Abstract
Due to the legalization of marijuana and the increased demand for cannabis and alcohol consumption, research efforts highlighting the biomedical consequences of the use of alcohol and cannabinoids are not only relevant to the substance abuse scientific field, but are also of public health interest. Moreover, an overview of the recent literature about alcohol and cannabinoids neuro-immunomodulatory effects highlighting their future therapeutic potentials will provide a significant contribution to science and medicine. Therefore, in the current review, we will first discuss briefly the prevalence of alcohol and marijuana abuse, followed by a discussion on the individual effects of alcohol and cannabinoids on the immune system; then, we will focus on the role of endocannabinoids on the alcohol-induced inflammatory effects. In addition, the review also incorporates cytokine array data obtained from human monocyte-derived dendritic cells, providing a different perspective on the alcohol and cannabinoid abuse divergent effects on cytokine production. The final section will highlight the therapeutic potential of cannabinoid receptors and the novel strategies to treat alcohol dependence as determined by in vitro, in vivo and clinical studies.
Keywords: Alcohol, Cannabinoids, Inflammation, Immune Modulation, Cytokines
Introduction
Statistical reports from the annual National Survey on Drug Use and Health (NSDUH) show that after alcohol, marijuana has the highest rate of abuse among all drugs [1]. Due to the recent public attention of the legalization of marijuana in the United States and the constant fight against alcohol abuse, research efforts highlighting the biomedical consequences of the use of these substances are relevant to the drug abuse field and of public health interest. One of the major components of health that affect the entire body and health outcome is the immune system; therefore, in this review, we will discuss the prevalence of alcohol and marijuana abuse, followed by the individual effects of alcohol and cannabinoids on the immune system; then, we will focus on the role of endocannabinoids on the alcohol-induced inflammatory effects. The ultimate objective of this review is a discussion of the combined effects of alcohol exposure and cannabinoid receptors on immune inflammation leading to a further discussion of novel therapeutic strategies to treat alcohol dependence using cannabinoid receptor targeting.
Alcohol abuse
In 2012, 3.3 million global deaths were attributed to alcohol consumption [2]. In the United States alone, 87.6 percent of people ages 18 or older reported that they drank alcohol during their lifetime, and among those who reported drinking, 24.6 percent were binge drinkers and 7.1 percent were engaged in heavy drinking [3]. Alcohol has been reported as the third leading preventable cause of death in the US [4] and has become a huge economic burden; for instance in 2006, alcohol misuse cost Americans over $ 200 billion [5]. Globally, alcohol misuse is the first leading risk factor for premature death and disability among people between the ages of 15 and 49 [6].
Even though alcohol abuse statistics are impressive in terms of death and cost, the widespread alcohol consumption is still prevalent and the above mentioned statistics are often disregarded. Moreover, the public health often has the assumption that alcohol use in general is common and not harmful; therefore, alcohol users seem to be consistently affected by the preventable harmful effects of alcohol. For instance, besides alcohol-induced liver cirrhosis [7] and other alcohol-related liver diseases [8], alcohol has been identified as a risk factor for cancer of the mouth, esophagus, pharynx, larynx, liver, and breast [9].
Although most of the statistics related to drinking alcohol indicate a harmful association with health and an increased risk of fatalities, it is relevant to point out that there are reports claiming beneficial effects of drinking alcohol. For instance, according to the dietary guidelines for Americans, moderate alcohol consumption is up to 1 drink per day for women and up to 2 drinks per day for men [10]. Some of the beneficial effects attributed to moderate alcohol consumption include decreased risk for heart disease, ischemic stroke, and diabetes [11,12]. Based on the statistics provided above and the harmful and beneficial aspects of alcohol drinking; broadening our understanding of the relationship between alcohol consumption and health is critical.
Since not all the effects of alcohol are damaging according to several positive associations of health and alcohol drinking, it is relevant to point out that compare with abstinence and heavy drinking, moderate alcohol consumption has been associated with higher intakes and blood concentrations of some micronutrients including antioxidants [13]. Furthermore, evidence has been provided that certain alcoholic drinks such as red wine possesses anti-oxidant activities due to its content of polyphenols [14]. Most recently, alcohol has also been associated with better memory as results suggest that light and moderate alcohol consumption in older people is associated with higher episodic memory and larger hippocampal volume [15].
Due to the past and current medical uses of prototypical substances of abuse, including alcohol and marijuana, these substances are gaining interest from the scientific community as recently reviewed not only for their medicinal benefits, but for their detrimental effects under Neuro-inflammatory conditions such as Neuro-HIV [16].
Marijuana abuse
As of 2013, cannabis was the most common illicit substance used by people admitted to treatment facilities [17]. In 2014, Marijuana was reported by NIDA as the most common illicit drug used in the United States [18]. As of march 2014, 20 states in the U.S. [19] had legalized the medicinal use of marijuana. Overall, cannabis has been considered the world's most widely used illicit substance; and in 2010, as much as 5 % of the world's population had abused marijuana [20]. Although in the U.S., marijuana is considered a Schedule I controlled substance, cannabis research and the medical use of cannabis have shown extreme promise for the treatment of numerous medical problems including pain, insomnia and anxiety [21]. For instance, CB2 agonists and fatty acid amide hydrolase inhibitors are among several molecular compounds in clinical trials for the treatment of neuropathic pain [22]. In addition, there are several patents claiming CB2 modulators as a new class of analgesics and supporting CB2 agonists’ promising role in the management of pain [23]. Other findings have indicated that activation of both, CB1 and CB2 receptors, has beneficial effects in Alzheimer experimental models by reducing harmful β-amyloid peptide accumulation and tau phosphorylation, as well as by promoting the brain's intrinsic repair mechanisms [24].
On the other side, studies have demonstrated that exocannabinoids, such as THC and synthetic cannabinoids, including “Spice”, interfere with the protective function of the Endocannabinoid System (ECS) present in the brain and required for neurogenesis. For instance, during cannabinoid abuse, significant impairments in neurocognitive and behavioral functioning are evident [25-27] and these effects are exacerbated in subjects with a compromised immune system as in the case of those with symptomatic HIV infection [28].
In summary, despite the increase evidence of the beneficial effects of marijuana and cannabinoid signaling in general, according to the National Institute on Drug Abuse (NIDA), cannabis has been associated with bi-directional effects on the neuronal, cardiovascular, endocrine, respiratory, and immune systems; therefore, there is a growing demand for cannabinoid research in order to elucidate and differentiate the positive and negative effects of marijuana use [29].
Alcohol and the immune system
Previous studies have demonstrated that alcohol abuse has immunosuppressive effects on the body and although alcohol has been shown to directly affect lymphocyte functions, a decreased in the function of antigen presenting cells seems to play a crucial role in ethanol-induced effects on cell-mediated immunity [30]. Furthermore, alcohol exposure has been shown to affect several aspects of the immune system including, but not limited to, monocyte/macrophage function [31], dendritic cell function [32-35], T and B cells function and cytokine/chemokine production [30-36].
Dendritic cells are crucial antigen presenting cells that link the cell-mediated innate immune response with the adaptive immune response [37,38]. Alcohol has been shown to impair the antigen presenting capacity of dendritic cells derive from Peripheral Blood Mononuclear Cells (PBMC) and may involve decreased IL-12, CD80, and CD86, and reduced dendritic cell differentiation [32]. In addition, both chronic and acute alcohol consumption have been shown to affect dendritic cell number and functions in rhesus macaques [39] and humans [35], interfering with their differentiation and antigen presentation [34].
Besides the cellular effects of alcohol, other humoral components of the immune system are also affected by alcohol consumption. For instance, acute alcohol has been shown to reduce pro-inflammatory cytokines such as TNF-α and IL-1β in rat macrophages [40] and in human blood monocytes [41]. In the context of other inflammatory conditions, alcohol may affect immune function including antigen presentation and disease progression [42].
A recent study analyzing gene expression profiles of rat cerebellum under acute alcohol intoxication revealed increases in the expression of genes involved in diverse cellular activities including immunological functions such as antigen processing, antigen presentation, immune response, and MHC protein complex among others [43]. Other animal studies with wild type, toll-like receptor 2 (TLR2)knockout, and toll-like receptor 4 (TLR4) knockout mice treated chronically with alcohol for 5 months demonstrated that ethanol activates the innate immune system by stimulating TLR4 signaling in glial cells, triggering the up-regulation of cytokines (IL-1β, IL-17, TNF-α) and chemokines (MCP-1, MIP-1α, CX3CL1) in the striatum and serum [44].
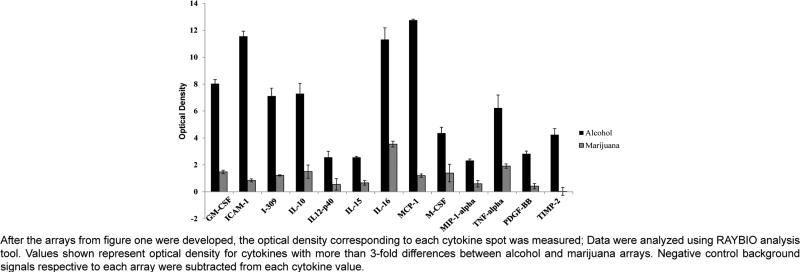
Overall, the effects of alcohol on cytokine and chemokine production are highly dependent on the duration and amount of alcohol use [36]. For instance, acute alcohol exposure, generally suppresses cytokine and chemokine production [45] while chronic alcohol use is frequently associated with activation of pro-inflammatory cytokines, particularly TNF- alpha [46]. Moreover, acute and chronic alcohol differentially modulate monocyte/macrophage activation and cytokine production [47]. Our recent publication using human Monocyte-Derived Dendritic Cells (MDDC) supports an increase of pro-inflammatory cytokine production after 0.1 % ethanol treatment in vitro [33] compare to untreated cells. Moreover, protein array profiles (Figure 2) of MDDC derived from alcohol user revealed a significant increase in the production of several pro-inflammatory cytokines and chemokines (MCP-1, ICAM-1, IL-16, GM-CSF, IL-10, IL-309, TNF-α, TIMP-2, MCSF, PDGF-β, MIP-1α, IL-12-p40, IL-15).
Figure 2.
Alcohol and Marijuana differentially modulate inflammatory cytokines
Other studies performed in adolescent rats have reported that alcohol-mediated immune-modulatory effects are dependent on the ethanol by volume concentration and exposure to alcoholic drinks containing high ethanol percentages can have more profound effects on immune responses than exposure to alcoholic drinks containing low percentages of ethanol [48]. In the context of other inflammatory conditions such as HIV, alcohol has been reported to cause concentration-dependent alterations in gene expression during acute binge drinking in the HIV-1 transgenic rat [49].
Additionally, reports focusing on the effects of chronic and acute alcohol consumption on the innate and adaptive immune responses have been extensively reviewed [30-36]. Most recently, during the 2013 Alcohol and Immunology Research Interest Group (AIRIG) meeting., emphasis has been given on the adverse effects of alcohol-induced inflammatory responses under diverse diseases and injury conditions, including discussions on the adverse effects of alcohol on liver inflammation, systemic effects, and alcohol's role in infection and immunology [50].
Cannabinoids and the immune system
The notion that cannabinoids have an effect on the immune system dates back to 1993, when the molecular characterization of a peripheral receptor for cannabinoids was identified in macrophages found in the marginal zone of the spleen [51], and human cannabinoid receptors and their gene transcripts were also identified in blood samples from normal human volunteers who reported no prior use of marijuana [52]. Since then, several reports with endocannabinoids, natural cannabinoids, and synthetic cannabinoids have demonstrated a major role of these compounds on inflammation and immunomodulation. For instance, the plant Cannabis sativa has been used for centuries in Asian medicine to reduce pain, inflammation, and asthma [53]. Over the years, the increase in popularity and recreational use of marijuana has caused a profound effect of marijuana smoke on immune defense mechanisms against bacterial and viral infections [54]. To date, there are reports of both anti and pro-inflammatory effects of cannabinoids. For instance, the main psychoactive cannabinoid, Δ-9- tetrahydrocannabinol (THC), and the main nonpsychoactive cannabinoid, cannabidiol , have been shown to decrease the Th17 inflammatory autoimmune phenotype [55]. The endocannabinoid, anandamide, has been shown to inhibit lymphocyte proliferation [56] and macrophage-mediated killing of tumor necrosis factor-sensitive cells [57]. The synthetic cannabinoid, CP55,940, has also been found to play a role in B cell activation and maturation [58]. And most recently, THC treatment of primary human monocytes during differentiation has been shown to reduce HIV-1 infection of subsequent macrophages [59].
Moreover, activation of CB2 has been shown to have both immune protective and neuro protective effects, for instance, CB2 agonists have been shown to attenuate leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions [60]. Overall, cannabinoid receptors and endogenous cannabinoids have been implicated in the regulation of the immune response and this topic has been widely reviewed [54-61].
Following the above review of the literature on alcohol, marijuana, and the immune system, we would like to give an overview of our own findings using inflammatory cytokine array profiles performed with MDDC whole cell lysates from blood donors who abuse alcohol or marijuana. Overall, there were higher levels of inflammatory cytokines produced by cells derived from the alcohol abusing patient while marijuana abuse caused lower activation of inflammatory cytokine production (Figure 1). A summary of the cytokines that were highly expressed on the alcohol abuse array compared to the marijuana abuse are depicted on (Figure 2) and include MCP-1, ICAM-1, IL-16, GMCSF, IL-10, IL-309, TNF-α, TIMP-2, MCSF, PDGF-β, MIP-1α, IL-12-p40, IL-15.
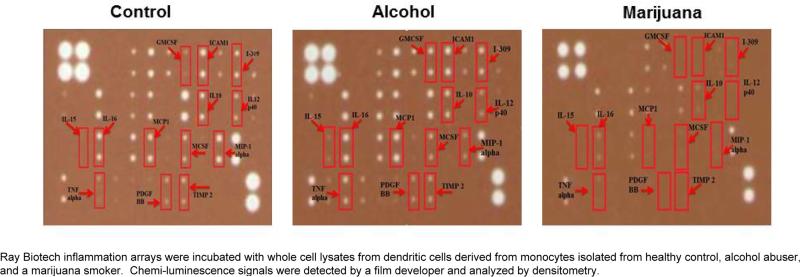
Figure 1.
Inflammatory profiles of non-substance, alcohol, and marijuana users
Materials and Methods
Participants
All procedures performed in the studies involving human participants were in accordance with Helsinki declaration [62] and approved by FIU's Institutional Review Board (IRB). Blood donors were recruited from the Borinquen Health Care Center, Inc., Miami. Consents were obtained consistent with Florida International University (FIU) and the National Institutes of Health (NIH) policies. Exclusion criteria were poly drug use, Hepatitis, HIV, other medical conditions, age < 18 and > 50 years, and pregnancy.
Cytokine array
Whole cell lysates (WCL) were extracted from dendritic cells derived from monocytes obtained from blood donors as previously described by us [33]. Ray Biotech inflammation arrays (catalog # AAHINF-3-8, Ray Biotech, Norcross, GA) were incubated with (WCL) from healthy control, alcohol abuser, and marijuana smoker. Chemi luminescence signals were detected by a film developer and analyzed by densitometry using Image J software. Data were analyzed using the RAYBIO Analysis Tool.
The endocannabinoid system
The discovery of cannabinoid receptor 1 (CB1) in the Central Nervous System (CNS) [63,64] the molecular characterization of a peripheral cannabinoid receptor 2 (CB2) [51-65], and the discovery of novel compounds that act as ligands of the brain receptors led to the assumption that other natural endogenous THC-like molecules called the endocannabinoids, were present in the CNS [66,67]. endocannabinoids such as anandamide, which was discovered by Mechoulam and colleagues [68] are recognized as endogenous ligands that act upon their cannabinoid receptors [69]. To date, two endogenous cannabinoids, anandamide and 2-arachiodonyl-glycerol (2-AG), play an important role in the modulation of physiological processes in the CNS [70]. The discovery of the above mentioned endocannabinoids and their receptors led to a new field of studies implicating them in many CNS dysfunctions including, but not limited to obesity [71], pain [72], osteoporosis [73], and addiction [74].
Recent studies suggest that cannabinoids, including endocannabinoids, which activate CB1 can increase neurotransmitter release by enhancing Ca(2+) influx in vitro; which demonstrates a crucial role of endocannabinoid -induced potentiation of neurotransmission [75]. Moreover, enhancement of endocannabinoid signaling can serve as a neuro-protective therapeutic modality by activating signaling pathways downstream from cannabinoid receptors, eventually promoting neuronal maintenance and function, and also subsequently supporting the endocannabinoid system as a target for novel therapeutic drugs [76,77].
Cannabinoid receptors, endocannabinoids, and alcoholism
Cannabinoids and endocannabinoids have been implicated with the rewarding effects of addictive substances, including, but not limited to nicotine, opiates, alcohol, and cocaine; therefore, they are part of natural regulatory mechanisms for drug reward and promising targets for the treatment of addictive disorders [78]. Various studies as previously reviewed and presented at the 2001 Research Society on Alcoholism symposium have revealed how endocannabinoids and the enzymes responsible for their synthesis and degradation have played an important role in complex physiological functions involving drug abuse and alcoholism [79,80].
The endocannabinoids system plays a crucial role in the dependence and withdrawal of substances such as alcohol, and changes in the levels of endogenous cannabinoids such as anandamide and 2-arachiodonyl-glycerol (2-AG) have been observed in regions of the brain responsible for reinforcement after long-term exposure to alcohol [81]. To date, scientists are still aiming to discover new ways in which the physiological processes of these endocannabinoids, natural cannabinoids, synthetic cannabinoids, and their receptors can be modified in order to achieve specific responses geared toward discovering their role in the motivation to consume alcohol [74]. A substantial amount of data suggesting a correlation between CB1 receptor agonist and antagonists and the motivation to consume alcohol have been reported in studies with rodent models. For instance, a down-regulation of CB1 receptor function and signal transduction caused by chronic ethanol administration have been reported, which are thought to result from the persistent stimulation of the receptors by the endogenous CB1 agonists, AEA and 2-AG, and these have been found to be also induced by chronic ethanol treatment [79]. There is also evidence of regulation of anandamide levels in the brain by acute administration of ethanol [82]. Further, electrophysiological evidence from in vivo animal studies, has demonstrated the involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit [83]. Other studies based on the rat Chronic Intermittent Ethanol (CIE) model for alcohol withdrawal and dependence have suggested a bidirectional effect of alcohol on CB1 by transiently down-regulating hippocampal CB1 followed by a long-term up-regulation, which may contribute to the long-term cognitive impairments observed during alcohol use disorders [84]. Along with other studies, this report also demonstrated a role of alcohol on the induction of endogenous cannabinoids [79,84,85].
Overall, these reports supported CB1 receptor blockage as crucial for decreasing alcohol consumption and supported the role of the endocannabinoid system in alcohol induced tolerance and dependence, suggesting that drugs targeted against the endo-cannabinoid system could be therapeutically useful in treating alcohol use disorders [86]. A more in depth review of the interactions between alcohol and the endocannabinoid system highlighting the implications for alcohol dependence and the comparative effects of alcohol and cannabis was recently published and provides additional relevant information [87].
Therapeutic potential of cannabinoid receptors and novel strategies to treat alcohol dependence
Animal models with CB1 receptor deletions have become prevalent in studies aiming to elucidate the effects of alcohol and endocannabinoid interactions in the CNS. For instance, in a study conducted involving CB1 receptor deficient mice exposed to chronic ethanol administration, the CB1 receptor deficient mice had a much higher inclination toward alcohol consumption than the wild-type controls, demonstrating a crucial role of CB1 in ethanol dependence and preference [88]. Furthermore, the CB1 antagonist, rimonabant (SR 141716), has been extensively studied and reviewed as a promising pharmacotherapy for alcohol dependence [89]. Besides the substantial evidence highlighted above using animal models, human studies have been reported on the use of rimonabant for the treatment of alcohol dependence. For instance, a 12-week double-blind, placebo-controlled clinical trial to assess the efficacy of rimonabant in the prevention of relapse to alcohol in recently detoxified alcohol-dependent patients was performed in 2008 [90].
Despite the evidence of CB1 antagonists to reduce alcohol dependence in animal models and human clinical trials, rimonabant was shown in a meta-analysis of four anti-obesity studies to increase the risk of psychiatric adverse events including depressed mood disorders and anxiety [91]. These findings of increased risk of suicide during treatment with rimonabant were later confirmed by the US Food and Drug Administration; therefore, leading to the recommendation to increase alertness to these severe psychiatric adverse reactions [92]. Besides the above mentioned side effects, other less conclusive reports show that rimonabant has no effect on alcohol consumption in heavy alcohol drinkers [93].
Overall, due to the detrimental CNS-related side effects of rimonabant, efforts are being shifted to the exploration of the blockade of CB1 receptors in peripheral tissues as in the case of obesity mouse models [94]. More recent approaches are considering the study of novel CB1 antagonists such as PF 514273 and their role on ethanol preference [95]. And lastly, studies targeting other receptors such as CB2 have gained popularity. For instance, the novel CB2 receptor agonist, HU- 910, has been found to exert a protective effect in various diseases associated with inflammation and tissue injury [96] and other reviews of the literature had focused on recent advances in studies involving CB2 activation in the setting of neuroinflammation, immunomodulation, and HIV infection [97]. Most recent efforts have been made for the design, synthesis, and evaluation of new amino alkylindole derivative compounds with dual CB1R antagonist/CB2R agonist activity with potential for the treatment of alcohol abuse [98].
Summary
In summary, while in recent years the scientific community has shifted gears into the support of CB2 receptor activation to mediate immunosuppressive effects by limiting inflammation and tissue injury in a vast majority of pathological conditions, there is still the paradigm claiming activation of CB2 capable of enhancing or even triggering tissue damage [99]. Therefore, other novel strategies that target the endocannabinoid system may prove valuable to treat not only substance abuse, but a wide range of neuroinflammatory conditions. For instance, Fatty Acid Amide Hydrolase (FAAH), the enzyme involved in the inactivation of the endocannabinoid anandamide is being considered as a therapeutic target for neuroprotection by Zhuang and colleagues. They are testing selective FAAH inhibitors and their binding with an anandamide carrier protein for their effective delivery to specific target sites in the brain [100]. In the context of other inflammatory conditions such as HIV, impaired neurogenesis in mice by HIV-1-Gp120 has been shown to be rescued by genetic deletion of FAAH [101].
Worldwide, chronic consumption of alcohol is one of the leading causes of severe injury and mortality, resulting in 3.3 million global deaths [2]; while, cannabis is one of the most common illicit substances used by people admitted to treatment facilities [17] and has been reported by NIDA as the most common illicit drug used in the United States [18]. Although the detrimental effects of addictive substances, including alcohol and marijuana are well known, it is relevant to highlight that they also have been reported to provide beneficial effects and are being exploited for medicinal use as highlighted above and as recently reviewed by Chang et al. [16].
In the context of alcohol abuse, alcohol and marijuana may have differential neuroimmune properties and modulatory effects; therefore, leading to the proposal of a potential role of cannabinoid receptors and endocannabinoids on the emancipation of alcohol-adverse effects and the possible treatment of alcohol use disorders. As our review of the literature points out, the endocannabinoid system is closely associated with the physiological responses involved in alcohol dependence and alcohol use disorders. Animal studies have suggested a bidirectional effect of alcohol on CB1, which may contribute to the long-term cognitive impairments observed during alcohol use disorders [84].
Along with other studies, this report also demonstrated a role of alcohol on the induction of endogenous cannabinoids [79,84,85]. Overall, the reviewed reports led to a suggestive role of cannabinoid receptor targeting as an effective tool for decreasing alcohol consumption and a role for the endocannabinoid system in alcohol- induced tolerance and dependence; therefore, therapeutic drugs targeted against the endocannabinoid system could be promising for the treatment alcohol use disorders [86].
We also discussed the role of animal models with CB1 receptor deletions as crucial in elucidating the effects of alcohol and endocannabinoid interactions in the CNS as shown by a much higher inclination toward alcohol consumption- ethanol dependence, and stress-induced increase of ethanol preference in the CB1 knockout mice [88]. In addition, we reviewed literature that highlights the cannabinoid CB1 receptor antagonist, rimonabant, (SR 141716), as a promising pharmacotherapy for alcohol dependence [89] as well as clinical data that showed rimonabant caused adverse unwanted psychiatric effects [91,92] or no effects on alcohol consumption [93]. Lastly, we presented studies targeting CB2 receptors and using novel CB2 agonists, which have been shown to exert a protective effect in various diseases associated with inflammation and tissue injury [96,97]. Other novel non-receptor-related strategies targeting the endocannabinoid system were also discussed to treat not only substance abuse, but a wide range of neuroinflammatory conditions [100,101].
Conclusion
Interestingly, our previous findings suggest that CB2 and GPR55 are highly up regulated in alcohol abusers and these receptors also play a crucial immune-regulatory role during alcohol treatment in vitro [33]. Furthermore, our own study highlighted in this review confirms that cannabinoids and alcohol exert differential and opposite inflammatory effects as shown by the cytokine array profiles performed with monocyte-derived dendritic cells from alcohol, marijuana, and control donors (Figures 1 and 2).
Based on our findings and on the current review of the past and current studies on alcohol and cannabinoid-induced neuroinflammation, it is clear that cannabinoids have been shown to have both beneficial and detrimental effects on different organ systems and the interactions of certain endocannabinoids and cannabinoid-based drugs have been shown to affect the motivation to consume alcohol; however, further studies must be conducted and substantial evidence must be acquired in order to use these novel endocannabinoid and exocannabinoid -based therapeutic approaches in the clinic for the treatment of alcohol use disorders.
Acknowledgements
NIAAA awards number K99AA021264 and R00AA021264, NIDA award number R01DA034547, Institute of Neuro-Immune Pharmacology, FIU.
Role of Funding Source
Research reported in this publication was partially supported by the National Institute on Alcohol Abuse and Alcoholism under award number K99AA021264 and R00AA021264, the National Institute on Drug Abuse under award number R01DA034547. All the authors who contributed to the design and research work of this study are affiliated with the Department of Immunology at the Herbert Wertheim College of Medicine and the Institute of Neuro-Immune Pharmacology at Florida International University.
Abbreviations
- ECS
endocannabinoid system
- CB1
Cannabinoid Receptor 1
- CB2
Cannabinoid Receptor 2
- THC
Δ9-tetrahydrocannabinol
- CNS
Central Nervous System
- EtOH
Alcohol
- MDDC
Monocyte-Derived Dendritic Cells
Footnotes
Citation: Madhavan PN, Gloria F, Gianna C, Karla M and Marisela Agudelo (2015) Alcohol Versus Cannabinoids: A Review of Their Opposite Neuro-Immunomodulatory Effects and Future Therapeutic Potentials. J Alcohol Drug Depend 3: 184. doi: 10.4172/2329-6488.1000184
References
- 1.Substance Abuse and Mental Health Services Administration, R MD . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46. Substance Abuse and Mental Health Services Administration; 2013. HHS (Publication No. (SMA) 13-4795) [Google Scholar]
- 2.World Health Organization W Global status report on alcohol and health. 2014 [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration National Survey on Drug Use and Health (NSDUH) 2012 [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention C Alcohol use and your health, in Fact Sheets- Alcohol Use and Your Health. 2014 [Google Scholar]
- 5.Centers for Disease Control and Prevention C Excessive drinking costs U.S. $223.5 Billion, in CDC Features N.C.f.C.D.P.a.H. Promotion. 2014 [Google Scholar]
- 6.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon YH, Yi HY. Liver Cirrhosis Mortality in the United States in Surveillance Report 93. 2012. National Institute on Alcohol Abuse and Alcoholism. 2009 [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AK, Guturu P, Hmoud B, Kuo YF, Salameh H, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. doi: 10.1097/TP.0b013e31827afb3a. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute, N. Cancer Trends Progress Report: 2011-2012 Update. 2012 [Google Scholar]
- 10.Services U.S.D.o.A.a.U.S.D.o.H.a.H, Dietary Guidelines for Americans. 2010 Available at: 31. [Google Scholar]
- 11.Agriculture, U.S.D.o Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. 2010:355–359. [Google Scholar]
- 12.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walmsley C, et al. Relationship between alcohol and nutrient intakes and blood status indices of older people living in the UK: further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/5. Public Health Nutrition. 1998;1:157–167. doi: 10.1079/phn19980025. [DOI] [PubMed] [Google Scholar]
- 14.Magrone T, Tafaro A, Jirillo F, Panaro MA, Cuzzuol P, et al. Red wine consumption and prevention of atherosclerosis: an in vitro model using human peripheral blood mononuclear cells. Curr Pharm Des. 2007;13:3718–3725. doi: 10.2174/138161207783018581. [DOI] [PubMed] [Google Scholar]
- 15.Downer B, et al. Effects of Alcohol Consumption on Cognition and Regional Brain Volumes among Older Adults. American Journal of Alzheimer’s disease and Other Dementias. 2014 doi: 10.1177/1533317514549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang SL, et al. Chapter Thirteen – Neuro-HIV and Use of Addictive Substances, in International Review of Neurobiology. 2014:403–440. doi: 10.1016/B978-0-12-801284-0.00013-0. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AJ, Conley JW, Gordon JM. Medical consequences of marijuana use: a review of current literature. Curr Psychiatry Rep. 2013;15:419. doi: 10.1007/s11920-013-0419-7. [DOI] [PubMed] [Google Scholar]
- 18.National Institute on Drug Abuse, N. [December 30th, 2014];Drug Facts: Marijuana, N.I.o.D. Abuse. 2014 [Google Scholar]
- 19.National Institute on Drug Abuse, N. [November 14, 2014];Is Marijuana Medicine? 2014 [Google Scholar]
- 20.Crime, U.N.O.o.D.a . World drug report. United Nations; New York: 2012. [Google Scholar]
- 21.Webb CW, Webb SM1. Therapeutic benefits of cannabis: a patient survey. Hawaii J Med Public Health. 2014;73:109–111. [PMC free article] [PubMed] [Google Scholar]
- 22.Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs. 2014;19:329–341. doi: 10.1517/14728214.2014.915025. [DOI] [PubMed] [Google Scholar]
- 23.Murineddu G, Asproni B. A survey of recent patents on CB2 agonists in the management of pain. Recent Pat CNS Drug Discov. 2012;7:4–24. doi: 10.2174/157488912798842214. [DOI] [PubMed] [Google Scholar]
- 24.Aso E, Ferrer I. Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front Pharmacol. 2014;5:37. doi: 10.3389/fphar.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crane N, et al. Effects of Cannabis on Neurocognitive Functioning: Recent Advances, Neurodevelopmental Influences, and Sex Differences. Neuropsychology Review. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, et al. Effects of chronic, heavy cannabis use on executive functions. J Addict Med. 2011;5:9–15. doi: 10.1097/ADM.0b013e31820cdd57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004;16:330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- 29.Khalsa J. Medical and Health Consequences of Marijuana, in Marijuana and the Cannabinoids, in WHO Drug Report. D.M.E. Sohly. Inc; New Jersey: 2012. pp. 237–252. [Google Scholar]
- 30.Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- 31.Jin M, Arya P, Patel K, Singh B, Silverstein PS, et al. Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin Exp Res. 2011;35:132–139. doi: 10.1111/j.1530-0277.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 33.Agudelo M, Yndart A, Morrison M, Figueroa G, Muñoz K, et al. Differential expression and functional role of cannabinoid genes in alcohol users. Drug Alcohol Depend. 2013;133:789–793. doi: 10.1016/j.drugalcdep.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- 35.Laso FJ, et al. Chronic Alcohol Consumption Is Associated with Changes in the Distribution, Immuno phenotype, and the Inflammatory Cytokine Secretion Profile of Circulating Dendritic Cells. Alcoholism: Clinical and Experimental Research. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 36.Molina PE, Happel KI, Zhang P, Kolls JK, Nelson S. Focus on: Alcohol and the immune system. Alcohol Res Health. 2010;33:97–108. [PMC free article] [PubMed] [Google Scholar]
- 37.Agudelo M, et al. Chapter 10: Role of Dendritic Cells in HIV Infection: DC-SIGN and Novel Therapeutic Approaches. Nova Publishers; 2010. Dendritic Cells: Types, Life Cycles and Biological Functions. pp. 167–177. [Google Scholar]
- 38.Markowicz S, Engleman EG. Granulocyte-macrophage colony-stimulating factor promotes differentiation and survival of human peripheral blood dendritic cells in vitro. The Journal of Clinical Investigation. 1990;85:955–961. doi: 10.1172/JCI114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, et al. Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques. Alcohol Clin Exp Res. 2009;33:1524–1531. doi: 10.1111/j.1530-0277.2009.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- 41.Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2010;55:159–166. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- 42.Haorah J, Heilman D, Diekmann C, Osna N, Donohue TM, Jr, et al. Alcohol and HIV decrease proteasome and immunoproteasome function in macrophages: implications for impaired immune function during disease. Cell Immunol. 2004;229:139–148. doi: 10.1016/j.cellimm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Wei G2, Wang Y3, Jing L3, Zhao Q4. Gene expression profile analysis of rat cerebellum under acute alcohol intoxication. Gene. 2015;557:188–194. doi: 10.1016/j.gene.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Pascual M, Baliño P, Aragón CM, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Pruett SB, et al. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Nagy LE. Stabilization of tumor necrosis factor-alpha mRNA in macrophages in response to chronic ethanol exposure. Alcohol. 2004;33:229–233. doi: 10.1016/j.alcohol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Mao X, Chang SL. Altered gene expression in the spleen of adolescent rats following high ethanol concentration binge drinking. Int J Clin Exp Med. 2011;4:252–257. [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S, Chang SL. Ethanol Concentration-Dependent Alterations in Gene Expression During Acute Binge Drinking in the HIV-1 Transgenic Rat. Alcoholism: Clinical and Experimental Research. 2013;7:1082–1090. doi: 10.1111/acer.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris NL, Ippolito JA, Curtis BJ, Chen MM, Friedman SL, et al. Alcohol and inflammatory responses: Summary of the 2013 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 52.Onaivi ES, Chaudhuri G, Abaci AS, Parker M, Manier DH, et al. Expression of cannabinoid receptors and their gene transcripts in human blood cells. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1063–1077. doi: 10.1016/s0278-5846(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 53.Mechoulam R. Cannabinoids as Therapeutic Agents. CRS Press; 1986. pp. 1–19. [PubMed] [Google Scholar]
- 54.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 55.Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, et al. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol. 2013;8:1265–1276. doi: 10.1007/s11481-013-9493-1. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz H, Blanco FJ. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;1:107–15. doi: 10.1016/0165-5728(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 57.Cabral GA, Toney DM, Fischer-Stenger K, Harrison MP, Marciano-Cabral F. Anandamide inhibits macrophage-mediated killing of tumor necrosis factor-sensitive cells. Life Sci. 1995;56:2065–2072. doi: 10.1016/0024-3205(95)00190-h. [DOI] [PubMed] [Google Scholar]
- 58.Agudelo M, et al. Cannabinoid Receptor 2 (CB2) Mediates Immunoglobulin Class Switching from IgM to IgE in Cultures of Murine-Purified B Lymphocytes. Journal of Neuroimmune Pharmacology. 2008;3:35–42. doi: 10.1007/s11481-007-9088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams J, et al. 9-Tetrahydrocannabinol Treatment during Human Monocyte Differentiation Reduces Macrophage Susceptibility to HIV-1 Infection. Journal of Neuro immune Pharmacology. 2014;9:369–379. doi: 10.1007/s11481-014-9527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez SH, et al. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte–Endothelial Cell Interactions and Blood–Brain Barrier Dysfunction under Inflammatory Conditions. The Journal of Neuroscience. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malfitano AM, Basu S, Maresz K, Bifulco M, Dittel BN. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin Immunol. 2014;26:369–379. doi: 10.1016/j.smim.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rickham PP. Human Experimentation. Code of Ethics of The World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 65.Kaminski NE. Evidence for a cannabinoid receptor in immunomodulation by cannabinoid compounds. Adv Exp Med Biol. 1993;335:115–120. doi: 10.1007/978-1-4615-2980-4_16. [DOI] [PubMed] [Google Scholar]
- 66.Devane WA, Breuer A, Sheskin T, Järbe TU, Eisen MS, et al. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35:2065–2069. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 67.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 68.Vogel Z, Barg J, Levy R, Saya D, Heldman E, et al. Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem. 1993;61:352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 69.Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 70.Di Marzo V, et al. Potential Biosynthetic Connections between the Two Canna bimimetic Eicosanoids, Anandamide and 2-Arachidonoyl-Glycerol, in Mouse Neuroblastoma Cells. Biochemical and Biophysical Research Communications. 1996;2279(1):281–288. doi: 10.1006/bbrc.1996.1501. [DOI] [PubMed] [Google Scholar]
- 71.Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 72.Buxbaum DM. Analgesic activity of 9 -tetrahydrocannabinol in the rat and mouse. Psychopharmacologia. 1972;25:275–280. doi: 10.1007/BF00422507. [DOI] [PubMed] [Google Scholar]
- 73.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci U S A. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marx J. Drug development. Drugs inspired by a drug. Science. 2006;311:322–325. doi: 10.1126/science.311.5759.322. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Xie H, Lei G, Li F, Pan J, et al. Regulatory effects of anandamide on intracellular Ca(2+) concentration increase in trigeminal ganglion neurons. Neural Regen Res. 2014;9:878–887. doi: 10.4103/1673-5374.131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang J, Adamson C, Butler D, Janero DR, Makriyannis A, et al. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 2010;86:615–623. doi: 10.1016/j.lfs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, et al. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- 78.Onaivi E. An endocannabinoid hypothesis of drug reward and drug addiction. Ann N Y Acad Sci. 2008;1139:412–421. doi: 10.1196/annals.1432.056. [DOI] [PubMed] [Google Scholar]
- 79.Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:287–299. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- 80.Hungund BL, Basavarajappa BS, Vadasz C, Kunos G, Rodriguez de Fonseca F, et al. Ethanol, endocannabinoids, and the cannabinoidergic signaling system. Alcohol Clin Exp Res. 2002;26:565–574. [PubMed] [Google Scholar]
- 81.González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, et al. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 82.Ferrer B, Bermúdez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, et al. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- 84.Mitrirattanakul S, et al. Bidirectional Alterations of Hippocampal Cannabinoid 1 Receptors and Their Endogenous Ligands in a Rat Model of Alcohol Withdrawal and Dependence. Alcoholism: Clinical and Experimental Research. 2007;31(5):855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 85.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Research. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 86.Nowak KL, Vinod KY, Hungund BL. Pharmacological manipulation of CB1 receptor function alters development of tolerance to alcohol. Alcohol Alcohol. 2006;41:24–32. doi: 10.1093/alcalc/agh217. [DOI] [PubMed] [Google Scholar]
- 87.Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, et al. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombo G, et al. The Cannabinoid CB1 Receptor Antagonist, Rimonabant, as a Promising Pharmacotherapy for Alcohol Dependence: Preclinical Evidence. Molecular Neurobiology. 2007;36:102–112. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 90.Soyka M, et al. Cannabinoid Receptor 1 Blocker Rimonabant (SR 141716) for Treatment of Alcohol Dependence: Results from a Placebo-Controlled, Double-Blind Trial. Journal of Clinical Psychopharmacology. 2008;28(3):317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- 91.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 92.Soyka M. Rimonabant and depression. Pharmacopsychiatry. 2008;41:204–205. doi: 10.1055/s-2008-1078744. [DOI] [PubMed] [Google Scholar]
- 93.George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, et al. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology (Berl) 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pina MM, Cunningham CL. Effects of the novel cannabinoid CB1 receptor antagonist PF 514273 on the acquisition and expression of ethanol conditioned place preference. Alcohol. 2014;48:427–431. doi: 10.1016/j.alcohol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horvath B, et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. British Journal of Pharmacology. 2012;165(8):2462–2478. doi: 10.1111/j.1476-5381.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rom S, Persidsky Y. Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol. 2013;8:608–620. doi: 10.1007/s11481-013-9445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vasiljevik T, et al. Design, Synthesis, and Biological Evaluation of Amino alkyl indole Derivatives as Cannabinoid Receptor Ligands with Potential for Treatment of Alcohol Abuse. Journal of Medicinal Chemistry. 2013;56(11):4537–4550. doi: 10.1021/jm400268b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuang J, Yang DP, Tian X, Nikas SP, Sharma R, et al. Targeting the Endocannabinoid System for Neuroprotection: A 19F-NMR Study of a Selective FAAH Inhibitor Binding with an Anandamide Carrier Protein, HSA. J Pharm Pharmacol (Los Angel) 2013;1 [PMC free article] [PubMed] [Google Scholar]
- 101.Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, et al. Impaired Neurogenesis by HIV-1-Gp120 is Rescued by genetic deletion of Fatty Acid Amide Hydrolase Enzyme. Br J Pharmacol. 2014 doi: 10.1111/bph.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]