Abstract
Introduction
Sepsis is a devastating condition that is generally treated as a single disease. Identification of meaningfully distinct clusters may improve research, treatment and prognostication among septic patients. We therefore sought to identify clusters among patients with severe sepsis or septic shock.
Methods
We retrospectively studied all patients with severe sepsis or septic shock admitted directly from the emergency department to the intensive care units (ICUs) of three hospitals, 2006–2013. Using age and Sequential Organ Failure Assessment (SOFA) subscores, we defined clusters utilizing self-organizing maps, a method for representing multidimensional data in intuitive two-dimensional grids to facilitate cluster identification.
Results
We identified 2533 patients with severe sepsis or septic shock. Overall mortality was 17 %, with a mean APACHE II score of 24, mean SOFA score of 8 and a mean ICU stay of 5.4 days. Four distinct clusters were identified; (1) shock with elevated creatinine, (2) minimal multi-organ dysfunction syndrome (MODS), (3) shock with hypoxemia and altered mental status, and (4) hepatic disease. Mortality (95 % confidence intervals) for these clusters was 11 (8–14), 12 (11–14), 28 (25–32), and 21 (16–26) %, respectively (p < 0.0001). Regression modeling demonstrated that the clusters differed in the association between clinical outcomes and predictors, including APACHE II score.
Conclusions
We identified four distinct clusters of MODS among patients with severe sepsis or septic shock. These clusters may reflect underlying pathophysiological differences and could potentially facilitate tailored treatments or directed research.
Keywords: Sequential organ failure assessment (SOFA), Sepsis, Multiple organ dysfunction syndrome (MODS), Cluster analysis, Phenotyping, Intensive Care Unit outcomes research
Introduction
Sepsis is a common and often lethal condition; even with improving mortality, approximately 20 % of patients still die of acute sepsis [1]. Severe sepsis and septic shock, the most severe forms of sepsis, are commonly associated with multiple organ dysfunction syndrome (MODS) [2]. Septic patients may differ from each other with regard to severity of disease, etiology of sepsis, and in the degree or type of MODS. Different etiologies of sepsis—e.g., pneumonia, urinary tract infection—are commonly associated with different clinical outcomes [3, 4]. Whether patterns of organ dysfunction define distinct clusters within severe sepsis and septic shock is not known. Identification of such clusters, if they represent distinct genetic or pathophysiological entities or patient groups with distinct natural histories, could support novel pathophysiological insights, allow improved power and efficiency for clinical studies, and increase personalization of treatments.
Historically, classification of sepsis has been limited to etiology and distinguishing severe sepsis from septic shock [3, 4], albeit with the recent addition of the predisposition, infection, response and organ dysfunction (PIRO) framework [5]. The complex physiological abnormalities associated with septic MODS have prevented sophisticated sepsis classification because there are so many relevant abnormalities [6]. Several investigations have probed the individual contributions of specific organ systems: liver [7], kidney [8], and cardiovascular [9]. These prior investigations evaluated associations between specific variables and clinical outcomes. These techniques poorly identify patterns in multiple covariates within complex data [10, 11]. Such patterns may lead to the discovery of clusters that could illuminate previously unrecognized pathophysiologic elements of disease, an important need [12].
Kohonen self-organizing maps (SOMs) are a kind of neural network used to simplify complex, multidimensional data [13]. SOMs group patients to facilitate identification of meaningful clusters. SOMs have identified clusters in asthma, COPD, and pain syndromes [14–16]. Given the large amount of physiological data associated with septic MODS, we applied SOMs to patients with severe sepsis or septic shock admitted to the intensive care units (ICUs) of three hospitals. An abstract of preliminary study results has been published [17].
Methods
We retrospectively studied patients with severe sepsis or septic shock, admitted directly from the emergency department (ED) to an ICU between January 1, 2006 and May 15, 2013 at three Utah hospitals: Intermountain Medical Center, LDS Hospital, and McKay-Dee Hospital. The Intermountain Healthcare Institutional Review Board (IRB #1014318) approved this study with waiver of informed consent.
All subjects admitted to the ICU from the ED, either directly or after emergency surgery, were screened as part of an ongoing effort to improve the quality of sepsis care. We excluded subjects younger than 18 years and those in whom data were incomplete. Trained coordinators reviewed each subject’s medical record to identify standard criteria for severe sepsis or septic shock, which includes a suspected infection plus at least two systemic inflammatory response syndrome criteria: temperature >38 or <36 °C, heart rate >90 min−1, respiratory rate >20 min−1 or PaCO2 <32 mmHg, white blood cell count >12,000 or <4000 mm−3 or >10 % immature forms [18], and the further requirement of organ dysfunction or persistent hypotension. The current study cohort overlaps partially with a larger, 11-hospital, cohort identified as part of the adoption of a surviving sepsis care bundle, the results of which (for 2004–2010) have been published [19]. We restricted our study to the three hospitals that had electronic data required to calculate acute physiology and chronic health evaluation II (APACHE II) [20] and sequential organ failure assessment (SOFA) scores; [2] in addition, we extended the cohort period through 2013.
We measured organ dysfunction using the SOFA score and its subcomponents. We measured comorbidities using the Elixhauser formula [21]. Our primary outcome was 30-day all-cause mortality. Our secondary outcome was ICU-free days at 30 days after admission (patients who died within 30 days were considered to have 0 ICU-free days). Three reviewers performed manual chart review to ascertain the etiology of sepsis, with an interobserver kappa = 0.71 from 83 randomly selected charts.
Calculating SOFA scores
For the admission SOFA score, we included data obtained up to 6 h before and 24 h after ICU admission. We included data up to 6 h prior to ICU admission in order to include values obtained in the ED, as clinicians may not repeat analyses during the first ICU day [22, 23]. We calculated SOFA scores for each patient according to standard methods [2] except urine output in the renal sub-score; we only used urine output to reclassify patients with a renal sub-score of 3 to a renal sub-score of 4 when urine output was <200 ml/24 h, due to less reliable charting of hourly urine output (Table 1). The SOFA central nervous system sub-score is based on the Glasgow coma scale (GCS), which is routinely measured in the study ICUs [24]. As per standard methodology, all patients undergoing mechanical ventilation through an endotracheal tube were assigned a GCS verbal score of 1, irrespective of the clinician’s estimate of the patient’s underlying verbal capacity [23]. When arterial blood gases were not available, PaO2/FiO2 was estimated from SpO2/FiO2, using the Ellis inversion [25] of the Severinghaus equation [26] to convert SpO2 to PaO2 as in prior studies [27].
Table 1.
SOFA Score
| SOFA sub-score | 0 | 1 | 2 | 3 | 4 |
| Respiratory PaO2/FiO2 (torr)a | >340 | ≤340 | ≤255 | ≤170b | ≤ 85b |
| Central nervous system Glasgow coma score | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Cardiovascular MAP (mmHg) vasopressor rate (μg/kg/min)c | ≥70 | <70 | Dopamine ≤5 or dobutamine >0 | Dopamine >5 or epinephrine ≤0.1 or norepinephrine ≤0.1 or phenylephrine ≤0.22 | Dopamine >15 or epinephrine >0.1 or norepinephrine >0.1 or phenylephrine >0.22 |
| Liver bilirubin (mg/dl) Coagulation platelet (× 103/μL) | <1.2 >150 | ≥1.2 ≤150 | ≥2.0 ≤100 | ≥6.0 ≤50 | ≥12.0 ≤20 |
| Renal creatinine (mg/dl) urine output (ml/day) | <1.2 | ≥1.2 | ≥2.0 | ≥3.5 | ≥5.0 or ≥3.5 and <200 mL/day |
MAP mean arterial pressure
PaO2/FiO2 ratio was adjusted for altitude (1500 m) per ARDS-Network methodology of adjusting the thresholds by a factor of (Patm/Psea level)
Requires the receipt of positive pressure ventilation
Statistical methods
We utilized SOMs, which are a nonparametric, neural network classification technique used to simplify datasets with large numbers of variables and/or observations [13]. While we describe technical details of the technique in the online Appendix, briefly SOMs group similar patients—based on multivariate distance—and display them on two-dimensional grids called “maps”. Patients close together on the maps are similar to each other in terms of their pattern of organ dysfunction, while patients far apart on the maps are different from each other.
We utilized SOFA sub-scores and age to generate the SOM grids. K-means clustering, a technique for grouping observations on the basis of similarity, was performed on the nodes within the SOM grids to assist with identification of distinct groupings, with manual adjustment to optimize contiguity, as is common with SOMs [28]. We validated the SOM clustering using a permutation technique (with 100 iterations) to compare quantitation (q)-errors for our SOM clustering versus randomly generated datasets [29], to assure that clusters were not identified merely by chance.
We then projected data (e.g., etiology of sepsis, comorbidities, ICU-free days) not utilized in the SOM construction process onto the two-dimensional SOM grids to explore the implications and substructure of the SOM-identified clusters. Because SOMs cannot calculate distance/similarity for incomplete cases, we excluded the few patients with missing data rather than risk the creation of spurious clusters based on imputation.
We used logistic and/or linear regression to explore associations among SOM cluster, SOFA scores, APACHE II scores, and clinical outcomes. Outcomes included 30-day mortality and ICU-free days at 30 days. (We did not incorporate ventilator-free and vasopressor-free days because they are highly collinear with ICU-free days.) In an initial analysis, we used simple multivariate regression to confirm that cluster membership was associated with mortality even after controlling for severity of illness and overall SOFA score. In further exploration of the relevance of cluster membership, we used two statistical methods. First, we calculated likelihood ratios for regression models with and without clusters included. Second, we employed multivariate adaptive regression splines (MARS) to detect statistical interaction between SOM cluster and other predictors. MARS performs an objective, automated feature selection strategy for regression that includes predictors which improve the squared error of a model. MARS thereby limits problems with simple forward or backward stepwise regression techniques [30]. We studied statistical interactions to evaluate the possibility that the relationship between overall severity and mortality differed among clusters, which would provide further evidence that the clusters were identifying meaningful differences among patients.
Summary statistics are reported as: mean/standard deviation, median/interquartile range, or count/percentage, as appropriate. We compared central tendencies with Chi square or Kruskall–Wallis statistic where appropriate. Two-tailed p < 0.05 was considered significant. We performed all analyses in the R Statistical Package, v.3.0.2 (www.r-project.org) using packages kohonen and earth.
Results
During the study period, 2687 patients were admitted to the ICUs from the ED with severe sepsis or septic shock. Of those, 2533 (94 %) patients had non-missing SOFA data and were included in the analysis, constituting the study cohort (Fig. 1). Four apparent clusters were identified by visual inspection of the SOM grids and k-means clustering of nodes. The resulting SOM grids are depicted in Figs. 2 and 3.
Fig. 1.
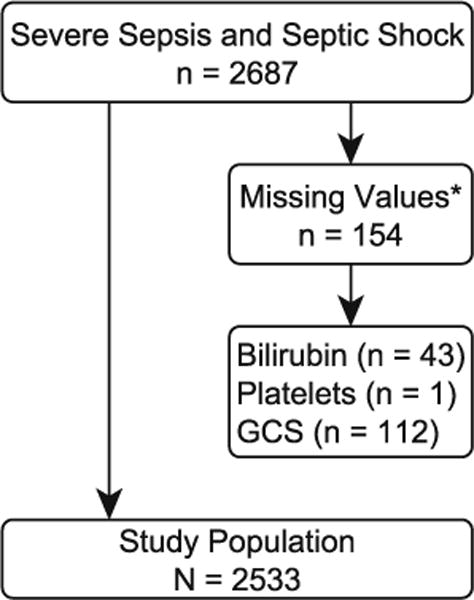
Study population, with subjects excluded due to missing data. Asterisk two patients were missing more than one value
Fig. 2.
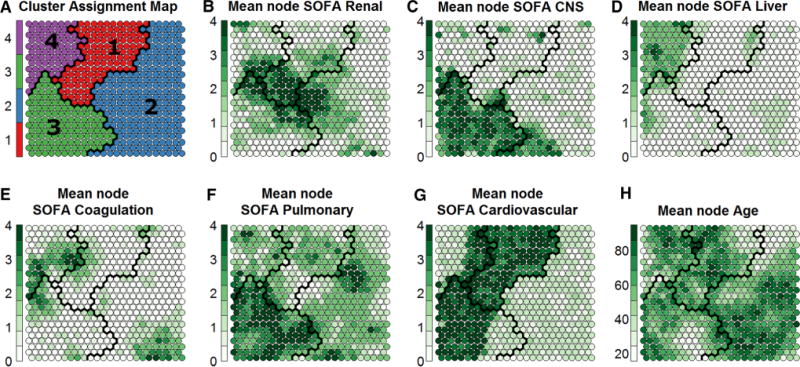
Kohonen Self-Organizing Maps. The maps depict both overall clusters and individual nodes (circles) to show the internal patterns within clusters. Nodes represent smaller groupings of patients: each node contains 0–20 patients who are extremely similar to each other. The four clusters are divided by black lines and depicted in (a). b–f Show those same clusters, but with depictions of different attributes of the nodes within each cluster. Within each node, the given value is represented by the darkness of the colour in the node. Each node is shaded from white to dark green, where darker colours represent higher average values (e.g., higher SOFA subscore) among the patients in the given node. The patterns visible in (b–f) suggest that the four clusters represent: (1) shock with elevated creatinine, (2) minimal multiple organ dysfunction syndrome, (3) shock with hypoxemia and altered mental status, and (4) hepatic disease
Fig. 3.
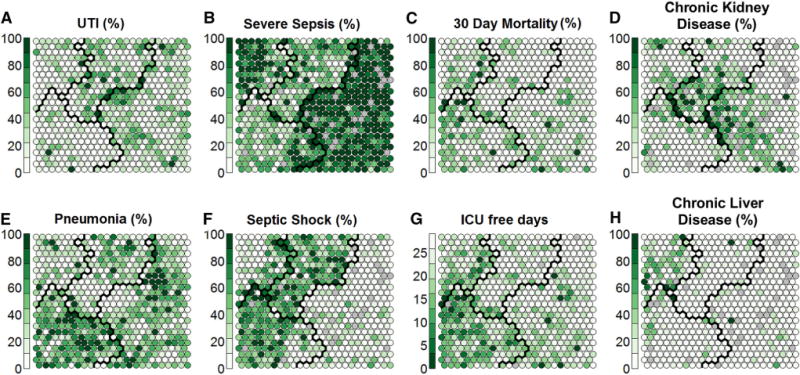
Distribution of variables not employed in clustering, across the nodes within each cluster. The clusters depicted here are the same as those depicted in Fig. 2. Each node on the map represents a group of patients and the value displayed represents the mean value of those patients. Within each node, the given value is represented by the darkness of the colour in the node. Each node is shaded from white to dark green, where darker colours represent higher average values (e.g., proportion of patients in that node with urinary infection as the cause of sepsis) among the patients in the given node. A cluster of patients with renal comorbidities is identified (d), which spreads over three clusters. In the pneumonia grid (e), two broad types are apparent: pneumonia with septic shock (f mainly in cluster 3) and pneumonia with severe sepsis (b mainly in cluster 2)
The identified clusters represent four distinct clusters: (1) shock with elevated creatinine, (2) minimal MODS, (3) shock with hypoxemia and altered mental status, and (4) hepatic disease. Mortality (95 % confidence intervals) for these clusters was 11 (8–14), 12 (11–14), 28 (25–32), and 21 (16–26) %, respectively (p < 0.0001 for comparison by Kruskal–Wallis). Further characteristics of the four clusters are presented in Table 2; all predictors differed significantly across clusters (all p < 0.001). Age, chronic obesity and chronic lung disease did not differ across clusters. Patients with chronic renal disease were mainly grouped within the center of the SOM grid, spanning all four clusters. The permutation technique (n = 100) confirmed reasonable quantitation-errors (q = 4.7) that were lower than would be observed by chance in the SOM classification.
Table 2.
Descriptive statistics of the clusters
| Cluster 1: shock with elevated creatinine | Cluster 2: minimal MODS | Cluster 3: shock with hypoxemia and altered mental status | Cluster 4: hepatic disease | Entire population | |
|---|---|---|---|---|---|
| Count | 523 | 1143 | 558 | 309 | 2533 |
| Age | 62 (52, 75) | 63 (53, 77) | 59 (49, 72) | 60 (48, 73) | 62 (51, 75) |
| Female | 56 % (294) | 53 % (604) | 49 % (274) | 41 % (127) | 51 % (1299) |
| Total SOFA N (IQR) (95 %CI) SOFA subscores n (IQR) (95 %CI) |
8.4 (7, 10) (8.3–8.6) | 5.2 (4,7) (5.1–5.3) | 12.1 (10,14) (11.9–12.3) | 9.8 (8,12) (9.5–10.2) | 8 (5,10) (7.8–8.1) |
| Hepatic SOFA | 0.3 (0, 0) (0.2–0.3) | 0.2 (0, 0) (0.2–0.2) | 0.5 (0,1) (0.4–0.5) | 2.1 (2, 2) (2.0–2.1) | 0.5 (0, 1) (0.5–0.5) |
| Renal SOFA | 1.7 (1, 3) (1.6–1.8) | 1 (0, 2) (1.0–1.1) | 1.6 (1, 2) (1.5–1.7) | 1.4 (0, 2) (1.2–1.5) | 1.3 (0, 2) (1.3–1.4) |
| Coagulation SOFA | 0.5 (0, 1) (0.5–0.6) | 0.5 (0, 1) (0.5–0.6) | 0.6 (0, 1) (0.5–0.6) | 1.6 (0, 3) (1.4–1.7) | 0.7 (0, 1) (0.6–0.7) |
| CNS SOFA | 0.7 (0, 1) (0.6–0.8) | 0.8 (0, 1) (0.7–0.8) | 3.2 (3, 4) (3.1–3.2) | 0.9 (0, 1) (0.8–1.0) | 1.3 (0, 2) (1.2–1.4) |
| Cardio SOFA | 3.5 (3, 4) (3.4–3.6) | 0.9 (1, 1) (0.9–0.9) | 3 (2, 4) (2.9–3.1) | 2 (1, 4) (1.9–2.2) | 2.1 (1, 4) (2.0–2.1) |
| Pulmonary SOFA | 1.7 (1, 2) (1.6–1.8) | 1.8 (1, 2) (1.7–1.8) | 3.3 (3, 4) (3.2–3.4) | 1.9 (1, 2) (1.8–2.0) | 2.1 (1, 3) (2.1–2.2) |
| Intubated | 3 % (18) | 6 % (67) | 76 % (426) | 9 % (29) | 21 % (540) |
| NIPPV | 9 % (46) | 11 % (122) | 17 % (94) | 8 % (26) | 11 % (288) |
| Dialysis | 5 % (25) | 1 % (14) | 4 % (21) | 2 % (6) | 3 % (66) |
| Max lactate (n = 2491) | 3.4 (1.6,4.1) (3.1–3.6) | 3.0 (1.6, 3.7) (2.8–3.1) | 5.0 (2.4, 6.5) (4.6–5.3) | 4.4 (2.3,5.7) (4.1–4.7) | 3.7 (1.8, 4.5) (3.6–3.8) |
| APACHE II (n = 2292) | 22 (17, 26) (21–23) | 20 (15, 24) (19–20) | 34 (29, 39) (33–35) | 22 (16, 27) (21–23) | 24 (17, 30) (23–24) |
| 30 day mortality % (n, 95 %CI) | 11 % (57, 8–14 %) | 12 % (142, 11–14 %) | 28 % (159, 25–32 %) | 21 % (65, 16–26 %) | 17 % (423, 15–18 %) |
| ICU-free days n (IQR) (95 %CI) | 24 (24, 28) (23–24) | 24 (24, 28) (23–24) | 15 (0, 25) (14–16) | 20 (15, 28) (18–21) | 21 (19,28) (21–22) |
| ICU length-of-stay days (n = 2528) n (IQR) (95 %CI) | 4.2 (2, 4.8) (3.9–4.6) | 3.9 (1.7, 4.3) (3.7–4.2) | 8.7 (3.1, 11.8) (8.0–9.3) | 5.8 (2, 6.3) (5.1–6.5) | 5.3 (1.9, 6) (5.0–5.5) |
| Severe sepsis | 46 % (239) | 89 % (1020) | 48 % (270) | 69 % (212) | 69 % (1741) |
| Septic shock | 54 % (284) | 11 % (123) | 52 % (288) | 31 % (97) | 31 % (792) |
| Etiology of sepsis | |||||
| Pneumonia | 29 % (153) | 39 % (451) | 57 % (318) | 25 % (76) | 39 % (998) |
| Urinary tract | 31 % (160) | 27 % (311) | 11 % (63) | 18 % (57) | 23 % (591) |
| Abdominal | 8 % (42) | 7 % (75) | 11 % (60) | 9 % (29) | 8 % (206) |
| Biliary | 2 % (10) | 2 % (19) | 2 % (9) | 12 % (38) | 3 % (76) |
| Soft tissue | 14 % (74) | 12 % (137) | 7 % (38) | 11 % (35) | 11 % (284) |
| Other | 16 % (84) | 13 % (150) | 13 % (70) | 24 % (74) | 15 % (378) |
| Elixhauser count | 4.9 (3, 6) | 4.5 (3, 6) | 5.2 (4,7) | 4.8 (3,6) | 4.8 (3, 6) |
Data are displayed as mean (interquartile range 25–75 %) or percent (count). All values have a significance of p < 0.001 when compared across groups APACHE II acute physiology and chronic health evaluation score 2nd version, SOFA sequential organ failure assessment
Patients with septic shock were largely in clusters 1 (shock with elevated creatinine) and 3 (shock with hypoxemia and altered mental status), whereas patients with severe sepsis were largely in clusters 2 (minimal MODS) and 4 (hepatic disease). There are two groupings of patients with pneumonia as their etiology of sepsis as seen in Fig. 3e. A group with severe pneumonia is located in cluster 3 (shock with hypoxemia and altered mental status), while a group with milder pneumonia is located in cluster 2 (minimal MODS).
Figure 2e highlights the fact that coagulation dysfunction occurs mostly concomitant with liver dysfunction in cluster 4. There does not appear to be a distinct population of coagulopathy in cluster 3 (shock with hypoxemia and altered mental status).
In an exploratory analysis, we evaluated whether cluster 4 (hepatic disease) was dominated by patients with chronic hepatic failure as defined by Elixhauser comorbidity scoring. Cluster 4 included both chronic liver disease as well as patients with acute hepatic injury pattern of sepsis from various causes (Fig. 3h). As evidence of an acute hepatic injury pattern, 41 % (126 patients) of the patients in cluster 4 did not have chronic liver disease, and they were distributed throughout cluster 4. In an additional exploratory analysis, we did not find significant changes in mortality among clusters when we restricted our analysis to patients who were “full code” at the time of admission.
Results of simple multivariate logistic regression of 30-day mortality are displayed in eTable 1, with p values from the likelihood ratio test. Results of simple multivariate linear regression of ICU-free days are displayed in eTable 2. Our ultimate logistic regression model of 30-day mortality included age, APACHE II score, SOFA score, peak lactate, urinary tract infection, pneumonia, or soft tissue infection as etiology of sepsis, and cluster membership after feature selection using MARS. The MARS algorithm identified an interaction between APACHE II and cluster membership. We therefore developed logistic regression models stratified by cluster membership to allow better visualization of the meaning of the interaction between APACHE II and cluster membership, the results of which are displayed in eTable 3, where we display only those predictors that were significantly associated with mortality. In eTable 4, we display the results of stratified linear regression analysis of ICU-free days, again displaying only those predictors that were significantly associated with outcome.
Discussion
We identified four distinct clusters of MODS in a large cohort of patients with severe sepsis or septic shock. The clusters were largely defined as: shock with renal dysfunction, minimal MODS, shock with hypoxemia and altered mental status, and hepatic dysfunction. These clusters differed substantially from each other in terms of the distribution of outcomes and the associations between severity scores and clinical outcomes. Septic shock tended to dominate clusters 1 (shock with elevated creatinine) and 3 (shock with hypoxemia and altered mental status) and severe sepsis to dominate clusters 2 (minimal MODS) and 4 (hepatic disease), suggesting that clusters exist within the traditional classification of sepsis into severe sepsis and septic shock. The clusters we identified do not simply mirror the traditional classification: patients with septic shock in cluster 1 (shock with elevated creatinine) had mortality similar to patients with severe sepsis in cluster 2 (minimal MODS), while patients with severe sepsis in cluster 4 (hepatic disease) had much higher mortality.
While severe pneumonia patients in septic shock represented a substantial proportion of cluster 3 (shock with hypoxemia and altered mental status) patients, and end-stage liver disease represented a substantial proportion of cluster 4 (hepatic disease) patients, the clusters we identified via SOMs were not merely surrogates for etiology of sepsis or the presence of end-stage liver disease. The presence of cluster 1 corroborates recent evidence suggesting a close association between hypotension and kidney failure among septic patients [31].
Because SOFA relies on GCS for evaluation of central nervous system (CNS) dysfunction, and GCS does not distinguish between decreased verbal ability related to mechanical ventilation from that due to CNS dysfunction, the altered mental status in cluster 3 may reflect mechanical ventilation rather than CNS dysfunction. However, in parallel work, we found that imputed verbal scores (the GCS component most affected by mechanical ventilation) did not substantially affect the association between SOFA and mortality [23]. A technique for identifying CNS dysfunction independent of mechanical ventilation is desirable for future studies in this area.
Prior studies have focused on sepsis subgroups on the basis of general severity (i.e., severe sepsis vs. septic shock) and etiology of sepsis (e.g., pulmonary vs. urinary, etc.) [3], with some early work on the PIRO system [5, 32]. The clusters we identified were largely independent of the etiology of sepsis, with a wide distribution of origins of sepsis throughout the clusters. We observed certain organ dysfunctions as predominating in specific clusters. Specific organ dysfunctions, such as liver [7] or kidney failure [33], have also been singled out as contributing to mortality in severe sepsis/septic shock.
Exploratory analyses of morbid obesity and pre-existing disease of the kidney, lung, and liver demonstrated that the clusters we identified were largely independent of obesity and other comorbidities. We also noted a cluster of patients with renal disease (and elevated SOFA sub-scores) that extended over clusters 1 (shock with elevated creatinine), 2 (minimal MODS), and 3 (shock with hypoxemia and altered mental status). These findings suggest that the clusters we identified are not surrogates for pre-existing comorbidities, nor are they simply surrogates for the overall severity of sepsis.
Clusters were independent of age despite age being utilized in the SOM mapping process, suggesting that clusters are unrelated to age. The presence of significant statistical interaction suggests that the clusters identify important heterogeneity in MODS among septic patients.
Within cluster 2 (minimal MODS), certain etiologies of sepsis are associated with worse outcome. As compared to abdominal, biliary, or “other” etiologies of sepsis, pneumonia, urinary tract, and soft tissue infection are associated with lower mortality. This association between etiology of sepsis and outcome was not present within other clusters.
Statistical learning techniques such as SOMs have been applied in many areas of biomedicine. While SOMs are applicable to classifying other complex diseases, we believe that they may be especially useful in the critically ill patient. The modern ICU collects vast quantities of laboratory results, vital signs, comorbidities, and imaging into an electronic medical record. These data are complex and multidimensional [6]. Clustering analyses could allow that complexity be reduced to a degree that could be useful for tailored therapy, study enrollment, or improved diagnostics, as well as the potential for improved pathophysiological understanding of MODS. Consistent with other studies of SOMs [14–16, 34], because SOMs have been shown to be superior to traditional clustering techniques [35], we did not compare SOM clustering with other cluster techniques.
We anticipate that the clustering performed in this current study will lay the groundwork for additional classifications that incorporate novel biomarkers (several of which have been evaluated recently [36]), including laboratory and physiological measurements and future efforts to understand pathophysiological differences among different clusters.
Our study has several limitations. While our cohort is large and from multiple centers, we do not include a separate validation cohort. We anticipate the need for prospective, external validation of our findings. This study is retrospective, and consequently may suffer from biased missingness and imprecision in measurement related to clinical rather than research measurements. However, because SOFA uses frequently obtained measures that are well established in clinical laboratories, we believe these risks are relatively minor. Additionally, the SOM technique cannot accommodate missing data. However, missing data were rare in this cohort, suggesting that this is not a substantial limitation. We also acknowledge that we used relatively simple measures of organ dysfunction, the sub-scores of the SOFA score, and we used only the admission SOFA score rather than sequential SOFA scores. We did so because those measures are commonly available in clinical practice, but we acknowledge that other physiological and laboratory measures might enhance the sophistication of these clusters. While other studies have not specified how they handled laboratory or physiological values obtained in the ER, we do not believe that our inclusion of data from the ER represents a substantial change in the usual calculation of the admission SOFA score. We also acknowledge that we did not collect data necessary to compare our findings with a PIRO-based approach, while noting that PIRO is not yet a reproducible system for classification [32].
In terms of generalizability, we acknowledge that our mortality is lower than described in some other recent cohorts [37], although it is very similar to the mortality observed in the recent multicenter ProCESS trial [38] and recent reports from Australasia [39]. Our choice to exclude patients who developed sepsis while in hospital (e.g., surgical site infections or hospital-associated pneumonia) does not allow us to describe such patients, who may have higher mortality than patients admitted directly from the ED.
Conclusions
We identified four distinct clusters of MODS within a cohort of critically ill septic patients admitted from the ED to the ICU using Kohonen self-organizing maps. The association between clinical predictors and mortality differed among cluster. These clusters may be useful in supporting tailored research and clinical therapies for septic patients.
Supplementary Material
Acknowledgments
The authors thank Allan J. Walkey, MD MSc and Allison Turnbull, DVM MPH PhD for close readings of the manuscript. This study was funded by National Institute of General Medical Sciences (1K23GM094465 to S.M.B.) and the Inter-mountain Research and Medical Foundation.
Footnotes
Take-home message: There appear to be four meaningful clusters of sepsis-associated multiple organ dysfunction syndrome. The distinct clusters have different associated mortality which is not explained by usual severity-of-illness scores.
Electronic supplementary material The online version of this article (doi:10.1007/s00134-015-3764-7) contains supplementary material, which is available to authorized users.
Conflicts of interest The author(s) declare that they have no competing interest.
Contributor Information
Daniel B. Knox, Email: danknoxmd@gmail.com, Pulmonary and Critical Care, Intermountain Medical Center, Salt Lake City, USA; Pulmonary and Critical Care, University of Utah School of Medicine, Salt Lake City, USA.
Michael J. Lanspa, Email: michael.lanspa@imail.org, Pulmonary and Critical Care, Intermountain Medical Center, Salt Lake City, USA; Pulmonary and Critical Care, University of Utah School of Medicine, Salt Lake City, USA.
Kathryn G. Kuttler, Email: kathryn.kuttler@imail.org, Pulmonary and Critical Care, Intermountain Medical Center, Salt Lake City, USA; Homer Warner Center for Informatics Research, Intermountain Healthcare, Salt Lake City, USA.
Simon C. Brewer, Email: simon.brewer@geog.utah.edu, Geography Department, University of Utah, Salt Lake City, USA.
Samuel M. Brown, Email: samuel.brown@imail.org, Pulmonary and Critical Care, Intermountain Medical Center, Salt Lake City, USA; Pulmonary and Critical Care, University of Utah School of Medicine, Salt Lake City, USA; Shock Trauma Intensive Care Unit, 5121 South Cottonwood Street, 84107 Murray, UT, USA, Tel.: 801-507-6556.
References
- 1.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17:R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis occurrence in acutely Ill patients I, sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 4.Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A, Cooperative antimicrobial therapy of septic shock database research G Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189:1204–1213. doi: 10.1164/rccm.201310-1875OC. [DOI] [PubMed] [Google Scholar]
- 5.Howell MD, Talmor D, Schuetz P, Hunziker S, Jones AE, Shapiro NI. Proof of principle: the predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39:322–327. doi: 10.1097/CCM.0b013e3182037a8e. [DOI] [PubMed] [Google Scholar]
- 6.Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Nieman GF, Chang S, Billiar TR, Kellum JA, Angus DC, Vodovotz Y. Sepsis: something old, something new, and a systems view. J Crit Care. 2012;27(314):e311–e312. doi: 10.1016/j.jcrc.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrmann V, Kneidinger N, Herkner H, Heinz G, Nikfardjam M, Bojic A, Schellongowski P, Angermayr B, Schoniger-Hekele M, Madl C, Schenk P. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37:1302–1310. doi: 10.1007/s00134-011-2248-7. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Bouchard J, Soroko SB, Ikizler TA, Paganini EP, Chertow GM, Himmelfarb J. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37:241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SM, Pittman JE, Hirshberg EL, Jones JP, Lanspa MJ, Kuttler KG, Litwin SE, Grissom CK. Diastolic dysfunction and mortality in early severe sepsis and septic shock: a prospective, observational echocardiography study. Crit Ultrasound J. 2012;4:8. doi: 10.1186/2036-7902-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Encode Project Consortium. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Presson AP, Yoon NK, Bagryanova L, Mah V, Alavi M, Maresh EL, Rajasekaran AK, Goodglick L, Chia D, Horvath S. Protein expression based multimarker analysis of breast cancer samples. BMC Cancer. 2011;11:230. doi: 10.1186/1471-2407-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Ranieri VM, Pesenti A. Sepsis: needs for defining severity. Intensive Care Med. 2015;41:551–552. doi: 10.1007/s00134-014-3598-8. [DOI] [PubMed] [Google Scholar]
- 13.Kohonen T. Self-organizing maps. Springer; Berlin: 2001. [Google Scholar]
- 14.Boudier A, Curjuric I, Basagana X, Hazgui H, Anto JM, Bousquet J, Bridevaux PO, Dupuis-Lozeron E, Garcia-Aymerich J, Heinrich J, Janson C, Kunzli N, Leynaert B, de Marco R, Rochat T, Schindler C, Varraso R, Pin I, Probst-Hensch N, Sunyer J, Kauffmann F, Siroux V. Ten-Year follow-up of cluster-based asthma phenotypes in adults. a pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013;188:550–560. doi: 10.1164/rccm.201301-0156OC. [DOI] [PubMed] [Google Scholar]
- 15.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, Rutten EP, Op ‘t Roodt J, Wouters EF, Franssen FM. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 16.Lotsch J, Ultsch A. A machine-learned knowledge discovery method for associating complex phenotypes with complex genotypes. Application to pain. J Biomed Inform. 2013;46:921–928. doi: 10.1016/j.jbi.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Knox DB, Lanspa MJ, Kuttler K, Brewer S, Brown SM. Phenotyping septic shock: use of kohonen self-organizing maps to identify patterns of organ dysfunction. Am J Respir Crit Care Med. 2014;189:A3791. [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign GuidelinesCommitteeincluding the Pediatric S Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 19.Miller RR, 3rd, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, Allen TL, Clemmer TP, Intermountain healthcare intensive medicine clinical P Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Grissom CK, Brown SM, Kuttler KG, Boltax JP, Jones J, Jephson AR, Orme JF., Jr A modified sequential organ failure assessment score for critical care triage. Disaster Med Public Health Preparedness. 2010;4:277–284. doi: 10.1001/dmp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knox DB, Lanspa MJ, Pratt CM, Kuttler KG, Jones JP, Brown SM. Glasgow coma scale score dominates the association between admission sequential organ failure assessment score and 30 day mortality in a mixed intensive care unit population. J Crit Care. 2014;29:780–785. doi: 10.1016/j.jcrc.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 25.Ellis RK. Determination of PO2 from saturation. J Appl Physiol. 1989;67:902. doi: 10.1152/jappl.1989.67.2.902. [DOI] [PubMed] [Google Scholar]
- 26.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 27.Dean NC, Jones JP, Aronsky D, Brown S, Vines CG, Jones BE, Allen T. Hospital admission decision for patients with community-acquired pneumonia: variability among physicians in an emergency department. Ann Emerg Med. 2012;59:35–41. doi: 10.1016/j.annemergmed.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartigan JA. Clustering algorithms. Wiley; New York: 1975. [Google Scholar]
- 29.Witten DM, Tibshirani R. A framework for feature selection in clustering. J Am Stat Assoc. 2010;105:713–726. doi: 10.1198/jasa.2010.tm09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res. 1995;4:197–217. doi: 10.1177/096228029500400303. [DOI] [PubMed] [Google Scholar]
- 31.Poukkanen M, Wilkman E, Vaara ST, Pettila V, Kaukonen KM, Korhonen AM, Uusaro A, Hovilehto S, Inkinen O, Laru-Sompa R, Hautamaki R, Kuitunen A, Karlsson S, Group FS. Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: data from the prospective observational FINNAKI study. Crit Care. 2013;17:R295. doi: 10.1186/cc13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tafelski S, Nachtigall I, Stengel S, Wernecke K, Spies CD. Comparison of three models for sepsis patient discrimination according to PIRO: predisposition, infection, response and organ dysfunction. Minerva Anestesiol. 2014 (in press) [PubMed] [Google Scholar]
- 33.Joannidis M, Metnitz PG. Epidemiology and natural history of acute renal failure in the ICU. Crit Care Clin. 2005;21:239–249. doi: 10.1016/j.ccc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, Gaston BM, Bleecker ER, Moore WC, NationalInstitutesof Health, National Heart L, Blood Institute Severe Asthma Research P Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the national institutes of health/national heart, lung, and blood institute severe asthma research program. J Allergy Clin Immunol. 2011;127(382–389):e381–381. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangiameli P, Chen SK, West D. A comparison of SOM neural network and hierarchical clustering methods. Eur J Oper Res. 1996;93:402–417. [Google Scholar]
- 36.Barnay-Verdier S, Fattoum L, Borde C, Kaveri S, Gibot S, Marechal V. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37:957–962. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- 37.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 38.Investigators ProCess. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 40.Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, Miller RR, Hirshberg EL, Grissom CK, Morris AH. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143:664–671. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


