For over two decades sterol regulatory element-binding proteins (SREBPs) have been heavily scrutinized because of their central importance to lipid metabolism and membrane biology In 1993, Brown and Goldstein at the University of Texas Southwestern identified a nuclear protein that bound the sterol regulatory element of the low-density lipoprotein receptor1,2 and controls its transcription.3 Concurrently the Spiegelman laboratory at Harvard identified SREBP-1c as a major determinant of adipocyte determination and differentiation (aka ADD1).4 More than 3,000 PubMed articles now document the exciting life cycle of SREBPs, many of which flow from these investigators and their protégés, including the study in the current issue of HEPATOLOGY.5 The stream is unlikely to abate as new SREBP insights are uncovered in diabetes, cancer, and other areas.6
Two genes, SREBF1 and SREBF2, encode for three SREBP proteins: SREBP-1a, SREBP-1c, and SREBP-2. All are basic helix-loop-helix leucine zipper (bHLH-LZ) transcription factors. SREBP-1a and SREBP-1c are derived from distinct transcriptional start sites in the SREBF1 gene, but SREBP-1c is the master regulator of de novo lipogenesis in the liver. In response to falling membrane cholesterol concentrations, SREBP-2 induces the enzymes of the mevalonate pathway to promote cholesterol synthesis and uptake. In their inactive state, SREBPs reside in the endoplasmic reticulum (ER) membrane in association with Scap and Insig proteins. Here they sense membrane levels of sterols, and also respond to insulin, unsaturated fatty acids, and carbohydrates. With positive stimulation, the precursor SREBP (pSREBP) is transferred to the Golgi, where site-specific proteolytic cleavage occurs, releasing the activated transcription factor, which then translocates to the nucleus (nSREBP) to exert effects on target gene expression.
SREBP actions are especially important in the liver, where insulin is the major hormone to stimulate hepatic lipogenesis, a potential contributor to nonalcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH). Feeding leads to a profound increase in the activity of hepatic SREBP-1c through insulin release, and this effect depends on liver X receptors (LXRs)7 and S6 kinase.8 But the pathologic significance of hepatic SREBP-1c to the development of metabolic syndrome is unclear, since SREBP activation in obesity produces steatosis and hypertriglyceridemia without insulin resistance,9 while deletion of LXRs in obesity eliminates steatosis and produces profound improvement in hepatic insulin sensitivity.10
Terminating SREBP-1c activity is regulated in several ways (Fig. 1). Primarily, negative feedback comes from sterols themselves—SREBP downstream products—that inhibit the transfer of SREBP-1c to the Golgi and subsequent proteolysis to the nuclear form. AMP kinase (AMPK) phosphorylates SREBP-1c, also preventing nuclear translocation.11 The catalytic function of the phosphatase Lipin1 limits SREBP-1 activity,12 but whether this interaction is direct or requires p53 is unknown. During fasting, SIRT1 deacetylation of SREBP-1a/1c leads to ubiquitin-proteasome degradation,13 but the identity of the specific E3 ubiquitin ligase remains unknown. And fasting, or at least the absence of insulin, allows GSK3 to phosphorylate SREBP-1c, leading to ubiquitinylation by SCF(Fbw7) and eventual degradation.14
Fig. 1.
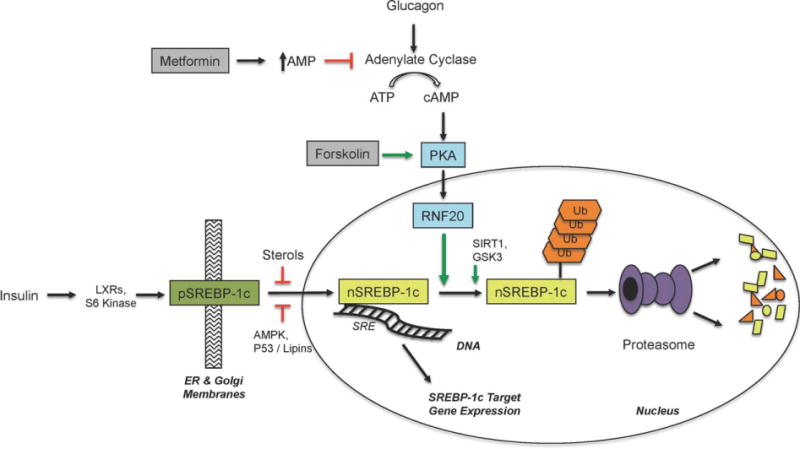
Regulatory pathways for SREBP-1c signaling. Insulin, in an LXR and S6 kinase-dependent manner, promotes the site-specific cleavage of pSREBP-1c. This releases transcriptionally active nSREBP-1c, which goes to the nucleus and binds sterol response elements (SRE) in the DNA to modulate target gene expression. Numerous other stimuli and regulators of SREBP-1c gene expression are not shown for brevity. Multiple types of sterols, AMPK signaling, lipins, and p53 can inhibit the cleavage/translocation event. SIRT1 and GSK3 are known to promote SREBP-1c degradation by the ubiquitin-proteasome pathway. In the current work, RNF20 is shown to cause the proteasomal degradation of nSREBP-1c, and this can be induced by PKA signaling, forskolin, or glucagon. Metformin, through PKA inhibition, is predicted to block SREBP-1c degradation, although formal demonstration of this remains to be seen. AMP, adenosine monophosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; RNF20, Ring finger protein 20; LXRs, Liver X receptors; AMPK, AMP-activated protein kinase; pSREBP-1c, precursor SREBP-1c; nSREBP-1c, nuclear SREBP-1c; Ub, ubiquitin; SRE, sterol response element.
In this issue of HEPATOLOGY Lee et al.5 identify a new route for the demise of SREBP-1c during fasting. In primary hepatocytes, activation of protein kinase A (PKA) by glucagon or forskolin greatly enhanced the degradation of nSREBP-1c, while proteasome inhibition diminished it. Primary hepatocytes were transduced with a tagged nSREBP-1c followed by affinity purification and mass spectrometry to identify SREBP-1c interacting proteins. These experiments identified a novel interaction with RNF20, a RING-finger containing E3 ubiquitin ligase. RNF20 had not previously been implicated in liver physiology or metabolic disease; its major function appeared to be the monoubiquitinylation of histones as part of the DNA damage response.15 But RNF20 specifically ubiquitinylates and promotes the degradation of nSREBP-1c in a PKA-dependent manner. Remarkably, it does this without affecting the stability of other lipogenic transcription factors such as LXRα or peroxisome proliferator activated receptor gamma (PPARγ). Overexpressing RNF20 impaired endogenous nSREBP-1c target gene expression even in the face of insulin or synthetic LXR agonists. Conversely, small interfering RNA (siRNA) knockdown of RNF20 specifically increased SREBP-1c transcript levels but not those of SREBP-1a or SREBP-2. This correlated with increased neutral lipid accumulation in primary hepatocytes, thereby showing that RNF20 negatively regulates hepatic lipogenesis.
The authors then elegantly demonstrate RNF20 regulation of SREBP-1c in vivo. In livers from fasted, fed, or fasted/refed mice, levels of SREBP-1c are inversely correlated with RNF20. Forced hepatic overexpression of RNF20 in normal mice significantly reduces nSREBP-1c accumulation, diminishes the downstream targets of SREBP-1c, and cuts hepatic triglyceride accumulation in half. Finally, RNF20 overexpression not only improves the hepatic steatosis found in obese leptin receptor-deficient mice, but it also modestly improves the glucose tolerance curves of these animals.
RNF20 now adds another dimension by which the ubiquitin-proteasome system impacts the lifespan of SREBP-1c. But is RNF20 also the E3 ligase that mediates SIRT1-dependent SREBP-1c degradation? How does PKA signaling increase RNF20 levels? What other proteins (if any) does RNF20 ubiquitinylate and is there a role for de-ubiquitinylation in stabilizing SREBP-1c? This provocative article may raise more questions than it answers, but it also helps connect the dots in other areas and points the way to new investigations. First, it is possible that stimulating RNF20 will provide clinical benefit for NAFLD/NASH by reducing hepatic lipogenesis. The current work potentially explains why metformin is not an effective NAFLD/NASH drug, since PKA signaling promotes SREBP-1c degradation and metformin was recently shown to antagonize glucagon action and inhibit PKA activity (Fig. 1).16 Second, because p53 has well-known roles in genotoxicity and oncogenesis it will be critical to determine whether RNF20 mediates p53 suppression of SREBP-1c.17 Since RNF20 has a role in DNA damage responses, one can imagine that the PKA-RNF20-SREBP-1c axis will impact these pathways too. If so, this would further strengthen the link between SREBPs, metabolic remodeling, and cancer.
Abbreviations
- bHLH-LZ
basic helix-loop-helix leucine zipper
- LXR
liver X receptors
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PKA
protein kinase A
- SREBP
sterol regulatory element-binding protein
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Wang X, Briggs MR, Hua X, Yokoyama C, Goldstein JL, Brown MS. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. II. Purification and characterization. J Biol Chem. 1993;268:14497–14504. [PubMed] [Google Scholar]
- 2.Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem. 1993;268:14490–14496. [PubMed] [Google Scholar]
- 3.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 4.Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Lee GY, Jang H, Choe SS, Koo SH, Kim JB. RNF20 regulates hepatic lipid metabolism through PKA-dependent SREBP1c degradation. Hepatology. 2014;60:844–857. doi: 10.1002/hep.27011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16:414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci USA. 2012;109:16184–16189. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver x receptors in obesity and insulin resistance. Cell Metab. 2013;18:106–117. doi: 10.1016/j.cmet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1. J Biol Chem. 2009;284:5885–5895. doi: 10.1074/jbc.M807906200. [DOI] [PubMed] [Google Scholar]
- 15.Shiloh Y, Shema E, Moyal L, Oren M. RNF20-RNF40: a ubiquitin-driven link between gene expression and the DNA damage response. FEBS Lett. 2011;585:2795–2802. doi: 10.1016/j.febslet.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH. Metabolic regulation by p53 family members. Cell Metab. 2013;18:617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


