Abstract
Calreticulin (CRT) is a highly conserved and abundant multifunctional protein that is encoded by a small gene family and is often associated with abiotic/biotic stress responses in plants. However, the roles played by this protein in salt stress responses in wheat (Triticum aestivum) remain obscure. In this study, three TaCRT genes were identified in wheat and named TaCRT1, TaCRT2 and TaCRT3-1 based on their sequence characteristics and their high homology to other known CRT genes. Quantitative real-time PCR expression data revealed that these three genes exhibit different expression patterns in different tissues and are strongly induced under salt stress in wheat. The calcium-binding properties of the purified recombinant TaCRT1 protein were determined using a PIPES/Arsenazo III analysis. TaCRT1 gene overexpression in Nicotiana tabacum decreased salt stress damage in transgenic tobacco plants. Physiological measurements indicated that transgenic tobacco plants showed higher activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) than non-transgenic tobacco under normal growth conditions. Interestingly, overexpression of the entire TaCRT1 gene or of partial TaCRT1 segments resulted in significantly higher tolerance to salt stress in transgenic plants compared with their WT counterparts, thus revealing the essential role of the C-domain of TaCRT1 in countering salt stress in plants.
Introduction
The Calreticulin protein (CRT) was first identified as a high affinity Ca2+-binding protein from the endoplasmic reticulum (ER) of rabbit skeletal muscle in 1974 [1]. In 1989, rabbit [2] and mouse [3] forms of this protein were cloned. Since then, CRT genes have been isolated from various other species including humans [4], sheep [5], nematodes [6], fruit flies [7], spinach [8], barley [9], tobacco (Nicotiana benthamiana) [10], corn [11–13], Chinese cabbage (Brassica pekinensis) [14], Arabidopsis thaliana [15], castor beans [16], rice [17], wheat [18,19], sea bass [20] and Anopheles stephensi [21]. CRT genes are present in all studied multicellular eukaryotes and are highly conserved [22]. However, these genes have not been found in yeast or prokaryotes, suggesting that CRT genes evolved shortly before plants and animals diverged during the evolutionary process.
A typical CRT protein comprises three distinct subdomains: a conserved globular N-domain including a cleavable signal sequence, which directs the protein to the ER, a P-domain, which exhibits high affinity, low capacity Ca2+-binding ability and a C-terminal domain, which includes a (K/H)DEL ER retrieval signal [22,23] and exhibits low affinity, high capacity Ca2+-binding ability. Inside the cells, CRTs are mainly present in the ER [24] but also in the nucleus [25], the nuclear envelope [12], the cytosol [26], the spindle apparatus of dividing cells [10], the cell surface [27], mitochondria [28] and plasmodesmata [29,30], indicating that CRTs are important for several cellular functions.
Extensive studies of mammalian CRTs have defined more than 40 physiological functions inside and outside the ER [22,23,31,32], including intracellular Ca2+ storage, the regulation of ER Ca2+ homeostasis [33–35], involvement in Ca2+-dependent signal pathways [36–39], molecular chaperone activity in the ER [40–45], control of cell adhesion [24,46], angiogenesis [47], functions related to the immune system and apoptosis [48] as well as roles in pathogenesis [49].
Despite the elucidation of CRT functions in animal cells, the role of plant CRTs is less clear [50]; however, plant CRTs have been shown to bind to calcium in the same way as their animal homologs [9,16,17,51]. In addition, plant CRTs exhibit calcium-storing functions in the ER of plant cells [35,52]. Recently, it was demonstrated that plant CRTs are able to modulate intracellular Ca2+ homeostasis and to function in the ER quality control (ERQC) of N-glycosylated proteins [53–55]. Moreover, plant CRTs exhibit unique features, as demonstrated by newly obtained data. For example, plant CRTs might play a role in the response to some phytohormone stimuli [10,17,56], pollen-pistil interactions [57], the regulation of root and shoot regeneration processes [17] and plant immunity [43,54]. In addition, plant CRTs respond to a variety of stress-mediated stimuli, e.g., cold and gravi-stimulation [58–60], indicating that plant CRTs play multiple roles in plant development and stress responses.
In the ‘Hanxuan 10’ variety of wheat, Jia et al. [18] isolated a full-length cDNA that encodes a CRT3 isoform of the calreticulin protein family (named TaCRT). The subcellular location of this isoform was determined to be the cytoplasm and the nucleus by transiently expressing GFP fused with TaCRT in onion epidermal cells. The CRT3 isoform transcript is up-regulated in wheat seedlings by PEG-induced drought stress. Moreover, TaCRT overexpression resulted in an enhanced drought resistance to several water deficit conditions in tobacco.
In this study, three new full-length cDNAs encoding wheat CRT1, CRT2 and CRT3 isoforms were isolated and named TaCRT1, TaCRT2 and TaCRT3-1, respectively. These isoforms were obtained from spike tissues of the ‘Wangshuibai’ hexaploid wheat cultivar using reverse transcription PCR (RT-PCR). The expression patterns of these isoforms were determined in various organs and under salinity stress conditions in wheat. The calcium-binding properties of purified recombinant TaCRT1 protein were determined through PIPES/Arsenazo III analysis. Interestingly, transgenic tobacco plants overexpressing the entire TaCRT1 or partial TaCRT1 segments exhibited significantly greater tolerance to salt stress than their WT counterparts, revealing that the C-domain of TaCRT1 is essential for the response to salt stress in plants.
Materials and Methods
Plant Materials and Growth Conditions
The plants used in this study included the hexaploid wheat cv. Wangshuibai and Nicotiana tabacum cv. SamSun. Wangshuibai plants were planted in a field at the experimental station of Nanjing Agricultural University, Nanjing, China, unless otherwise indicated. Tobacco plants were planted in a controlled environment chamber (150 μmol photons m-2 s-1, 16 h light/8 h dark per day at 25/16°C). Wheat roots, stems, leaves, glumes, inner bracts, outer bracts, pistils, stamens, rachises and awn tissues were collected from field-planted wheat 15 d after anthesis. Six-day-old seedlings were transferred to Petri dishes containing 250 mM NaCl (Nanjing Chemical Reagent) to inflict osmotic stress conditions. The seedlings were grown at room temperature under 16 h of light daily. The root tissues were harvested at 0.5, 3, 6, 9, 12, and 24 h after the transfer to Petri dishes.
Isolation and Sequence Analysis of TaCRTs
The plant CRT proteins were queried against a wheat dbEST (1,071,054 ESTs, the 177th release of GenBank, 2010) using the function tBlastn. Using the criteria of >95% identity and E<e-100, EST hits for wheat CRT genes were retrieved and used in contig assembly with the parameter settings of > 40 bp overlap and > 95% identity after removing possible vector sequence contamination. Genomic DNA was extracted according to the procedure described by Ma et al. [61]. Total RNA was extracted from the wheat samples using Trizol reagent (Invitrogene, USA) following the manufacturer’s protocol and quantified using a spectrometer (Ultrospec 2100 pro, Amersham Pharmacia, England). First strand cDNA was synthesized from 3 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (Promega, USA) and oligo (dT15) primers according to the manufacturer’s instructions. RT-PCR was performed in a 25-μl mixture containing approximately 5 ng of template, 5 pmol of each primer, 5 nmol of each dNTP, 37.3 nmol MgCl2, and 0.5 U Taq DNA polymerase (TaKaRa, Kyoto, Japan). The PCR conditions used were as follows: 94°C for 3 min followed by 30 cycles of 94°C for 20 s, 58°C for 30 s, and 72°C for 1.5 min. The primers used for the RT-PCR are listed in S1 Table. The sequencing was performed by Invitrogen Corporation, Shanghai, China, and the nucleotide sequences were searched for in the NCBI databases.
Open reading frame (ORF) identification, protein translation prediction, molecular mass (MW) calculation and sequence alignment were conducted using Macvector 10.0 software (Accelrys, Oxford, USA). The signal peptide was predicted using WoLF PSORT (http://psort.nibb.ac.jp/), and the conserved domain was predicted using SMART (http://smart.embl-heidelberg.de). A phylogenetic analysis was conducted using the neighbor-joining algorithm included in MEGA4.0 software [62]. The robustness of the phylogenetic tree topology was assessed based on bootstrap values. TaCRT sequences were individually mapped over wheat genome at International Wheat Genome Sequencing Consortia (IWGSC, http://wheat-urgi.versailles.inra.fr/Seq-Repository) [63, 64].
Quantitative Real-time PCR Analysis
Quantitative real-time PCR (qRT-PCR) was performed using a SYBR-Green PCR Mastermix (ToYoBo, Osaka, Japan) and a Bio-Rad iCYCLER iQ5 (Bio-Rad, USA); the 25-μL reactions contained approximately 5 ng of cDNA template, 12.5 μL of SYBR-Green PCR Mastermix (ToYoBo, Osaka, Japan) and 10 pmol of each primer. Each sample was analyzed in triplicate. Data were normalized using the α-Tubulin gene from wheat as the reference [65]. Relative expression was estimated using the 2−ΔΔCt method [66]. Three biologically independent experiments were performed. The primer sequences used are listed in S2 Table.
Expression and Purification of Recombinant Proteins and Calcium-binding Analysis
An Escherichia coli expression plasmid was constructed using full-length cDNA from TaCRT1, which was amplified using the following primers: forward 5'-AAGGATCCATGGCGATCCGCCGTG-3', reverse 5'CCGTCGACCATCCATTTAGAGCTCATCGTG-3' (BamHI and SalI sites are underlined). The PCR products were digested with BamHI and SalI and inserted into a pET32a IPTG-inducible expression vector (Qiagen Inc., Chatsworth, CA). The construct was expressed in E. coli host strain BL21 (DE3). The bacteria were cultured at 37°C until OD600 = 0.6, at which point IPTG (Merck Chemicals, Shanghai, China) was added to a final concentration of 1 mM; the culture was then further cultured at 37°C for an additional 3 h. Induced E. coli cells were pelleted by centrifugation at 12,000 g for 5 min and resuspended in extraction buffer (50 mM sodium phosphate buffer, pH 8.0, 3 M NaCl and 1 mM PMSF). The recombinant protein was purified by capturing its 6x histidine tag on a Ni-NTA column (nickel nitrilotriacetic acid; Qiagen Inc.) under denaturing conditions. The protein was purified according to the protocol provided by the manufacturer. The calcium-binding capacity of the purified recombinant TaCRT1 protein was determined using PIPES/Arsenazo III analysis [67].
Construction of TaCRT1 Plant Expression Vectors and Plant Transformation
To generate cauliflower mosaic virus (CaMV) 35S-driven constructs, the ORF and segments of TaCRT were amplified using pfu DNA polymerase (Dingguo Biotech, China) and the primers listed in S3 Table. The amplified TaCRT1 included the ER import signal sequence (the SS region), the P-domain, the C-domain and the ER-retention signal HDEL. Restriction-digested PCR products were inserted into a modified pBI121 expression vector, which was kindly provided by Dr. Deyue Yu of Nanjing Agricultural University. The recombinant vector was then transformed into Agrobacterium tumefaciens strain LBA4404 by electroporation. The tobacco plants were transformed by the leaf disc method [68] using Agrobacterium tumefaciens strain LBA4404. Positive transgenic plants overexpressing TaCRT1 were first screened on agar solidified with Murashige and Skoog salts [69] and 1% sucrose (MS medium) containing 150 mg/L of kanamycin, and then by PCR (S1A Fig) with the primers presented in S3 Table. The expression of TaCRT1 or TaCRT1 segments in T2 transgenic lines was detected by RT-PCR (S1B Fig) with the same set of primers presented in S3 Table. Homozygous T2 transgenic plants were identified by analyzing the segregation of T3 seeds germination on MS medium containing 150 mg/L of kanamycin.
Salt Stress Treatments of Tobacco Plants
Seeds from homozygous T3 transgenic and non-transgenic tobacco plants were allowed to germinate in MS medium in the culturing room. Two-week-old seedlings were transferred into pots filled with a mixture of soil and vermiculite and given 16 h light/8 h dark per day at 25/16°C. Seedlings at the four-leaf stage were irrigated with saline water containing 150 mM or 250 mM NaCl every 2 d in the greenhouse for 20d to evaluate their salt tolerance. We use a less severe stress of 150 mM NaCl (according to Deng et al. [70]) for better evaluate the differences in root development and a more severe stress of 250 mM NaCl (according to Sheveleva et al. [71]) for better appreciate the differences in seedling development between transgenic and WT plants in response to salt stress. The length of the root and the weight of fresh roots were measured after the 20th day of the NaCl treatment.
Germination Assay
Seeds from T3 transgenic and non-transgenic tobacco plants were sown on filter paper in Petri dishes soaked with distilled water containing 150 mM NaCl. One hundred and fifty seeds were sown on three plates for each line. After stratification at 4°C for 2 d, the plates were moved to a chamber at room temperature and exposed to 15 h of light per day. Germination (emergence of the primary root) was scored daily until 6 days after sowing.
Leaf Disc Assays and Measurement of Chlorophyll Contents
The leaf discs of 1 cm diameter were cut from the healthy, fully expanded leaves of transgenic and WT plants grown under unstressed condition and floated on 400 mM NaCl for 4 days according to Negi et al. [72]. For measurement of chlorophyll contents, ten leaf discs were thoroughly homogenized in 95% ethanol (v/v) and centrifuged at 3,000 ×g for 2–3 min. The O.D. of each supernatant was recorded at 663 and 645 nm using a spectrophotometer (PGENERAL UV-1800S, Beijing, China), and the chlorophyll content was calculated per gram of fresh tissue according to Arnon [73].
Antioxidant Enzyme Activities
The activities of antioxidant enzymes such as peroxidase superoxide dismutase (SOD, EC 1.15.1.1), (POD, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.6), were measured in the roots from potted seedlings at the four-leaf stage of WT and transgenic tobacco under normal growth conditions. The activities of SOD and CAT were measured as described in Beyer and Fridovich [74] and Corbisier et al. [75], respectively. POD activity was measured spectrophotometrically by monitoring the increase in absorbance at 460 nm following the method of Polle et al. [76].
Statistical Analysis
The data sets were compared using the pairwise t test module included in Microsoft Office Excel 2008.
Results
Isolation and Characterization of CRT Genes from Wheat
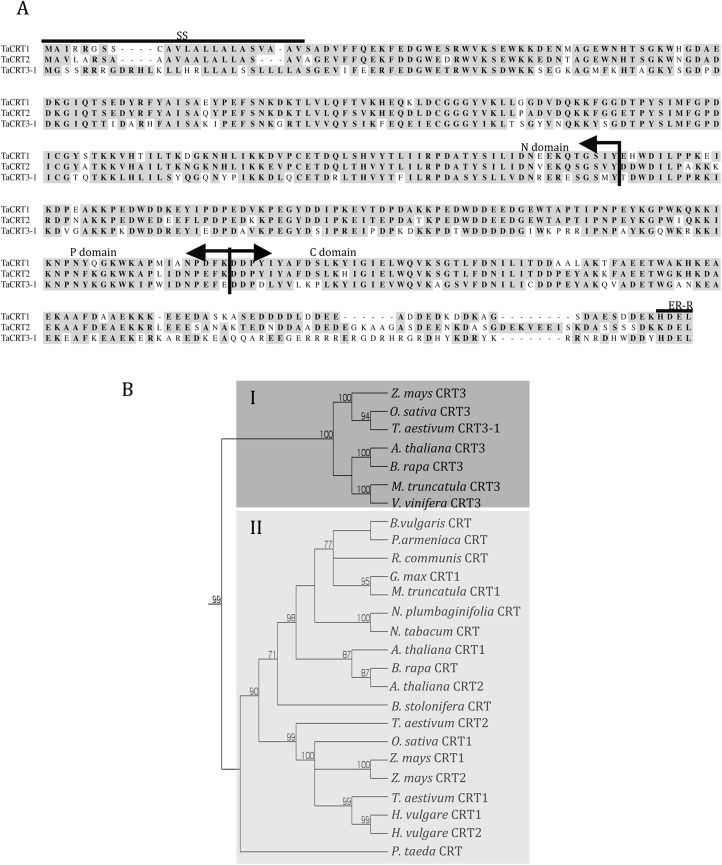
Publicly accessible wheat expressed sequence tag (EST) databases (http://www.ncbi.nlm.nih.gov/) were searched using the tBLASTn protocol with twenty-three plant CRT proteins (S4 Table) as queries. Three significant contigs with full-length ORFs (FL-ORFs) were obtained. Based on the sequence information, three pairs of gene-specific primers were designed. Three cDNA fragments (1473, 1473 and 1467 bp) covering FL-ORFs of corresponding CRT genes were obtained by PCR amplification from spike tissues of the Wangshuibai hexaploid wheat cultivar and were designated TaCRT1 (Accession number: AY836753), TaCRT2 and TaCRT3-1, respectively, according to their sequence similarity to the known CRT genes. BLAST searches against the wheat genome at IWGSC revealed that TaCRT1 was located on chromosome arm 2DL (with two additional homeologous copies on 2AL and 2BL, respectively), TaCRT2 on 5DL (with two additional homeologous copies on 4AL and 5BL, respectively) and TaCRT3-1 on 3AL (with two additional homeologous copies on 3BL and 3DL, respectively). The sizes of the ORFs were 1248, 1287 and 1287 bp, the corresponding protein MWs were 47.2, 48.3 and 50.3 kDa and the protein isoelectric points were 4.32, 4.34 and 8.73, respectively. The proteins encoded by these three genes presented the typical domain patterns of CRT proteins [23]: a conserved N-terminal domain (N-domain), a proline-rich region (P-domain), a highly acidic region (C-domain) at the C-terminus and a C-terminal region ending with the ER-retention signal HDEL (Fig 1A). The P- and C-domains play a critical role in determining the Ca2+ storage capacity of the ER, and the N-domain might participate in the chaperone functions of the protein [37]. Using the PSORT function (http://psort.nibb.ac.jp/), it was found that a potential N-terminal ER import signal sequence (SS region) exists in all three proteins (Fig 1A) and that the C-terminal regions of these three proteins end with the ER-retention signal HDEL [23].
Fig 1. Primary structure comparison among wheat CRT proteins (A) and phylogenetic analysis of plant CRT proteins (B).
Dark shading indicates conserved residues. SS, the ER import signal sequence; ER-R, the ER-retention signal HDEL. The protein sequences used to construct the phylogenetic tree are presented in S4 Table.
The amino acid sequences of the three wheat CRT proteins were then aligned with 23 plant CRT sequences and one M. fuscata CRT sequence. A phylogenetic tree was constructed based on the amino acid sequences and demonstrated the existence of two main clades (Fig 1B). TaCRT1 and TaCRT2 fell within one clade (group II in Fig 1B), and TaCRT3 was grouped into another clade (group I in Fig 1B); this finding is indicative of evolutionary conservation among the members of different clades. In each clade, CRT proteins from monocot and dicot species were clearly separated as sub-groups, which clustered distinctively. Within the first clade, TaCRT1/2 were grouped into the monocot sub-group together with CRT1/2 from barley, CRT1/2 from maize and CRT1 from rice, and these proteins were clearly related to CRT1/2 from barley. Interestingly, TaCRT2 was most closely related to CRT1 from rice; moreover, TaCRT3-1 was paired with an orthologous CRT3 from rice. Within the second clade, TaCRT3-1 was grouped into the monocot sub-group together with CRT3s from maize and rice. Dedicated nucleotide and amino acid sequence alignments showed, respectively, 95% (nucleotide) and 96% (amino acid) identity between TaCRT3-1 and a previously submitted wheat CRT (EF452301) [18], and 99% (nucleotide and amino acid) identity between TaCRT3-1 and another previously submitted wheat CRT (HM037186) [19]. This means that our TaCRT3-1 was identical to HM037186, and homeologous to EF452301, which has been showed to be involved in defense (yellow rust infection) responses and stress (dehydration) resistance, but TaCRT1/2 have never been studied in wheat so far.
Expression Patterns of TaCRT Genes
qRT-PCR was performed to determine the expression patterns of the TaCRT genes in different wheat tissues. The three TaCRT genes were expressed in all tested organs (Fig 2A); however, there was an obvious difference in transcript abundance. TaCRT1 and TaCRT2 transcripts were mainly detected in stems, leaves, pistils and awns at similar levels; TaCRT3-1 showed ~4- to 5-fold increase in stems, leaves and awns, and ~1.5- to 5-fold decrease in roots, glumes and pistils compared to TaCRT1/2, suggesting that TaCRT3-1 and TaCRT1/2 are functionally distinct during wheat growth.
Fig 2. Expression of TaCRT genes and their response to salt stress in wheat.
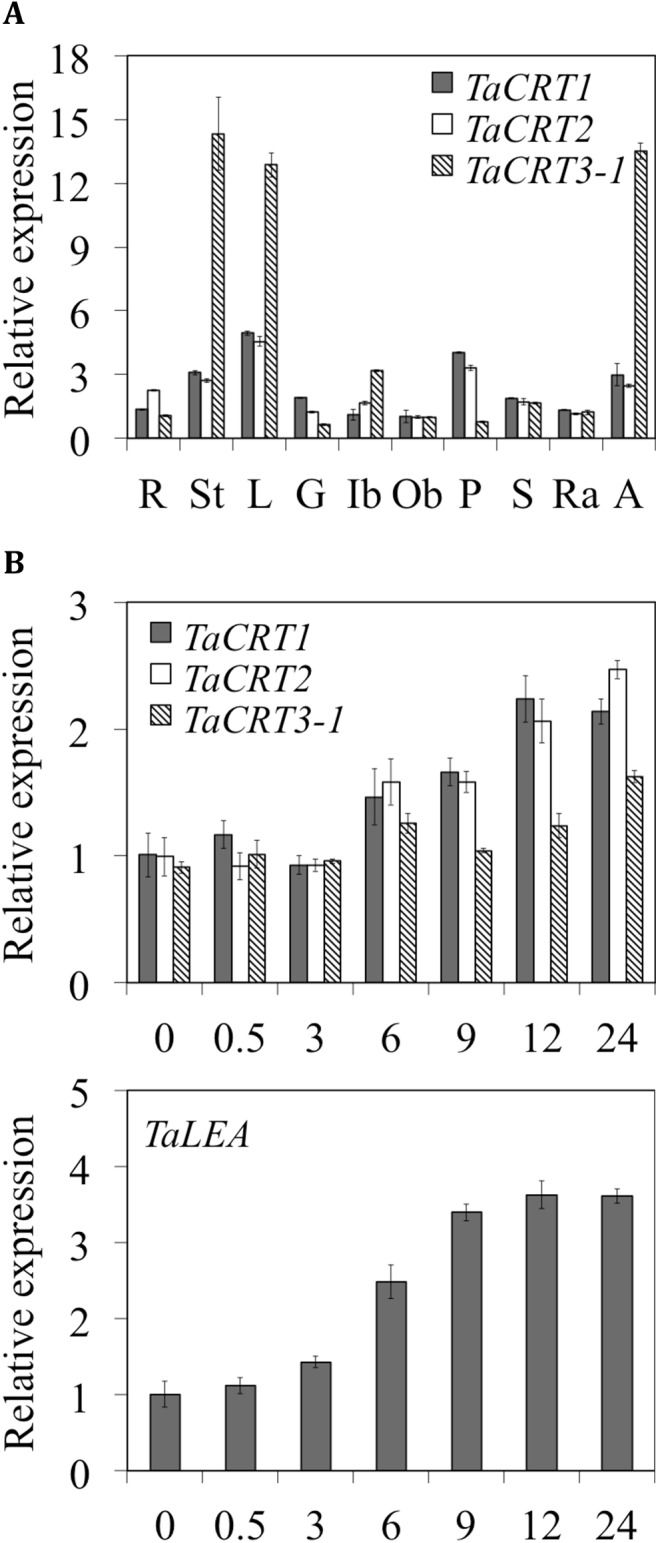
(A) Expression of TaCRT genes in various wheat tissues collected from field-grown plants at 15 days after anthesis, as measured using quantitative real-time PCR. R, seedling roots; L, leaves; St, spikes; G, glume; Ib and Ob, inner bract and outer bract, respectively; P and S, pistil and stamen, respectively; Ra and A, rachis and awn, respectively. (B) Expression patterns of TaCRT and TaLEA genes in wheat seedling roots after treatment with 250 mM. ‘0’ represents no treatment. Error bars represent the standard deviation of results obtained for three replicate experiments.
We examined the TaCRT expression patterns in response to treatment with 250 mM NaCl for various times. The transcript levels of the three TaCRT genes showed ~1.2- to 1.5-fold increase 6 h after the treatment, suggesting that these genes are up-regulated by NaCl stress (TaCRT1 appeared to be induced or up-regulated 0.5 h after the NaCl treatment; Fig 2B). However, under the NaCl treatment, the expression pattern of TaCRT3-1 was obviously different from those of TaCRT1 and TaCRT2 in roots. Likewise, the expression of TaCRT1 and TaCRT2 was more sensitive to NaCl stress and resulted in significantly higher transcript levels than those of TaCRT3 6–24 h after the NaCl stress.
The expression pattern of the wheat late embryogenesis abundant (LEA) protein gene TaLEA was analyzed in parallel on the same sets as a positive control (Fig 2C). LEA has previously been shown to be up-regulated by NaCl treatment in various plants [77–81].
TaCRT1 Encodes a Calcium-binding Protein
The complete ORF of TaCRT1 was cloned in a pET32a vector and expressed in E. coli. The recombinant protein was produced in E. coli after induction with IPTG. The 6xHis:TaCRT1 protein migrated on a SDS-PAGE gel with a mobility corresponding to a protein of approximately 70 kD (Fig 3A, lane 4), and the purified TaCRT1 protein, after thrombin cleavage, migrated with a mobility corresponding to a protein of approximately 50 kD (Fig 3A, lane 5), a weight that is slightly larger than the predicted molecular mass (47.2 kD). This difference might be due to glycosylation of the calreticulin. For the 6xHis-only control, the expected band at a position corresponding to 20 kDa was observed (Fig 3A, lane 2). The calcium binding assay showed that the TaCRT1 protein bound approximately 33.3 mol of Ca2+ per mol of protein (Fig 3B). Taken together, these results provide direct evidence that the gene product of TaCRT1 is an authentic calcium-binding protein.
Fig 3. Purification of the 6xHis:TaCRT1 protein (A) and Scatchard plot of calcium binding to the affinity-purified TaCRT1 protein (B).
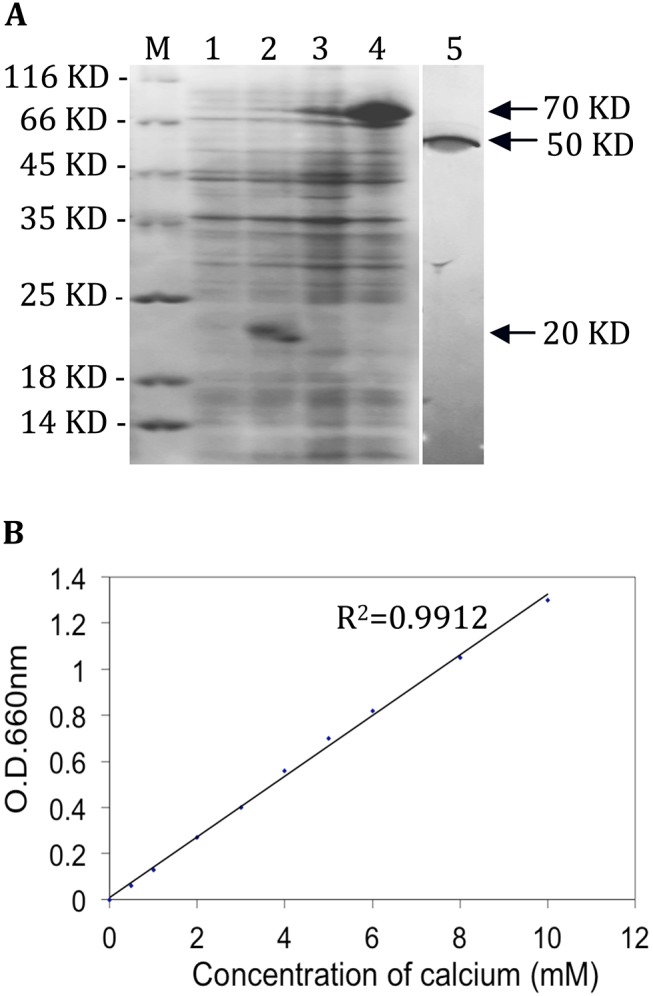
The 6xHis and 6xHis:TaCRT1 proteins isolated from non-induced cells (lanes 1 and 3) and IPTG-induced cells (lanes 2 and 4) and purified TaCRT1 proteins obtained after thrombin cleavage (lane 5) were resolved on an SDS-PAGE gel and stained with Coomassie Blue. Lanes 1–4 contain 2 μg of protein each.
TaCRT1 Overexpression Improves Salt Tolerance and Enhances Antioxidant Enzyme Activities in Tobacco
The growth of T3 transgenic tobacco (T6 and T10) and WT seedlings exposed to 150 mM NaCl stress was examined (Fig 4). The TaCRT1 transgenic seedlings exhibited significantly enhanced shoot growth (or leaf area) and root length (or root fresh weight) compared with WT plants after 20 days of exposure to 150 mM NaCl. In contrast, only small differences were observed between the transgenic and WT seedlings on day 0 (no treatment) or on day 20 under normal growth conditions (irrigated with water; Fig 4A and 4B).
Fig 4. Overexpression of TaCRT1 enhanced salt stress tolerance in transgenic tobacco plants.
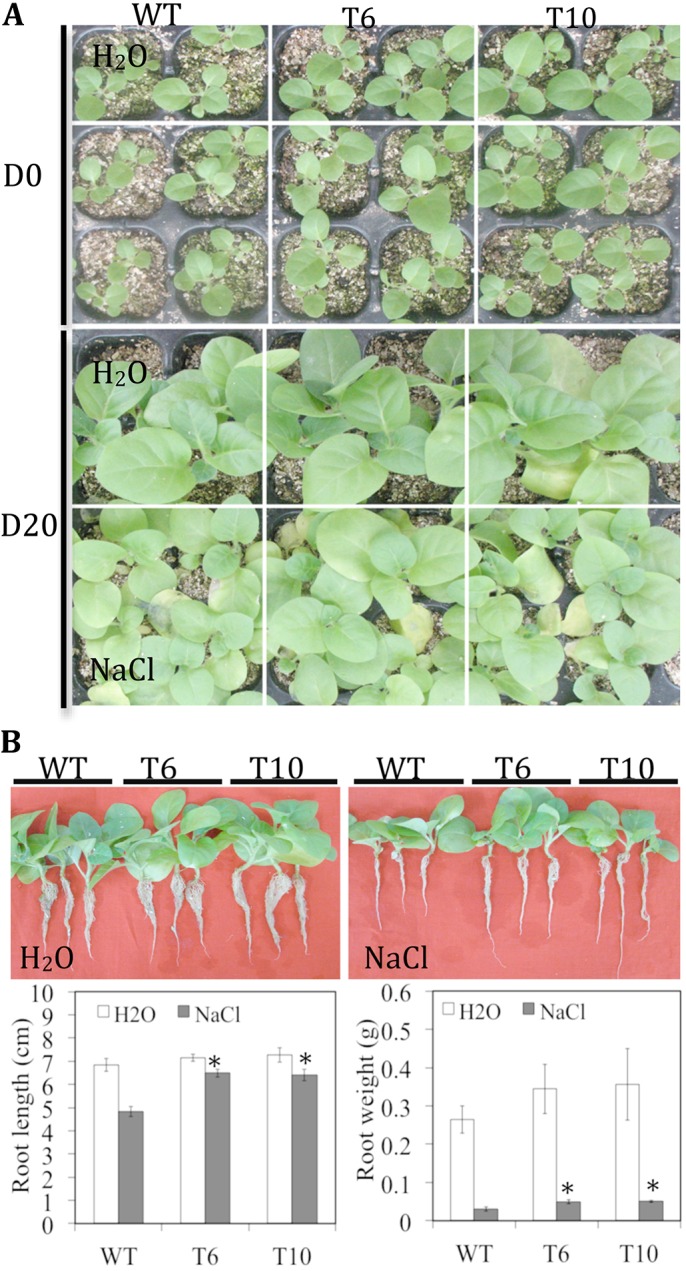
(A) Response of WT and transgenic tobacco lines to irrigation with 150 mM NaCl every 2 d. (B) Root length and fresh root weight of WT and transgenic tobacco lines treated with 150 mM NaCl. WT represents non-transgenic tobacco; T6 and T10 represent transgenic tobacco lines. Error bars represent the standard deviation of results obtained for three replicate experiments; asterisks indicate significant differences from WT plants at P = 0.05.
In addition, the germination of T3 transgenic (T6 and T10) tobacco seeds and WT seeds were tested in the presence of NaCl. The TaCRT1 transgenic seeds exhibited superior germination efficiency over WT seeds in the presence of 150 mM NaCl (Fig 5A). The mean germination rate from day 0 to day 6 after sowing showed that the germination of both WT and transgenic seeds was delayed at least one day or more by the treatment with 150 mM NaCl, compared with the germination of seeds in unsalted water (Fig 5B). Moreover, the TaCRT1 transgenic seeds exhibited greater tolerance to 150 mM NaCl (beginning from day 3) than WT seeds, as demonstrated by their higher germination rate.
Fig 5. Germination rates of TaCRT1-overexpressing tobacco plants and WT plants under salt stress and activities of antioxidant enzymes in the roots of transgenic and non-transgenic tobacco.
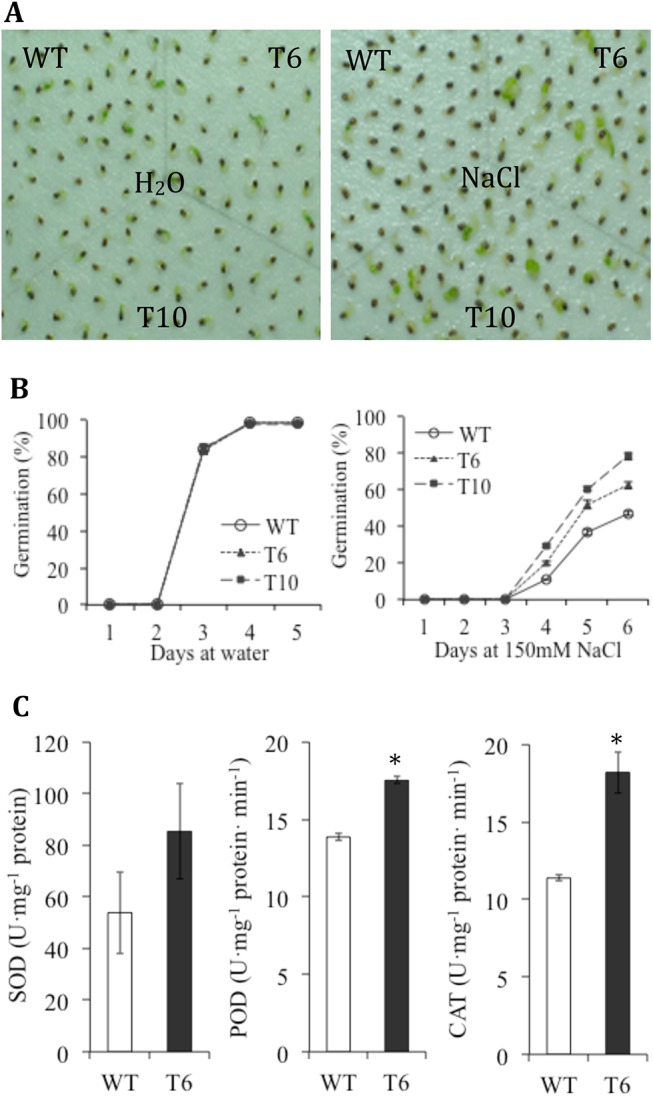
(A) Germination at 25°C ambient temperature of seeds sown on filter papers saturated with water or with 150 mM NaCl. Photographs were taken 6 d after sowing. (B) Germination rate from day 0 to day 6 after sowing. WT represents non-transgenic tobacco; T6 and T10 represent transgenic tobacco lines. Error bars represent the standard deviation of results obtained for three replicate experiments. (C) Activities of antioxidant enzymes SOD, POD and CAT in the roots from potted WT and transgenic (T6) tobacco under normal growth conditions.
The activities of antioxidant enzymes such as SOD, POD and CAT were measured in the roots from potted WT and transgenic tobacco (T6) under normal growth conditions. The transgenic line showed higher and SOD, POD and CAT activities in roots than WT plants (Fig 5C), but the difference was statistically not significant for SOD activity. These results suggested that the overexpression of TaCRT1 can enhance the antioxidant enzyme activities in tobacco.
Overexpression of Partial TaCRT1 Segments Increases the Salinity Tolerance in Transgenic Tobacco Plants
The growth of T3 transgenic tobacco seedlings of T6 (entire TaCRT1), T19 (omission of N-domain), T27 (omission of N- and P-domain) and WT seedlings was examined under exposure to 250 mM NaCl stress (Fig 6A). In each case, the growth of the transgenic seedlings was indistinguishable from that of WT seedlings grown under normal conditions. The results showed that the transgenic lines overexpressing the entire TaCRT1 gene or partial TaCRT1 segments exhibited better tolerance to salinity stress than their WT counterparts.
Fig 6. TaCRT1 segments in transgenic tobacco exhibited greater chlorophyll content.
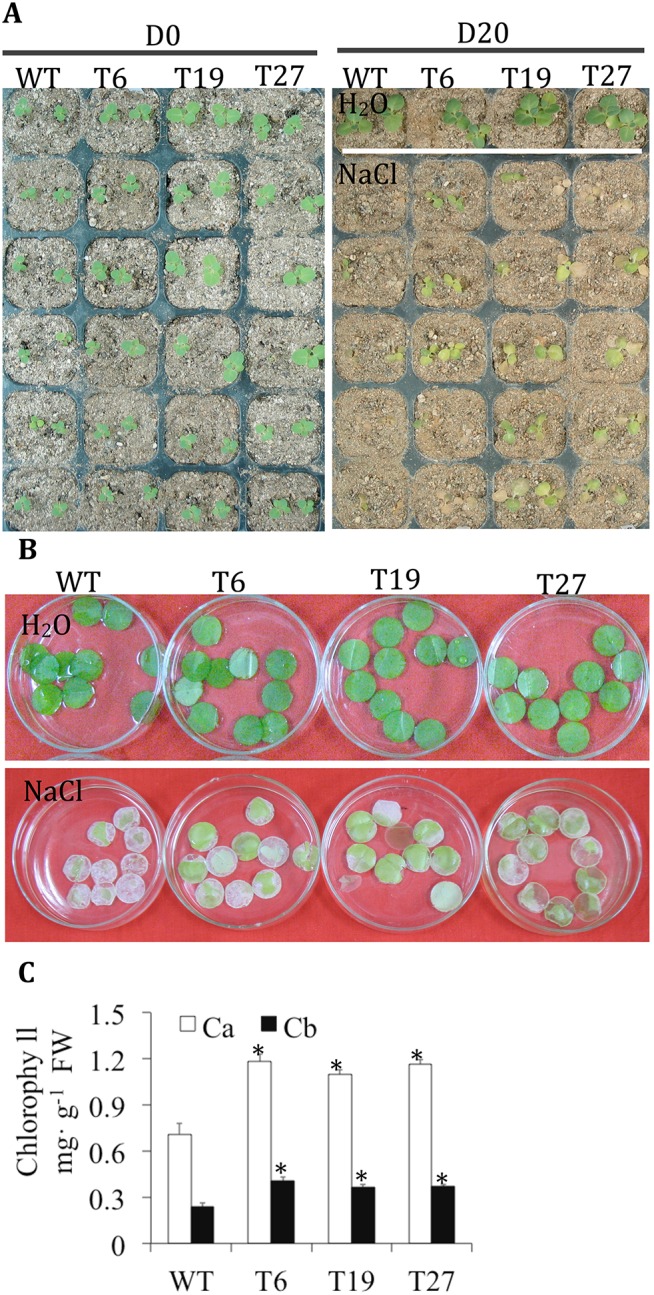
(A) Phenotypes of transgenic tobacco lines under irrigation with 250 mM NaCl every 2 d. (B) Response of leaf discs obtained from WT and transgenic tobacco lines floating on a 400 mM NaCl solution. (C) Chlorophyll content of leaf discs obtained from WT and transgenic tobacco lines. WT represents non-transgenic tobacco; T6, T19 and T27 represent transgenic tobacco lines. D0 represents no treatment, D20 represents NaCl treatment for 20 d; Ca: chlorophyll a; Cb: chlorophyll b. Error bars represent the standard deviation of results obtained for three replicate experiments; asterisks indicate significant differences from WT plants at P = 0.05.
Leaf disc assays were also performed to determine the salinity tolerance of WT and T3 transgenic plants (T6, T19 and T27). Leaf discs from WT plants began to turn yellow after 3 days and were completely bleached after 5 days of treatment in a 400 mM NaCl solution; in contrast, leaf discs from T6, T19 and T27 plants exhibited greater tolerance under the same conditions (Fig 6B). The contents of chlorophyll a and b in the T6, T19 and T27 transgenic plants were significantly higher than the chlorophyll contents of WT plants (Fig 6C).
Discussion
CRT is a widespread ER protein involved in many plant cellular processes, such as protein folding and calcium homeostasis [53, 55]. Dicot CRT genes have been well characterized, but monocot CRT genes remain poorly understood. In this study, three cDNAs from wheat, namely TaCRT1, TaCRT2 and TaCRT3-1, which encode different CRT proteins, were identified (Fig 1A). The derived proteins contained domain patterns that are typical of plant CRTs [23]. Comparison of the putative wheat CRT sequences with CRTs from other plant species revealed that plant CRTs can be divided into two distinct groups: the CRT1/2 group and the CRT3 group (Fig 1B), as described in previous studies [56].
TaCRT1/TaCRT2 and TaCRT3-1 have orthologous isoforms in other species (Fig 1B). Sequence analysis showed that TaCRT1/TaCRT2 exhibits higher sequence identity with CRT1/CRT2 of barley and maize and with CRT1 of rice, whereas TaCRT3-1 exhibits high sequence identity with CRT3s of monocots such as rice and maize and with CRT3s of dicots such as Arabidopsis, rapeseed, grape and M. truncatula (Fig 1B). These findings suggest that at least three different CRT genes occur in the hexaploid wheat genome and that the isolated cDNAs of TaCRT1, TaCRT2 and TaCRT3-1 encode wheat CRT1, CRT2 and CRT3 isoforms, respectively. Because wheat is hexaploid, each of the three cloned wheat CRT genes has three homeologous copies in the wheat genome: TaCRT1 on chromosome arm 2DL with two additional homeologous copies on 2AL and 2BL, respectively, TaCRT2 on 5DL with two homeologous copies on 4AL and 5BL, respectively, and TaCRT3-1 on 3AL with two homeologous copies on 3BL and 3DL, respectively. One of the three TaCRT2 homeologous copies was located on a 4AL/5AL translocated chromosomal segment [82].
Similarly, our analysis suggests that the TaCRT1/TaCRT2 and TaCRT3-1 isoforms result from an early duplication event in wheat that might predate the evolutionary split of plants into dicots and monocots [83], possibly even the split between the animal and plant kingdoms [84]. In addition, Fig 2B shows that the CRT1/2 genes evolved faster than the CRT3 gene in plants.
Interestingly, CRTs were implicated in plant growth and development [8] due to their role in regulating calcium signaling and assisting protein folding [85]. In addition, the expression of CRT genes is up-regulated by a wide range of developmental and environmental stimuli, including cold [86, 87] and pathogens [88]. Jia et al. [18] found that the expression of a CRT3 gene (EF452301) was significantly enhanced by PEG-induced drought stress in wheat seedlings. An et al. [19] found that the expression of another CRT3 gene (HM037186) could be induced by Puccinian striiformis infection and cold treatment, but suppressed by dehydration in wheat seedlings. In this study, wheat CRT1/CRT2 and CRT3 differed in their expression patterns during plant development (Fig 2A) and were all up-regulated by NaCl salt stress (Fig 2B). Moreover, the CRT1 and CRT2 were induced at higher levels than CRT3 under the same salt stress (Fig 2B). The up-regulation of CRTs is considered a conserved self-protection mechanism that was acquired during a long-term evolutionary process and that is likely to facilitate the survival of plants under unfavorable osmotic conditions [18].
In plants, the Arabidopsis AtCRT1a protein and several other CRT proteins have already been shown bind calcium [53,55]. The cDNA of TaCRT1 was expressed in E. coli, and the purified protein was confirmed to possess Ca2+-binding properties (Fig 3A and Fig 3B), as expected for a typical CRT protein [22,23]. The result showing that TaCRT1 possesses a Ca2+-binding function provides direct evidence that TaCRT1 can sequester Ca2+. The highly conserved primary structure of TaCRT1/2 and TaCRT3 proteins among higher plants suggests that their fundamental functions might be conserved during the evolution of plants.
The overexpression of TaCRT1 in tobacco enhanced seed germination (Fig 5) and significantly improved shoot growth (or leaf area) and root length (or root fresh weight) compared with WT plants under NaCl stress (Fig 4). In addition, similar phenotypes were observed in transgenic lines overexpressing partial TaCRT1 segments that contained only the C-domain or the P- and C-domain (Fig 6A). Transgenic lines overexpressing the entire TaCRT1 gene or partial TaCRT1 gene segments exhibited better tolerance to the NaCl stress than their WT counterparts, revealing that the C-domains play essential roles in the response to NaCl stress, at least in plants. These results are consistent with those obtained in a previous study, which demonstrated that expression of the high-capacity calcium-binding domain of CRT increased the storage of bio-available calcium in plants [52]. Likewise, transgenic Arabidopsis plants expressing the C-domain of maize CRT exhibited significant resistance to drought, salt and heavy metal stresses [52]. To further consolidate our present results, the partial TaCRT1 segments could be expressed in E. coli and the purified proteins might be also used for calcium-binding analysis to confirm their capacity of calcium-binding, respectively.
Salt stress can induce an increase in the production of cytotoxic reactive oxygen species (ROS) and oxidative damage in plants [89], and there is a constant need for efficient mechanisms to avoid oxidative damage to cells [90]. One of the most important plant strategies is to reduce the oxidative damage through improved antioxidant capacity. Antioxidant enzymes, such as SOD, POD and CAT etc. catalyze the scavenging of ROS and combat the oxidative damages induced by stresses [91]. A correlation between antioxidant capacity and salinity tolerance has been demonstrated in several plant species [92]. Our results showed that the transgenic tobacco overexpressing TaCRT1 gene displayed higher SOD, POD and CAT activities in roots than non-transgenic tobacco (Fig 5C). These suggested that TaCRT1 might confer the salt stress tolerance by enhancing the activities of antioxidant enzymes, which in turn protected transgenic tobacco against ROS-mediated damage under salt stress.
Calcium is an essential second messenger that mediates plant responses to developmental and environmental clues, increasing evidence supports that calcium levels are altered in plant cells in response to salt stress [93]. In plants, CRT plays important roles in a variety of cellular processes including regulating the Ca2+ homeostasis and protein folding [50]. The results here presented demonstrated that TaCRT1 encodes a calcium-binding protein containing the three-domain structure typical of calreticulin protein (Fig 1A). This suggested that the activities of antioxidant enzymes might be enhanced by Ca2+ signaling mediated by TaCRT1 protein.
Jia et al. [18] showed that transgenic tobacco plants overexpressing a wheat CRT3 gene (EF452301) exhibited enhanced drought resistance by their capacity to maintain higher water use efficiency, water retention ability, relative water content, and lower membrane damaging ratio under water deficit condition. Tsou et al. [94] showed that the overexpression of maize CRT1 could improve tolerance to both salt and drought stresses in Arabidopsis, and meanwhile increase the total plant Ca2+ by ~25% and the expression level of calcineurin B-like protein-interacting protein kinases 6 (CIPK6), which is a member of the CIPK gene family. Deng et al. [95] showed that the overexpression of wheat CIPK14 exhibited higher CAT activity, while decreased amounts of H2O2 and malondialdehyde, and less ion leakage under salt stresses, which then lead to salinity and cold tolerance in tobacco. Deng et al. [70] showed that the overexpression of wheat CIPK29 can also result in an increased salt tolerance, accompanied by an increase of the expression level and activities of CAT and POD under salt stress in tobacco. The work presented here suggested a link between TaCRT1 and antioxidant enzymes. The overexpression of TaCRT1 could increase Ca2+ level, which would trigger the Ca2+ signaling downstream targets, like CIPK genes. CIPK genes might then regulate appropriate downstream responses such as the changes in the expression of protein kinases, transcription factors, and antioxidant enzymes, etc. Further studies are needed to examine if overexpression of TaCRT1 could also improve tolerance to other stresses, such as drought and cold in tobacco or other plants.
Our study also showed that the leaf discs of transgenic tobacco plants tolerate salt better than those of non-transgenic plants (Fig 6B). Moreover, chlorophyll content was significantly higher in transgenic plants than in WT plants (Fig 6C). These results suggest that TaCRT1 overexpression might increase the chlorophyll content in leaves. This study constitutes the first demonstration of an increase in chlorophyll content triggered by the overexpression of CRT genes. Our results are consistent with the study of Wyatt et al. [52], in which they found that overexpression of the C-domain of maize CRT1 could delay the loss of chlorophyll in transgenic Arabidopsis plants on media lacking external Ca2+.
In the present study, we used the dicot tobacco (N. tabacum), one of the most-studied hosts for developmental and molecular genetic analysis, as host plant for TaCRT1 overexpression analysis. Future studies with transgenic monocot wheats overexpressing TaCRT1 or partial TaCRT1 segments will help to consolidate the results of present work and elucidate better the in vivo mechanism of TaCRT1-mediated salinity tolerance in plants. As TaCRT2 and TaCRT1 have high sequence similarity to each other (Fig 1) and showed similar expression patterns clearly different from TaCRT3-1 (Fig 2), further studies by overexpressing TaCRT2 in tobacco or wheat will allow to know if the two genes had the same role in plant salinity tolerance.
In conclusion, three CRT genes, TaCRT1, TaCRT2 and TaCRT3-1, were identified in hexaploid wheat. TaCRT1 overexpression in tobacco improved salt tolerance, as manifested by a higher seed germination rate, significantly enhanced shoot growth (or leaf area) and root length (or root fresh weight) and greater chlorophyll content compared with WT plants. These results suggest that CRT genes are potential targets for improving environmental stress resistance in agricultural crops such as wheat and tobacco through genetic manipulation.
Supporting Information
(A): PCR with genomic DNA of young leaves. (B): RT-PCR with root tissues. WT, non-transgenic tobacco; P, positive plasmid DNA; T6, T10, T19, T27, T3 transgenic tobacco plants.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Shengwei Ma for his help with the bioinformation analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was partially supported by NFSC program (31360344), Agricultural Scientific and Technological Research Projects of Guizhou province (No. NY[2013]3009), Science & technology special project of Guizhou Academy of Agricultural Sciences (no. [2014]014), Engineering Technology Research Center Fund of Guizhou Province (No. [2012]4006), and Grand Science and Technology Special Project of Guizhou (no. [2013]6005).
References
- 1. Ostwald TJ, MacLennan DH. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. Journal Biology Chemistry 1974; 249(3): 974–979. [PubMed] [Google Scholar]
- 2. Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. Journal Biology Chemistry 1989; 264(36): 21522–21528. [PubMed] [Google Scholar]
- 3. Smith MJ, Koch GL. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J 1989; 8(12): 3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCauliffe DP, Lux FA, Lieu TS, Sanz I, Hanke J, Newkirk MM, et al. Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. Journal of Clinical Investigation 1990; 85(5): 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dar MA, Wahiduzzaman, Islam A, Hassan MI, Ahmad F. Purification and characterization of calreticulin: a Ca(2+)-binding chaperone from sheep kidney. Applied Biochemistry and Biotechnology 2014; 174(5): 1771–1783. 10.1007/s12010-014-1150-5 [DOI] [PubMed] [Google Scholar]
- 6. Smith MJ. A C. elegans gene encodes a protein homologous to mammalian calreticulin. DNA Sequence 1992a; 2(4): 235–240. [DOI] [PubMed] [Google Scholar]
- 7. Smith MJ. Nucleotide sequence of a Drosophila melanogaster gene encoding a calreticulin homologue. DNA Sequence 1992b; 3(4): 247–250. [DOI] [PubMed] [Google Scholar]
- 8. Menegazzi P, Guzzo F, Baldan B, Mariani P, Treves S. Purification of calreticulin-like protein(s) from spinach leaves. Biochemical and Biophysical Research Communications 1993; 190(3): 1130–1135. [DOI] [PubMed] [Google Scholar]
- 9. Chen F, Hayes PM, Mulrooney DM, Pan A. Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell 1994; 6(6): 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denecke J, Carlsson LE, Vidal S, Hoglund AS, Ek B, van Zeijl MJ, et al. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo . The Plant Cell 1995; 7(4): 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwiatkowski BA, Zielinska-Kwiatkowska AG, Migdalski A, Kleczkowski LA, Wasilewska LD. Cloning of two cDNAs encoding calnexin-like and calreticulin-like proteins from maize (Zea mays) leaves: identification of potential calcium-binding domains. Gene 1995; 165(2): 219–222. [DOI] [PubMed] [Google Scholar]
- 12. Napier RM, Trueman S, Henderson J, Boyce JM, Hawes C, Fricker MD, et al. Purification, sequencing and functions of calreticulin from maize. Journal of Experimental Botany 1995; 46(291): 1603–1613. 10.1093/jxb/46.10.1603 [DOI] [Google Scholar]
- 13. Dresselhaus T, Hagel C, Lörz H, Kranz E. Isolation of a full-length cDNA encoding calreticulin from a PCR library of in vitro zygotes of maize. Plant Molecular Biology 1996; 31(1): 23–34. [DOI] [PubMed] [Google Scholar]
- 14. Lim CO, Kim HY, Kim MG, Lee SI, Chung WS, Park SH, et al. Expressed sequence tags of Chinese cabbage flower bud cDNA. Plant Physiology 1996; 111(2): 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson DE, Glaunsinger B, Bohnert HJ. Abundant accumulation of the calcium-binding molecular chaperone calreticulin in specific floral tissues of Arabidopsis thaliana . Plant Physiology 1997; 114(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coughlan SJ, Hastings C, Winfrey R Jr. Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Molecular Biology 1997; 34(6): 897–911. [DOI] [PubMed] [Google Scholar]
- 17. Li Z, Komatsu S. Molecular cloning and characterization of calreticulin, a calcium-binding protein involved in the regeneration of rice cultured suspension cells. European Journal of Biochemistry 2000; 267(3): 737–745. [DOI] [PubMed] [Google Scholar]
- 18. Jia XY, Xu CY, Jing R, Li RZ, Mao XG, Wang JP, et al. Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. Journal of Experimental Botany 2008; 59(4): 739–751. 10.1093/jxb/erm369 [DOI] [PubMed] [Google Scholar]
- 19. An YQ, Lin RM, Wang FT, Feng J, Xu YF, Xu SC. Molecular cloning of a new wheat calreticulin gene TaCRT1 and expression analysis in plant defense responses and abiotic stress resistance. Genetics and Molecular Research 2011; 10(4): 3576–3585. 10.4238/2011.November.10.1 [DOI] [PubMed] [Google Scholar]
- 20. Pinto RD, Moreira AR, Pereira PJ, Dos Santos NM. Molecular cloning and characterization of sea bass (Dicentrarchus labrax, L.) calreticulin. Fish Shellfish Immunology 2013; 34(6): 1611–1618. 10.1016/j.fsi.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 21. Borhani DN, Basseri HR, Naddaf SR, Heidari M. Molecular characterization of calreticulin from Anopheles stephensi midgut cells and functional assay of the recombinant calreticulin with Plasmodium berghei ookinetes. Gene 2014; 550(2): 245–252. 10.1016/j.gene.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 22. Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: oneprotein, one gene, many functions. Biochemical Journal 1999; 344(Pt 2): 281–292. [PMC free article] [PubMed] [Google Scholar]
- 23. Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochemical Journal 2009; 417(3): 651–666. 10.1042/BJ20081847 [DOI] [PubMed] [Google Scholar]
- 24. Opas M, Szewczenko-Pawlikowski M, Jass GH, Mesaeli N, Michalak M. Calreticulin modulates cellular adhesiveness via regulation of expression of vinculin. The Journal of Cell Biology 1996; 135(6 Pt 2): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brünagel G, Shah U, Schoen RE, Getzenberg RH. Identification of calreticulin as a nuclear matrix protein associated with human colon cancer. Journal of Cellular Biochemistry 2003; 89(2): 238–243. [DOI] [PubMed] [Google Scholar]
- 26. Afshar N, Black BE, Paschal BM. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Molecular and Cellular Biology 2005; 25(20): 8844–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123(2): 321–334. [DOI] [PubMed] [Google Scholar]
- 28. Shan H, Wei J, Zhang M, Lin L, Yan R, Zhu Y. Calreticulin is localized at mitochondria of rat cardiomyocytes and affected by furazolidone. Molecular and Cellular Biochemistry 2014; 397(1–2): 125–130. 10.1007/s11010-014-2179-z [DOI] [PubMed] [Google Scholar]
- 29. Laporte C, Vetter G, Loudes AM, Robinson DG, Hillmer S, Stussi-Garaud C, et al. Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. The Plant Cell 2003; 15(9): 2058–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen D, Texada DE, Duggan C, Liang C, Reden TB, Kooragayala LM, et al. Surface calreticulin mediates muramyl dipeptide-induced apoptosis in RK13 cells. Journal of Biological Chemistry 2005; 280(23): 22425–22436. [DOI] [PubMed] [Google Scholar]
- 31. Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 2010; 24(3): 665–683. 10.1096/fj.09-145482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. International Journal of Biochemistry & Cell Biology 2012; 44(6): 842–846. [DOI] [PubMed] [Google Scholar]
- 33. Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biological Chemistry 1996; 271(16): 9332–9339. [DOI] [PubMed] [Google Scholar]
- 34. Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, et al. Calreticulin is essential for cardiac development. Journal Cell Biology 1999; 144(5): 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Persson S, Wyatt SE, Love J, Thompson WF, Robertson D, Boss WF. The Ca2+ status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiology 2001; 126(3): 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 1995; 82(5): 765–771. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, et al. Functional specialization of calreticulin domains. Journal Cell Biology 2001; 154(5): 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. Journal Biological Chemistry 2002; 277(48): 46696–46705. [DOI] [PubMed] [Google Scholar]
- 39. Gelebart P, Opas M and Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. International Journal of Biochemistry & Cell Biology 2005; 37(2): 260–266. [DOI] [PubMed] [Google Scholar]
- 40. Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman My. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biological Chemistry 1994; 269(3): 1744–1749. [PubMed] [Google Scholar]
- 41. Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biological Chemistry 1995(9); 270: 4741–4747. [DOI] [PubMed] [Google Scholar]
- 42. Jin H, Hong Z, Su W, Li J. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(32): 13612–13617. 10.1073/pnas.0906144106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, et al. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(37): 15973–15978. 10.1073/pnas.0905532106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang Y, Dey S, Matsunami H. Calreticulin: roles in cell-surface protein expression. Membranes (Basel) 2014; 4(3): 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakono M, Seko A, Takeda Y, Ito Y. PDI family protein ERp29 forms 1:1 complex with lectin chaperone calreticulin. Biochemical and Biophysical Research Communications 2014; 452(1): 27–31. 10.1016/j.bbrc.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 46. Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends in Cell Biology 2001; 11(3): 122–129. [DOI] [PubMed] [Google Scholar]
- 47. Ding H, Hong C, Wang Y, Liu J, Zhang N, Shen C, Wei W, Zheng F. Calreticulin promotes angiogenesis via activating nitric oxide signalling pathway in rheumatoid arthritis. Clinical and Experimental Immunology 2014; 178(2): 236–244. 10.1111/cei.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waterhouse NJ and Pinkoski MJ. Calreticulin: raising awareness of apoptosis. Apoptosis 2007; 12(4): 631–634. [DOI] [PubMed] [Google Scholar]
- 49. Qiu Y, Michalak M. Transcriptional control of the calreticulin gene in health and disease. International Journal of Biochemistry & Cell Biology 2009; 41(3): 531–538. [DOI] [PubMed] [Google Scholar]
- 50. Jia XY, He LH, Jing RL, Li RZ. Calreticulin: conserved protein and diverse functions in plants. Physiologia Plantarum 2009; 136(2): 127–138. 10.1111/j.1399-3054.2009.1223.x [DOI] [PubMed] [Google Scholar]
- 51. Navazio L, Baldan B, Dainese P, James P, Damiani E, Margreth A, et al. Evidence that spinach leaves express calreticulin but not calsequestrin. Plant Physiology 1995; 109(3): 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wyatt SE, Tsou PL, Robertson D. Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Research 2002; 11(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 53. Christensen A, Svensson K, Persson S, Jung J, Michalak M, Widell S, et al. Functional characterization of Arabidopsis calreticulin1a: A key alleviator of endoplasmic reticulum stress. Plant Cell Physiology 2008; 49(6): 912–924. 10.1093/pcp/pcn065 [DOI] [PubMed] [Google Scholar]
- 54. Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Haweker H, et al. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 2009; 28(21): 3439–3449. 10.1038/emboj.2009.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Christensen A, Svensson K, Thelin L, Zhang W, Tintor N, Prins D, et al. Higher plant calreticulins have acquired specialized functions in Arabidopsis . PLOS One 2010; 5(6): e11342 10.1371/journal.pone.0011342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Persson S, Rosenquist M, Svensson K, Galvão R, Boss WF, Sommarin M. Phylogenetic analyses and expression studies reveal two distinct groups of calreticulin isoforms in higher plants. Plant Physiology 2003; 133(3): 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lenartowska M, Karas K, Marshall J, Napier R, Bednarska E. Immunocytochemical evidence of calireticulin-like protein in pollen tubes and styles of Petunia hybrida Hort. Protoplasma 2002; 219(1–2): 23–30. [DOI] [PubMed] [Google Scholar]
- 58. Li Z, Onodera H, Ugaki M, Tanaka H, Komatsu S. Characterization of calreticulin as a phosphoprotein interacting with cold-induced protein kinase in rice. Biological & Pharmaceutical Bulletin 2003; 26(2): 256–261. [DOI] [PubMed] [Google Scholar]
- 59. Komatsu S, Yamada E, Furukawa K. Cold stress changes the concanavalin A-positive glycosylation pattern of proteins expressed in the basal parts of rice leaf sheaths. Amino Acids 2009; 36(1): 115–123. 10.1007/s00726-008-0039-4 [DOI] [PubMed] [Google Scholar]
- 60. Heilmann I, Shin J, Huang J, Perera IY, Davies E. Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini. Plant Physiology 2001; 127(3): 1193–1203. [PMC free article] [PubMed] [Google Scholar]
- 61. Ma ZQ, Sorrells ME. Genetic analysis of fertility restoration in wheat using restriction fragment length polymorphisms, Crop Science 1995; 35(4): 1137–1143. [Google Scholar]
- 62. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 2007; 24(8): 1596–1599. [DOI] [PubMed] [Google Scholar]
- 63. International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014; 345(6194): 1251788. 10.1126/science.1251788 [DOI] [PubMed] [Google Scholar]
- 64. Chapman JA, Mascher M, Buluç A, Barry K, Georganas E, Session A, et al. A whole-genome shotgun approach for assembling and anchoring the hexaploid bread wheat genome. Genome Biology 2015; 16: 26 10.1186/s13059-015-0582-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Konishi S, Sasakuma T, Sasanuma T. Identification of novel Mlo family members in wheat and their genetic characterization. Genes & Genetic Systems 2010; 85(3): 167–175. [DOI] [PubMed] [Google Scholar]
- 66. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001; 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- 67. Leary NO, Pembroke A, Duggan PF. Single stable reagent (Arsenazo III) for optically robust measurement of calcium in serum and plasma. Clinical Biochemistry 1992; 38(6): 904–908. [PubMed] [Google Scholar]
- 68. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science 1985; 227(4691): 1229–1231. [DOI] [PubMed] [Google Scholar]
- 69. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 1962; 15(3): 473–497. 10.1111/j.1399-3054 [DOI] [Google Scholar]
- 70. Deng X, Hu W, Wei S, Zhou S, Zhang F, Han J, et al. TaCIPK29, a CBL-interacting protein kinase gene from wheat, confers salt stress tolerance in transgenic tobacco. PLoS One 2013; 8(7): e69881 10.1371/journal.pone.0069881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sheveleva E, Chmara W, Bohnert HJ, Jensen RG. Increased salt and drought tolerance by D-Ononitol production in transgenic Nicotiana tabacum L. Plant Physiology 1997; 115(3): 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Negi NP, Shrivastava DC, Sharma V, Sarin NB. Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Reports 2015; 34(7): 1109–1126. 10.1007/s00299-015-1770-4 [DOI] [PubMed] [Google Scholar]
- 73. Arnon DI. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris . Plant Physiology 1949; 24(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry 1987; 161(2): 559–566. [DOI] [PubMed] [Google Scholar]
- 75. Corbisier P, Houbion A, Remacle J. A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Analytical Biochemistry 1987; 164(1): 240–247. [DOI] [PubMed] [Google Scholar]
- 76. Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiology 1994; 106(1): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang X, Lu S, Jiang C, Wang Y, Lv B, Shen J, et al. RcLEA, a late embryogenesis abundant protein gene isolated from Rosa chinensis, confers tolerance to Escherichia coli and Arabidopsis thaliana and stabilizes enzyme activity under diverse stresses. Plant Molecular Biology 2014; 85(4–5): 333–347. 10.1007/s11103-014-0192-y [DOI] [PubMed] [Google Scholar]
- 78. Wu Y, Liu C, Kuang J, Ge Q, Zhang Y, Wang Z. Overexpression of SmLEA enhances salt and drought tolerance in Escherichia coli and Salvia miltiorrhiza . Protoplasma 2014; 251: 1191–1199. 10.1007/s00709-014-0626-z [DOI] [PubMed] [Google Scholar]
- 79. Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M. A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant Cell Environment 2014; 37(9): 2024–2035. [DOI] [PubMed] [Google Scholar]
- 80. Liang J, Zhou M, Zhou X, Jin Y, Xu M, Lin J. JcLEA, a novel LEA-like protein from Jatropha curcas, confers a high level of tolerance to dehydration and salinity in Arabidopsis thaliana . PLoS One 2013; 8: e83056 10.1371/journal.pone.0083056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao W, Bai S, Li Q, Gao C, Liu G, Li G, et al. Overexpression of TaLEA gene from Tamarix androssowii improves salt and drought tolerance in transgenic poplar (Populus simonii × P. nigra). PLoS One 2013; 8(12): e67462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jiang Y, Jiang Q, Hao C, Hou J, Wang L, Zhang H, et al. A yield-associated gene TaCWI, in wheat: its function, selection and evolution in global breeding revealed by haplotype analysis. Theoretical and Applied Genetics 2015; 128(1): 131–143. 10.1007/s00122-014-2417-5 [DOI] [PubMed] [Google Scholar]
- 83. Soltis PS, Soltis DE, Chase DW. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 1999; 402(6760): 402–404. [DOI] [PubMed] [Google Scholar]
- 84. Persson S, Rosenquist M, Sommarin M. Identification of a novel calreticulin isoform (Crt2) in human and mouse. Gene 2002; 4(1–2): 151–158. [DOI] [PubMed] [Google Scholar]
- 85. Nardi MC, Feron R, Navazio L, Mariani P, Pierson E, Wolters-Arts M, et al. Expression and localization of calreticulin in tobacco anthers and pollen tubes. Planta 2006; 223(6): 1263–1271. [DOI] [PubMed] [Google Scholar]
- 86. Borisjuk N, Sitailo L, Adler K, Malysheva L, Tewes A. Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta 1998; 206(4): 504–514. [DOI] [PubMed] [Google Scholar]
- 87. Sharma A, Isogai M, Yamamoto T, Sakaguchi K, Hashimoto J, Komatsu S. A novel interaction between calreticulin and ubiquitin-like nuclear protein in rice. Plant Cell Physiology 2004; 45(6): 684–692. [DOI] [PubMed] [Google Scholar]
- 88. Qiu Y, Xi J, Du L, Roje S, Poovaiah BW. A dual regulatory role of Arabidopsis calreticulin-2 in plant innate immunity. Plant Journal 2012; 69(3): 489–500. 10.1111/j.1365-313X.2011.04807.x [DOI] [PubMed] [Google Scholar]
- 89. Ding M, Hou P, Shen X, Wang M, Deng S, Sun J, et al. Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Molecular Biology 2010; 73(3): 251–269. 10.1007/s11103-010-9612-9 [DOI] [PubMed] [Google Scholar]
- 90. Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. Journal of Experimental Botany 2012; 63(11): 4033–4044. 10.1093/jxb/ers126 [DOI] [PubMed] [Google Scholar]
- 91. Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stress. Plant, Cell and Environment 2010; 33(4): 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- 92. Türkan I, Demiral T. Recent developments in understanding salinity tolerance. Environmental and Experimental Botany 2009; 67(1): 2–9. [Google Scholar]
- 93. Boudsocq M, Sheen J. Calcium Sensing and Signaling In Pareek A, Sopory S, Bohnert H, Govindjee, eds, Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation Springer, Dordrecht, The Netherlands, 2010; pp 75–90 [Google Scholar]
- 94. Tsou PL, Lee SY, Allen NS, Winter-Sederoff H, Robertson D. An ER-targeted calcium-binding peptide confers salt and drought tolerance mediated by CIPK6 in Arabidopsis . Planta 2012; 235(3): 539–552. 10.1007/s00425-011-1522-9 [DOI] [PubMed] [Google Scholar]
- 95. Deng X, Zhou S, Hu W, Feng J, Zhang F, Chen L, et al. Ectopic expression of wheat TaCIPK14, encoding a calcineurin B-like protein-interacting protein kinase, confers salinity and cold tolerance in tobacco. Physiologia Plantarum 2013; 149(3): 367–377. 10.1111/ppl.12046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A): PCR with genomic DNA of young leaves. (B): RT-PCR with root tissues. WT, non-transgenic tobacco; P, positive plasmid DNA; T6, T10, T19, T27, T3 transgenic tobacco plants.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.