Abstract
Despite the anatomical overlap between the brain’s fear/threat and olfactory systems, a very limited number of investigations have considered the role of odors and the central olfactory system in the pathophysiology of PTSD. The goal of the present study was to assess structural differences in primary and secondary olfactory cortex between combat veterans with and without PTSD (CV+PTSD, CV−PTSD, respectively). An additional goal was to determine the relationship between gray matter volume (GMV) in olfactory cortex and the distressing properties of burning-related odors. A region of interest voxel-based morphometric (VBM) approach was used to measure GMV in olfactory cortex in a well-characterized group of CV+PTSD (n=20) and CV−PTSD (n=25). Prior to the MRI exam, combat-related (i.e., burning rubber) and control odors were systematically sampled and rated according to their potential for eliciting PTSD symptoms. Results showed that CV+PTSD exhibited significantly reduced GMV in anterior piriform (primary olfactory) and orbitofrontal (secondary olfactory) cortices compared to CV−PTSD (both p<.01). For the entire group, GMV in bilateral anterior piriform cortex was inversely related to burning rubber odor-elicited memories of trauma (p<.05). GMV in orbitofrontal cortex was inversely related to both clinical and laboratory measures of PTSD symptoms (all p<.05). In addition to replicating an established inverse relationship between GMV in anxiety-associated brain structures and PTSD symptomatology, the present study extends those findings by being the first report of volumetric decreases in olfactory cortex that are inversely related to odor-elicited PTSD symptoms. Potential mechanisms underlying this finding are discussed.
Keywords: odor threat, anxiety, PTSD, trauma, VBM, MRI
Graphical Abstract
Introduction
Over the past 20 years, a large literature of neuroimaging studies investigating the structural brain changes associated with PTSD has emerged. While important initial investigations revealed PTSD-related volumetric reductions in hippocampus (Bremner et al., 1995, Gurvits et al., 1996, Stein et al., 1997), subsequent studies have described gray matter volume (GMV) reductions extending throughout limbic and paralimbic structures, as well as prefrontal cortical regions (Aupperle et al., 2013, Chen et al., 2006, Geuze et al., 2008, Keding and Herringa, 2015, Kuhn and Gallinat, 2013, Nardo et al., 2010, Rauch et al., 2003, Yamasue et al., 2003). To date, an inverse relationship between limbic/paralimbic GMV and PTSD severity, as well as the severity of the individual symptom clusters of re-experiencing, avoidance/numbing, and hyperarousal, has predominated (Araki et al., 2005, Kroes et al., 2011, Lindauer et al., 2004, Shucard et al., 2012, Thomaes et al., 2010, Villarreal et al., 2002, Yamasue, Kasai, 2003); yet, other reports of positive associations between PTSD and early life trauma severity, and hippocampal/amygdala volume have also been reported (Baldacara et al., 2014, Weber et al., 2013).
Although the full range of pathophysiological processes that underlie PTSD-related decreases in brain volume are not completely understood, one mechanism by which severe stress may contribute to brain atrophy (e.g., hippocampal) has a great deal of support in both animal and human research. Evidence for the “glucocorticoid hypothesis” suggests that chronic stress, accompanied by dysregulation of glucocorticoids (i.e., cortisol), leads to hippocampus vulnerability and potential structural insult that could negatively impact cognitive function including learning and memory (Lindauer et al., 2006, Lupien et al., 1998, McEwen, 2000). In addition to increased baseline levels of salivary cortisol (Young et al., 2004), trauma-exposed individuals also exhibit memory-triggered augmented cortisol responses (Dekel et al., 2013) that can modulate regional brain activity (Liberzon et al., 2007). These studies suggest that traumatic reminders and re-experiencing of cues that resemble or symbolize the original trauma [termed conditioned stimuli, which from a fear conditioning perspective is believed to underlie the fear-related symptoms of PTSD (Briscione et al., 2014)] may contribute to the chronicity of glucocorticoid dysregulation and subsequent changes in brain structure and function.
Situated at the junction of the temporal and frontal corticies, the primary olfactory (piriform) cortex, along with the extended olfactory circuit, shares common neuroanatomy with the brain’s fear/threat circuit (LeDoux, 2012, Price, 1990), including many of the same limbic/paralimbic structures identified in the pathophysiology of PTSD (e.g., amygdala, hippocampus and surrounding cortex, anterior insula, and orbitofrontal cortex). Emerging evidence suggests that the olfactory system is particularly susceptible to insult and thus may serve as a sensitive indicator of structural integrity in these limbic/paralimbic and frontal neural networks. For example, olfactory dysfunction is reported in laboratory animals following exposure to environmental toxins (Blechinger et al., 2007), chronic stress (Mo et al., 2014), as well as mechanical percussion-induced closed head injury (Siopi et al., 2012); this type of dysfunction is also considered a prodromal marker of human neurodegeneration (Devanand et al., 2000, Lerche et al., 2014). Given that glucocorticoid receptors are widely distributed from the olfactory bulb throughout the olfactory cortex (Morimoto et al., 1996), and that hypercortisolemia can negatively affect olfactory structure (Kratskin et al., 1999) and function (Ezeh et al., 1992), the olfactory system and/or olfactory function is likely impaired by chronic stress and subsequent psychiatric conditions including PTSD.
Despite the anatomical overlap between the olfactory and fear/threat systems, as well as its vulnerability to insult, a limited number of investigations (Hinton et al., 2004, Vermetten and Bremner, 2003, Vermetten et al., 2007) have considered the role of the central olfactory system in their study of PTSD. To our knowledge, only one study assessed olfactory-related brain volume and reported reduced olfactory bulb volume in women with a history of childhood maltreatment (Croy et al., 2013). Other studies designed to assess clinical olfactory function in PTSD have been inconsistent (Croy et al., 2010, Dileo et al., 2008, Vasterling et al., 2000, Vasterling et al., 2003). However, recent preliminary data from our laboratory suggest that trauma-exposed combat veterans with and without PTSD may have a decreased ability to detect odors (Cortese et al., 2014), in addition to a self-reported reduction in general odor sensitivity (Cortese et al., 2015). It remains to be seen the degree to which this reduced sensitivity to odor is the result of generalized hyposmia, damage to the olfactory mucosa/receptors, perceptual difficulties mediated by cortical damage, or some combination of these factors.
Given our promising preliminary olfactory findings and the notion that re-experiencing trauma-related triggers may be associated with decreased GMV, as well as the fact that trauma-related differences in GMV along the olfactory pathway have not been adequately studied, we sought to assess GMV in combat veterans utilizing a region of interest (ROI) approach focused along the central olfactory pathway. We hypothesized that PTSD-related reductions in GMV in primary and secondary olfactory structures would be inversely related not only to general PTSD symptomology, but also to specific subjective ratings of odor hedonics and, most importantly, to increased odor-elicited re-experiencing of combat trauma.
Methods and Materials
Participants
Combat veterans with PTSD (CV+PTSD: n=23) and without PTSD (CV−PTSD: n=25) were recruited from the Ralph H. Johnson Veterans Affairs (VA) Medical Center, as well as the greater Charleston, South Carolina community via advertisement to participate in a larger study investigating odor-elicited anxiety. To meet eligibility for this study, participants were required to 1) have served in a combat zone in Iraq or Afghanistan [Operation Enduring Freedom (OEF), Iraqi Freedom (OIF), or New Dawn (OND)]; 2) meet current (past month) or lifetime DSM-IV primary diagnosis of combat-related PTSD [assessed by the Clinician Administered PTSD Scale (CAPS)] (Blake et al., 1995), or have no history of any DSM-IV disorder including alcohol or other substance-use disorder [assessed by the Mini International Neuropsychiatric Interview (MINI)] (Sheehan et al., 1998); 3) have no history of self-reported head injury/trauma (e.g., blast exposure; given the association between head trauma and olfactory dysfunction); 4) be psychiatric medication-free; 5) be able to undergo an MRI exam (contraindications such as shrapnel injuries, pregnancy, and claustrophobia excluded); 6) be right handed; and 7) pass a urine drug screen (CLIAwaived ™, San Diego, CA). All study procedures were approved by the Institutional Review Board at the Medical University of South Carolina and the Research and Development (R&D) Committee at the Charleston VA. All participants provided informed consent prior to the start of any study procedures.
Three CV+PTSD were not included in the analyses. One participant had a large nasal polyp that was discovered during the MRI exam, while another was unable to tolerate the scan due to claustrophobia. The third participant was excluded due to a suspected head injury/concussion. The final sample consisted of 20 CV+PTSD and 25 CV−PTSD.
Procedure
Odor sampling
Prior to the MRI exam, combat veterans systematically sampled and provided self-report ratings for a total of 4 odor cues. Odor intensity (i.e., “the odor was strong”) and negative valence (i.e., “the odor was unpleasant”), as well as baseline and odor-elicited ratings along the 3 symptom clusters of PTSD including re-experiencing (i.e., “the odor triggered memories of my trauma”), avoidance and numbing (i.e., “the odor made me feel numb”), and hyperarousal (i.e., “the odor made me feel anxious”) were acquired on 100mm visual analog scales (VAS) with anchor points of “not at all” to “extremely”. The odor cues (ScentAir™, Charlotte, NC) were selected based upon survey data collected in our laboratory (Cortese, Leslie, 2015) and included burning rubber (BR), a trauma-related “burning” odor cue; lavender (LAV), a relatively pleasant non-trauma-related control odor cue; cigarette smoke (SMK), a “burning” odor that was generally not identified as an odor related to combat experiences in our sample; and propylene glycol (PG) which served as the odorless control as well as the base oil for preparing the other odor cues. Similar to previously published methods, the odor cues were prepared and pilot tested for an average intensity rating of 50mm (Khan et al., 2007).
All odor sampling studies were carried out in the MUSC Department of Psychiatry Sleep Laboratory, which provided a dimly-lit, climate-controlled, well-ventilated, video-/audio-equipped testing room with an adjacent control/monitoring room. Each session, which lasted about 1 hour, was conducted using Superlab 4 (Cedrus Corp., San Pedro, CA) stimulus presentation software that delivered all pre-recorded odor-sampling instructions in order to ensure consistent testing across participants. The session began with baseline self-reports after which the first odor flask and VAS was delivered. The automated instructions for sampling the odor began shortly after the study assistant exited the room and directed participants to sniff the odor a total of 4 times on a 6-s breathing cycle (3-s inhale and 3-s exhale), providing a total of 12 seconds of odor inhalation. Once sampled, participants completed the odor VAS, the study assistant removed all items from the testing room, 10 minutes of nature slides were presented, and the entire sequence of events was repeated for the next odor until all 4 odors were sampled. All odors were presented in a counterbalanced design to offset potential order effects.
VBM data acquisition
The MRI study was conducted within 1 week from odor sampling. The entire session, acquired on a 3 T Siemens (Erlangen, Germany) TIM Trio scanner equipped with a standard head coil, included a localizer scan, a high-resolution anatomical scan, 2 odor cue-reactivity functional scans, and a resting-state functional scan (functional imaging reports forthcoming). The high-resolution, T1-weighted structural images were acquired with a ~6-minute, magnetization-prepared rapid gradient-echo (MP-RAGE) sequence. Image acquisition parameters were: repetition/echo time =2250/4 ms; flip angle =9°; matrix=256 × 256; voxel size=1.0 mm3, which yielded 176 contiguous, sagittal slices.
VBM data processing
Structural images were pre-processed using the VBM8 toolbox (dbm.neuro.uni-jena.de/vbm8) for SPM12 (www.fil.ion.ucl.ac.uk/spm). Data were preprocessed according to default toolbox settings: bias correction; tissue classification/segmentation (Rajapakse et al., 1997); partial volume estimation (Tohka et al., 2004); denoising/filtering (Manjon et al., 2010, Rajapakse, Giedd, 1997); warping to the DARTEL IXI-550 template in Montreal Neurologic Institute (MNI) space; resampling to a 1.5 mm3 voxel-size using high-dimensional affine and nonlinear transforms (Ashburner, 2007); voxelwise modulation by nonlinear transform components only (Ashburner and Friston, 2001); and finally, smoothing of normalized gray matter (GM) tissue maps with a 6mm3 FWHM Gaussian filter. First principle eigenvariates (i.e., weighted means) were extracted for each subject from a priori ROIs that were determined from an fMRI meta-analysis for the statistical localization of the human olfactory cortex (Seubert et al., 2013a). The regions included bilateral anterior piriform cortex (aPC), lateral amygdala (AMYG), head of the hippocampus (HPC), anterior insula (aINS), and orbitofrontal cortex (OFC). Each ROI was created in MARSBAR (marsbar.sourceforge.net) as a 5mm-radius sphere centered around the coordinates provided by Seubert and colleagues (Seubert, Freiherr, 2013a): bilateral aPC (±22, −2 −14), bilateral AMYG (±26, −6, −16), bilateral HPC (±24, −10, −24), bilateral aINS (±38, 14, −8), and bilateral OFC (−28, 34, −12; 26, 34 −12).
Statistical analyses
Gray matter volume (GMV) in μL/voxel was calculated by multiplying mean voxel intensity value by voxel volume for each olfactory ROI (Radua et al., 2014), and was then exported, along with participant demographics, clinical variables, and odor ratings, into an IBM SPSS Statistics 22 data editor (www-01.ibm.com/software/analytics/spss/products/statistics/index.html). Separate ANCOVA, with age as a covariate, was used to determine significant group differences in GMV for each olfactory ROI. Repeated-measures ANOVA was used to determine group (CV+PTSD versus CV−PTSD) differences in ratings of odor intensity and pleasantness, as well as ratings of odor-elicited changes in mood. Pearson’s correlation was utilized to demonstrate the relationship between participant characteristics, odor ratings, and gray matter volume within olfactory ROIs.
Results
Participant characteristics
The 20 combat veterans with PTSD (CV+PTSD) comprised 16 veterans that met DSM-IV criteria (APA, 1994) for current combat-related PTSD and 4 veterans that met current subclinical PTSD (i.e., met criterion A and 2/3 symptom clusters) and met diagnostic criteria for lifetime PTSD related to their combat experiences. Of the 20 CV+PTSD, 6 met diagnostic criteria for secondary depression, 3 had comorbid panic disorder and 1 had comorbid generalized anxiety disorder, but no other Axis I disorders. The 25 combat veterans without PTSD (CV−PTSD) had no history of any DSM-IV disorder. All participants, regardless of group, were psychiatric medication-free, right-handed, had no history of blast exposure/head injury, and did not abuse alcohol or other drugs (caffeine and cigarette use was permitted). Table 1 provides a summary of demographic and clinical variables, showing that the CV+PTSD and CV−PTSD were well-matched on age, sex, race, education, cigarette use and combat exposure (Keane et al., 1989), but were significantly different on CAPS total score.
Table 1.
Demographic and clinical characteristics of study participants
| CV+PTSD (n=20) | CV−PTSD (n=25) | χ2 or t | p | |
|---|---|---|---|---|
| Sex - n (%) male | 19 (95.0) | 24 (96.0) | 0.03 | ns |
| Race - n (%) minority | 6 (30.0) | 3 (12.0) | 2.76 | ns |
| Smokers - n (%) regular users | 7 (35.0) | 4 (16.0) | 4.67 | ns |
| Employment - n (%) employed | 12 (60.0) | 15 (60.0) | 0.23 | ns |
| Age in years (mean ± SD) | 30.5±8.6 | 30.6±7.1 | 0.00 | ns |
| Education in years (mean ± SD) | 14.4±1.1 | 14.8±2.3 | 0.50 | ns |
| Combat Exposure (mean ± SD) | 20.8±7.5 | 20.0±10.0 | 0.31 | ns |
| CAPS total score (mean ± SD) | 59.4±23.1 | 14.8±12.4 | 8.30 | <.001 |
CV+PTSD = combat veteran with PTSD, CV−PTSD = combat veteran without PTSD
CAPS=Clinician Administered PTSD Scale
Combat Exposure Scale (Keane et al., 1989)
Odor ratings
Repeated-measures ANOVA revealed a main effect of odor as well as a diagnosis by odor interaction for odor intensity [F(3,43)=58.7, p<.001; F(3,43)=2.91, p<.05, respectively] and odor unpleasantness [F(3,43)=47.9, p<.001; F(3,43)=3.32, p<.05, respectively]. Figure 1 demonstrates that the CV+PTSD, compared to CV−PTSD, rated the “burning” odors (BR and SMK), but not the pleasant odor (LAV) or odorless control (PG), to have significantly greater intensity and unpleasantness. Figure 2 represents the findings for odor-elicited PTSD symptomatology. The assessment of odor-elicited re-experiencing revealed both a main effect of odor [F(3,43)=7.92, p<.001] and an odor by diagnosis interaction [F(3,43)=3.18, p<.05], indicating that CV+PTSD, but not the CV−PTSD, rated BR and SMK to elicit significantly more memories of trauma than LAV and PG. The assessment of odor-elicited avoidance revealed no main effect of odor or odor by diagnosis interaction (p>.1). And finally, the assessment of odor-elicited hyperarousal revealed a main effect of odor [F(3,43)=5.18, p<.01], but no diagnosis by odor interaction [F(3,43)=0.38, p=.77], indicating that the combined group of veterans rated BR (M=13.6, SD=20.5) and SMK (M=14.4, SD=17.0) as more anxiety-inducing than either LAV (M=5.2, SD=13.6) or PG (M=8.8, SD=16.6).
Figure 1.
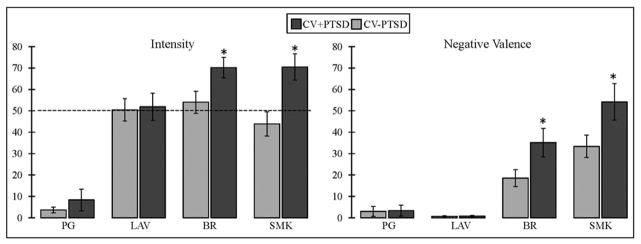
Figure 1 illustrates participant ratings of odor intensity and negative valence (unpleasantness) on 100mm visual analog scales. Combat veterans with PTSD (CV+PTSD) compared to combat controls (CV−PTSD) rated burning rubber (BR) and cigarette smoke (SMK), but not lavender (LAV) or the odorless control (PG), as significantly more intense and unpleasant. The dashed line represents the average odor intensity of LAV, BR, and SMK, acquired in healthy adult civilians during the formulation of odor cues. *=p<.05
Figure 2.
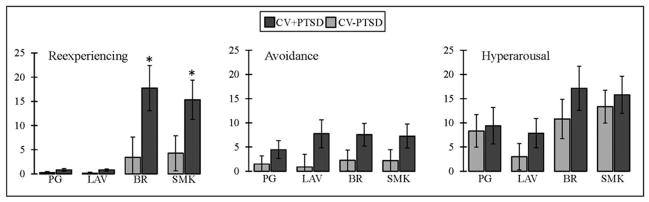
Figure 2 displays odor-elicited PTSD symptomatology, measured on 100mm visual analog scales, in combat veterans. Combat veterans with PTSD (CV+PTSD) compared to combat controls (CV−PTSD) rated burning rubber (BR) and cigarette smoke (SMK), but not lavender (LAV) or an odorless control (PG), as eliciting significantly more memories of trauma. *=p<.05
Gray matter volume (GMV)
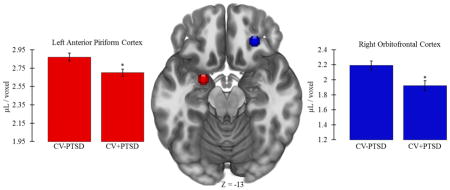
One-way ANCOVA, controlling for age, revealed that CV+PTSD, compared to CV−PTSD, had significantly less GMV in left aINS [CV+PTSD: M=2.59, SD=0.34; CV−PTSD: M=2.76, SD=0.27; F(1,42)=4.25, p<.05] and right aINS [CV+PTSD: M=3.29, SD=0.49; CV−PTSD: M=3.52, SD=0.31; F(1,42)=4.73, p<.05], and less GMV in left AMYG that approached significance [CV+PTSD: M=3.06, SD=0.20; CV−PTSD: M=3.18, SD=0.22; F(1,42)=3.96, p=.053]. PTSD-related GMV reductions that remained significant after Bonferroni correction for multiple ROI comparisons were found in left aPC [F(1,42)=8.48, p<.01] and right OFC [F(1,42)=8.90, p<.01; Figure 3].
Figure 3.
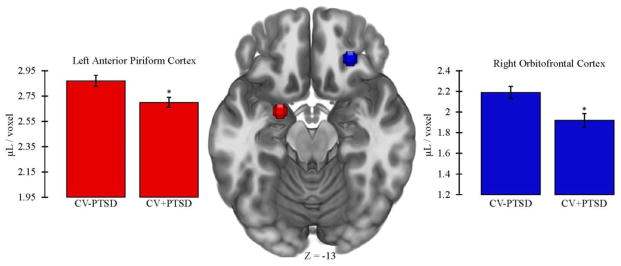
Figure 3 demonstrates that combat veterans with PTSD (CV+PTSD) compared to combat controls (CV−PTSD) had significantly reduced GMV in primary and secondary olfactory cortex (i.e., left anterior piriform and right orbitofrontal). These differences remained significant after Bonferroni correction for multiple brain region comparisons. *=p<.01
Relationship between olfactory GMV and demographic/clinical measures
Pearson’s correlations between regional GMV and demographic and clinical measures for the entire group of combat veterans are provided in Table 2. While regional (i.e., left and right anterior insula and right piriform cortex) decreases in GMV were related to advancing age (all p<.05), no relationship between GMV and education was revealed for any olfactory regions. Olfactory GMV was also not related to the degree of combat exposure, but was inversely related to PTSD symptomology in several regions (see Table 2 for specific details).
Table 2.
Pearson correlations between regional gray matter volume and demographic/clinical characteristics in combat veterans
| Brain Region | Age | Education | Combat Exposure | CAPS (reexperiencing) | CAPS (avoidance) | CAPS (hyperarousal) | CAPS (total score) | |
|---|---|---|---|---|---|---|---|---|
| left | ||||||||
| piriform cortex | −0.238 | 0.018 | −0.196 | −0.241 | −0.166 | −0.397** | −0.282 | |
| lateral amygdala | −0.123 | 0.040 | −0.213 | −0.107 | −0.089 | −0.312* | −0.178 | |
| hippocampus (head) | −0.072 | 0.032 | −0.199 | 0.066 | −0.076 | −0.182 | −0.074 | |
| anterior insula | −0.408** | 0.027 | 0.157 | −0.248 | −0.198 | −0.411** | −0.302* | |
| orbitofrontal cortex | −0.270 | 0.016 | −0.042 | −0.027 | −0.120 | 0.065 | −0.039 | |
| right | ||||||||
| piriform cortex | −0.318* | −0.152 | −0.189 | −0.021 | 0.046 | −0.105 | −0.022 | |
| lateral amygdala | −0.132 | −0.012 | −0.251 | 0.103 | 0.052 | −0.102 | 0.021 | |
| hippocampus (head) | −0.102 | 0.137 | −0.238 | 0.134 | 0.062 | −0.102 | 0.034 | |
| anterior insula | −0.387** | 0.166 | 0.074 | −0.261 | −0.163 | −0.365* | −0.276 | |
| orbitofrontal cortex | −0.157 | 0.143 | −0.122 | −0.428** | −0.502** | −0.368* | −0.476** | |
Combat veterans (n=45) are collapsed across PTSD diagnosis.
p<.05
p<.01
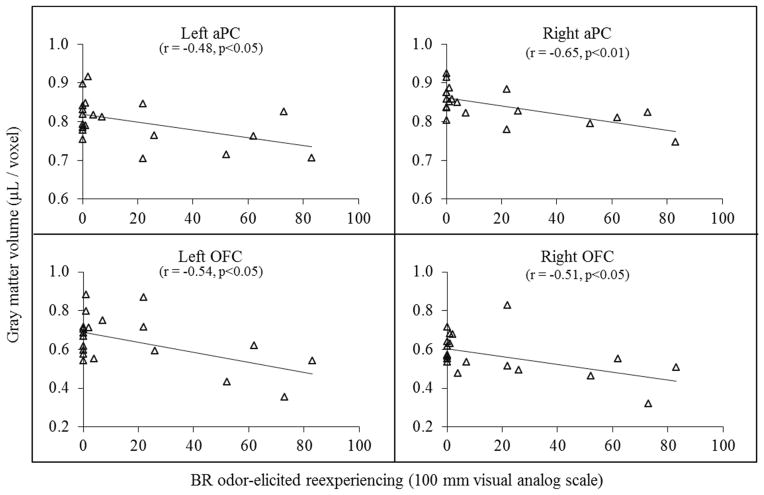
Relationship between olfactory GMV and burning rubber odor-elicited ratings
Pearson’s correlations between regional GMV and burning rubber odor-elicited ratings for the entire group of combat veterans (n=45) are provided in Table 3. There was no significant relationship between olfactory GMV and ratings of odor intensity or negative valence. However, GMV in bilateral aINS and OFC was inversely related to burning rubber odor-elicited ratings of re-experiencing (“memories”), avoidance (“numb”), and hyperarousal (“anxiety”) (all p<.05). GMV in bilateral aPC was inversely related to burning rubber odor-elicited ratings of re-experiencing (“memories”) as well (p<.05; see Table 3 for specific details and Figure 4 for scatter plots in CV+PTSD). In contrast, no significant relationships, nor any trends, were noted for regional GMV and lavender odor-elicited ratings of intensity, negative valence, re-experiencing (“memories”), avoidance (“numb”), and hyperarousal (“anxiety”) (all p>0.1).
Table 3.
Pearson correlations between gray matter volume and odor-elicited ratings in combat veterans
| Brain Region | odor intensity | odor negative valence | odor-elicited reexperiencing (memories) | odor-elicited avoidance (numb) | odor-elicited hyperarousal (anxiety) | |
|---|---|---|---|---|---|---|
| left | ||||||
| piriform cortex | −0.129 | 0.163 | −0.305* | −0.175 | 0.025 | |
| lateral amygdala | −0.151 | 0.189 | −0.289 | −0.133 | 0.039 | |
| hippocampus (head) | −0.087 | 0.222 | −0.257 | −0.166 | 0.017 | |
| anterior insula | −0.104 | −0.025 | −0.330* | −0.393** | −0.386** | |
| orbitofrontal cortex | −0.240 | 0.108 | −0.452** | −0.312* | −0.428** | |
| right | ||||||
| piriform cortex | −0.106 | 0.125 | −0.300* | −0.127 | −0.020 | |
| lateral amygdala | −0.091 | 0.147 | −0.220 | −0.134 | 0.002 | |
| hippocampus (head) | −0.103 | 0.255 | −0.253 | −0.185 | −0.056 | |
| anterior insula | −0.083 | −0.031 | −0.496** | −0.377* | −0.395** | |
| orbitofrontal cortex | −0.138 | 0.086 | −0.514** | −0.427** | −0.445** | |
Combat veterans (n=45) are collapsed across PTSD diagnosis.
p<.05
p<.01
Figure 4.
Figure 4 illustrates the inverse relationship between burning rubber (BR) odor-elicited reexperiencing (i.e. ratings for “the odor triggered memories of my trauma”) and gray matter volume (GMV) in bilateral anterior piriform (aPC) and orbitofrontal cortices (OFC) in combat veterans with PTSD (CV+PTSD; n=20).
Discussion
Our recent survey findings revealed a PTSD-related sensitivity to specific odors, with a significantly greater percentage of combat veterans with PTSD, compared to healthy combat veterans and civilians, reporting “burning” odors to be distressing (Cortese, Leslie, 2015). The present laboratory results are consistent with these and reveal that burning odors (burning rubber and smoke), but not lavender, were rated as more intense and unpleasant, as well as significantly more effective at eliciting memories of trauma in the combat veterans with PTSD compared to the healthy combat veterans. Moreover, burning rubber odor-elicited memories of trauma were inversely related to GMV in bilateral anterior piriform and orbitofrontal cortices.
With a direct connection to the olfactory bulb, anterior piriform cortex (i.e., primary olfactory cortex) is one of the first brain regions to process odor information (Price, 1990). Gray matter volume in piriform cortex is related to general olfactory function, with smaller volumes indicative of odor-detection deficits (Bitter et al., 2010, Peng et al., 2013, Wattendorf et al., 2009). While odor-detection deficits in PTSD would require additional study to confirm, preliminary work in our laboratory (Cortese, Leslie, 2014) revealed mild hyposmia in combat trauma-exposed veterans with and without PTSD. In our study, we reported significantly higher odor-detection thresholds in OEF/OIF/OND combat veterans, regardless of PTSD status, compared to published age norms (Pierce et al., 1996). These data, in addition to other findings of a self-reported reduction in general odor sensitivity in combat veterans (Cortese, Leslie, 2015), led us to question whether indigenous environmental factors and/or war-related chemical hazards in Iraq and Afghanistan (e.g., sand, dust, particulates, toxins released from burn pits, etc.) could cause irreversible damage to the olfactory system. Given that deployment-related insults to peripheral olfactory structures (e.g., olfactory mucosa/epithelium, receptors, and/or nerve damage due to environmental exposure) in OEF/OIF/OND military personnel could potentially produce atrophy of downstream olfactory targets, this specific hypothesis should be tested in future investigations. At this point however, we have no reason to believe that the combat veterans with PTSD had different environmental exposures than the healthy combat veterans in our study, given their similar deployment histories and combat experiences.
An alternative mechanism for our findings of significantly reduced GMV in primary (left anterior piriform) and secondary (right orbitofrontal) olfactory cortex in combat veterans with PTSD, compared to healthy combat veterans, relates to odor-triggered fear and anxiety and subsequent brain insult. It is well known that odors are effective retrieval cues for stressful events (Wiemers et al., 2013), and that odors trigger involuntary memories of traumatic events and precipitate trauma-related flashbacks (Kline and Rausch, 1985, Vermetten and Bremner, 2003). It is therefore reasonable to speculate that trauma odors may play a significant role in PTSD-related brain atrophy, and perhaps contribute to the particular damage of the olfactory cortex, which is known to be especially vulnerable to insult (Devanand, Michaels-Marston, 2000, Lerche, Seppi, 2014).
Situated on the ventral surface of the frontal lobe, the OFC is a relatively large and complex region thought to have numerous functional roles (Price, 2007, Rolls, 2004b) including, but not limited to, emotion regulation (Golkar et al., 2012, Kross et al., 2009), decision making (Fellows, 2007, Gourley et al., 2013), sensory integration (Rolls, 2004a), as well as a critical role in olfactory functioning (Gottfried, 2006). Like the piriform cortex, a positive relationship between gray matter volume in OFC and general olfactory performance has been reported (Seubert et al., 2013b). Many, but not all (Anderson et al., 2003), have also reported that the right OFC, in particular, is involved in odor learning as well as the affective evaluation of odors (Gottfried et al., 2002, Li et al., 2010). Consistent with a hypothesis of PTSD-related brain atrophy due to the chronic triggering of symptoms by a trauma-associated odor, we found reduced gray matter volume in right OFC that was strongly related to increased CAPS scores (total and symptom clusters), as well as laboratory ratings of burning rubber-elicited re-experiencing (i.e., memories of trauma), avoidance (i.e., numbing), and hyperarousal (i.e., anxiety). In fact, GMV in right OFC related to each of the clinical and odor-elicited measures of PTSD, while showing no relationship to odor intensity or negative valence ratings for burning rubber. These results are not surprising, given that odor-elicited activity in OFC is highly influenced by experience (Gottfried, 2007, Li et al., 2006).
In the present study, a significant group difference in GMV of bilateral aINS did not survive correction for multiple comparisons. However, correlational results revealed a clear inverse relationship between GMV in aINS and burning rubber odor-elicited, as well as CAPS-measured, PTSD symptomatology in the overall group. This finding is consistent with a prior report of an inverse relationship between posttraumatic symptom severity and GMV in anterior insula (Herringa et al., 2012), but extends it to an association with odor-elicited anxiety in particular. With direct connections between piriform cortex and anterior insula, as well as bidirectional connections via the mediodorsal nucleus of the thalamus (Ray and Price, 1992), the anterior insula has major roles in chemoreception (i.e. gustation and olfaction) and is consistently active during odor processing (Seubert, Freiherr, 2013a). Anterior insula has also been strongly implicated in interoceptive awareness and homeostatic regulation, as well as the processing of emotions (Craig, 2009, Critchley, 2005). Together, these diverse roles may allow the anterior insula to shape the emotional perceptions and bodily experience of odor-related cues.
While a major strength of this study includes a well-characterized group of non-medicated veteran participants with similar combat experiences, there are some limitations. Our findings should be considered preliminary, given group sizes that were comparatively small for VBM studies. However, while single case VBM studies are extremely susceptible to high false positive rates and thus should be avoided, a recent study revealed no significant impact of sample size (n=8, 12, and 16) on detecting false positive group differences (Scarpazza et al., 2015). We also did not include a comparison group of non-deployed veterans. Therefore, our methodology does not allow us to determine if healthy veterans also had combat-related, but less severe, reductions of GMV in olfactory cortex. A finding of olfactory GMV reductions in both veterans groups would lend support to speculation that unknown environmental toxins (e.g., volatile chemicals released from burn pits) in the combat zone, at least in part, may lead to brain atrophy of olfactory cortex. Another limitation relates to the retrospective design of the investigation. Through prospective testing of olfactory function and GMV assessment, we could examine whether baseline deficits in olfactory function, as well as pre-deployment reductions in olfactory GMV, are biomarkers for combat trauma-related PTSD.
A rich literature of imaging studies have endorsed PTSD-related differential patterns of brain function (Etkin and Wager, 2007, Fonzo et al., 2010, Hopper et al., 2007, Rauch et al., 1996, Sartory et al., 2013, Shin et al., 2001, Sripada et al., 2012), as well as structure (Araki, Kasai, 2005, Herringa, Phillips, 2012, Kroes, Whalley, 2011, Lindauer, Vlieger, 2004, Thomaes, Dorrepaal, 2010, Villarreal, Hamilton, 2002, Yamasue, Kasai, 2003). Our results, therefore, replicate an established relationship between PTSD symptomatology and gray matter volume in fear-, threat-, and anxiety-associated brain structures. We extend those findings by being the first to report, to our knowledge, on odor-elicited, PTSD-related gray matter volume changes in primary and secondary olfactory cortex.
Highlights.
Combat veterans commonly report odors associated with their traumatic experiences.
Volumetric imaging revealed insult of the olfactory cortex in combat-related PTSD.
Olfactory cortical atrophy was inversely related to odor-elicited PTSD symptoms.
Acknowledgments
Funding for this study was provided by NIMH Grant K01 MH090548 (BMC). The authors thank Drs. Anouk Grubaugh and Ron Acierno for their help with study recruitment.
Role of funding source
Funding for this study was provided by NIMH Grant K01 MH090548 (BMC).
NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Financial Disclosures
All authors declare that they have no biomedical financial interests or potential conflicts of interest.
Contributors
BMC and TWU conceived and developed the study. BMC and KL collected the data. All authors contributed to the data analysis, manuscript composition, and illustrations. BMC wrote the first draft of the manuscript. All authors have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Araki T, Kasai K, Yamasue H, Kato N, Kudo N, Ohtani T, et al. Association between lower P300 amplitude and smaller anterior cingulate cortex volume in patients with posttraumatic stress disorder: a study of victims of Tokyo subway sarin attack. Neuroimage. 2005;25:43–50. doi: 10.1016/j.neuroimage.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–43. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Connolly CG, Stillman AN, May AC, Paulus MP. Deployment and post-deployment experiences in OEF/OIF veterans: relationship to gray matter volume. PLoS One. 2013;8:e75880. doi: 10.1371/journal.pone.0075880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacara L, Zugman A, Araujo C, Cogo-Moreira H, Lacerda AL, Schoedl A, et al. Reduction of anterior cingulate in adults with urban violence- related PTSD. Journal of affective disorders. 2014;168:13–20. doi: 10.1016/j.jad.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Bitter T, Bruderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O. Gray and white matter reduction in hyposmic subjects--A voxel-based morphometry study. Brain Res. 2010;1347:42–7. doi: 10.1016/j.brainres.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. Journal of traumatic stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blechinger SR, Kusch RC, Haugo K, Matz C, Chivers DP, Krone PH. Brief embryonic cadmium exposure induces a stress response and cell death in the developing olfactory system followed by long-term olfactory deficits in juvenile zebrafish. Toxicology and applied pharmacology. 2007;224:72–80. doi: 10.1016/j.taap.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–81. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in psychiatry. 2014;5:88. doi: 10.3389/fpsyt.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Cortese BM, Leslie K, Grubaugh A, Yang QX, Uhde TW. Olfactory function and odor cue-reactivity in combat veterans with and without PTSD. American College of Neuropsychopharmacology (ACNP); Phoenix, Arizona: 2014. [Google Scholar]
- Cortese BM, Leslie K, Uhde TW. Differential odor sensitivity in PTSD: Implications for treatment and future research. Journal of affective disorders. 2015;179:23–30. doi: 10.1016/j.jad.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of comparative neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Croy I, Negoias S, Symmank A, Schellong J, Joraschky P, Hummel T. Reduced olfactory bulb volume in adults with a history of childhood maltreatment. Chemical senses. 2013;38:679–84. doi: 10.1093/chemse/bjt037. [DOI] [PubMed] [Google Scholar]
- Croy I, Schellong J, Joraschky P, Hummel T. PTSD, but not childhood maltreatment, modifies responses to unpleasant odors. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2010;75:326–31. doi: 10.1016/j.ijpsycho.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dekel S, Ein-Dor T, Gordon KM, Rosen JB, Bonanno GA. Cortisol and PTSD symptoms among male and female high-exposure 9/11 survivors. Journal of traumatic stress. 2013;26:621–5. doi: 10.1002/jts.21839. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157:1399–405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Dileo JF, Brewer WJ, Hopwood M, Anderson V, Creamer M. Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychological medicine. 2008;38:523–31. doi: 10.1017/S0033291707001456. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh PI, Myers LJ, Hanrahan LA, Kemppainen RJ, Cummins KA. Effects of steroids on the olfactory function of the dog. Physiology & behavior. 1992;51:1183–7. doi: 10.1016/0031-9384(92)90306-m. [DOI] [PubMed] [Google Scholar]
- Fellows LK. The role of orbitofrontal cortex in decision making: a component process account. Annals of the New York Academy of Sciences. 2007;1121:421–30. doi: 10.1196/annals.1401.023. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–81. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, et al. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012;7:e48107. doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA. Smell: central nervous processing. Advances in oto-rhino-laryngology. 2006;63:44–69. doi: 10.1159/000093750. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. What can an orbitofrontal cortex-endowed animal do with smells? Annals of the New York Academy of Sciences. 2007;1121:102–20. doi: 10.1196/annals.1401.018. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–37. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, Dileone RJ, Taylor JR. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci. 2013;38:2382–8. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–9. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R, Phillips M, Almeida J, Insana S, Germain A. Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res. 2012;203:139–45. doi: 10.1016/j.pscychresns.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DE, Pich V, Chhean D, Pollack MH, Barlow DH. Olfactory-triggered panic attacks among Cambodian refugees attending a psychiatric clinic. General hospital psychiatry. 2004;26:390–7. doi: 10.1016/j.genhosppsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of traumatic stress. 2007;20:713–25. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Keane T, Fairbank J, Caddell J, Zimering R, Taylor K, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–5. [Google Scholar]
- Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40:537–45. doi: 10.1038/npp.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, et al. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–23. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline NA, Rausch JL. Olfactory precipitants of flashbacks in posttraumatic stress disorder: case reports. The Journal of clinical psychiatry. 1985;46:383–4. [PubMed] [Google Scholar]
- Kratskin IL, Kimura Y, Hastings L, Doty RL. Chronic dexamethasone treatment potentiates insult to olfactory receptor cells produced by 3-methylindole. Brain Res. 1999;847:240–6. doi: 10.1016/s0006-8993(99)02076-4. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Whalley MG, Rugg MD, Brewin CR. Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. Eur Psychiatry. 2011;26:525–31. doi: 10.1016/j.eurpsy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65:361–6. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–4. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- LeDoux J. RETHINKING THE EMOTIONAL BRAIN. Neuron. 2012;73:653–76. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche S, Seppi K, Behnke S, Liepelt-Scarfone I, Godau J, Mahlknecht P, et al. Risk factors and prodromal markers and the development of Parkinson’s disease. Journal of neurology. 2014;261:180–7. doi: 10.1007/s00415-013-7171-0. [DOI] [PubMed] [Google Scholar]
- Li W, Lopez L, Osher J, Howard JD, Parrish TB, Gottfried JA. Right Orbitofrontal Cortex Mediates Conscious Olfactory Perception. Psychological science. 2010;21:1454–63. doi: 10.1177/0956797610382121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52:1097–108. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, Taylor SF. Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. Am J Psychiatry. 2007;164:1250–8. doi: 10.1176/appi.ajp.2007.06081367. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry. 2006;59:171–7. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–63. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. Journal of magnetic resonance imaging : JMRI. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–31. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- Mo C, Renoir T, Hannan AJ. Effects of chronic stress on the onset and progression of Huntington’s disease in transgenic mice. Neurobiology of disease. 2014;71:81–94. doi: 10.1016/j.nbd.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neuroscience research. 1996;26:235–69. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Nardo D, Hogberg G, Looi JC, Larsson S, Hallstrom T, Pagani M. Gray matter density in limbic and paralimbic cortices is associated with trauma load and EMDR outcome in PTSD patients. Journal of psychiatric research. 2010;44:477–85. doi: 10.1016/j.jpsychires.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Peng P, Gu H, Xiao W, Si LF, Wang JF, Wang SK, et al. A voxel-based morphometry study of anosmic patients. The British journal of radiology. 2013;86:20130207. doi: 10.1259/bjr.20130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JD, Jr, Doty RL, Amoore JE. Analysis of position of trial sequence and type of diluent on the detection threshold for phenyl ethyl alcohol using a single staircase method. Perceptual and motor skills. 1996;82:451–8. doi: 10.2466/pms.1996.82.2.451. [DOI] [PubMed] [Google Scholar]
- Price JL. Olfactory system. In: GP, editor. The Human Nervous System. San Diego, CA: Academic Press; 1990. pp. 979–98. [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Radua J, Canales-Rodriguez EJ, Pomarol-Clotet E, Salvador R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage. 2014;86:81–90. doi: 10.1016/j.neuroimage.2013.07.084. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE transactions on medical imaging. 1997;16:176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–6. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–7. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. The Journal of comparative neurology. 1992;323:167–97. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004a;281:1212–25. doi: 10.1002/ar.a.20126. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and cognition. 2004b;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, et al. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS One. 2013;8:e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpazza C, Tognin S, Frisciata S, Sartori G, Mechelli A. False positive rates in Voxel-based Morphometry studies of the human brain: should we be worried? Neurosci Biobehav Rev. 2015;52:49–55. doi: 10.1016/j.neubiorev.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Djordjevic J, Lundstrom JN. Statistical localization of human olfactory cortex. Neuroimage. 2013a;66:333–42. doi: 10.1016/j.neuroimage.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundstrom JN. Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex. 2013b;23:2448–56. doi: 10.1093/cercor/bhs230. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shucard JL, Cox J, Shucard DW, Fetter H, Chung C, Ramasamy D, et al. Symptoms of posttraumatic stress disorder and exposure to traumatic stressors are related to brain structural volumes and behavioral measures of affective stimulus processing in police officers. Psychiatry Res. 2012;204:25–31. doi: 10.1016/j.pscychresns.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Siopi E, Calabria S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. Journal of neurotrauma. 2012;29:354–61. doi: 10.1089/neu.2011.2055. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of psychiatry & neuroscience : JPN. 2012;37:241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological medicine. 1997;27:951–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. The Journal of clinical psychiatry. 2010;71:1636–44. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Sutker PB. Olfactory identification in combat-related posttraumatic stress disorder. Journal of traumatic stress. 2000;13:241–53. doi: 10.1023/A:1007754611030. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Tomlin H, Rice J, Sutker PB. Olfactory functioning in Gulf War-era veterans: relationships to war-zone duty, self-reported hazards exposures, and psychological distress. Journal of the International Neuropsychological Society : JINS. 2003;9:407–18. doi: 10.1017/S1355617703930062. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Olfaction as a traumatic reminder in posttraumatic stress disorder: case reports and review. The Journal of clinical psychiatry. 2003;64:202–7. doi: 10.4088/jcp.v64n0214. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacology bulletin. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–25. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Welge-Lussen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, et al. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci. 2009;29:15410–3. doi: 10.1523/JNEUROSCI.1909-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Killgore WD, Rosso IM, Britton JC, Schwab ZJ, Weiner MR, et al. Voxel-based morphometric gray matter correlates of posttraumatic stress disorder. Journal of anxiety disorders. 2013;27:413–9. doi: 10.1016/j.janxdis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Wolf OT. Odors as effective retrieval cues for stressful episodes. Neurobiology of learning and memory. 2013 doi: 10.1016/j.nlm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–43. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55:621–6. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]