels of CRF expression (Groenink et al., 2003; Tovote et al., 2005; van Gaalen et al., 2002). Interestingly, CRF administration in the LA has a different effect 24 hours after training, but prior to testing, such that it improves fear conditioning (Isogawa et al., 2012). Given the timing, this result indicates that CRF can enhance the retention of fearful memories. Notably, the effects of exogenously applied CRF on fear learning appear specific to the LA, as similar infusions into the CE did not alter the consolidation or retention of fear conditioning (Isogawa et al., 2012).
Another critical aspect of fear learning is fear extinction, a process where it is learned that cues associated with a traumatic event no longer predict that event (Maren and Quirk, 2004; Myers and Davis, 2002). In the laboratory, after the acquisition of fear conditioning, extinction training is initiated when the CS (e.g., tone) is presented alone and no longer paired with the US (e.g., footshock). The subject then learns that the CS no longer predicts the US and freezing decreases and potentially disappears. The acquisition of extinction learning requires the BLA, while the consolidation of extinction depends on the infralimbic region of the prefrontal cortex (reviewed in Pape and Pare, 2010; Quirk and Mueller, 2007; Sotres-Bayon and Quirk, 2010). The role of CRF in extinction has recently been examined in male rats (Abiri et al., 2014). Both endogenous and exogenous increases in CRF in the BLA prior to extinction training impaired extinction recall 24 hours later. Conversely, administering a non-selective CRF receptor antagonist into the BLA had the opposite effect (i.e., it improved extinction recall; Abiri et al., 2014). Similarly, treatment with a CRF1 receptor antagonist rescued an extinction deficit seen in mice with increased CRF in the amygdala due to the GABA(A)α1 receptor deletion in CRF neurons (Gafford et al., 2012). Although the role of CRF during extinction has been evaluated for the BLA, no studies, to our knowledge, have examined the role of CRF in the infralimbic cortex during extinction, despite high expression of CRF1 receptors there (Justice et al., 2008; Van Pett et al., 2000).
As noted, PTSD is thought to result, at least in part, from enhanced memories of cues associated with trauma, as well as from a resistance to extinguishing responses to those cues (Blechert et al., 2007; Orr et al., 2000; Pitman, 1989; Wessa and Flor, 2007). The preclinical work suggests that excessive CRF release could contribute to these mnemonic changes, depending on the timing of the CRF elevation. Although high levels of CRF in the amygdala around the time of fear conditioning disrupt consolidation (Isogawa et al., 2012), later increases in CRF enhance retention and impair extinction (Abiri et al., 2014; Isogawa et al., 2012). If similar effects hold true in humans, it would be unlikely that CRF hypersecretion prior to a traumatic event contributes to PTSD. However, if the trauma itself induces CRF hypersecretion, this increase in CRF could drive mnemonic changes that contribute to PTSD symptoms. Future studies in clinical populations will be needed to test this idea.
Fear conditioning studies in rodents not only reveal potential mechanisms that contribute to stress-related psychiatric disorders, but they also suggest a possible pharmacotherapy. A drug that can enhance extinction learning (i.e., the partial NMDA agonist, D-Cycloserine) has been shown to improve exposure therapy in patients with phobias and PTSD (de Kleine et al., 2013; Norberg et al., 2008; Rothbaum et al., 2014). If CRF1 receptor antagonists improve extinction in humans as they do in rodents (Abiri et al., 2014; Gafford et al., 2012), these compounds may represent a new pharmaceutical approach that can be used as an adjunctive treatment to facilitate the effects of exposure therapy in patients with PTSD.
CRF, Declarative Memory, and the Hippocampus
Declarative memory is the conscious recollection of facts and events, and requires medial temporal lobe structures, particularly the hippocampus (Squire and Zola-Morgan, 1991). The hippocampus is not only required for the formation of declarative memories, but it is also necessary for their consolidation (Squire and Alvarez, 1995). Interestingly, patients with stress-related psychiatric disorders have impaired declarative memory (Bremner et al., 2004; Burt et al., 1995; Dresler et al., 2011; Samuelson, 2011). These deficits are not surprising given that the hippocampus is actually smaller in adults with PTSD and depression (Karl et al., 2006; Kitayama et al., 2005; MacQueen et al., 2003; Sheline, 2000; Sheline et al., 1996). In addition to problems with declarative memory, patients with PTSD and depression also show deficits in another type of memory mediated by the hippocampus, spatial learning (Moser et al., 1995; O'Keefe and Nadel, 1979; Richards and Ruff, 1989; Tempesta et al., 2012; Veiel, 1997). Collectively, these findings indicate that a number of memory functions involving the hippocampus are disrupted in stress-related psychiatric disorders.
The hippocampus is also involved in responding to stressors. It mediates the endocrine limb of the stress response, regulating glucocorticoid negative feedback of the HPA axis (McEwen and Gianaros, 2011; Sapolsky et al., 1985). Additionally, in preclinical models, stressor exposure alters the physiology and morphology of hippocampal neurons (for review see, Buwalda et al., 2005; Howland and Wang, 2008; Leuner and Shors, 2012; McEwen, 1999; McLaughlin et al., 2007). These cellular changes in the hippocampus can translate into changes in learning and memory (Conrad et al., 1996; McEwen et al., 1997; Watanabe et al., 1992), indicating that the hippocampus mediates stress and learning interactions (Bangasser and Shors, 2007, 2010). The mechanisms by which stress alters hippocampal structure and function involve a variety of stress hormones, neuropeptides, and neurotransmitters, including CRF (for review see, Joels and Baram, 2009; McEwen, 1999; McLaughlin et al., 2007). CRF is well positioned to alter hippocampal function, as it is actually produced locally within the hippocampus by interneurons (Chen et al., 2001; Chen et al., 2004; Yan et al., 1998). Additionally, CRF1 and CRF2 receptors are both present in the hippocampus (Primus et al., 1997; Van Pett et al., 2000), however, their distribution is different (Joels and Baram, 2009). On hippocampal pyramidal neurons, CRF1 receptors are located on the soma and at excitatory synapses in dendritic spines, while CRF2 receptors are found in axons (Chen et al., 2004; Chen et al., 2000; Joels and Baram, 2009). Given this anatomy, it is not surprising that non-human animal studies have identified a critical role for CRF in the modulation of mnemonic processes that require the hippocampus.
Anecdotally, it is easy to think of examples of how stress can impair declarative learning. However, the effect of CRF on hippocampal-dependent learning is not always detrimental. In fact, acute CRF exposure can enhance memory. Specifically, CRF can enhance the acquisition of hippocampal dependent contextual and spatial tasks when infused centrally or directly into the hippocampus, respectively (Blank et al., 2003; Koob and Bloom, 1985). Memory consolidation also is improved by infusion of a moderate dose of CRF into the hippocampus of male rodents (Hung et al., 1992; Lee et al., 1992; Ma et al., 1999; Radulovic et al., 1999). Enhancements in learning following CRF administration have been linked to changes in intracellular signaling and the modulation of growth factors (Hung et al., 1992; Lee et al., 1992; Ma et al., 1999), and may also be attributable to the fact that CRF applied to hippocampal slices enhances long-term-potentiation (LTP), a putative learning mechanism (Blank et al., 2002; Blank et al., 2003). Actually, a moderate amount of CRF activation of the hippocampus may be required for optimal learning, as CRF1 and CRF2 receptor knockout mice do not perform as well on hippocampus-dependent learning tasks as wild type animals (Contarino et al., 1999; Risbrough et al., 2009).
In contrast to the beneficial effects of moderate amounts of acute CRF on learning, too much CRF, especially if elevated chronically, has a detrimental effect on mnemonic processes mediated by the hippocampus. For instance, five hours of multimodal stress in adult male rats impairs learning that requires the hippocampus, an effect associated with a spine loss on apical dendrites in the CA3 region of the hippocampus (Chen et al., 2010). These mnemonic and morphological effects are prevented by CRF1 receptor antagonism (Chen et al., 2010). Although the time course of this stressor is acute, its severity likely increases CRF above levels that would be beneficial, suggesting that too much CRF can remodel hippocampal neurons and cause memory impairments. Chronically elevated levels of CRF also can impair hippocampal learning and alter neuronal morphology. Memory impairments were first demonstrated in male mice overexpressing CRF throughout their lifetime, which have spatial learning deficits that are not attributable to sensory or motor effects (Heinrichs et al., 1996). CRF overexpression, when restricted to adulthood, also impairs hippocampal-dependent contextual learning in female, but not male mice (Toth et al., 2014). In contrast, this type of learning is not affected by lifetime CRF overexpression (Toth et al., 2014). These studies highlight that the timing of expression, nature of the task, and the sex of the animal can affect the degree to which genetic CRF overexpression impairs hippocampal-dependent memory. Another manipulation that can persistently elevate CRF is chronic stress (Chappell et al., 1986; Imaki et al., 1991). In adult male rodents, this type of stress has detrimental effects on spatial and recognition learning, which require the hippocampus (Broadbent et al., 2010; Clark et al., 2000), and these effects are mediated by CRF1 receptors (Wang et al., 2011a). When compared to adults, young animals are perhaps even more vulnerable to the effects of high levels of CRF exposure. Chronic early-life stress impairs spatial learning in adulthood and this effect is mimicked by increasing CRF levels early in development (Brunson et al., 2001; Wang et al., 2011b). Mechanistically these impairments may result from a CRF-induced reduction in LTP, as well as CA3 neuronal and spine damage (Brunson et al., 2001; Wang et al., 2011b). Unfortunately, the deleterious effects of early CRF exposure on memory only worsen with age (Brunson et al., 2001; Ivy et al., 2010). They are, however, ameliorated by removing or antagonizing CRF1 receptors (Ivy et al., 2010; Wang et al., 2011b). Thus, CRF1 receptor antagonists may be a potential treatment for mnemonic deficits caused by early-life stress.
When taken together, these preclinical studies indicate that although hippocampal-dependent memory may benefit from acute, moderate elevations in CRF, this type of memory is disrupted by high, chronically elevated levels of CRF, an effect which is more severe if it occurs early in life. Unfortunately, the link between chronically high levels of CRF and memory deficits has yet to be studied in humans. In light of the preclinical findings, however, it is likely that the persistently high levels of CRF in patients with PTSD and depression contribute to their deficits in declarative and spatial memory.
CRF and Executive Functions
Executive functions are a collection of processes aimed at regulating thought, action, and emotion. These functions include attention, working memory, cognitive flexibility, inhibitory control, and planning. Executive functions are impaired in patients with stress-related psychiatric disorders. For example, compared to healthy and trauma exposed controls, PTSD patients have impaired attention and working memory (Koso and Hansen, 2006; Vasterling et al., 1998; Vasterling et al., 2002). Depressed patients also have difficulty with executive functions, such as trouble with cognitive flexibility and sustaining attention (Farrin et al., 2003; Fossati et al., 1999; McDermott and Ebmeier, 2009). Because executive functions regulate other cognitive domains, these deficits disrupt a number of cognitive abilities, and thus understanding how executive functions become impaired could improve daily function for patients with stress-related psychiatric disorders.
Broadly speaking, the prefrontal cortex (PFC) mediates executive functions (Funahashi and Andreau, 2013; Robbins, 1996). More specifically, different aspects of executive function are associated with different divisions within the PFC. For example, the right inferior frontal cortex controls inhibition, while the dorsolateral PFC is critical for working memory (Arnsten and Jin, 2014; Aron et al., 2014). Some executive functions engage other cortical regions in addition to the PFC, as exemplified by the fact that the anterior cingulate and parietal cortices work with the PFC to regulate attention (Fink et al., 1997; Han and Marois, 2014; Petersen and Posner, 2012). Moreover, these cortical structures do not operate in isolation, but instead interact heavily with basal forebrain and brainstem ascending modulatory systems, including the cholinergic, noradrenergic, dopaminergic, and serotonergic systems. In a top-down fashion, the PFC can regulate these modulatory systems (Amat et al., 2005; Jodo et al., 1998; Zaborszky et al., 1997). Conversely, these neurotransmitter systems influence the PFC so that information about state (e.g., arousal, alertness, and motivation) can alter executive functions (for review see, Chandler et al., 2014; Robbins and Arnsten, 2009; Sarter et al., 2001).
The CRF dysregulation observed in stress-related psychiatric disorders could contribute to impaired executive function because CRF is poised to modulate both cortical and subcortical structures involved in these processes. Studies in rodents have found that CRF1 receptors are highly expressed in cortical areas, including the PFC (Fig. 2; Primus et al., 1997; Van Pett et al., 2000). CRF receptors are also present in the modulatory regions that project to the PFC to regulate executive function. Specifically, CRF1 receptors are present on cholinergic neurons in the basal forebrain that release acetylcholine into the PFC (Chen et al., 2000; Sauvage and Steckler, 2001; Zaborszky et al., 2012). The ventral tegmental area, which releases dopamine in cortical regions, contains both CRF1 and CRF2 receptors (Sauvage and Steckler, 2001; Ungless et al., 2003). CRF1 receptors are also found on noradrenergic neurons in the locus coeruleus (LC; Curtis et al., 2006; Reyes et al., 2008) that are the source of norepinephrine for the cortex (Aston-Jones et al., 1995), while both CRF1 and CRF2 receptor subtypes are found on serotonergic and non-serotonergic neurons in the dorsal raphe (Reyes et al., 2006; Waselus et al., 2009). Local infusions of CRF into LC and the dorsal raphe increase norepinephrine and serotonin release in the PFC, respectively, revealing a mechanism by which CRF modulation of brainstem structures can affect cortical processing (Curtis et al., 1997; Forster et al., 2008; Smagin et al., 1995). Collectively, these findings reveal that direct and indirect (via subcortical projection systems) modulation of frontal-executive functions by CRF is possible (Fig. 2).
Figure 2.
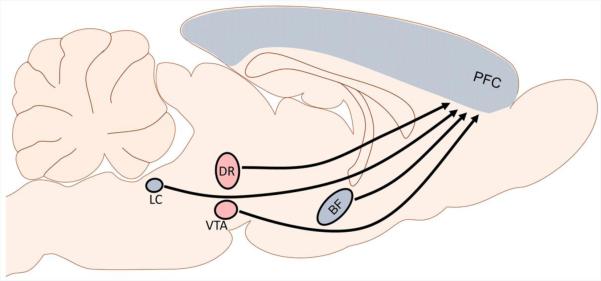
Schematic depicting the circuitry by which CRF can regulate executive functions. CRF could regulate the PFC directly or alter executive functions indirectly by activating basal forebrain and brainstem ascending systems. Regions with CRF1 receptors are shaded in blue, while those with both CRF1 and CRF2 receptor subtypes are shaded in red. BF: basal forebrain; DR: dorsal raphe; LC: locus coeruleus; PFC: prefrontal cortex; VTA: ventral tegmental area
Some executive functions, such as planning, cannot be studied in rodent models, yet for most executive functions (e.g., attention, inhibition, cognitive flexibility, working memory) there are relevant non-human animal tasks. Surprisingly, direct effects of CRF in the PFC have not been evaluated with any of these tasks, to our knowledge. However, executive functions have been examined following brain-wide changes in CRF levels, as well as following site specific infusions of CRF into subcortical modulatory regions. Here we will highlight studies that have investigated the role of CRF in attention, cognitive flexibility, and working memory.
CRF and Attention
Patients with PTSD and depression have attentional disruptions and show impairments on the continuous performance task that measures sustained and selective attention (Canpolat et al., 2014; Vasterling et al., 2002). In rodents, these attentional processes are often tested with the 5-choice serial reaction time task (5-CSRTT), which models some aspects of the continuous performance task (Robbins, 2002). In this task, a rodent is required to monitor the location of a light briefly presented in one of five spatial locations. In order to obtain a reward, the rodent must poke with its nose the lit aperture within several seconds of light offset (Bari et al., 2008; Robbins, 2002). Performance measures on the task include accuracy (i.e., correct responses), incorrect responses, and omissions (i.e., trials during which no response is made; Bari et al., 2008). Additionally, aspects of inhibitory control can be measured by counting premature responses (nose pokes that occur prior to the light presentation) or perseverative responses (repeated nose pokes to a previously lit aperture; Robbins, 2002). Alterations in these different performance measures reflect disruptions in different cortical and sub-cortical systems (Robbins, 2002).
The effects of global increases in CRF on 5-CSRTT performance have been evaluated in several ways. For example, attentional impairments have been examined in male mice that overexpress CRF throughout development (Stenzel-Poore et al., 1992; van Gaalen et al., 2003). On the 5-CSRTT, these CRF overexpressing mice take longer to acquire the task contingencies than wild type controls (van Gaalen et al., 2003), which likely reflects mnemonic deficits that are separate from attentional impairments. However, disrupted attentional processes are observed once the task is acquired, as CRF overexpressing mice have impaired accuracy, longer correct response latencies, and increased omissions (van Gaalen et al., 2003). These performance measures improve to wild type levels if attentional demands are decreased (i.e., when the light stimulus duration is increased), suggesting that these effects are due to disruptions in attention, not other performance issues (e.g., sensory or motor impairments). In addition to CRF overexpressing mice, the central effects of CRF on 5-CSRTT performance also have been examined in male rats. Central CRF administration affects accuracy in a dose-dependent fashion, such that a low dose of CRF increases accuracy, while higher doses impair accuracy (Ohmura et al., 2009; Van't Veer et al., 2012). High doses of CRF also increase omissions and the latency to make a correct response, effects similar to that observed in CRF overexpressing mice (Van't Veer et al., 2012). Collectively, these findings demonstrate that high levels of CRF can impair attention.
As noted, a number of brain regions work in concert to mediate attentional processes. Because the aforementioned studies examined the effects of central changes in CRF, the precise structures targeted by CRF to alter attention remain unknown. However, previous studies have linked specific deficits in 5-CSRTT performance to certain brain regions and neurotransmitter systems (Robbins, 2002). Medial PFC lesions impair accuracy and increase correct response latencies (Muir et al., 1996). This pattern of performance is observed following high levels of CRF (Van't Veer et al., 2012; van Gaalen et al., 2003), and thus suggests that CRF may be directly affecting the medial PFC. Altering cholinergic and dopaminergic function in the PFC also affects accuracy (Granon et al., 2000; Robbins, 2002; Robbins et al., 1998), which suggests that CRF could also act at the level of the basal forebrain or ventral tegmental area to alter attention. High levels of CRF also increased omissions (Van't Veer et al., 2012; van Gaalen et al., 2003), and omissions are often mediated by changes in the dopaminergic system (Baunez and Robbins, 1999; Cole and Robbins, 1989; Robbins, 2002). Thus, the combined pattern of decreased accuracy and increased omissions could indicate that the disruptions in attention are primarily driven by the effects of CRF on dopaminergic neurons in ventral tegmental area. Because a goal of modern pharmacology is to develop drugs that target certain brain regions, identifying the location(s) where CRF acts to disrupt attention would help guide the development of novel treatments to mitigate stress-induced attentional impairments.
CRF and Cognitive Flexibility
Another executive function mediated by the PFC is cognitive flexibility, which is the ability to shift between rules or concepts (Sohn et al., 2000; Stuss et al., 2000). Some patients with depression and PTSD show impairments in tests of cognitive flexibility, such as the Wisconsin Card Sorting Test that examines the ability to shift between different cognitive strategies in response to changing contingencies (Channon, 1996; LaGarde et al., 2010; Merriam et al., 1999, but see Vasterling et al., 2002). A rodent cognitive flexibility task that is analogous to the Wisconsin Card Sorting Test is attentional set shifting (Birrell and Brown, 2000). In this task, rats are trained to discriminate between two pots in order to receive a buried food reward (only one pot is baited with food). These pots are distinguishable by cues in two stimulus dimensions (e.g., odor and digging medium). During the task, rats perform a series of discriminations including reversals, intradimensional shifts (e.g., shifting from one set of odor cues to another set of odor cues), and extradimensional shifts (e.g., shifting from odor cues to digging medium). Much like its role in cognitive flexibility in humans, the PFC is also involved in aspects of set shifting in rats. Specifically, lesions to the medial PFC selectively disrupt the extradimensional shift, suggesting that this region is critical for inhibiting responses to old cues and shifting the strategy to respond to newly relevant cues (Birrell and Brown, 2000). Cognitive flexibly is mediated by norepinephrine in the medial PFC, such that increasing norepinephrine neurotransmission in this region improves performance on the extradimensional shift (Lapiz and Morilak, 2006). This exemplifies the important role that brainstem modulatory systems play in executive function.
CRF modulation of attentional set shifting has been investigated in male rats (Snyder et al., 2012). In this study, CRF injections into the LC affected performance on the extradimensional shift in an inverted U-shaped dose-response relationship, such that low and high doses of CRF have no effect on performance, while a moderate dose improves extradimensional shifting. The moderate dose of CRF in the LC also activates the medial PFC, as assessed by immunoreactivity for the immediate early gene cFOS (Snyder et al., 2012). As noted, CRF in the LC can increase norepinephrine release in cortical regions (Curtis et al., 1997; Smagin et al., 1995), so the improvement in the extradimensional shift likely results from increased cortical norepinephrine induced by CRF activation of the LC. Interestingly, this enhanced extradimensional shifting actually may be an adaptive response that promotes behavioral flexibility under moderately stressful conditions (Snyder et al., 2012).
In contrast to the effects in the LC, central administration of CRF impairs several aspects of the attentional set shifting task, including the reversal, intradimensional shift, and extradimensional shift (Snyder et al., 2012). The difference between the central and LC-specific effects of CRF is likely due to the fact that central CRF is acting on a number of different brain regions, some of which may have opposing effects to that of the LC, and this discrepancy has implications for treatment. Currently, pharmacotherapies targeting the CRF system (e.g., CRF antagonists) are aimed at causing a global decrease in CRF effects. Presumably these drugs would eliminate any positive effects of CRF in the LC on cognitive flexibility. Thus, this study highlights the need for more selective treatments that perhaps target CRF dysregulation in specific brain regions. In order to develop such compounds, clearly more basic research is needed to identify the brain regions where positive versus negative effects of CRF secretion occur, and to identify the molecular and cellular differences between these regions that would allow them to be more selectively targeted pharmacologically.
CRF and Working Memory
Working memory requires storing and manipulating multiple pieces of transitory information in order to carry out complex cognitive tasks. This type of memory is mediated by the dorsolateral PFC along with other cortical structures (Barbey et al., 2013; Levy and Goldman-Rakic, 2000; Nee et al., 2013). Much like other executive functions, working memory is disrupted in stress-related psychiatric disorders (Rose and Ebmeier, 2006; Vasterling et al., 2002). Although there are many working memory tasks for non-human animals, to our knowledge, the effect of CRF on working memory has not been investigated in rodents.
Working memory deficits in humans have been linked to the interaction between the environmental factor, early life stress, and differences in the CRF1 receptor gene. Early life stress is a risk factor for the development of both cognitive deficits and stress-related psychiatric disorders in adulthood (for review see, Gutman and Nemeroff, 2002; Heim and Binder, 2012; Pechtel and Pizzagalli, 2011). However, only a portion of people who experience early life stress suffer impairments as adults, suggesting that genetic factors moderate the effect of stressful environmental influences. The SNPs, rs110402 and rs242924, on the CRF1 receptor gene have been shown to confer resilience to the detrimental effects of early life stress on working memory and depression. Specifically, while moderate exposure to early life stress impaired working memory in healthy adult subjects who were homozygous GG for both rs110402 and rs242924, it took severe stressor exposure to impair memory in those with the less common combination of homozygous AA and TT alleles for these genes, respectively, suggesting that this combined genotype is protective (Fuge et al., 2014). These SNPs also moderate the effects of early life stress on HPA axis dysregulation and depression, such that subjects with one copy of either the rs110402 A-allele or rs242924 T-allele are protected as adults from the endocrine and psychiatric consequences of certain types of childhood trauma (Bradley et al., 2008; Heim et al., 2009; Polanczyk et al., 2009; Tyrka et al., 2009). Despite these interesting findings, the nature of the relationship between working memory deficits and depression in patients homozygous GG for rs110402 and rs242924 genotype is, at this point, unclear. It could be that working memory deficits are a risk factor for depression in people with this genotype, or alternatively that depression causes greater cognitive disruptions in patients with this genotype. Surprisingly, the consequences of these SNPs for CRF1 receptor function also are unknown. It is tempting, however, to speculate that these SNPs alter CRF signaling in brain regions implicated in depression and working memory, such as the dorsolateral PFC. Although more research is clearly needed, the current data do highlight the important role of CRF1 receptors in moderating the effects of early life stress on executive function and mood.
Sex Differences in the CRF system
Women are twice as likely as men to suffer from PTSD and depression (Breslau, 2002; Kendler et al., 1995; Kessler, 2003; Kessler et al., 1993; Tolin and Foa, 2006). Despite this reliably reported epidemiological difference, the neurobiological basis for the sex bias in these disorders remains largely unknown. However, preclinical research suggests that sex differences in the CRF system contribute to increased susceptibility to stress in females, which could account for the higher rates of stress-related disorders in women (for review see, Bangasser, 2013; Bangasser and Valentino, 2012; Valentino et al., 2012).
Most preclinical research has focused on how sex differences in the CRF system can impact anxiety and endocrine responses to stress (Babb et al., 2013; Bale and Vale, 2003; Duncko et al., 2001; Iwasaki-Sekino et al., 2009). Cognition may also be altered by sex differences in the CRF system because sex differences in CRF expression and its receptors are found within regions critical for memory formation. Adult female rats have greater CRF expression in the CE and more CRF1 receptor binding in the BLA than males (Iwasaki-Sekino et al., 2009; Weathington and Cooke, 2012), effects that could increase fear consolidation and impair extinction in females. Sex differences in CRF1 receptor binding also are found in the hippocampus, such that binding increases during puberty in females, but not males in the CA3 region (Weathington et al., 2014). Given that chronically elevated levels of CRF impair declarative memory via CRF1 receptor activation (Wang et al., 2011a), this sex difference in binding could render females more vulnerable to these negative mnemonic effects, especially after puberty. In support of this, CRF overexpression that was restricted to adulthood impaired hippocampal-dependent contextual conditioning in female but not male mice (Toth et al., 2014). Unfortunately, the extent of sex differences in the effects of CRF on fear conditioning and declarative memory are largely unknown because most of the studies conducted thus far have used only male subjects, or were otherwise not designed to detect sex differences.
Executive functions mediated by the cortex also could be differentially altered by CRF in males versus females due to complex sex differences in the cortical CRF system. Not only do females have greater cortical CRF1 receptor binding than males (Weathington et al., 2014), but there are also sex differences in cortical CRF1 receptor signaling (Bangasser et al., 2010; Valentino et al., 2013). For example, CRF receptors are G-protein coupled receptors that preferentially bind Gs to activate the cyclic AMP and protein kinase A (PKA) signaling cascade leading to a variety of downstream cellular changes (Grammatopoulos et al., 2001; Hauger et al., 2009; Hillhouse and Grammatopoulos, 2006; Jedema and Grace, 2004). However, cortical CRF1 receptors of females are more highly coupled to the Gs protein than those of males (Bangasser et al., 2010). This sex difference can lead to increased PKA signaling in females under conditions of excessive CRF release (Valentino et al., 2013). It is clear from these studies that cortical CRF1 receptors are functionally very different in males and females. However, whether these differences result in sex differences in CRF’s modulation of executive functions remains unknown.
Sex differences in CRF are also observed in brainstem structures that regulate cognition. In the dorsal raphe-serotonin system, local infusions of CRF increase depressive-like behavior and HPA axis activity in male but not female mice (Howerton et al., 2014). This could indicate that males are more sensitive to CRF-induced changes in cognition mediated by serotonin, an idea that has yet to be explored. Sex differences in CRF modulation of the LC-norepinephrine system also have been identified. CRF activates LC neurons to a greater degree in female than male rats, an effect linked to sex differences in CRF-induced PKA signaling (Bangasser et al., 2010; Curtis et al., 2006). Given that higher LC neuronal activity increases norepinephrine release in target regions, such as the cortex (Curtis et al., 1997; Smagin et al., 1995), this sex difference could differentially alter executive function in males versus females. CRF1 receptors in the LC are also trafficked differently in male and females following stress and CRF hypersecretion (Bangasser et al., 2010; Bangasser et al., 2013). Specifically, these manipulations increase CRF1 receptor internalization in LC dendrites only in males. As internalized receptors can no longer be activated by CRF, this compensatory mechanism can explain why male CRF overexpressing mice can maintain their LC neuronal response to CRF hypersecretion at wild type level (Bangasser et al., 2013). In contrast, LC neurons of female CRF overexpressing mice fire roughly three times faster than their wild type counterparts. This high level of neuronal firing in females under conditions of CRF hypersecretion is expected to result in high levels of norepinephrine release in the cortex, an effect associated with “going off task” (Aston-Jones and Cohen, 2005). Thus, females may be less likely to sustain or focus attention during conditions of high CRF release. Collectively, these sex differences in brainstem modulatory systems could impact several aspects of executive function that are disrupted in stress-related psychiatric disorders. However to date, only male subjects have been included in basic research studies examining the effects of CRF on executive function.
Clearly more preclinical work that includes both male and female subjects is required to elucidate the extent to which sex differences in the CRF system result in sex differences in cognition. Clinical studies are also needed, as researches have only recently begun to systematically investigate sex difference in cognition in patients with stress-related psychiatric disorders. For instance, new studies in PTSD patients reveal that women with this disorder show enhanced fear conditioning, while men with PTSD have deficits in extinction recall (Inslicht et al., 2013; Shvil et al., 2014). Women also have greater impairments in declarative memory than men following acute tryptophan depletion, a manipulation that mimics low levels of serotonin thought to be found in depression (Sambeth et al., 2007). In the future, it would also be useful to examine whether sex differences in cognition in patient populations are associated with CRF dysregulation, as this could help guide treatment.
Implications for Treatment
First-line pharmacotherapies for stress-related psychiatric disorders are selective serotonin reuptake inhibitors (SSRIs; Altshuler et al., 2001; Krystal et al., 2011). In fact, these compounds are the only drug approved by the Food and Drug Administration for the treatment of PTSD, and thus are widely prescribed (Krystal et al., 2011; Mohamed and Rosenheck, 2008). Yet SSRIs are not very effective at treating male combat veterans with PTSD (Friedman et al., 2007; Hertzberg et al., 2000). Similarly SSRIs and other approved pharmacotherapies are unable to adequately treat 20-30% of depressed patients (Fava, 2003; Rush et al., 2006). Thus, there is a need to develop more effective compounds. Given that CRF hypersecretion is found in patients with PTSD and depression (Baker et al., 2005; Baker et al., 1999; Banki et al., 1992; Bremner et al., 1997; Nemeroff et al., 1984; Sautter et al., 2003), CRF receptor antagonists are one class of drug being designed to treat these disorders (Holsboer and Ising, 2008; Ising et al., 2007; Nemeroff, 1996; Zobel et al., 2000). Although the main focus has been to ameliorate the anxiety and depressive symptoms (Zobel et al., 2000), the studies detailed here suggest that CRF receptor antagonists may also treat some of the cognitive alterations that characterize these disorders. In fact, these drugs could potentially outperform SSRIs in this regard, because there is evidence that SSRIs fail to treat the cognitive impairments in patients with remitted depression (Smith et al., 2006). Of course, the existing data indicate that the effects of CRF on cognition depend on the timing, dose, brain region, and sex, so simply blocking CRF receptors may not be sufficient. Instead, identifying new ways to target the actions of these or related compounds may be required to maximize their benefits.
Conclusions
It has long been thought that CRF hypersecretion contributes to the changes in emotion and mood observed in PTSD and depression. The studies reviewed here suggest that the cognitive deficits that disrupt daily function in these patients could also be caused by CRF hypersecretion. More clinical and preclinical studies are needed to further our understanding of the circuits and mechanisms by which CRF can contribute to changes in cognition. It is notable, however, that the existing literature that covers multiple cognitive domains consistently implicates CRF1 receptors in mediating the deleterious effects of CRF on cognition. Therefore, efforts to develop safe and effective CRF1 receptor antagonists and related compounds may not only improve the affective symptoms of these disorders, but they may also treat their associated cognitive disruptions, thus greatly improving the quality of life for patients with PTSD and depression.
Highlights.
Corticotropin releasing factor (CRF) is hypersecreted in PTSD and depression.
Preclinical studies reveal that high levels of CRF disrupt many cognitive processes.
CRF dysregulation could impair cognition in patients with PTSD and depression.
Acknowledgments
The authors acknowledge the support of PHS grant MH092438. Special thanks to D. E. Waxler for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abiri D, Douglas CE, Calakos KC, Barbayannis G, Roberts A, Bauer EP. Fear extinction learning can be impaired or enhanced by modulation of the CRF system in the basolateral nucleus of the amygdala. Behavioural Brain Research. 2014;271:234–239. doi: 10.1016/j.bbr.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Cohen LS, Moline ML, Kahn DA, Carpenter D, Docherty JP. The Expert Consensus Guideline Series. Treatment of depression in women. Postgraduate medicine. 2001:1–107. [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, A.P.A.D.S.M.T.F. Diagnostic and statistical manual of mental disorders : DSM-5. 2013.
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Jin LE. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Progress in molecular biology and translational science. 2014;122:211–231. doi: 10.1016/B978-0-12-420170-5.00008-8. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: Disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD., Jr. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser DA. Sex differences in stress-related receptors: "micro" differences with "macro" implications for mood and anxiety disorders. Biology of sex differences. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877–904. doi: 10.1038/mp.2010.66. 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neurosci Biobehav Rev. 2010;34:1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1992;2:107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49:1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nature protocols. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Afzal N, Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2004;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Canpolat S, Kirpinar I, Deveci E, Aksoy H, Bayraktutan Z, Eren I, Demir R, Selek S, Aydin N. Relationship of asymmetrical dimethylarginine, nitric oxide, and sustained attention during attack in patients with major depressive disorder. TheScientificWorldJournal. 2014;2014:624395. doi: 10.1155/2014/624395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Litvin Y, Nunes-de-Souza RL, Blanchard DC, Blanchard RJ. Effects of intra-PAG infusion of ovine CRF on defensive behaviors in Swiss-Webster mice. Behav Brain Res. 2007;176:222–229. doi: 10.1016/j.bbr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Waterhouse BD, Gao WJ. New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Frontiers in neural circuits. 2014;8:53. doi: 10.3389/fncir.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: The Wisconsin Card Sorting Test. J Affect Disorders. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proceedings of the National Academy of Sciences. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired Recognition Memory in Rats after Damage to the Hippocampus. The Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Commons KG, Valentino RJ. Cellular basis for the effects of substance P in the periaqueductal gray and dorsal raphe nucleus. J Comp Neurol. 2002;447:82–97. doi: 10.1002/cne.10228. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain research. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Kleine RA, Rothbaum BO, van Minnen A. Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. European journal of psychotraumatology. 2013;4 doi: 10.3402/ejpt.v4i0.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. The Journal of clinical endocrinology and metabolism. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- Dresler M, Kluge M, Pawlowski M, Schussler P, Steiger A, Genzel L. A double dissociation of memory impairments in major depression. J Psychiatr Res. 2011;45:1593–1599. doi: 10.1016/j.jpsychires.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89. doi: 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The Neuroscience of Mammalian Associative Learning. Annual Review of Psychology. 2004;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Farrin L, Hull L, Unwin C, Wykes T, David A. Effects of depressed mood on objective and subjective measures of attention. The Journal of neuropsychiatry and clinical neurosciences. 2003;15:98–104. doi: 10.1176/jnp.15.1.98. [DOI] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain : a journal of neurology. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. Pt 10. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Res. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711–720. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- Fuge P, Aust S, Fan Y, Weigand A, Gärtner M, Feeser M, Bajbouj M, Grimm S. Interaction of Early Life Stress and Corticotropin-Releasing Hormone Receptor Gene: Effects on Working Memory. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. Journal of physiology, Paris. 2013;107:471–482. doi: 10.1016/j.jphysparis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. Journal of neurochemistry. 2001;76:509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Groenink L, Pattij T, De Jongh R, Van der Gugten J, Oosting RS, Dirks A, Olivier B. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol. 2003;463:185–197. doi: 10.1016/s0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Neurobiology of early life stress: rodent studies. Semin Clin Neuropsychiatry. 2002;7:89–95. doi: 10.1053/scnp.2002.31781. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Marois R. Functional fractionation of the stimulus-driven attention network. J Neurosci. 2014;34:6958–6969. doi: 10.1523/JNEUROSCI.4975-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, Ressler KJ, Binder EB. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Stenzel-Poore MP, Gold LH, Battenberg E, Bloom FE, Koob GF, Vale WW, Pich EM. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74:303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Hertzberg MA, Feldman ME, Beckham JC, Kudler HS, Davidson JR. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2000;12:101–105. doi: 10.1023/a:1009076231175. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disorders. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75:873–883. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung HC, Chou CK, Chiu TH, Lee EH. CRF increases protein phosphorylation and enhances retention performance in rats. Neuroreport. 1992;3:181–184. doi: 10.1097/00001756-199202000-00015. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Marmar CR, Neylan TC. Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res. 2013;47:64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, Learned-Coughlin SM, Holsboer F, Grigoriadis DE. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Isogawa K, Bush DE, Ledoux JE. Contrasting Effects of Pretraining, Posttraining, and Pretesting Infusions of Corticotropin-Releasing Factor into the Lateral Amygdala: Attenuation of Fear Memory Formation but Facilitation of its Expression. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal Dysfunction and Cognitive Impairments Provoked by Chronic Early-Life Stress Involve Excessive Activation of CRH Receptors. The Journal of Neuroscience. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kasckow JW, Baker D, Geracioti TD., Jr Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22:845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disorders. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Federation proceedings. 1985;44:259–263. [PubMed] [Google Scholar]
- Koso M, Hansen S. Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. European psychiatry : the journal of the Association of European Psychiatrists. 2006;21:167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Rosenheck RA, Cramer JA, Vessicchio JC, Jones KM, Vertrees JE, Horney RA, Huang GD, Stock C. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. Jama. 2011;306:493–502. doi: 10.1001/jama.2011.1080. [DOI] [PubMed] [Google Scholar]
- LaGarde G, Doyon J, Brunet A. Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Res. 2010;177:144–149. doi: 10.1016/j.psychres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee EHY, Hung HC, Lu KT, Chen WH, Chen HY. Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides. 1992;13:927–937. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic P. Segregation of working memory functions within the dorsolateral prefrontal cortex. In: Schneider W, Owen A, Duncan J1, editors. Executive Control and the Frontal Lobe: Current Issues. Springer Berlin Heidelberg; 2000. pp. 23–32. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Ma YL, Chen KY, Wei CL, Lee EH. Corticotropin-releasing factor enhances brain-derived neurotrophic factor gene expression to facilitate memory retention in rats. The Chinese journal of physiology. 1999;42:73–81. [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in neurosciences. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L. Cognitive impairment in major depression. Eur J Pharmacol. 2010;626:83–86. doi: 10.1016/j.ejphar.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic Mechanisms of Associative Memory in the Amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7(Suppl 3):S323–328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci. 2002;3:122–131. doi: 10.1038/nrn728. [DOI] [PubMed] [Google Scholar]
- Melchior M, Caspi A, Milne BJ, Danese A, Poulton R, Moffitt TE. Work stress precipitates depression and anxiety in young, working women and men. Psychological Medicine. 2007;37:1119–1129. doi: 10.1017/S0033291707000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck RA. Pharmacotherapy of PTSD in the U.S. Department of Veterans Affairs: diagnostic- and symptom-guided drug selection. J Clin Psychiatry. 2008;69:959–965. doi: 10.4088/jcp.v69n0611. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The Cerebral Cortex of the Rat and Visual Attentional Function: Dissociable Effects of Mediofrontal, Cingulate, Anterior Dorsolateral, and Parietal Cortex Lesions on a Five-Choice Serial Reaction Time Task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A Meta-analysis of Executive Components of Working Memory. Cerebral Cortex. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Newman SC, Bland RC. Life events and the 1-year prevalence of major depressive episode, generalized anxiety disorder, and panic disorder in a community sample. Comprehensive Psychiatry. 1994;35:76–82. doi: 10.1016/0010-440x(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, Normann C. Learning as a Model for Neural Plasticity in Major Depression. Biological Psychiatry. 2010;68:544–552. doi: 10.1016/j.biopsych.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A Meta-Analysis of D-Cycloserine and the Facilitation of Fear Extinction and Exposure Therapy. Biological Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. Précis of O'Keefe & Nadel's The hippocampus as a cognitive map. Behavioral and Brain Sciences. 1979;2:487–494. [Google Scholar]
- Ohmura Y, Yamaguchi T, Futami Y, Togashi H, Izumi T, Matsumoto M, Yoshida T, Yoshioka M. Corticotropin releasing factor enhances attentional function as assessed by the five-choice serial reaction time task in rats. Behav Brain Res. 2009;198:429–433. doi: 10.1016/j.bbr.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- Pape H-C, Pare D. Plastic Synaptic Networks of the Amygdala for the Acquisition, Expression, and Extinction of Conditioned Fear. Physiological reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli D. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem. 2011;95:86–91. doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2007;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards PM, Ruff RM. Motivational effects on neuropsychological functioning: comparison of depressed versus nondepressed individuals. Journal of consulting and clinical psychology. 1989;57:396–402. doi: 10.1037//0022-006x.57.3.396. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. discussion 1470-1461. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, Everitt BJ. Neural systems underlying arousal and attention. Implications for drug abuse. Ann N Y Acad Sci. 1998;846:222–237. [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disorders. 2006;90:149–161. doi: 10.1016/j.jad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Duncan EJ, Rizzo A, Ressler KJ. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Blokland A, Harmer CJ, Kilkens TOC, Nathan PJ, Porter RJ, Schmitt JAJ, Scholtissen B, Sobczak S, Young AH, Riedel WJ. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: A pooled analysis of nine studies. Neuroscience & Biobehavioral Reviews. 2007;31:516–529. doi: 10.1016/j.neubiorev.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Samuelson KW. Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues in clinical neuroscience. 2011;13:346–351. doi: 10.31887/DCNS.2011.13.2/ksamuelson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain research. 1985;350:169–173. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- Shabsigh R, Rowland D. The diagnostic and statistical manual of mental disorders, fourth edition, text revision as an appropriate diagnostic for premature ejaculation. J Sex Med. 2007;4:1468–1478. doi: 10.1111/j.1743-6109.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, Milad MR, Neria Y. Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiol Learn Mem. 2014;113:101–108. doi: 10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Swiergiel AH, Dunn AJ. Corticotropin-releasing factor administered into the locus coeruleus, but not the parabrachial nucleus, stimulates norepinephrine release in the prefrontal cortex. Brain Res Bull. 1995;36:71–76. doi: 10.1016/0361-9230(94)00166-x. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Muir WJ, Blackwood DHR. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disorders. 2006;8:40–46. doi: 10.1111/j.1399-5618.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current opinion in neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Current opinion in neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The Medial Temporal Lobe Memory System. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl O, Meyer M, Jahn O, Ogren SO, Spiess J. Corticotropin-releasing factor receptor 1 and central heart rate regulation in mice during expression of conditioned fear. J Pharmacol Exp Ther. 2005;312:905–916. doi: 10.1124/jpet.104.075820. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neuroscience and biobehavioral reviews. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tempesta D, Mazza M, Iaria G, De Gennaro L, Ferrara M. A specific deficit in spatial memory acquisition in post-traumatic stress disorder and the role of sleep in its consolidation. Hippocampus. 2012;22:1154–1163. doi: 10.1002/hipo.20961. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Toth M, Gresack JE, Bangasser DA, Plona Z, Valentino RJ, Flandreau EI, Mansuy IM, Merlo-Pich E, Geyer MA, Risbrough VB. Forebrain-Specific CRF Overproduction During Development is Sufficient to Induce Enduring Anxiety and Startle Abnormalities in Adult Mice. Neuropsychopharmacology. 2014;39:1409–1419. doi: 10.1038/npp.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Ronnenberg A, Ogren SO, Spiess J, Stiedl O. Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor-overexpressing mice. Neuroscience. 2005;134:1113–1122. doi: 10.1016/j.neuroscience.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of Childhood Maltreatment with the Corticotropin-Releasing Hormone Receptor Gene: Effects on Hypothalamic-Pituitary-Adrenal Axis Reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends in pharmacological sciences. 2013;34:437–444. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA., Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore M, Holsboer F, Steckler T. Reduced attention in mice overproducing corticotropin-releasing hormone. Behavioural Brain Research. 2003;142:69–79. doi: 10.1016/s0166-4328(02)00381-9. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr., Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. Journal of clinical and experimental neuropsychology. 1997;19:587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–799. doi: 10.1038/mp.2008.38. 741. [DOI] [PubMed] [Google Scholar]
- Wang X-D, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, Harbich D, Mayer B, Wurst W, Holsboer F, Deussing JM, Baram TZ, Müller MB, Schmidt MV. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiology of Disease. 2011a;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Muller MB, Schmidt MV. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011b;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008;7:14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Waters AM, Peters R-M, Forrest KE, Zimmer-Gembeck M. Fear acquisition and extinction in offspring of mothers with anxiety and depressive disorders. Developmental Cognitive Neuroscience. 2014;7:30–42. doi: 10.1016/j.dcn.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Cooke BM. Corticotropin-Releasing Factor Receptor Binding in the Amygdala Changes Across Puberty in a Sex-Specific Manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM. Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol. 2014;522:1284–1298. doi: 10.1002/cne.23475. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R Gene Expression after Electrical Foot Shock Stress in the Rat Amygdala and Hypothalamus. Journal of Veterinary Medical Science. 2004;66:1323–1327. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- Yan XX, Baram TZ, Gerth A, Schultz L, Ribak CE. Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Experimental brain research. 1998;123:334–340. doi: 10.1007/s002210050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, van den Pol A, Gyengesi E. The basal forebrain cholinergic projection system in mice. The mouse nervous system. 2012:684–714. [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]


