Abstract
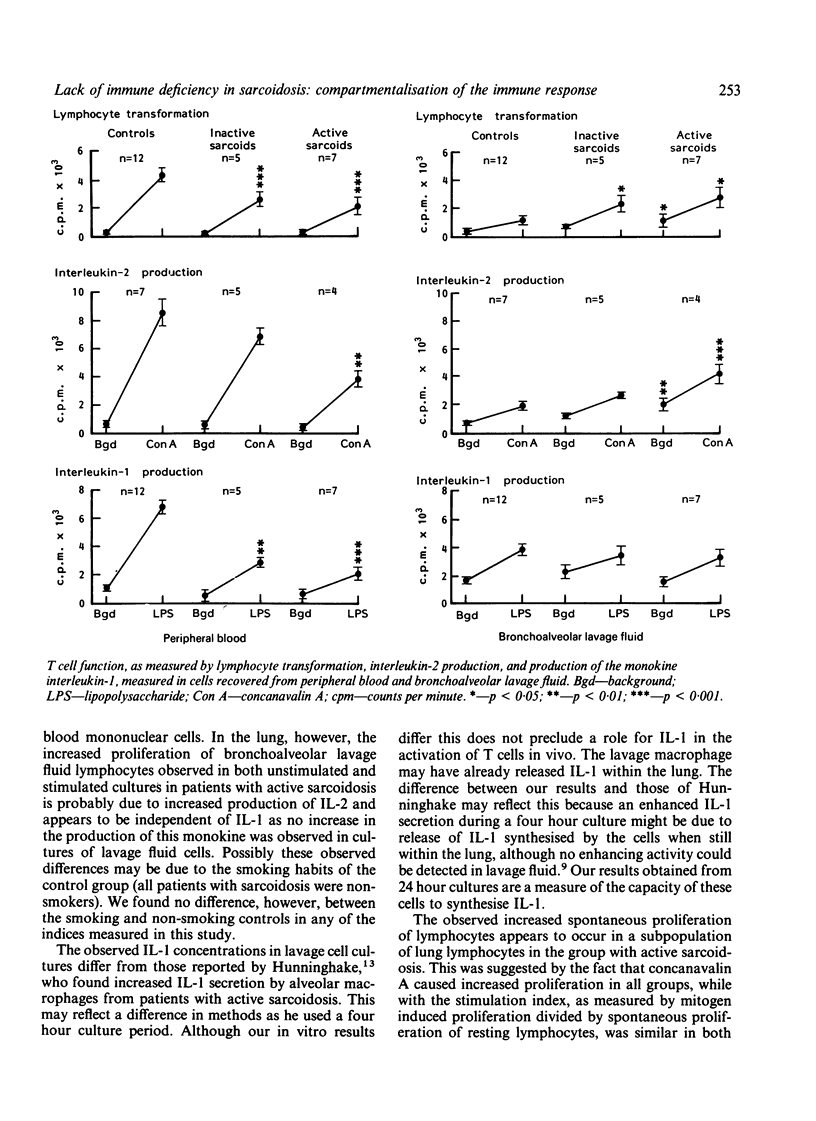
The original findings of peripheral anergy in sarcoidosis led to the conclusion that sarcoidosis was a disease associated with immune deficiency, but patients with sarcoidosis do not appear to suffer from repeated infections suggestive of immune suppression. With the technique of bronchoalveolar lavage it is now possible to examine the local immune response within the lung, the most commonly affected organ in sarcoidosis. In this study three different indices of cell mediated immunity (lymphocyte transformation, interleukin-2 production, and interleukin-1 production) have been examined by comparison of cells recovered by lavage with those collected from peripheral blood. It was found that in vitro anergy was confined to peripheral blood cells, where all three markers of the immune response used in this study was impaired in the 12 patients with sarcoidosis group when compared with results in the 12 controls, with the most depressed responses seen in those patients classified as having active disease (lymphocyte proliferation 45% (SD 17%); interleukin-2 production 44% (13%), and interleukin-1 production 31% (10%) of control levels). By contrast, T lymphocytes recovered from the lungs of patients with sarcoidosis showed a greater response than did those from controls in terms of lymphocyte transformation and interleukin-2 production; these differences were greatest in those with active disease (lymphocyte proliferation 209% (27%) and interleukin-2 production 202% (19%) of control levels). Interleukin-1 production by cells of the monocyte lineage recovered from the lung gave similar results to those of the control and sarcoid groups. It is concluded that the anergy seen in the peripheral blood compartment possibly reflects redistribution of T lymphocytes rather than a generalised immune deficiency.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goodwin J. S., DeHoratius R., Israel H., Peake G. T., Messner R. P. Suppressor cell function in sarcoidosis. Ann Intern Med. 1979 Feb;90(2):169–173. doi: 10.7326/0003-4819-90-2-169. [DOI] [PubMed] [Google Scholar]
- Greening A. P., Nunn P., Dobson N., Rudolf M., Rees A. D. Pulmonary sarcoidosis: alterations in bronchoalveolar lymphocytes and T cell subsets. Thorax. 1985 Apr;40(4):278–283. doi: 10.1136/thx.40.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsmanheimo M. Correlation of tuberculin-induced lymphocyte transformation with skin test reactivity and with clinical manifestations of sarcoidosis. Cell Immunol. 1974 Mar 15;10(3):329–337. doi: 10.1016/0008-8749(74)90125-7. [DOI] [PubMed] [Google Scholar]
- Hudspith B. N., Brostoff J., McNicol M. W., Johnson N. M. Anergy in sarcoidosis: the role of interleukin-1 and prostaglandins in the depressed in vitro lymphocyte response. Clin Exp Immunol. 1984 Aug;57(2):324–330. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fulmer J. D., Young R. C., Jr, Gadek J. E., Crystal R. G. Localization of the immune response in sarcoidosis. Am Rev Respir Dis. 1979 Jul;120(1):49–57. doi: 10.1164/arrd.1979.120.1.49. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Keogh B. A., Hunninghake G. W., Line B. R., Crystal R. G. The alveolitis of pulmonary sarcoidosis. Evaluation of natural history and alveolitis-dependent changes in lung function. Am Rev Respir Dis. 1983 Aug;128(2):256–265. doi: 10.1164/arrd.1983.128.2.256. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Hunninghake G. W., Line B. R., Crystal R. G. The alveolitis of pulmonary sarcoidosis. Evaluation of natural history and alveolitis-dependent changes in lung function. Am Rev Respir Dis. 1983 Aug;128(2):256–265. doi: 10.1164/arrd.1983.128.2.256. [DOI] [PubMed] [Google Scholar]
- Paganelli R., Aiuti F., Beverley P. C., Levinsky R. J. Impaired production of interleukins in patients with cell-mediated immunodeficiencies. Clin Exp Immunol. 1983 Feb;51(2):338–344. [PMC free article] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]


