Abstract
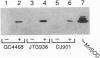
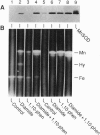
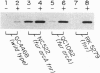
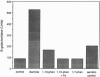
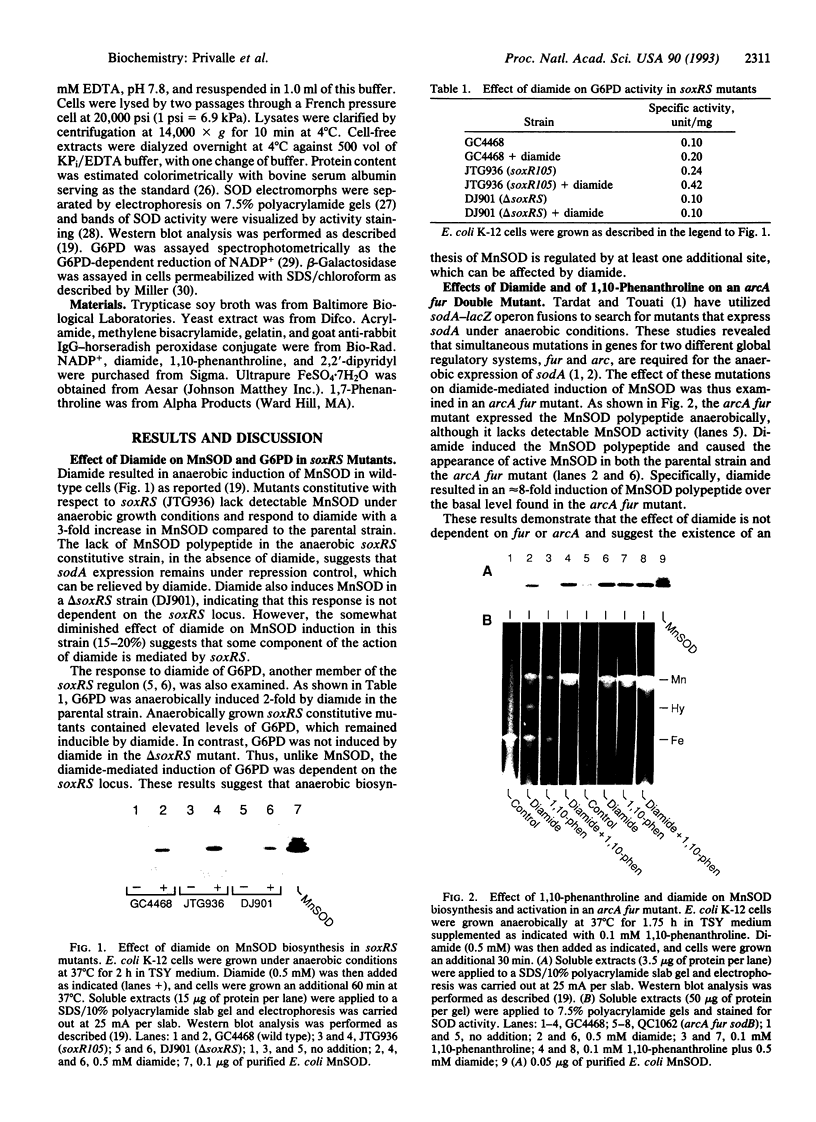
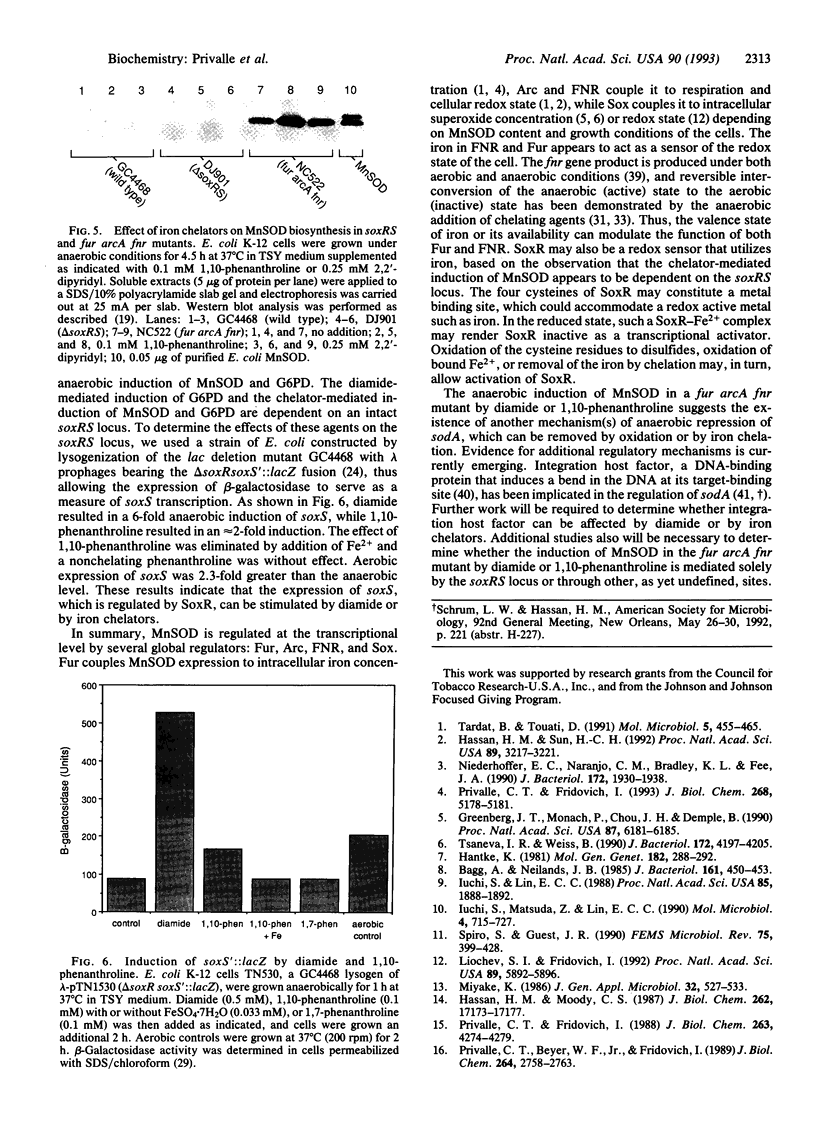
Transcriptional regulation of the sodA gene, a member of the soxRS regulon encoding the manganese-containing superoxide dismutase (MnSOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) of Escherichia coli, was examined in a variety of regulatory mutants. Diamide, an oxidant that causes the anaerobic biosynthesis of the MnSOD polypeptide and also facilitates insertion of manganese at the active site, was found to anaerobically induce MnSOD in both soxRS and fur arcA fnr strains. Metal chelating agents also caused anaerobic induction of MnSOD in a fur arcA fnr triple mutant; however, this induction of MnSOD and of glucose-6-phosphate dehydrogenase (G6PD) by 1,10-phenanthroline was dependent on an intact soxRS locus. A strain of E. coli bearing a fusion of the soxS promoter to lacZ was used to demonstrate that both diamide and 1,10-phenanthroline caused anaerobic activation of soxS transcription. These results indicate that (i) both diamide and 1,10-phenanthroline induce the soxRS regulon anaerobically by stimulation of soxS transcription; (ii) diamide, but not metal chelators, also induces MnSOD biosynthesis by a soxRS-independent mechanism, perhaps mediated by effects on fur, arcA, or fnr-mediated repression of sodA; and (iii) the soxRS locus contains a metal-binding component and is responsive to the redox status of the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amábile-Cuevas C. F., Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991 Aug 25;19(16):4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Engel P., Trageser M., Unden G. Reversible interconversion of the functional state of the gene regulator FNR from Escherichia coli in vivo by O2 and iron availability. Arch Microbiol. 1991;156(6):463–470. doi: 10.1007/BF00245393. [DOI] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Controls on the biosynthesis of the manganese-containing superoxide dismutase of Escherichia coli. Effects of thiols. J Biol Chem. 1987 Dec 25;262(36):17591–17595. [PubMed] [Google Scholar]
- Green J., Trageser M., Six S., Unden G., Guest J. R. Characterization of the FNR protein of Escherichia coli, an iron-binding transcriptional regulator. Proc Biol Sci. 1991 May 22;244(1310):137–144. doi: 10.1098/rspb.1991.0062. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Moody C. S. Regulation of manganese-containing superoxide dismutase in Escherichia coli. Anaerobic induction by nitrate. J Biol Chem. 1987 Dec 15;262(35):17173–17177. [PubMed] [Google Scholar]
- Hassan H. M., Sun H. C. Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3217–3221. doi: 10.1073/pnas.89.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Ballard B. T., Walsh C. T. The MerR metalloregulatory protein binds mercuric ion as a tricoordinate, metal-bridged dimer. Science. 1990 Feb 23;247(4945):946–948. doi: 10.1126/science.2305262. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Matsuda Z., Fujiwara T., Lin E. C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990 May;4(5):715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Reznikoff W. S. Fnr mutants that activate gene expression in the presence of oxygen. J Bacteriol. 1991 Jan;173(1):16–22. doi: 10.1128/jb.173.1.16-22.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn S., Imlay J. A. Toxicity, mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Cell Sci Suppl. 1987;6:289–301. doi: 10.1242/jcs.1984.supplement_6.19. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S. M., Hassan H. M. Use of site-directed mutagenesis to identify an upstream regulatory sequence of sodA gene of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2618–2622. doi: 10.1073/pnas.87.7.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer E. C., Naranjo C. M., Bradley K. L., Fee J. A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990 Apr;172(4):1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Beyer W. F., Jr, Fridovich I. Anaerobic induction of ProMn-superoxide dismutase in Escherichia coli. J Biol Chem. 1989 Feb 15;264(5):2758–2763. [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. Effects of diazenedicarboxylic acid bis(N,N'-dimethylamide) (diamide). J Biol Chem. 1990 Dec 15;265(35):21966–21970. [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic inductions of active forms of superoxide dismutases in Escherichia coli. Free Radic Res Commun. 1991;12-13 Pt 1:419–428. doi: 10.3109/10715769109145812. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Inductions of superoxide dismutases in Escherichia coli under anaerobic conditions. Accumulation of an inactive form of the manganese enzyme. J Biol Chem. 1988 Mar 25;263(9):4274–4279. [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem. 1993 Mar 5;268(7):5178–5181. [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Transcriptional and maturational effects of manganese and iron on the biosynthesis of manganese-superoxide dismutase in Escherichia coli. J Biol Chem. 1992 May 5;267(13):9140–9145. [PubMed] [Google Scholar]
- Ross W., Park S. J., Summers A. O. Genetic analysis of transcriptional activation and repression in the Tn21 mer operon. J Bacteriol. 1989 Jul;171(7):4009–4018. doi: 10.1128/jb.171.7.4009-4018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone J. R., Hassan H. M. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4269–4273. [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spiro S., Roberts R. E., Guest J. R. FNR-dependent repression of the ndh gene of Escherichia coli and metal ion requirement for FNR-regulated gene expression. Mol Microbiol. 1989 May;3(5):601–608. doi: 10.1111/j.1365-2958.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Tardat B., Touati D. Two global regulators repress the anaerobic expression of MnSOD in Escherichia coli::Fur (ferric uptake regulation) and Arc (aerobic respiration control). Mol Microbiol. 1991 Feb;5(2):455–465. doi: 10.1111/j.1365-2958.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Trageser M., Unden G. Role of cysteine residues and of metal ions in the regulatory functioning of FNR, the transcriptional regulator of anaerobic respiration in Escherichia coli. Mol Microbiol. 1989 May;3(5):593–599. doi: 10.1111/j.1365-2958.1989.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991 May;173(9):2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]