Abstract
Tissue-engineered blood vessels (TEBVs) are promising in regenerating a live vascular replacement. However, the vascular cell source is limited, and it is crucial to develop a scaffold that accommodates new type of vascular progenitor cells and facilitates in vivo lineage specification of the cells into functional vascular smooth muscle cells (VSMCs) to regenerate vascular tissue. In the present study, integration-free human induced pluripotent stem cells (hiPSCs) were established from patient peripheral blood mononuclear cells through episomal vector nucleofection of reprogramming factors. The established hiPSCs were then induced into mesoderm-originated cardiovascular progenitor cells (CVPCs) with a highly efficient directed lineage specification method. The derived CVPCs were demonstrated to be able to differentiate into functional VSMCs. Subcutaneous implantation of CVPCs seeded on macroporous nanofibrous poly(l-lactide) scaffolds led to in vivo VSMC lineage specification and matrix deposition inside the scaffolds. In summary, we established integration-free patient-specific hiPSCs from peripheral blood mononuclear cells, derived CVPCs through directed lineage specification, and developed an advanced scaffold for these progenitor cells to further differentiate in vivo into VSMCs and regenerate vascular tissue in a subcutaneous implantation model. This study has established an efficient patient-specific approach towards in vivo regeneration of vascular tissue.
Keywords: Human induced pluripotent stem cell, Cardiovascular progenitor cell, Vascular smooth muscle cell, Macroporous nanofibrous scaffold, Tissue-engineered vascular tissue
1. Introduction
Cardiovascular disease remains the leading cause of mortality and impaired quality of life worldwide [1]. Vascular transplantation or grafting has been widely used to treat cardiovascular disease including aortic aneurysm and peripheral vascular disease [2]. However, suitable autologous blood vessels are limited for many patients, mostly due to widespread atherosclerotic vascular disease, useful vessels having been exhausted in previous procedures or the availability of autografts with matched size. Synthetic vascular grafts, made of expanded polytetrafluoroethylene, polyethylene terephthalate or polyurethane, have been successfully used to replace diseased vessels with a diameter greater than 6 mm in a high-flow and low-resistance circulation[3]. However, the grafts smaller than 6 mm in diameter are susceptible to thrombosis and stenosis, leading to very poor patency rate. Coating the intimal surface with heparin and other anticoagulant materials has been attempted, but these approaches are less-than-optimal [4]. Another inherent deficiency of synthetic grafts is the lack of growth potential, which leads to multiple operations in pediatric patients as they grow [5].
Tissue-engineered blood vessels (TEBVs) are promising in regenerating a live vascular replacement, where vascular cells seeded in three-dimensional biodegradable scaffolds are induced to develop functional vascular tissue. However, the vascular cell source is limited [6]. The efforts to construct TEBVs using mature vascular smooth muscle cells (VSMCs) have been reported, especially at the early stage of TEBV development [7]. Unfortunately, mature VSMCs isolated from autologous explanted vascular segments suffered from both inadequate cell numbers and decreased functionality over extensive in vitro expansion. Adult mesenchymal stem cells, with potent proliferative capability and differentiation potential into vascular cells, have been used to regenerate vascular tissues [8–11]. However, recent studies revealed poor engraftment of mesenchymal stem cells in the in situ regenerated vascular tissues [12, 13]. With the unlimited potential to differentiate into various cell types, pluripotent stem cells have become a promising cell source in tissue engineering [14]. In one of our previous studies [15], we successfully established human induced pluripotent stem cells (hiPSCs) from donor fibroblasts through lentiviral transfection of Yamanaka Factors and induced the established hiPSCs into functional SMCs through an embryonic body (EB)-mediated route. The derived SMCs demonstrated the capabilities of vascular tissue formation in a subcutaneous implantation model. However, many issues need to be further addressed. First, an integration-free reprogramming is necessary as viral integration may lead to tumorigenesis [16]. Second, a directed lineage specification method is critical as EB route is time-consuming and results in a heterogeneous population of SMCs from diverse germ layers, which may not reflect the physiological properties of VSMCs in native blood vessels [17]. To address these issues, we aim to develop a novel procedure to generate patient-specific, integration-free hiPSCs and their defined mesoderm-originated cardiovascular progenitor cell (CVPC) and VSMC derivatives. The capabilities of the derived CVPCs in lineage specification into VSMCs and vascular tissue formation in pre-designed three-dimensional biodegradable scaffolds were investigated.
2. Materials and methods
2.1. Generation of integration-free hiPSCs with episomal vectors nucelofection
The procedure is illustrated in Fig. 1A. The use of the peripheral blood was approved by the Institutional Review Board of The University of Michigan and written informed consent was obtained from patients with aortic aneurysm diseases. Peripheral blood mononuclear cells (PBMCs) from three patients were obtained by density gradient centrifugation with lymphocyte separation medium (MP Biomedicals, Santa Ana, CA, USA). PBMCs were cultured under PBMC culture medium for 4–6 days. To generate integration-free hiPSCs, 1×106 cells were nucleofected with 20 µg episomal vectors (EV) plasmid DNA using a Human CD34 Cell Nucleofector Kit (Lonza, Walkersville, MD, USA) following a previous report [18]. The mixture of EVs contained 10 µg EV SFFV-OS, 5 µg EV SFFV-BCL-XL and 5 µg EV SFFV-MK. Immediately after nucleofection, the cells were cultured in plates pretreated with Retronectin (Lonza, Walkersville, MD, USA). Then the cells were transferred onto mouse embryonic fibroblast layers the next day. The alkaline phosphatase-positive hiPSC colonies were observed at 3–4 wk after nucleofection. Some hiPSCs colonies were picked for long-term culture. The hiPSCs were manually passaged every 5–7 days. At least three hiPSC lines were established for each donor. For long-term feeder-free culture, the cells were cultured and maintained on Matrigel-coated surface with TeSR-E8 medium.
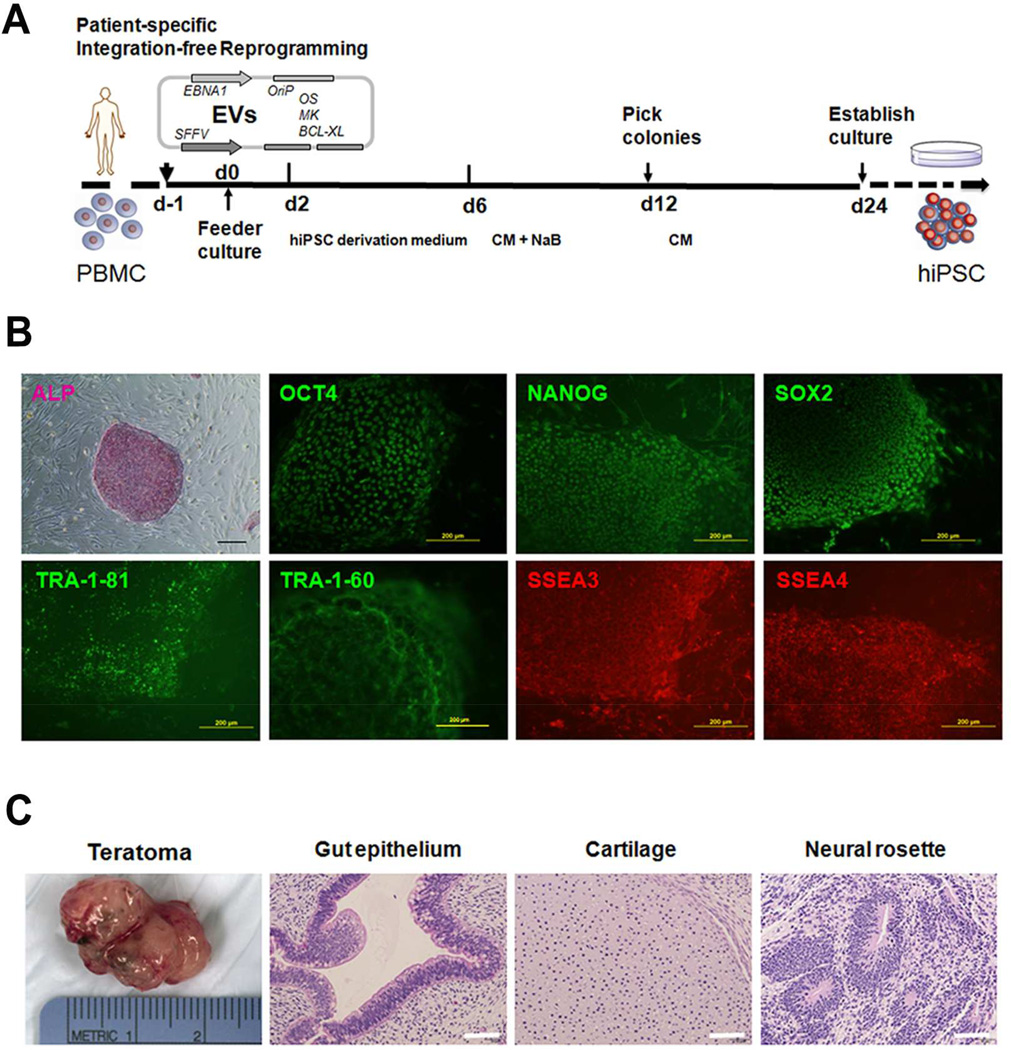
Fig. 1.
Characterization of patient-specific human induced pluripotent stem cells (hiPSCs) established by integration-free reprogramming of peripheral blood mononuclear cells (PBMCs). (A) Schematic diagram of hiPSC generation procedure. EVs: episomal vectors; O: OCT4; S: SOX2; M: MYC; K: KLF4; BCL-XL: isoform of BCL2L1; CM: conditioned medium; NaB: sodium butyrate. (B) Established hiPSC line was observed with alkaline phosphatase (ALP) staining and immunofluorescence staining of pluripotency markers including OCT4, NANOG, SOX2, TRA-1–81, TRA-1–60, SSEA3 and SSEA4. Scale bars = 200 µm. (C) Hematoxylin and eosin (H–E) staining of hiPSC-derived teratoma showing formation of all three germ layer tissues including gut epithelium, cartilage, and neural rosettes. Scale bars = 50 µm.
2.2. Characterization of hiPSCs
Alkaline Phosphatase Staining Kit II (Stemgent, Cambridge, MA, USA) was used to stain for alkaline phosphatase. Briefly, the cells were fixed at room temperature for 5 min, washed with phosphate buffered saline (PBS) with Tween 20, and incubated with Substrate Solution for 5–15 min before the reaction was stopped. Immunofluorescence staining was applied to characterize marker expression. Briefly, hiPSCs or their derivatives were fixed in ice-cold 4% paraformaldehyde for 20 min, permeabilized in 0.5% Triton X-100, and blocked with 5% normal goat serum. The cells were then incubated overnight with a panel of primary antibodies: rabbit anti-OCT4, rabbit anti-NANOG, rabbit anti-SOX2, mouse anti-TRA-1–81, mouse anti-TRA-1–60 (1:100, Stemgent, Cambridge, MA, USA); rat anti-SSEA3, mouse anti-SSEA4 (1:100, Developmental Studies Hybridoma Bank, Iowa City, IA, USA); mouse anti-α-SMA, mouse anti-CNN1 (1:500, Sigma, St. Louis, MO, USA); rabbit anti-SM22α (1:500, Abcam, Cambridge, MA, USA); rabbit anti-MESP1 (1:100, Aviva Systems Biology, San Diego, CA, USA) and mouse anti-ISL1 (1:100, Developmental Studies Hybridoma Bank, Iowa City, IA, USA). After washing with PBS, Alexa 488 or Alexa 594 conjugated secondary goat anti-mouse, rat or rabbit IgG or IgM antibodies were added and incubated for 1 h. The antibodies used were listed in Supplemental Table S1. The cell nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Grand Island, NY, USA). The samples were observed under an Olympus BX53 fluorescence microscope. The percentage of SMC marker positive cells was quantified from three independent cultures (n =3).
2.3. Teratoma formation assay
NOD.CB17-Prkdcscid/J mice (Stock# 001303, Jackson Laboratory, Bar Harbor, ME, USA) were used for teratoma assay. 1 × 106 hiPSCs were suspended in 50 µ1 of PBS and mixed with 50 µ1 of ice-cold Matrigel [19]. The cell/Matrigel mixture was then subcutaneously injected intra-muscularly into hind limbs. After 8 wk, the formed teratoma was dissected and fixed with 4% paraformaldehyde for 24 h. Paraffin-embedded tissue samples were sliced and stained with hematoxylin and eosin (H–E). Images were captured with an Olympus IX71 microscope. The animal procedures were performed according to the protocol approved by the University of Michigan Committee for Use and Care of Laboratory Animals.
2.4. Lineage specification of hiPSCs into CVPCs and VSMCs
The procedure was illustrated in Fig. 2A. The hiPSCs were digested with Versene (Life Technologies, Grand Island, NY, USA) into single cells and plated onto Matrigel-coated culture dishes at a density of 5 × 104 cells/cm2 in CVPC induction medium (CIM), which contained DMEM/F12, 1% penicillin/streptomycin, 1× B27 supplement (without vitamin A), 400 µM 1-thioglycerol (Sigma, St. Louis, MO, USA), 50 µg/mL ascorbic acid (Sigma, St. Louis, MO, USA), 25 ng/mL BMP4 (R&D Systems, Minneapolis, MN, USA), and 3 µM GSK3 inhibitor CHIR99021 (Stemgent, Cambridge, MA, USA) [19]. To enhance cell viability, 10 µM ROCK inhibitor Y27632 (Stemgent, Cambridge, MA, USA) was added only during the first day of induction [20]. The CIM was replenished every 24 h. For VSMCs terminal differentiation, CVPCs was induced with basal CIM containing DMEM/F12, 1% penicillin/streptomycin, 1 × B27 supplement (without vitamin A) and 400 µM 1-thioglycerol, supplemented with 10 ng/mL PDGF-BB (PeproTech, Rocky Hill, NJ, USA) and 2 ng/mL TGF-β1 (PeproTech, Rocky Hill, NJ, USA) for 3–9 d.
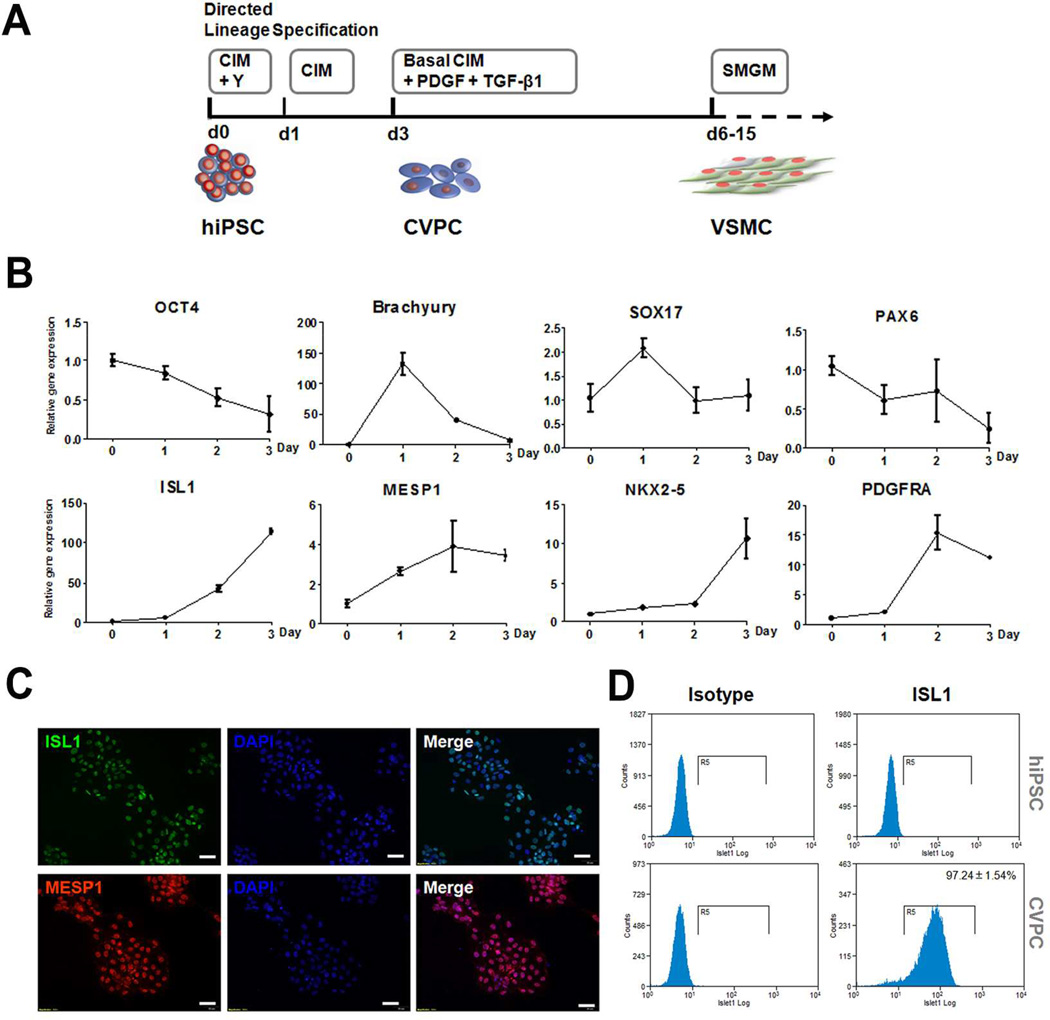
Fig. 2.
Derivation of vascular smooth muscle cells (VSMCs) from human induced pluripotent stem cells (hiPSCs) through directed lineage specification and cardiovascular progenitor cell (CVPC) intermediate. (A) Schematic diagram of the procedure. CIM: basal cardiovascular progenitor induction medium plus BMP4, CHIR and ascorbic acid; Y: ROCK inhibitor Y27632; SMGM: smooth muscle cell growth medium. (B) Characterization of CVPC lineage specification process by qRT-PCR, depicting the down-regulation of pluripotency markers of OCT4, diverse expression pattern of germ layer marker of brachyury (mesoderm), SOX17 (endoderm) and PAX6 (ectoderm), and up-regulation of CVPC markers of ISL1, MESP1, NKX2–5 and PDGFRA. (C) Immunofluorescence staining showing the protein expression of CVPC markers of ISL1 and MESP1 at day 3 of directed lineage specification. Scale bars = 100 µm. (D) Flow cytometry measurement of the percentage of ISL1-positive cells in derived CVPC population at day 3 of directed lineage specification. Data are presented as mean ± SEM (n = 3).
Primary human aortic smooth muscle cells were obtained from Lonza and cultured in complete smooth muscle growth medium 2 (SmGM2, Lonza, Walkersville, MD, USA).
2.5. Contractility assay
Differentiated cells at day 6 were stimulated with 1 mM carbachol. The change of cell surface area at 30 min was analyzed using ImageJ software (NIH, Bethesda, MD, USA).
2.6. Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). 0.5 µg of RNA was transcribed into cDNA with TaqMan Reverse Transcription Reagents (Life Technologies, Grand Island, NY, USA) in a 20 µl reaction volume. The primer sequences were listed in Supplemental Table S2. The qRT-PCR was performed on a StepOne Plus Real-Time System (Life Technologies, Grand Island, NY, USA) using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Comparative cycle threshold (Ct) method was used to quantify gene expression levels. The housekeeping gene GAPDH was used as internal control.
2.7. Flow cytometry
A Fixation/Permeabilization Kit (eBiosciences, San Diego, CA, USA) was used. 0.5 × 106 cells were suspended in 400 µl of Fixation/Permeabilization Solution at room temperature for 30 min. The cells were washed twice with Wash Buffer. After being blocked with Blocking Solution for 30 min, the cells were incubated with the primary anti-ISL1 antibody at room temperature for 1 h, washed twice with Wash Buffer, and incubated with FITC-conjugated secondary antibody at room temperature for 1 h. The cells were washed twice and the re-suspended cells in PBS were analyzed by flow cytometry. Isotype IgG2b was used as the negative control.
2.8. Fabrication of 3D macroporous nanofibrous poly(l-lactide) (PLLA) scaffolds
PLLA with an inherent viscosity of approximately 1.6 dl/g (Boehinger Ingelheim, Ingelheim, Germany) was dissolved in tetrahydrofuran. The solution (10% wt/v) was cast into an assembled sugar template (formed from bound sugar spheres, 60–150 µm in diameter) under a mild vacuum. The polymer-sugar composite was phase-separated at −20 °C overnight. The composite was then immersed into cyclohexane for 2 d to exchange tetrahydrofuran. The resulting composites were freeze-dried for 2 d to remove the solvent. Sugar spheres were leached out using distilled water, resulting in macroporous nanofibrous scaffolds with the pore size of 60–150 µm. The scaffolds were punched into circular disks with dimensions of 5 mm in diameter and 1 mm in thickness.
2.9. Lineage specification of CVPCs inside 3D scaffolds
The 3D nanofibrous PLLA scaffolds were pre-wetted in 70% ethanol for 30 min, washed three times with PBS for 30 min each, and incubated in 50 µg/ml human plasma fibronectin (Sigma, St. Louis, MO, USA) overnight. hiPSC-derived CVPCs were labeled with a PKH67 Green Fluorescent Cell Linker Kit for general cell membrane labeling (Sigma, St. Louis, MO, USA) following the manufacturer’s instructions. 5×105 labeled cells in 20 µl suspension were seeded into each scaffold using a pipet. After 2 h of incubation, the cell/scaffold constructs were subcutaneously implanted into 6–8 wk old female nude mice (Charles River Laboratories, Wilmington, MA, USA). The animal procedures were performed according to the protocol approved by the University of Michigan Committee for Use and Care of Laboratory Animals. Surgery was performed under general inhalation anesthesia with isofluorane. Two midsagittal incisions were made on the dorsa and two pockets were created for each incision. One cell-scaffold construct was implanted into each subcutaneous pocket. The incisions were closed with staples. After 2 wk, the mice were euthanized and the implants were harvested for analysis.
2.10. Scanning electron microscopy (SEM) observation
The cell-seeded scaffold samples were fixed in fixative with 2.5% glutaraldehyde and 2% paraformaldehyde at 4 °C overnight, and post-fixed in 1% osmium tetroxide at room temperature for 1 h. The samples were dehydrated in increasing concentrations of ethanol. After treated with hexamethyldisilazane, the samples were sputter-coated with gold and observed under a scanning electron microscope (Phillips XL30 FEG).
2.11. Immunofluorescence observation of implants
The implants were washed in PBS, fixed with 4% paraformaldehyde in PBS for 15 min. The samples were cryosectioned at a thickness of 5 µm. Sections were incubated with primary antibodies against SM22α at 1:100 dilutions. Following PBS rinse, sections were incubated with Alexa 594 conjugated secondary goat anti- rabbit IgG antibody. Isotype rabbit IgG was used as a negative control. The samples were observed under a confocal microscope. The percentage of SM22α-positive cells in fluorescence-labeled donor cell populations was quantified for three implants (n =3).
2.12. Statistics
Numerical data were reported as mean ± SEM from independent cell cultures. The Student’s t-test was applied to test the significance between the groups. The value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Generation of integration-free hiPSCs and characterization of their pluripotency
The procedure for integration-free reprogramming is illustrated in Fig. 1A. The peripheral blood mononuclear cells isolated from the patient was cultured and nucleofected with episomal vectors carrying OCT4, SOX2, KLF4, MYC and BCL-XL. After 2 wk of transfection, the colonies with clear borders appeared. The colonies were passaged and expanded for several times to establish integration-free hiPSC lines. For all three donors, at least three hiPSC lines were successfully established for each donor. Fig. 1B shows representative hiPSCs culture data of one cell line. Strong positive staining of alkaline phosphatase was observed. The cells showed positive expression of pluripotency markers, including nuclear expression of OCT4, NANOG and SOX2, and cell surface expression of TRA-1–81, TRA-1–60, SSEA3 and SSEA4.
To further validate the pluripotency of established hiPSC lines, a teratoma formation assay was performed (Fig. 1C). The established hiPSCs developed teratoma after being injected into SCID mice with the formation of typical tissue types of all three germ layers.
3.2. Directed lineage specification of hiPSCs into CVPCs and VSMCs
The lineage specification procedure is illustrated in Fig. 2A and representative results of one hiPSC line are shown in Fig. 2B–D and Fig. 3. Y27632, a Rho-associated coiled-coil kinase (ROCK) inhibitor, was added to the culture for the first day of induction to improve cell survival. A cocktail of BMP4, ascorbic acid and GSK3 inhibitor CHIR99021 was used to induce CVPCs. Gene expression profiling revealed the step-wise lineage specification process (Fig. 2B). A continuous loss of pluripotency marker OCT4 expression was observed through the three days of CVPC induction. The early mesoderm marker brachyury was rapidly elevated to more than 100 folds at day 1 of induction and then dropped down. In contrast, the endoderm marker SOX17 was only transiently and slightly increased at day 1 and then dropped to baseline. The ectoderm marker PAX6 expression was maintained at about the baseline level during the induction. Correlating with the transiently elevated expression pattern of mesoderm marker, the CVPC markers of ISL1, MESP1, NKX2–5 and PDGFRA significantly increased through the induction. These gene expression profile data reveled that mesoderm specification preceded CVPC induction, and hiPSCs were induced into CVPCs through a mesoderm intermediate. The immunofluorescence staining data further showed that most of the derived CVPCs were ISL1 and MESP1 positive (Fig. 2C), and the flow cytometry assay showed that 97.24 ± 1.54% of the derived CVPCs were ISL1 positive in contrast to essentially negative in un-differentiated hiPSCs (Fig. 2D).
Fig. 3.
Differentiation of cardiovascular progenitor cells (CVPCs) into vascular smooth muscle cells (VSMCs). (A) Immunofluorescence staining showed that the VSMC markers of α-SMA, SM22α and CNN1 were up-regulated upon differentiation. Scale bars = 100 µm. (B) qRT-PCR demonstrated the elevated gene expression levels of α-SMA, SM22α and CNN1 during the differentiation process. Graphed data are presented as mean ± SEM (n = 3).
To investigate the potential of VSMC differentiation of CVPCs, the derived CVPCs were then further induced with a cocktail of PDGF-BB and TGF-β1. After three to nine days of induction, the cells showed positive staining of VSMC markers α-SMA, SM22α and CNN1 (Fig. 3A). Ten times of VSMC induction experiments were performed and efficient differentiation was achieved for each experiment. Gene expression profiling revealed that the highest expression of most VSMC markers occurred at day 6 (Fig. 3B). Therefore, the day 6 induction culture was used in further VSMC function evaluation experiments. Quantitation of day 6 induction culture also showed that 70.94 ± 6.98 % cells were α-SMA-positive, 94.13 ± 4.22 % cells were SM22α-positive and 94.70 ± 3.02 % cells were CNN1-positive. To validate the SMC phenotype in long-term culture, the day 6 derived VSMCs were also passaged and cultured in smooth muscle culture medium. Gene expression profile study showed that the hiPSC-derived VSMCs maintained a comparable level of SMC marker gene expression, in comparison to primary human aortic smooth muscle cells cultured in identical smooth muscle culture medium (Supplementary Fig. 1).
3.3. Functional assessment of hiPSC-derived VSMCs
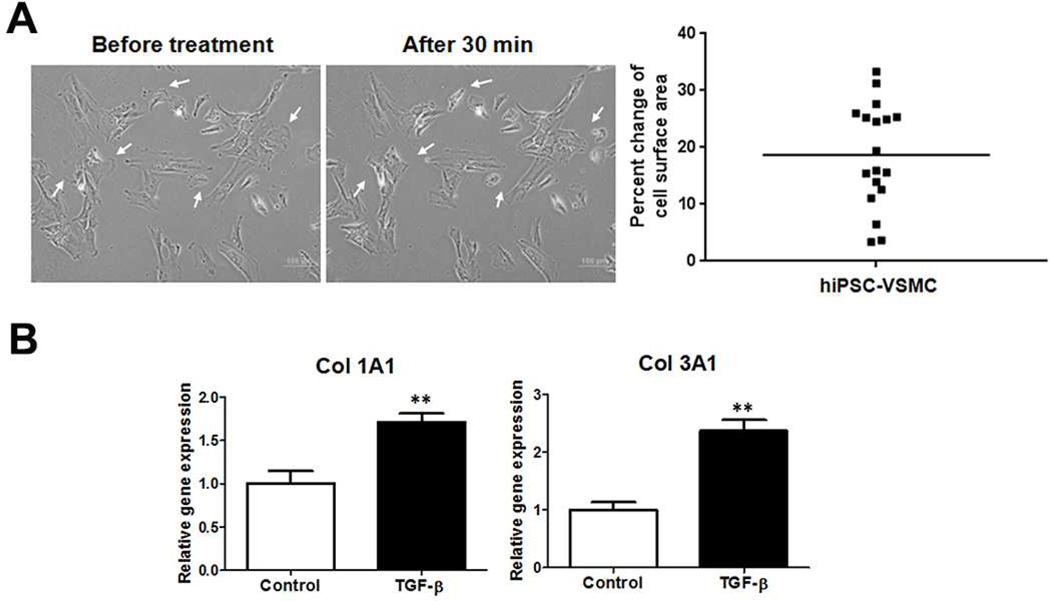
The contractility of hiPSC-derived VSMCs under muscarinic agonist treatment was measured. The differentiated cells at day 6 were treated with 1 mM carbachol. More than 50% of total tested VSMCs contracted at 30 min (Fig. 4A). The contracting cells exhibited 18.64 ± 2.15 % change of cell surface area. This result is comparable to the contractility of primary VSMCs isolated from patient’s aorta tissue (20.17 ± 3.00 %), as we described in a previous report [15]. The matrix gene expression of hiPSC-derived VSMCs under growth factor treatment was assayed. Under the TGF-β1 treatment for 24 h, both the collagen 1A1 and 3A1gene expression levels were significantly enhanced, as compared to un-treated control (Fig. 4B). These data indicated that the hiPSC-derived VSMCs attained the functionality of the primary VSMCs.
Fig. 4.
Functional assays of the human induced pluripotent stem cells (hiPSC)-derived vascular smooth muscle cells (VSMCs). (A) Contraction response of hiPSC-derived VSMCs to 1 mM carbachol treatment. The images showed the cells prior to treatment and the cells after 30 min of treatment. Arrows indicated the contracting cells. Scale bars = 100 µm. The change of cell surface area was calculated and plotted (cell number n = 18, representative data of three independent experiments). (B) Gene expression profile of col 1A1 and col 3A1 under 5 ng/ml of TGF-β1 treatment for 24 h. Graphed data are presented as mean ± SEM (n = 3). *, P < 0.05; **, P < 0.01 vs vehicle control.
3.4. Lineage specification of CVPCs in 3D macroporous nanofibrous scaffolds
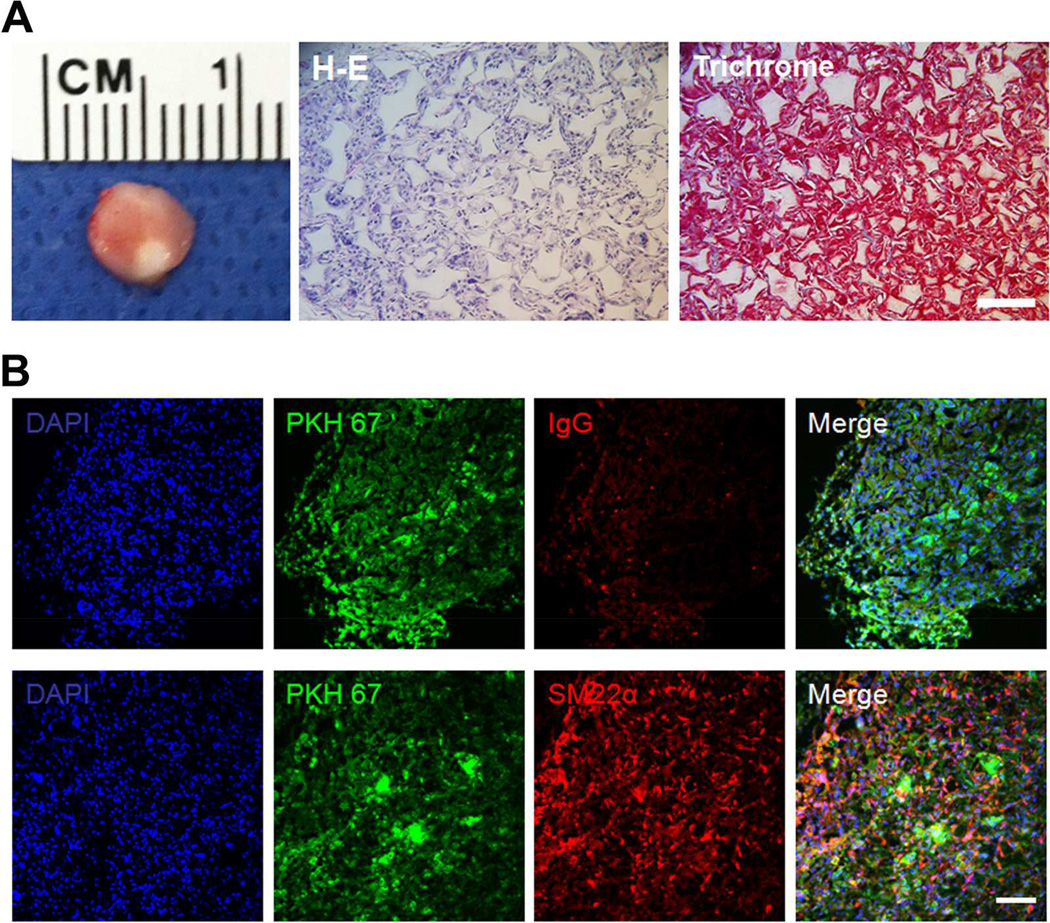
Next we investigated whether the CVPCs can be directly harnessed to regenerate vascular tissues in a subcutaneous implantation model, since stem cells have been well documented to better engraft into neo tissue compared to terminally-differentiated derivatives. Given the thin layer characteristic of vascular media tissue, fully-interconnected macroporous scaffolds with small pore size were used to facilitate vascular tissue formation. Our pilot study showed that pore sizes smaller than 30–60 µm impeded the cell infiltration (Supplementary Fig. 2), therefore in this study a pore size of 60–150 µm were developed to support cell seeding and growth. The porosity of the scaffolds was as high as 95.68 ± 0.5 % (n = 4), which can facilitate uniform tissue formation. After 2 h of seeding, hiPSC-derived CVPCs attached well on the interconnected pores of the scaffolds (Fig. 5). For subcutaneous implantation, CVPCs were pre-labeled with PKH67 green fluorescent dye for donor cell tracing. After 2 wk of implantation in nude mice, the constructs were retrieved and subjected to histological observations. Fig. 6A shows the histology of a representative implant, with uniform cell growth inside the entire scaffold (H-E staining) and significant collageneous matrix deposition (trichrome staining). No teratoma tissue formation was observed. Immunofluorescence staining revealed that the majority of implanted CVPCs differentiated into SM22α-positive VSMCs, depicted by the co-localization of SMC marker SM22α and PKH67 fluorescence label (Fig. 6B). Quantitation data showed that 83.71 ± 10.32 % of fluorescence-labeled donor cells were SM22α-positive. In contrast, cTnT-positive cardiomyocytes were not observed in the implants, likely due to the lack of proper chemical factors for cardiomyocyte differentiation (such as BMP4 and Wnt antagonist) in the subcutaneous healing microenvironment. These data collectively demonstrated the feasibility of in vivo direct regeneration of vascular tissues with CVPCs seeded in a favorable 3D scaffold and the engraftment of differentiated VSMCs inside the constructs in a subcutaneous implantation model.
Fig. 5.
Seeding of human induced pluripotent stem cells (hiPSC)-derived cardiovascular progenitor cells (CVPCs) into three-dimensional macroporous nanofibrous PLLA scaffolds. Scanning electron microscopic images showed the morphology of (A) the scaffolds and (B) hiPSC-derived CVPCs seeded in the scaffolds for 2 h.
Fig. 6.
In vivo differentiation of human induced pluripotent stem cells (hiPSCs)-derived cardiovascular progenitor cells (CVPCs) in three-dimensional scaffolds. The hiPSCs-derived CVPCs were pre-labeled with PKH67 green fluorescent dye, seeded into macroporous nanofibrous PLLA scaffolds for 2 h and the cell-scaffold constructs were subcutaneously implanted into nude mice. After 2 wk of implantation, the constructs were retrieved for histological observation with hematoxylin and eosin (H–E) staining and trichrome staining (A), and immunofluorescence co-staining of SM22α (B). Isotype IgG was used as negative control. Scale bar = 100 µm.
4. Discussion
Functional VSMCs are the key component in the regenerated TEBVs [21]. In a previous report, we found that tubular macroporous nanofibrous PLLA scaffolds support growth and vascular tissue formation of primary human aortic VSMCs [22]. The nanofibrous feature, which mimics the architecture of extracelluar matrix, promotes cell-material interaction and tissue regeneration [23]. As an abundant cell source, autologous mesenchymal stem cells were explored to differentiate into SMC-like cells. However, the engraftment of mesenchymal stem cells and their derivatives in the regenerated vascular tissues is poor. Recent studies showed that bone marrow mesenchymal stem cells or mononuclear cells exerted their pro-regeneration function primarily through anti-coagulation [12] or paracrine factor secretion and facilitated recruitment of host vascular cells [13] in in situ regeneration animal models, rather than direct constitution of the regenerated blood vessel wall. Therefore it is crucial to seek for a better stem cell source that can directly reconstruct the vascular tissue in situ, which is especially important for regenerating long TEBVs in human bodies such as peripheral vascular bypass, wherein acellular biodegradable grafts are far from ideal due to limited transverse host vascular cell migration from native blood vessels over an extended distance. With the potential to differentiate into various cell types, patient-specific hiPSCs has brought substantial excitement to the field of tissue engineering and regenerative medicine [14]. In a recent report, we successfully established hiPSCs from donor fibroblasts with lentiviral transfection of Yamanaka Factors and induced the established hiPSCs into SMCs through an EB-mediated route [15]. However, many issues need to be further addressed due to concerns over the safety and efficiencies of the procedure. To eliminate genotoxicity inherent to viral vectors, efforts have been made to generate hiPSCs without the integration of exogenous DNA into cellular genomes, by delivering reprogramming factors in the form of mRNA [24] or recombinant protein [25], and more recently by expressing the factors with non-integral episomal vectors [26]. In the present study, integration-free patient-specific hiPSCs were derived from peripheral blood mononuclear cells using our advantageous episomal vector nucleofection protocol to specifically reprogram non-lymphoid cell sub-population of peripheral blood mononuclear cells with an improved reprogramming efficiency. In one of our previous report, qPCR analysis of iPSCs after 10 passages showed that the average copy number of residual EV plamids decreased to less than 0.01 copy per cell, suggesting that EV plasmids were depleted from almost all cells [18]. Further study is warranted to exclude the accident plasmid integration for the established cell line before clinic application, although the incidence of integration is very low. Compared to widely used skin fibroblasts, peripheral blood mononuclear cells can be more easily isolated from patients, and contain less somatic mutations due to frequent replenishment and less exposure to environmental mutagens. The established hiPSCs were then efficiently differentiated into mesoderm-derived CVPCs and VSMCs through a step-wise directed specification route. Given that the media layer of most blood vessels are originated from mesoderm-derived VSMCs [17], our main interest is to derive a homogenous VSMC population from a mesoderm origin. Moreover, origin-defined cell derivatives can better model the progression of diseases such as aortic aneurysm. The procedure that we developed recapitulates the natural vascular development process and could be easily scaled up to produce large numbers of mesoderm-derived CVPCs and functional VSMCs with reproducible properties.
A biomimetic porous scaffold is critical to facilitate vascular cells or their progenitors to grow, differentiate, and ultimately form the functional vascular tissues. The nanofibrous scaffold in this study is designed to be highly porous and the pores are highly interconnected to promote uniform cell infiltration and growth, which is extremely important for the construction of vascular wall tissue, as defect in the relative thin tissue layers may cause vessel dissection and rupture under blood pressure. The pore surface is engineered with nanofibrous features to promote the desired function of attached cells [27]. Pore size is another important parameter for successful tissue regeneration. A smaller pore size is preferred given the thin layer characteristics of the vascular graft wall tissue. However, the pore size bellow 60 µm impeded efficient vascular cell seeding and infiltration into the scaffolds. In this study, the porous scaffold with a pore size scale of 60–150 µm was demonstrated to allow for efficient CVPCs seeding and uniform growth inside the scaffolds while supporting the VSMC lineage specification in a subcutaneous implantation model. To the best of our knowledge, this study is the first report demonstrating hiPSC-derived CVPCs can be harnessed to directly regenerate vascular tissue. In this study, a disc shaped scaffold was used to conveniently investigate the capability of the porous nanofibrous structure to support SMC differentiation and facilitate vascular tissue formation. In future in situ vascular regeneration studies, the mechanical properties of the tubular scaffold will be enhanced to resist the blood pressure and improve the suture retention strength [22, 28–31], where a multilayer tubular scaffold will be constructed. We will further evaluate our scaffold and patient-specific cell systems for blood vessel replacement and the modeling of disease progression of various subtypes of aortic aneurysm.
5. Conclusions
Large numbers of mesoderm originated CVPCs and VSMCs were efficiently obtained with our established integration-free reprogramming and directed lineage specification protocols. The hiPSC-derived CVPCs regenerated vascular tissue in an advantageous macroporous nanofibrous PLLA scaffold. Taken together, our study has established an efficient patient-specific approach towards in vivo regeneration of vascular tissue.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the financial support from the National Institute of Health (HL114038: PXM and YEC), University of Michigan Frankel Cardiovascular Center Inaugural Grant (BY), and University of Michigan MCubed Grant (BY, YEC, and PXM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laslett LJ, Alagona P, Jr, Clark BA, 3rd, Drozda JP, Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 3.Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28:5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Kurobe H, Maxfield MW, Breuer CK, Shinoka T. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med. 2012;1:566–571. doi: 10.5966/sctm.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. 2008;81:161–166. [PMC free article] [PubMed] [Google Scholar]
- 6.Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012;18:405–425. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 8.Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- 9.Kashiwakura Y, Katoh Y, Tamayose K, Konishi H, Takaya N, Yuhara S, Yamada M, Sugimoto K, Daida H. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22alpha promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation. 2003;107:2078–2081. doi: 10.1161/01.CIR.0000070082.64414.B5. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Tjwa M, Moons L, Fons P, Noel A, Ny A, Zhou JM, Lennartsson J, Li H, Luttun A, Ponten A, Devy L, Bouche A, Oh H, Manderveld A, Blacher S, Communi D, Savi P, Bono F, Dewerchin M, Foidart JM, Autiero M, Herbert JM, Collen D, Heldin CH, Eriksson U, Carmeliet P. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest. 2005;115:118–127. doi: 10.1172/JCI19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, Yang B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35:8960–8969. doi: 10.1016/j.biomaterials.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tubsuwan A, Abed S, Deichmann A, Kardel MD, Bartholoma C, Cheung A, Negre O, Kadri Z, Fucharoen S, von Kalle C, Payen E, Chretien S, Schmidt M, Eaves CJ, Leboulch P, Maouche-Chretien L. Parallel assessment of globin lentiviral transfer in induced pluripotent stem cells and adult hematopoietic stem cells derived from the same transplanted beta-thalassemia patient. Stem Cells. 2013;31:1785–1794. doi: 10.1002/stem.1436. [DOI] [PubMed] [Google Scholar]
- 17.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su RJ, Baylink DJ, Neises A, Kiroyan JB, Meng X, Payne KJ, Tschudy-Seney B, Duan Y, Appleby N, Kearns-Jonker M, Gridley DS, Wang J, Lau KH, Zhang XB. Efficient generation of integration-free ips cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS One. 2013;8:e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, Yang HT. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 2013;23:1119–1132. doi: 10.1038/cr.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 21.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010;31:7971–7977. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowey SN, Huang X, Chou BK, Ye Z, Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012;7:2013–2021. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Hu J, Ma PX. Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev. 2012;64:1129–1141. doi: 10.1016/j.addr.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31:4313–4321. doi: 10.1016/j.biomaterials.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 29.de Valence S, Tille JC, Giliberto JP, Mrowczynski W, Gurny R, Walpoth BH, Moller M. Advantages of bilayered vascular grafts for surgical applicability and tissue regeneration. Acta Biomater. 2012;8:3914–3920. doi: 10.1016/j.actbio.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Nieponice A, Soletti L, Guan J, Hong Y, Gharaibeh B, Maul TM, Huard J, Wagner WR, Vorp DA. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng Part A. 2010;16:1215–1223. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012;18:1148–1153. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.