Abstract
The retinoid (visual) cycle is a complex enzymatic pathway essential for regeneration of the visual chromophore, 11-cis-retinal, a component of rhodopsin that undergoes activation by light in vertebrate eyes. Pathogenic mutations within genes encoding proteins involved in the retinoid cycle lead to abnormalities in retinoid homeostasis and numerous congenital blinding diseases of humans. Thus, elucidation of disease-specific changes in enzymatic activities and retinoid content of the retina can provide important insights into the mechanisms of disease initiation and progression. Here, we use the protein RPE65 as an example to describe generally applicable methods for determining the stability and enzymatic activity of proteins and their mutants involved in retinoid metabolism. Additionally, we introduce a range of analytical techniques involving high-performance liquid chromatography and mass spectrometry to detect and quantify retinoids and their derivatives in eye extracts. Biochemical protocols combined with advanced mass spectrometry should facilitate fundamental biological studies of vision.
Keywords: RPE65, retinoid isomerization, mass spectrometry, A2E, retinal dimer
1. Introduction
Visual phototransduction relies upon light-induced isomerization of the visual chromophore (11-cis-retinal) coupled to rhodopsin (1). Maintaining continuous vision and preserving the health of photoreceptors require an adequate continuing supply of the chromophore. Unlike invertebrates where 11-cis-retinal is regenerated by a photochemical process, vertebrates have evolved a complex enzymatic pathway called the retinoid cycle to achieve this objective (2). This pathway operates in both the photoreceptor cells and the retinal pigment epithelium (RPE), allowing reformation of 11-cis-retinal from all-trans-retinal by several chemical transformations. The classical vertebrate retinoid cycle contributes primarily to regeneration of rhodopsin in rod cells; however, RPE65-based chromophore production may also be important for cone function (3).
The retinoid cycle consists of a complex chain of enzymatic reactions employing over 20 known proteins (Fig. 13.1). Accordingly, it is susceptible to diseases resulting from mutations in genes encoding retinoid cycle proteins that cause impaired vision. In many cases, the disease is caused by loss of catalytic or retinoid-binding function (2, 4, 5). One approach to understanding the etiologies of visual impairments is to examine the enzymatic activity and stability of retinoid cycle proteins and their mutants. Substantial progress has been made in understanding the pathological basis of inactivating mutations in RPE65. Based on screening mutations in the RPE65 gene from patients with Leber’s congenital amaurosis (LCA), autosomal recessive retinitis pigmentosa (arRP), and autosomal dominant/recessive cone–rod dystrophy (CORD), more then 90 different variants were found (6, 7) (summarized in (8, 9)). Many of these were evaluated further by biochemical studies in heterologous expression systems to gain more insight into the disease mechanisms involved. The most common approach used to express RPE65 and its mutants was to generate expression vectors or recombinant adenoviruses to induce transient expression. Although both methods permit robust expression of RPE65 and detection of its enzymatic activity, quantification of 11-cis-retinol production or protein stability requires careful control of multiple variables such as transfection efficiency, viral titer, and protein expression levels. To overcome these obstacles, Bereta et al. employed an advanced bicistronic retroviral-based expression system developed initially by Kitamura and colleagues (10). Insertion of RPE65 and its mutants’ cDNA into a multi-cloning site located upstream of the internal ribosomal entry site (IRES) and an enhanced green fluorescence protein (EGFP) sequence led to expression of both the protein of interest and the EGFP from the same mRNA. This novel arrangement offers two important advantages. First is that expression of the retrovirally transduced cDNA becomes stable after integration. Transduced cells can be visualized due to EGFP co-expression and, notably, they can be sorted or subcloned based on fluorescence intensity to unify and optimize the protein expression level. The last feature is especially important for experiments focused on comparisons of enzymatic properties or stability of wild-type and mutated proteins. To ensure equivalent mRNA levels for wildtype and mutated RPE65, populations of cells characterized by identical fluorescence signals for both cell lines are used. In the example shown in Fig. 13.2, a Gly244Val mutant of RPE65 evidenced a lower protein level as determined by immunoblotting. Considering that both cell lines, i.e., the expressing wild-type and mutated protein, had similar levels of RPE65 transcripts, the observed difference in protein level clearly suggests lower stability and accelerated degradation of the Gly244Val variant (8). The methodology described here significantly simplifies interpretation of such results and can be applied to any protein of interest.
Fig. 13.1.
Schematic representation of the retinoid cycle components and formation of lipofuscin chromophores in roddominant animals (reviewed in (2)). Concomitantly with inactivation of metarhodopsin II, all-trans-retinal is released from the opsin into the lipid membranes of outer disc segments. The first catalytic step in the retinoid cycle involves enzymatic reduction of all-trans-retinal to all-trans-retinol (vitamin A) by members of the short-chain dehydrogenase/reductase (SDR) superfamily. The all-trans-retinol dehydrogenases of both rod and cone outer segments are integral membrane proteins that utilize NADPH as a cofactor. RDH8 as well as ATP-binding cassette transporter 4 (ABCR) play an important role in clearing all-trans-retinal from photoreceptor outer segments (ROS). Delay in this process leads to rapid condensation of retinal with phosphatidylethanolamine (PE) and formation of N-retinylidene-PE (N-ret-PE) that subsequently can be converted into A2E and retinal dimer that can trigger photoreceptor degeneration. Newly formed all-trans-retinol is transported across the interphotoreceptor matrix to retinal pigment epithelium (RPE) cells. There it binds to cellular retinol-binding protein-1 (CRBP1) and is esterified by lecithin:retinol acyl transferase (LRAT) that catalyzes the transfer of an acyl group from the SN1 position of phosphatidylcholine onto retinol. RPE cells store retinyl esters in lipid-droplet-like structures called retinosomes (RESTs). RESTs appear to be metabolically active because retinyl esters can be mobilized in response to light exposure and higher demand for the chromophore. Thermodynamically unfavorable isomerization of planar all-trans-retinoid to the sterically constrained 11-cis conformation is the key enzymatic step in chromophore regeneration. This reaction is catalyzed by RPE-specific 65-kDa protein (RPE65). The final catalytic step in the retinoid cycle is oxidation of 11-cis-retinol carried out by other SCAD family dehydrogenases in the RPE: 11-cis-ROL dehydrogenase type 5 (RDH5) and type 11 (RDH11). The product of this reaction, 11-cis-retinal, is bound by cellular retinal-binding protein (CRBP) that enhances transport of this chromophore through the interphotoreceptor matrix back to photoreceptor rod cells, thereby completing the retinoid cycle.
Fig. 13.2.
Expression and enzymatic activity of RPE65 and RPE65(Gly244Val)-mutated protein. (a) Graphic representation of pMXs-IG retroviral vector used to generate the NIH3T3 cell line stably expressing RPE65 and its mutant. This vector contains the ampicillin-resistance gene (Amp r), murine leukemia retroviral long terminal repeats (5′ and 3′LTR), retroviral package signal (ψ), and multi-cloning site (MCS) for cloning of the gene of interest. Presence of an internal ribosome entry site (IRES) results in expression of the chosen protein and EGFP from a common mRNA. (b) Distribution of EGFP fluorescence intensity in LRAT/RPE65 and LRAT/RPE65(Gly244Val) expressing cell lines after sorting by a FACSAria cell sorter. Similar intensities of EGFP fluorescence indicate comparable levels of RPE65 mRNA in the tested cells. Cells expressing LRAT were prepared with pMXs-IP vector that carries a puromycin resistance gene instead of EGFP. (c) An example of expression levels of RPE65 and its mutant variant analyzed by immunoblotting. With similar mRNA levels, the lower signal from the RPE65(Gly244Val) variant protein indicates its low stability compared to wild-type protein. (d) HPLC separation of retinoids extracted from cultured RPE65-expressing cells incubated with all-trans-retinol. Saponification of the sample significantly increases 11-cis-retinol levels due to hydrolysis of 11-cis-retinyl esters. Peaks were identified based on their elution times and characteristic UV absorbance spectra: (1) retinyl esters, (2) 11-cis-retinol, (3) 13-cis-retinol, and (4) all-trans-retinol.
Abnormalities in the retinoid cycle that lead to blinding diseases are often manifested by disruption of retinoid homeostasis within the eye. Thus, detection of abnormalities in retinoid metabolism provides important insights into possible mechanisms of disease formation and progression. Several examples of defective retinoid metabolism in the eye are exhibited by animal models of human retinal diseases. For example, Rpe65−/− mice evidence severely attenuated rod and cone responses and progressive retinal degeneration due to inadequate regeneration of 11-cis-retinals leading to high levels of all-trans-retinyl esters which accumulate in lipid vacuoles (11, 12). Lrat−/− mice manifest undetectable levels of retinyl esters within the RPE and severely attenuated rod and cone visual responses (13, 14) and Rdh5−/− mice exhibit enhanced accumulation of cis-retinyl esters in their RPE (15). Interestingly, deficits in proteins without enzymatic activity toward retinoids also have been shown to attenuate retinoid metabolism in the eye. Lack of retinal-specific ATP-binding cassette transporter member 4 (ABCA4) causes increased retinal levels in rod outer segments and progressive accumulation of toxic retinal derivatives, e.g., lipofuscin chromophores, within RPE cells (16, 17). Another example is Rlbp1−/− mice (retinaldehyde-binding protein (CRALBP) knockout mice) that show delayed rates of rhodopsin regeneration and recovery of chromophore following a bleach in addition to accumulation of retinyl esters (18). Retinoid cycle impairment in Rlbp1 −/− mice is manifested by delayed dark adaptation. Quantification of chromophore levels within the retina is especially important because it provides information about the overall performance of the retinoid cycle. This approach has been widely used in combination with electroretinography to investigate potential delays in 11-cis-retinal production and rhodopsin regeneration.
Analytical methods for retinoid separation have been gradually developed nearly 100 years since “fat-soluble factor B” (vitamin A) was proven to have an impact on the health and growth of young rats (19). Compounds soluble in organic solvents, including retinol and its esters, were initially separated on alumina- and silica-based stationary phases. A pivotal breakthrough was the development of modern high-performance liquid chromatography (HPLC) techniques in the early 1970s together with commercially available small size stationary phases (20, 21). The relatively mild conditions of HPLC make this technique suitable for separating and analyzing labile light-, heat-, and oxygen-sensitive retinoids. Today, retinoids can be separated under numerous chromatographic conditions (summarized in (22, 23)) optimized for normal and reverse-phase columns. Selection of the most appropriate methodology depends on the chemical properties of the particular retinoid as well as the source of the biological sample. The pool of retinoids present in a vertebrate eye is composed of retinyl esters, retinal, retinol, and their geometric (9-cis-, 11-cis-, 13-cis-, and all-trans-) isomers. Accordingly, the currently preferable method for separating these three classes of compounds, as well as their isomers, is normal-phase HPLC wherein hexane/ethyl acetate mixtures are used as the mobile phase (24). This methodology provides unique flexibility in tuning chromatographic conditions to allow identification and quantification of key retinoids, including 11-cis-retinol and 11-cis-retinal. Moreover, use of highly hydrophobic hexane for sample preparation simplifies the tissue extraction procedure to a one-step process without sacrificing overall analytical performance (25).
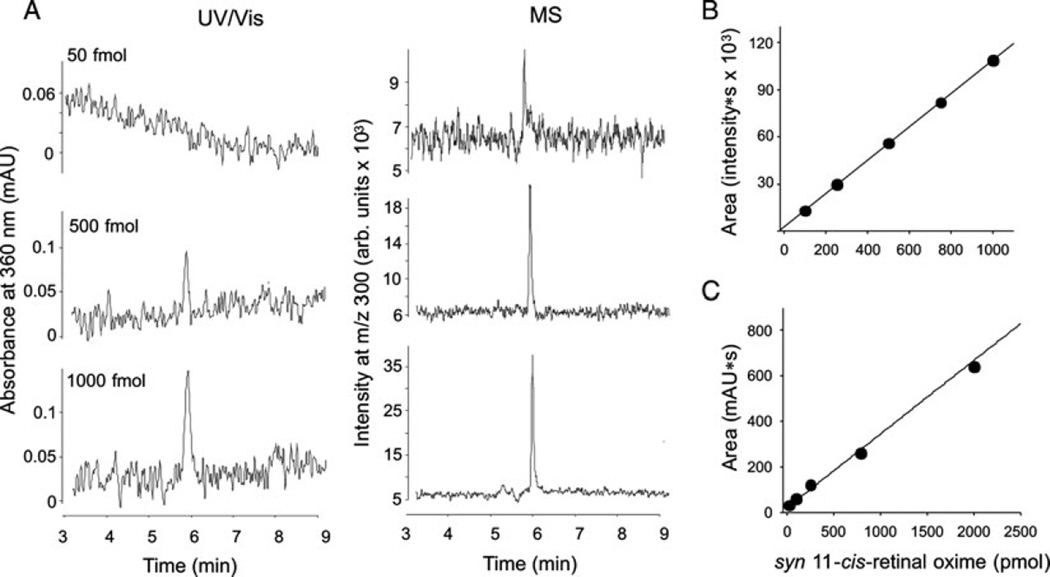
A common method for retinoid detection, identification, and quantification is based on the spectral properties of these compounds. The conjugated polyene chain of retinoids contributes to their relatively strong absorption at UV and visible wavelengths. Thus, absorbance spectra provide information about the number of conjugated double bonds. Even more important, slight differences in wavelengths of maximum absorbance and shapes of the spectra allow precise identification of retinoid isomers. HPLC with UV detection is both economical and easy, providing a limit of retinoid quantification down to ~2 pmol through photodiode array detection (Fig. 13.3). The great advantage is that the standard curves used for quantification are characterized by excellent linearity over a wide range of concentrations (2–1500 pmol) for all tested retinoids and their isomers (correlation coefficient R2 for syn 11-cis-retinal oxime is 0.9995). But a limitation of this method is the low selectivity of its UV–Vis absorbance, which mandates carefully designed chromatographic conditions and precise identification of the compounds being analyzed. This becomes especially challenging when multiple geometric isomers of retinoids or complex mixtures of retinyl esters with different length fatty acid chains are examined.
Fig. 13.3.
Detection and quantification of syn 11-cis-retinal oxime. (a) Comparison of syn 11-cis-retinal oxime detection and quantification limits obtained by UV/Vis and mass spectrometry. The limit of detection is defined as a signal to noise ratio of 3:1 whereas the quantification limit corresponds to ratio of 10:1. Panels (b) and (c) represent calibration curves for mass spectrometry and UV/Vis spectrometry, respectively.
A complementary technique allowing precise molecular identification and quantification is mass spectrometry, especially when it is combined with high-performance liquid chromatography (LC-MS). Several experimental approaches have been developed over last 15 years to detect and quantify retinoic acid, retinol, and retinyl esters in a variety of tissues. The greatest advantage of LC-MS is its sensitivity that reaches the limits of retinoid detection and quantification, depending on applied methodology and class of instrument, in the 10–50 fmol and 20–200 fmol ranges, respectively. Moreover, instruments that have the capacity to perform MSn analyses provide definitive mass and structural identification. These also can be used to enhance the specificity and sensitivity of analyses done in a selected ion or selected reaction monitoring mode.
Here we describe methodology that employs mass spectrometry for detection of the chromophores as well as products of retinal conjugation extracted from mouse eyes. We found that application of LC-MS technology is especially important for quantification of 11-cis-retinal in samples containing low amounts of this chromophore. Prominent examples are evaluation of 11-cis-retinal production in retinas of animal models of human ocular diseases treated with gene therapy, determination of retinoid composition in subregions of the retina, or detection of changes in the chromophore levels after light exposure in Nrl−/− mice (26). LC-MS also turns out to be an excellent tool for studying side products of retinoid metabolism, especially cytotoxic lipofuscin chromophores (Fig. 13.4). Correlations of the relative abundance of di-retinoid-pyridium-ethanolamine (A2E) or retinal dimer with age and progressive retinal degeneration may provide important insights into the mechanism(s) of human age-related macular degeneration (AMD) and Stargardt disease (16, 27).
Fig. 13.4.
Example of retinal dimer (a) and A2E (b) detection in mice lacking both the ATP-binding cassette transporter 4 and the retinol dehydrogenase 8. These mice develop severe RPE/photoreceptor dystrophy at an early age. Progression of retinal degeneration is correlated with a rapid increase in lipofuscin chromophore accumulation (17). Representative mass spectra and fragmentation patterns for the retinal dimer and A2E are shown on the right side of each panel.
Here, we specify LC-MS conditions for the detection and quantification of retinoids optimized for normal-phase HPLC. Experimental conditions are also described for LC-MS-based detection of A2E and retinal dimer in eye extracts.
2. Materials
2.1. RPE65 Heterologous Expression and Activity Assay
- Primers:
- RPE65 – GCAGATGAATTCACCATGTCGTCCCAGCC AGCAGG and CGTCTAGCGGCCGCTCAGGGCT GGGCACCATTGG.
- LRAT – GAGGTGAATTCAGCTACTCAGGGATGAAG AACCCCATGCTG and ACTGACGCGGCCGCATGA AGTTAGCCAGCCATCCATAG.
Cell growth medium (GM) consists of Dulbecco’s modified Eagle’s medium, pH 7.2, with 4 mM L-glutamine, 4,500 mg/l glucose, and 110 mg/l sodium pyruvate (HyClone Laboratories, Inc., Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 units/ml streptomycin.
0.067 M phosphate-buffered saline (PBS) composed of NaCl (9 g/l), Na2HPO4 (0.8 g/l), NaH2PO4 (0.14 g/l).
2 M CaCl2 solution.
25 mM chloroquine diphosphate salt solution in PBS.
1 M potassium phosphate dibasic (K2HPO4).
5 M sodium chloride (NaCl).
1 M potassium chloride (KCl).
100 mM D dextrose.
1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.05.
5 mg/ml polybrene solution.
4 M potassium hydroxide solution in methanol.
Hexane (HPLC grade) (Fisher Scientific, Fair Lawn, NJ).
1 M hydroxylamine, pH 7.4.
4 mM all-trans-retinol solution in N,N-dimethylformamide (DMF).
Transfection buffer composed of 50 mM HEPES, pH 7.05, containing 10 mM KCl, 12 mM dextrose, 280 mM NaCl, and 1.5 mM Na2HPO4.
2.2. Retinoid Extraction, Detection, and Quantification
Ethyl acetate (HPLC grade) (Acros, Morris Plains, NJ).
Ethanol (ACS grade) (Pharmco-Aaper, Brookfield, CT).
Acetonitrile (HPLC grade) (Fisher Scientific, Fair Lawn, NJ).
Isopropanol (HPLC grade) (Fisher Scientific, Fair Lawn, NJ).
Trifluoroacetic acid (TFA) (HPLC grade) (Supelco, Bellefonte, PA).
Liquid nitrogen.
Teflon syringe filter (National Scientific Company, Rockwood, TN).
Synthetic, purified standards of 11-cis-retinal oximes, all-trans-13,14-dihydro-retinal oxime, di-retinoid-pyridiumethanolamine (A2E), and retinal dimer.
Extraction buffer composed of 50% ethanol in 0.067 M phosphate-buffered saline, pH 7.4, containing 40 mM hydroxylamine.
10% ethyl acetate in hexane.
1% isopropanol in hexane.
10% isopropanol in acetonitrile, 0.1% TFA.
Agilent-Si, 5 µm, 4.5 × 250 mm column (Agilent Technologies, Santa Clara, CA).
C18 reverse-phase column, 4.5 × 250 mm (Phenomenex, Torrance, CA).
Other materials as in Section 2.1.
3. Methods
3.1. Evaluating Enzymatic Activity of RPE65 and Its Mutants
3.1.1. Stable Transduction and Expression of RPE65 and LRAT in NIH3T3 Cells
Both RPE65 and LRAT are essential for robust production of 11-cis-retinol in cell/tissue cultures. Thus, RPE65 should be expressed in a cell line stably producing LRAT (28).
Amplify RPE65 cDNA by PCR introducing EcoRI and NotI restriction sites at the beginning and the end of this sequence, respectively.
Clone amplified cDNAs into pMXs-IG retroviral vectors. Confirm the sequence of selected clones by DNA sequencing.
Twenty-four hours prior to transfection, seed 1.5 × 106 Phoenix-Eco retroviral producer cells on a 6-cm plate and culture them in 6 ml of GM. Maintain cells at 37°C in 5% CO2.
Just prior to transfection, replace media with 6 ml of warm media containing 25 µM chloroquine.
Generate a transfection mixture by adding 1 ml of transfection buffer to an equal volume of 250 mM CaCl2 solution containing 15 µg of plasmid DNA. Mix and incubate for 1 min. Add mixture dropwise to the cells and place the cells in 32°C, 5% CO2 incubator (see Note 1).
Replace the medium with fresh GM at both 8 and 24 h after transfection.
Harvest released retrovirus by collecting cell medium 48 h post-transfection; centrifuge at 500×g for 5 min to remove detached cells. Aliquot the retrovirus-containing medium into 0.5-ml portions, freeze in liquid nitrogen, and store at −80°C.
Twenty-four hours prior to transduction, suspend 2 × 105 of NIH3T3 fibroblasts expressing LRAT in 6 ml of GM and then place the suspension in a 6-cm dish.
For transduction, remove 2.5 ml of the medium and mix it with 0.5 ml of viral supernatant. Add polybrene to a final concentration of 5 µg/ml and replace the remaining media with the virus mix. Incubate for 24 h at 32°C in 5% CO2 before replacing the medium with fresh GM. Split the cells upon reaching confluence and repeat the infection cycle if necessary.
Sort the cells based on their expression of EGFP protein by using the FACSAria automated sorter. Prepare cell suspensions containing 5 × 106 cells per 0.5 ml of GM + 5% serum.
3.1.2. Cell Culture Retinoid Isomerization Assay
Twenty hours prior to the experiment, plate NIH3T3 cells expressing LRAT and RPE65 in 6-well culture plates at a density of 1 × 106 cells per well.
Replace GM with 2 ml of fresh GM containing 10 µM of all-trans-retinol delivered in DMF. Protect cells from white light by covering the plates with aluminum foil.
After 16 h of incubation, collect medium and cells separately. Add 2 ml of 4MKOH in methanol, homogenize the sample in a glass–glass homogenizer, and incubate at 50°C for 2 h to hydrolyze retinyl esters.
Add 4 ml of hexane and extract retinoids by vigorous shaking for 5 min.
Separate the organic and aqueous phases by centrifugation (4000×g, 5 min); carefully collect the hexane phase and place it in a glass test tube (see Note 2).
Dry the hexane extract by using a SpeedVac or a stream of inert gas. Redissolve retinoids in 250 µl of hexane and place the mixture in an HPLC vial. The sample now is ready to be analyzed by HPLC under conditions described in Section 3.2.1.
3.2. Sample Preparation and Chromatography Conditions for Retinoid Separation
All procedures described in this and the next section should be carried out in a dark room under a dim red light safe lamp. All chromatographic separations of retinoids described were performed with an Agilent 1100 series binary liquid chromatograph gradient system equipped with a diode array detector and HP Chemstation software. This allowed identification of retinoid isomers by their specific retention times and absorption maxima relative to chemically synthesized standards.
3.2.1. Extraction, Detection, and Quantification of Retinyl Esters, Retinols, and Retinals
Normal-phase chromatography offers better resolution than reverse-phase chromatography for separating retinoid isomers. The former technique is suitable for determining the chromophore levels in eye extracts and quantifying in vitro 11-cis-retinol production in retinoid isomerization assays. UV absorption detection offers high analytical specificity because only a limited number of compounds extractable from tissues share a spectral absorbance range with retinoids. Retinoid analyses were developed for normal-phase chromatography by using Agilent-Si, 5 µm, 4.5 × 250 mm column. Because retinals with a reactive aldehyde group may couple with amine groups of lysine and phosphoethanolamine, hydroxylamine was used to convert retinals into their stable oxime derivatives (syn- and anti-). This strategy ensures efficient extraction and accurate quantification of these reactive compounds (see Notes 3 and 4).
Collect a whole mouse eye, put it in an Eppendorf tube, and immediately flash freeze it in liquid nitrogen. Frozen eye lenses are easier to homogenize.
Transfer the eye into a glass–glass homogenizer, add 0.5 ml of ice-cold extraction buffer, and homogenize immediately.
Incubate the sample for 20 min at room temperature with occasional shaking.
Add 2 ml of hexane and extract retinoids. Prepare sample for HPLC as described in Section 3.1.2, points 5 and 6.
Set wavelengths at which diode array detector will monitor retinoid elution. These usually are 325 and 360 nm for retinyl esters/retinols and retinals/retinal oximes, respectively.
Elute retinoids with an isocratic flow of 10% ethyl acetate in hexane at flow rate of 1.4 ml/min for 30 min to achieve baseline separation of 9-cis-, 11-cis-, 13-cis-, and all-trans-retinal oximes, as well as the corresponding isomers of retinol. Because of a significantly higher hydrophobicity of retinyl esters, resolution of their isomers and acyl chain variants can be achieved with a lower ethyl acetate concentration (0.5%) in hexane at the same flow rate (see Note 5). Detect retinal oximes at 360 nm.
Quantify 11-cis-retinal oximes and 11-cis-retinol based on standard curves that correlate integrated peak areas calculated from chromatograms with known amounts of synthetic standards injected onto the column.
3.2.2. Extraction and HPLC Separation of A2E
Mechanically homogenize a mouse eye in 1 ml of acetonitrile with a kinematic polytron homogenizer (PT 1200).
Extract the sample twice with 1 ml of acetonitrile. Centrifuge at 13,000×g for 2 min.
Collect and transfer the solvent to a glass test tube and dry it down in a SpeedVac.
Redissolve the sample in 150 µl of acetonitrile and 0.1% TFA and filter the mixture through a teflon syringe filter.
Load the sample (up to 100 µl) onto a C18 reverse-phase HPLC column, 4.5 × 250 mm and analyze with a linear gradient of acetonitrile in water (80–100%), 0.1% TFA for 30 min. Monitor elution of A2E at 435 nm (see Note 6).
Quantify A2E based on known concentrations of pure synthetic A2E standard.
3.2.3. Extraction and Chromatographic Conditions for Retinal Dimer
Homogenize a mouse eye as described in Section 3.2.1.
Extract retinal dimer with 3 ml of a hexane:ethyl acetate mixture (2:1).
Evaporate the solvent; redissolve dried extracts in 150 µl of 10% isopropanol in acetonitrile with 0.1% TFA.
Pass the solution through a teflon syringe filter prior to loading the sample on the HPLC.
Analyze the sample on a Phenomenex C18 reverse-phase column, 4.5 × 250 mm, with a gradient of isopropanol in acetonitrile (0–25%) in the presence of 0.1% formic acid for 20 min at a flow rate of 1 ml/min.
Monitor elution of retinal dimer at 430 nm and quantify it based on the standard curve derived from a synthesized standard.
3.3. LC-MS Analysis of 11-cis-Retinal and Lipofuscin Chromophores
Acquire mass spectra by using a LXQ high-throughput linear ion trap mass spectrometer interfaced with an atmospheric pressure chemical ionization (APCI) source and a series 1100 HPLC system consisting of a vacuum degasser, a binary pump, an autosampler with a cooled sample tray, a thermostatically controlled column compartment, and a diode array detector. The APCI source was chosen for development of LC-MS methodology because of its wide dynamic range and capacity to operate at the high flow rates required for HPLC retinoid separation (29).
3.3.1. The Chromophore
Tune the mass spectrometer to ensure optimal operating parameters by using flow injection of the retinoid standard in a mobile phase and a flow rate corresponding to that for its elution from the HPLC column. Positive-ion APCI is more sensitive for retinoid analyses and can be used for all applications. Optimal mass spectrometer parameters for chromophore detection include sheath, aux, and sweep gas flow set at 20, 5, and 0, respectively; capillary temperature and voltage of 275°C and 2 V; vaporized temperature 380°C; tube lens voltage 70 V; and corona discharge current 5 µA. The base peak at m/z = 300 corresponding to retinal oximes [M + H]+ is recorded by using the selected ion monitoring (SIM) mode.
Separate retinal oximes on an Agilent-Si, 5 µm, 4.5 × 250 mm normal-phase column equilibrated with 1% isopropanol in hexane at a fixed flow rate of 1.4 ml/min.
Prepare calibration curves by using external or internal standard methodology. To ensure the highest precision for the external standard quantification technique, spike eye extracts from Rpe65−/− mice (having undetectable levels of 11-cis-retinoids) with known amounts of 11-trans-retinal oximes and extract as described in Section 3.2.1. Construct a calibration curve by plotting the magnitude of the detector response as a function of the external standard concentration (see Note 7). The standard curve should evidence linearity over a 0.1–500 pmol range. Alternatively, to reduce errors generated by potential system instability or variations in source conditions, use commercially available all-trans-13,14-dihydro-retinal oximes or all-trans-3,4-dihydro-retinal oximes (m/z = 288 [M + H]+) that differ from natural retinal oximes only by saturation of a double bond, as internal standards. Add and mix a fixed amount of internal standard to each sample prior to extraction. After samples are prepared and analyzed, the quantity of the target compound can be determined from the calibration curve that displays the ratio of the target compound and the standard detector responses as a function of the amount of retinal oxime injected on the column.
Inject a sample and perform online HPLC separation of retinoids followed by APCI positive ionization mode mass spectrometric detection.
Confirm identification of the detected product by referring to the characteristic fragmentation pattern of its parent ion.
Determine the areas under peaks of interest. Quantify analytes in relation to internal standards by using one of the methods described above.
3.3.2. Identification of A2E in a Mouse Eye
Optimize conditions for A2E detection by using a synthetic standard and the mobile phase required for optimal A2E resolution on a C-18 column. Optimum APCI conditions include sheath, aux, and sweep gas flow fixed at 20, 5, and 0 units, respectively; capillary temperature and voltage of 275°C and 46 V; vaporized temperature 450°C; tube lens voltage 105 V; and corona discharge current 5 µA.
Inject 100 µl of an acetonitrile mouse eye extract onto a reverse-phase column as described in Section 3.2.2 and perform retinoid separation. For the LC-MS application, reduce concentration of TFA in the mobile phase to 0.05%.
Carry out analysis by using the SIM mode at m/z = 592.4 [M + H]+.
Confirm detection of A2E and its isomers by monitoring the unique fragmentation pattern of the parent ion.
3.3.3. Detection of Retinal Dimer by Mass Spectrometry
Tune the mass spectrometer by using the purified synthetic retinal dimer standard under chromatographic conditions required for its elution.
Separate retinal dimer extracted from a mouse eye according to conditions described in Section 3.2.3.
Use the SIM mode to detect the mass at m/z = 551.4 that corresponds to the retinal dimer parent ion [M+H]+ (see Note 8).
Confirm detection of retinal dimer by recording the MS2 fragmentation pattern of the parent ion.
Acknowledgment
This research was supported in part by grants EY009339 and P30 EY11373 from the National Institutes of Health and the Foundation Fighting Blindness.
Footnotes
All buffers for transfecting cells need to be sterilized by filtration (0.2 µm filters) and stored at −80°C. The pH of HEPES buffer should be adjusted carefully to 7.05 ± 0.05. This parameter is critical for transfection efficiency.
Collect organic (hexane) phase carefully. Even small traces of water in a sample prepared for normal-phase HPLC may destroy a silica column.
Retinoids are sensitive to light, heat, and oxygen. So they should be protected from light and stored in organic solvents rather than in dried form.
Hydroxylamine solution is unstable so it should always be freshly prepared. Adjust pH of 1 M stock solution to 7.4 by adding concentrated HCl before use.
Isopropanol used for retinoid separation in LC-MS experiments provides better sensitivity compared to ethyl acetate utilized under standard conditions. The disadvantage of this approach is that 1% isopropanol in hexane does not permit chromatographic separation of 11-cis- and 13-cis-retinol isomers.
We found that TFA is required for chromatography of A2E. Although TFA may reduce mass spectrometer signal intensity, it should not be replaced with formic acid.
Ionization of retinoids does not depend on the geometry of these molecules. Thus, more available all-trans isomers can be used as standards for mass spectrometry-based retinoid quantification (23).
Retinal dimer is extremely sensitive to oxidation. Protection from light and excess air is required to prevent formation of a variety of epoxy and furano retinal dimer derivatives.
References
- 1.Palczewski K. G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, Wilson JM, Aguirre GD, Stone EM, Palczewski K. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. USA. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson DA, Gal A. Vitamin A metabolism in the retinal pigment epithelium: Genes, mutations, and diseases. Prog. Retin. Eye Res. 2003;22:683–703. doi: 10.1016/s1350-9462(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 5.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: The interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog. Retin. Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 6.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 7.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc. Natl. Acad. Sci. USA. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bereta G, Kiser PD, Golczak M, Sun W, Heon E, Saperstein DA, Palczewski K. Impact of retinal disease-associated RPE65 mutations on retinoid isomerization. Biochemistry. 2008;47:9856–9865. doi: 10.1021/bi800905v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrault I, Rozet JM, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, Dufier JL, Munnich A, Kaplan J. Leber congenital amaurosis. Mol. Genet. Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: Powerful tools in functional genomics. Exp. Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 11.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 12.Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell. Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batten ML, Imanishi Y, Tu D, Doan T, Zhu L, Pang J, Glushakova L, Moise AR, Baehr W, Van Gelder RN, Hauswirth WW, Rieke F, Palczewski K. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driessen CA, Winkens HJ, Hoffmann K, Kuhlmann LD, Janssen BP, Van Vugt AH, Van Hooser JP, Wieringa BE, Deutman AF, Palczewski K, Ruether K, Janssen JJ. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cisretinols and cis-retinyl esters. Mol. Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 17.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, Huang J, Possin DE, Crabb JW. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron. 2001;29:739–748. doi: 10.1016/s0896-6273(01)00248-3. [DOI] [PubMed] [Google Scholar]
- 19.McCollum EV, Davis M. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 1913;15:167–175. [Google Scholar]
- 20.Vecchi J, Vesely J, Oesterhelt G. Applications of high-pressure liquid chromatography and gas chromatography to problems in vitamin A analysis. J. Chromatogr. 1973;83:447–453. doi: 10.1016/s0021-9673(00)97061-4. [DOI] [PubMed] [Google Scholar]
- 21.Rotmans JP, Kropf A. The analysis of retinal isomers by high speed liquid chromatography. Vision Res. 1975;15:1301–1302. doi: 10.1016/0042-6989(75)90181-9. [DOI] [PubMed] [Google Scholar]
- 22.Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal. Biochem. 2008;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Hooser JP, Liang Y, Maeda T, Kuksa V, Jang GF, He YG, Rieke F, Fong HK, Detwiler PB, Palczewski K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Hooser JP, Garwin GG, Saari JC. Analysis of visual cycle in normal and transgenic mice. Methods Enzymol. 2000;316:565–575. doi: 10.1016/s0076-6879(00)16750-3. [DOI] [PubMed] [Google Scholar]
- 26.Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat. Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 27.Fishkin NE, Sparrow JR, Allikmets R, Nakanishi K. Isolation and characterization of a retinal pigment epithelial cell fluorophore: An all-trans-retinal dimer conjugate. Proc. Natl. Acad. Sci. USA. 2005;102:7091–7096. doi: 10.1073/pnas.0501266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Breemen RB, Nikolic D, Xu X, Xiong Y, van Lieshout M, West CE, Schilling AB. Development of a method for quantitation of retinol and retinyl palmitate in human serum using high-performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J. Chromatogr. A. 1998;794:245–251. doi: 10.1016/s0021-9673(97)01138-2. [DOI] [PubMed] [Google Scholar]