Abstract
Background
Acinetobacter baumannii strains co-producing carbapenemase and 16S rRNA methylase are highly resistant to carbapenems and aminoglycosides.
Methods
Ninety-three isolates of multidrug-resistant A. baumannii were obtained from an intensive care unit in a hospital in Vietnam. Antimicrobial susceptibility tests and whole genome sequencing were performed. Multilocus sequence typing and the presence of drug resistant genes were determined and a maximum-likelihood phylogenetic tree was constructed by SNP alignment of whole genome sequencing data.
Results
The majority of isolates belonged to clonal complex 2 (ST2, ST570 and ST571), and carried carbapenemase encoding genes blaOXA-23 and blaOXA-66. Two isolates encoded carbapenemase genes blaNDM-1 and blaOXA-58 and the 16S rRNA methylase encoding gene armA and did not belong to clonal complex 2 (ST16).
Conclusion
A. baumannii isolates producing 16S rRNA methylase ArmA and belonging to clonal complex 2 are widespread, and isolates co-producing NDM-1 and ArmA are emerging, in medical settings in Vietnam.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-015-1171-x) contains supplementary material, which is available to authorized users.
Keywords: Multidrug-resistance, Acinetobacter baumannii, 16S rRNA methylase ArmA, Metallo-β-lactamase NDM-1, Intensive care unit
Background
Metallo-β-lactamases (MBLs) confer reduced susceptibility to carbapenems, cephalosporins, and all penicillins except monobactams [1]. Acquired MBLs are produced by several Gram-negative bacterial strains, including Acinetobacter spp., Pseudomonas aeruginosa, and several Enterobacteriaceae [1]. MBLs are categorized by their amino acid sequences into various types [2–4], including AIM [5], DIM [6], FIM [7], GIM [8], IMPs [9], KHM [10], NDMs [11], SMB [12], SIM [13], SPM [14], TMBs [15] and VIMs [16]. The most prevalent MBLs are IMP-, VIM-, and NDM-type enzymes [1, 2, 17]. NDM-1 was initially isolated from Klebsiella pneumoniae and Escherichia coli in 2008 in Sweden [11]. Between 2009 and 2012, 950 isolates of NDM-1-producing bacteria, including 36 A. baumannii isolates, were reported worldwide [18]. Subsequently, at least 13 NDM variants (www.lahey.org/studies) have been reported in several countries [4, 19–30].
Aminoglycosides are effective antibiotics for the treatment of infectious diseases caused by Gram-negative bacteria. These agents block bacterial protein synthesis by binding to the 30S ribosomal subunit [31]. Methylation of 16S rRNA by 16S rRNA methylases, however, makes Gram-negative bacteria highly resistant to all clinically important aminoglycosides [32]. In 2003, clinical isolates of highly aminoglycoside-resistant Gram-negative bacteria producing 16S rRNA methylases were identified in France [33] and Japan [34]. Since then, 16S rRNA methylase-producing Gram-negative bacteria have been isolated in other parts of the world, including Asian countries, such as Afghanistan, Bangladesh, China, Hong Kong, India, Japan, Korea, Oman, and Pakistan [35].
Methods
Bacterial samples and drug susceptibility tests
From 2011 to 2013, 93 clinical isolates of A. baumannii were obtained from respiratory tract samples taken from patients hospitalized in an intensive care unit (ICU) in Cho Ray Hospital in Ho Chi Minh City, Vietnam.
MICs of amikacin, arbekacin, ciprofloxacin, colistin, imipenem, meropenem, and tigecycline were determined using the microdilution method, as described [36].
Whole genome sequences
Genomic DNA from the 93 multidrug-resistant isolates were extracted using DNeasy Blood & Tissue kits (QIAGEN, Tokyo, Japan) and sequenced by MiSeq (Illumina, San Diego, CA). MiSeq data, including total length, number of contig, N50, average contig length and % GC content, were shown in Additional file 1: Table S1. To identify SNPs among these genomes, all reads of each isolate were aligned against the A. baumannii TYTH-1 sequence (Accession no. CP003856) using CLC genomics workbench, version 5.5 (CLC bio, Tokyo, Japan). SNP concatenated sequences were aligned by MAFFT (http://mafft.cbrc.jp/alignment/server/). A maximum-likelihood phylogenetic tree was constructed from the SNP alignment with PhyML 3.0 [37]. The probability of node branching was evaluated with 100 bootstrappings. Raw reads of all isolates were assembled into more than 500 bp contigs by CLC genomics workbench. Contigs around drug-resistant genes were annotated using the BLAST database (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome). Multilocus sequence typing (MLST) based on contig data was deduced using CLC genomics workbench, and matched against the Institut Pasteur MLST (http://pubmlst.org/abaumannii/) databases. The result of STs according to PubMLST (http://pubmlst.org/abaumannii/) scheme was shown in Additional file 2: Table S2. Annotations using the RAST server (http://rast.nmpdr.org/) were performed to compare numbers of prophages and resistance factors. All raw read data of the 93 isolates have been deposited at GenBank as accession numbers DRX032164 to DRX032256.
Ethical approval
The study protocol was carefully reviewed and approved by the ethics committee of Cho Ray Hospital (approval number: 1644/QD-BVCR), the ethics committee of the National Center for Global Health and Medicine (No. 1268), and the Biosafety Committee of the National Center for Global Health and Medicine (approval number: 27-M-52), respectively. Individual informed consent was waived by the ethics committee listed above because this study used currently existing sample collected during the course of routine medical care and did not pose any additional risks to the patients.
Results
Drug susceptibility tests
The majority of the A. baumannii isolates tested were highly resistant to carbapenems, aminoglycosides, and ciprofloxacin, but sensitive to colistin and tigecycline (Table 1). MICs were 0.5 – > 512 μg/mL (MIC50 > 512 μg/mL and MIC90 > 512 μg/mL) to amikacin, 32– > 512 μg/mL (MIC50 > 512 μg/mL and MIC90 > 512 μg/mL) to ciprofloxacin, 0.125–16 μg/mL (MIC50 = 0.5 μg/mL and MIC90 = 1 μg/mL) to colistin, 8–128 μg/mL (MIC50 = 32 μg/mL and MIC90 = 64 μg/mL) to imipenem, 4 to 128 μg/mL (MIC50 = 32 μg/mL and MIC90 = 64 μg/mL) to meropenem, and < 0.125–16 μg/mL (MIC50 = 1 μg/mL and MIC90 = 8 μg/mL) to tigecycline. The isolate NCGM321 was particularly resistant to carbapenems and aminoglycosides, with MICs of > 512 μg/mL to amikacin, > 512 μg/mL to arbekaein, 512 μg/mL to ciprofloxacin, 0.25 μg/mL to colistin, 128 μg/mL to imipenem, 64 μg/mL to meropenem, and 2 μg/mL to tigecycline.
Table 1.
MIC50 and MIC90 values and antimicrobial resistance of the 93 A. baumannii isolates
| Antimicrobial agents | Breakpoint for resistance (mg/L) | % Resistant | Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) |
|---|---|---|---|---|---|
| Imipenem | ≥8 | 100 | 8–128 | 32 | 64 |
| Meropenem | ≥8 | 99 | 4–128 | 32 | 64 |
| Amikacin | ≥64 | 87 | 0.5 – > 512 | >512 | >512 |
| Ciprofloxacin | ≥4 | 100 | 32 – > 512 | >512 | >512 |
| Colistin | ≥4 | 5 | 0.125–16 | 0.5 | 1 |
| Tigecycline | - | - | ≤0.125–16 | 1 | 8 |
Molecular epidemiology and drug resistant genes
Phylogenic analysis based on SNP concatenation showed that the 93 isolates belonged to seven clades, ST2 (28 isolates), ST16 (two isolates), ST23 (seven isolates), ST215 (seven isolates), ST570 (19 isolates), ST571 (28 isolates), and ST575 (11 isolates) (Fig. 1). The isolates in Clades ST2, ST570, and ST571 belonged to worldwide clonal lineage II (CC2, European Clone II) [38]. All isolates tested contained intrinsic blaADC. No novel blaADC gene was detected. None of the intrinsic blaADC genes contained ISAba1, which is responsible for the overexpression of these genes [39]. The intrinsic blaADC genes encoded clade-specific blaOXA-51-like variants, with the 71 isolates belonging to Clades ST2, ST215, ST570, and ST571 having blaOXA-66, the 11 isolates belonging to ST575 having blaOXA-144, the seven isolates belonging to ST23 having blaOXA-68, and the two isolates belonging to ST16 having blaOXA-51 (Table 2). The 2 isolates belonging to ST16 also contained the blaNDM-1, blaOXA-58, and blaVEB-1 genes. Of the all 93 isolates tested, 71 had blaTEM-1, 56 had blaOXA-23, and three had blaVEBs.
Fig. 1.
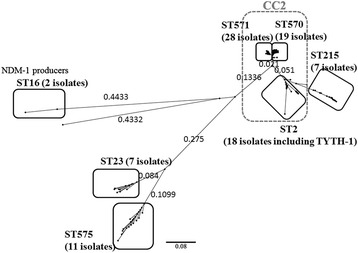
Molecular phylogeny of the 93 Acinetobacter baumannii strains from patient isolates. Phylogenic analysis based on SNP concatenation revealed seven clades, ST2, ST16, ST23, ST214, ST570, ST571, and ST575. Isolates in Clades ST2, ST570, and ST571 belonged to clonal complex 2 (CC2)
Table 2.
MLST and drug resistance genes in A. baumannii isolates
| MLST | No. of isolatesa | Carbapenemase and ESBL encoding genes | Aminoglycoside-resistance genes |
|---|---|---|---|
| ST2 (CC2) | 17 | bla OXA-66, bla OXA-23 (9/17), bla PER-1 (1/17), bla TEM-1 (16/17) | armA, aac(6’)-Ib-cr (13/17), aac(3)-Ia(1/17), aadA1 (14/17), aph(3’)-Ia (14/17) |
| ST16 | 2 | bla NDM-1, bla OXA-51, bla OXA-58, bla VEB-1, bla TEM-1 (1/2) | armA (1/2), aac(3)-Iid, aadA1, aadB, aph(3’)-Via |
| ST23 | 7 | bla OXA-23 (2/7), bla OXA-68, bla PER-1, bla TEM-1 (1/23) | armA (3/7), aac(6’)-Ib-cr (4/7), aadA1 (3/7), aadB (1/7), aph(3’)-Ia (1/7), aph(3’)-Via (5/7), aph(3’)-Vib (1/7), aphA6 (1/7) |
| ST215 | 7 | bla OXA-23 (4/7), bla OXA-66, bla TEM-1 | armA (6/7), aac(6’)-Ib (1/7), aac(6’)-Ib-cr (5/7), aac(3)-Ia (4/7), aadA1, aph(3’)-Ia (5/7) |
| ST570 (CC2) | 19 | bla OXA-23 (9/19), bla OXA-66, bla TEM-1 (18/19) | armA, aac(6’)-Ib (7/19), aac(6’)-Ib-cr (11/19), aac(3)-Ia, aadA1, aph(3’)-Ia (7/19) |
| ST571 (CC2) | 28 | bla OXA-23 (22/28), bla OXA-66, bla PER-1 (5/28), bla TEM-1 | armA, aac(3)-Iid (4/28), aadA1, aadB (2/28), aph(3’)-Via (1/28), aph(3’)-Vib (4/28) |
| ST575 | 11 | bla OXA-23 (9/11), bla OXA-144, bla PER-1, bla TEM-1 | aadB (5/11), aph(3’)-Via (3/11), aph(3’)-Vib (4/11), aphA6 (4/11) |
| ST577 | 1 | bla OXA-23, bla OXA-66, bla TEM-1 | armA, aac(6’)-Ib, aac(3)-Ia, aadA1 |
| ST578 | 1 | bla OXA-51, bla OXA-58, bla VEB-7 | aac(3)-Ib, aadA1, aph(3’)-Via |
aTotal number of isolates belonging to the same sequence type
Among the 93 isolates, 77 had armA, 77 had aadA1, 34 had aac(6’)-Ib-cr, 28 had aph(3’)-Ia, 18 had aac(3)-Ia, 12 had aph(3’)-Via, 10 had aph(3’)-Vib, seven had aac(3)-Iid, five had aphA6, and one had aac(3)-Ib. No plasmid was detected in any of the 93 isolates, indicating that all drug resistance genes were located on chromosomes.
Genomic environments surrounding armA, blaOXA-23blaNDM-1, blaOXA-51-like, and blaOXA-58
The genetic environment surrounding armA in NCGM346 belonging to Clade ST571 (Accession no. LC030435) is shown in Fig. 2a. This genetic environment, from nt 1 to nt 17,473, was more than 99.99 % homologous to the analogous region of A. baumannii strains MDR-TJ isolated in China [40] and NCGM253 isolated in Japan [41]. The sequence surrounding armA from nt 5838 to nt 9879 was identical to the transposon Tn1548 (Accession no. EU014811) detected in an A. baumannii isolate from North America [42] and included the ISCR1 insertion sequence. Putative transposase genes were located both upstream (tnpU) and downstream (tnpD) of armA (Fig. 2a). Four additional isolates, NCGM165, NCGM169, NCGM175, and NCGM194, belonging to Clades ST570, ST215, ST23, and ST2, respectively, had the same genetic organization surrounding armA as the NCGM346 isolate. None of these five isolates contained plasmids, indicating that armA is chromosomally encoded in each.
Fig. 2.
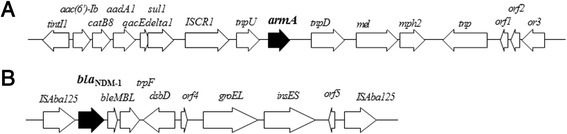
Genetic environments of armA and bla NDM-1 in A. baumannii NCGM346 (a) and NCGM321 (b). orf1, gene encoding a hypothetical protein; orf2, gene encoding a DNA-binding protein; orf3, gene encoding a DNA replication protein; orf4 and orf5, genes encoding hypothetical proteins
The genetic environment surrounding blaNDM-1 in NCGM321 belonging to Clade ST16 (Accession no. LC032101) is shown in Fig. 2b. The blaNDM-1 gene was located between two copies of ISAba125 and was carried by the Tn125 composite transposon. The genetic environment surrounding blaNDM-1 was 100 % homologous to those of A. baumannii strain IOMTU433 isolated in Nepal (accession no. AP014649), A. baumannii ZW85-1 plasmid pAbNDM-1 isolated in China (accession no. JN377410), Acinetobacter lwoffii WJ10621 plasmid pNDM-BJ01 isolated in China (accession no. JQ001701), and A. baumannii 161/07 isolated in Germany (accession no. HQ857107). The genetic environment surrounding blaNDM-1 in NCGM328, the second isolate belonging to Clade ST16, was identical to the genetic environment surrounding blaNDM-1 in NCGM321.
The genetic environment surrounding blaOXA-23 in NCGM346 belonging to Clade ST571 was ISAba1-blaOXA-23-yeeA (yeeA: ATPase encoding gene) and was more than 99 % identical with chromosome sequences of A. baumannii strains IOMTU433 (accession no. AP014649) and NCGM237 [41]. The genetic organization surrounding blaOXA-23 in four additional isolates, NCGM165, NCGM169, NCGM175, and NCGM194, belonging to Clades ST570, ST215, ST23, and ST2, respectively, was identical to that surrounding blaOXA-23 in NCGM346.
The genetic environment surrounding blaOXA-51-like in NCGM346 belonging to Clade ST571 was fxsA-blaOXA-66-orf6-orf7-ruvC-orf8-gueG-bioB, where orf6 encodes the enzyme phosphinothricin N-acetyltransferase, orf7 encodes an XRE family transcriptional regulator, and orf8 encodes a hypothetical protein. The same genetic organization surrounding blaOXA-51-like was observed in four additional isolates, NCGM165, NCGM169, NCGM175, and NCGM194, belonging to Clades ST570, ST215, ST23, and ST2, respectively.
The genetic environment surrounding blaOXA-58 in NCGM328 belonging to Clade ST16 was ISAba3-blaOXA-58-orf9-orf10-ISAba3, where orf9 encodes a transposon-related protein and orf10 encodes a hypothetical protein. The structure was the same as a part of Acinetobacter spp. M131 plasmid pM131-2 (accession no. JX101647).
Structures of the genomic resistance islands of CC2 isolates
The resistance island (RI) of the isolate NCGM196 belonging to Clade ST2 contained two Tn6021-like copies and one Tn5393-like copy. The resistance genes in the RI included sul1, which encodes sulfonamide resistance protein, and tetB and tetR, which regulate tetracycline resistance, as well as the streptomycin resistance genes strA and strB. The RI structure of the other ST2 isolate (NCGM194) was identical to that of A. baumannii MDT-TJ [40] and TYTH-1 [43]. RIs of the isolates belonging to Clades ST570 (NCGM165) and ST571 (NCGM346) were identical to those of AbaR4 [44], a compound transposon containing a Tn6022 backbone.
Prophages and resistance factors
The A. baumannii isolates had several transposable elements, phages/prophages and resistance factors. The isolates belonging to international clone 2, including NCGM165 (ST570), NCGM194 (ST2) and NCGM346 (ST571), had fewer phages/prophages than the isolates belonging to other clones, including NCGM169 (ST215), NCGM175 (ST23) and NCGM328 (ST16). The isolates NCGM165, NCGM194, NCGM346, NCGM169, NCGM175, and NCGM328 contained 10, 18, 10, 8, 7, and 6 transposable elements, respectively; 54, 60 80, 57, 57 and 57 resistance factors, respectively; and 11, 8, 8, 27, 49 and 32 phages/prophages, respectively.
Discussion
To our knowledge, this is the first report of A. baumannii isolates co-producing NDM-1 and ArmA emerging in a medical setting in Vietnam. Enterobacteriaceae producing only NDM-1 had been reported in Vietnam [18, 45, 46], including NDM-1-producing K. pneumoniae isolated from environmental samples [45] and NDM-1-producing Enterobacteriaceae isolated from samples in a Vietnamese surgical hospital [47]. There have been no reports of A. baumannii co-producing NDM-1 and ArmA and belonging to international clone 2, although NDM-1 producers belonging to international clone2 were reported in East Africa in 2013 [48]. It is important to continue the surveillance of NDM-1-producing pathogens, including A. baumannii, in medical settings in Vietnam.
The high prevalence of Gram-negative bacteria producing ArmA in Vietnam may result from the inadequate use of aminoglycosides in that country. An analysis of patients hospitalized in Vietnam showed that 67.4 % received antibiotics, with 18.9 % receiving aminoglycosides, although 30.8 % of the prescribed antibiotics were considered inappropriate [49]. This latter rate was higher than the rates of inappropriately prescribed antibiotics in Malaysia (4.0 %) [50], Turkey (14.0 %) [51], Hong Kong (20.0 %) [52] and European countries (17.8–32.0 %) [53, 54].
A similar genetic environment surrounding blaNDM-1 has been reported in A. baumannii stains isolated in China [55], Colombia (accession no. CP010399), France [22], Germany [56] and the United States (accession no. CP010370); in A. lwoffii isolated in China [57]; in E. coli isolated in Colombia (accession no. CP010373); in K. pneumoniae isolated in Colombia (CP010391) and the United States [58]; and in Providencia rettgeri isolated in Canada [59]. A similar environment surrounding armA was reported in A. baumannii strains isolated in China [40], Japan [41], and Nepal (accession no. AP014649). The genetic organization of blaNDM-1 has spread worldwide, whereas that of armA has spread in Asian countries.
A. baumannii isolates belonging to international clone 2 must have been disseminated throughout medical settings in Vietnam, since 69.9 % of all isolates tested belonged to this clone (ST2, ST570, and ST571). Epidemiological studies of A. baumannii isolates obtained from a hospital in Hanoi are currently ongoing to clarify whether A. baumannii isolates belonging to international clone 2 are disseminating throughout Vietnam. The isolates belonging to Clades ST16, ST23, and ST215 were not identified as belonging to any previously described international clones [38]. To date, one A. baumannii isolate belonging to Clade ST16 was isolated in 2001 in the Netherlands, 3 isolates belonging to ST23 were isolated in the Netherlands (in 1964) and Sweden (in 2006 and 2007), and 6 isolates belonging to Clade ST215 were isolated in 2008 in China. Clones ST570, ST571, and ST575 were novel STs. Of the isolates belonging to CC2, those in Clades ST570 and ST571 may have evolved in a unique manner in Vietnam because the structures of resistant islands in ST570 and ST571 isolates were different from those in ST2 isolates.
Conclusions
This study showed that 16S rRNA methylase ArmA-producing A. baumannii isolates belonging to clonal complex 2 have spread, and that NDM-1-and ArmA-co-producers not belonging to clonal complex 2 are emerging, in medical settings in Vietnam.
Acknowledgements
This study was supported by Japan Initiative for Global Research Network on Infectious Diseases (J-GRID), and a grant of the Research Program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and Development, and a grant (26-A-103) from International Health Cooperation Research.
Abbreviations
- MBLs
Metallo-β-lactamases
- MLST
Multilocus sequence typing
- IP-MLST
Institut Pasteur MLST
Additional files
Assembly summary report of 93 A. baumannii isolates using CLC Genomics Workbench version 5.5. (XLSX 15 kb)
MLST analysis on PubMLST scheme in 93 A. baumannii isolates. (XLSX 14 kb)
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
TT: Performed whole genome sequencing, analyzed data and drafted the manuscript. TMA: Performed whole genome sequencing. KS: Performed drug-susceptibility tests. TTTN: Performed clinical bacterial analyses. LTAT and NTS: Designed protocols and supervised this study at CRH. NO and TK: Designed protocols and supervised this study. All authors read and approved the final manuscript.
Contributor Information
Tatsuya Tada, Email: ttada@ri.ncgm.go.jp.
Tohru Miyoshi-Akiyama, Email: takiyam@ri.ncgm.go.jp.
Kayo Shimada, Email: kshima@ri.ncgm.go.jp.
Tran Thi Thanh Nga, Email: ngatrancrh@gmail.com.
Le Thi Anh Thu, Email: letathu@yahoo.com.
Nguyen Truong Son, Email: truongson_cr@yahoo.com.vn.
Norio Ohmagari, Email: nohmagari@hosp.ncgm.go.jp.
Teruo Kirikae, Phone: (81) 3 3202 7181, Email: tkirikae@ri.ncgm.go.jp.
References
- 1.Bush K. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis. 2001;32(7):1085–1089. doi: 10.1086/319610. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18(2):306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis. 2011;11(5):381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 5.Yong D, Toleman MA, Bell J, Ritchie B, Pratt R, Ryley H, et al. Genetic and biochemical characterization of an acquired subgroup B3 metallo-beta-lactamase gene, blaAIM-1, and its unique genetic context in Pseudomonas aeruginosa from Australia. Antimicrob Agents Chemother. 2012;56(12):6154–6159. doi: 10.1128/AAC.05654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogalski TM, Gilbert MM, Devenport D, Norman KR, Moerman DG. DIM-1, a novel immunoglobulin superfamily protein in Caenorhabditis elegans, is necessary for maintaining bodywall muscle integrity. Genetics. 2003;163(3):905–915. doi: 10.1093/genetics/163.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollini S, Maradei S, Pecile P, Olivo G, Luzzaro F, Docquier JD, et al. FIM-1, a new acquired metallo-beta-lactamase from a Pseudomonas aeruginosa clinical isolate from Italy. Antimicrob Agents Chemother. 2013;57(1):410–416. doi: 10.1128/AAC.01953-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castanheira M, Toleman MA, Jones RN, Schmidt FJ, Walsh TR. Molecular characterization of a beta-lactamase gene, blaGIM-1, encoding a new subclass of metallo-beta-lactamase. Antimicrob Agents Chemother. 2004;48(12):4654–4661. doi: 10.1128/AAC.48.12.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, et al. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38(1):71–78. doi: 10.1128/AAC.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiguchi J, Morita K, Kitao T, Watanabe N, Okazaki M, Miyoshi-Akiyama T, et al. KHM-1, a novel plasmid-mediated metallo-beta-lactamase from a Citrobacter freundii clinical isolate. Antimicrob Agents Chemother. 2008;52(11):4194–4197. doi: 10.1128/AAC.01337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachino J, Yoshida H, Yamane K, Suzuki S, Matsui M, Yamagishi T, et al. SMB-1, a novel subclass B3 metallo-beta-lactamase, associated with ISCR1 and a class 1 integron, from a carbapenem-resistant Serratia marcescens clinical isolate. Antimicrob Agents Chemother. 2011;55(11):5143–5149. doi: 10.1128/AAC.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Yum JH, Yong D, Lee HM, Kim HD, Docquier JD, et al. Novel acquired metallo-beta-lactamase gene, blaSIM-1, in a class 1 integron from Acinetobacter baumannii clinical isolates from Korea. Antimicrob Agents Chemother. 2005;49(11):4485–4491. doi: 10.1128/AAC.49.11.4485-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zavascki AP, Gaspareto PB, Martins AF, Goncalves AL, Barth AL. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-beta-lactamase in a teaching hospital in southern Brazil. J Antimicrob Chemother. 2005;56(6):1148–1151. doi: 10.1093/jac/dki390. [DOI] [PubMed] [Google Scholar]
- 15.El Salabi A, Borra PS, Toleman MA, Samuelsen O, Walsh TR. Genetic and biochemical characterization of a novel metallo-beta-lactamase, TMB-1, from an Achromobacter xylosoxidans strain isolated in Tripoli, Libya. Antimicrob Agents Chemother. 2012;56(5):2241–2245. doi: 10.1128/AAC.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, et al. Cloning and characterization of blaVIM, a new integron-borne metallo-beta-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43(7):1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby GA, Munoz-Price LS. The new beta-lactamases. N Engl J Med. 2005;352(4):380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 18.Berrazeg M, Diene S, Medjahed L, Parola P, Drissi M, Raoult D, et al. New Delhi Metallo-beta-lactamase around the world: an eReview using Google Maps. Euro Surveill. 2014;19(20):20809. doi: 10.2807/1560-7917.es2014.19.20.20809. [DOI] [PubMed] [Google Scholar]
- 19.Pillai DR, McGeer A, Low DE. New Delhi metallo-beta-lactamase-1 in Enterobacteriaceae: emerging resistance. CMAJ. 2011;183(1):59–64. doi: 10.1503/cmaj.101487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaase M, Nordmann P, Wichelhaus TA, Gatermann SG, Bonnin RA, Poirel L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J Antimicrob Chemother. 2011;66(6):1260–1262. doi: 10.1093/jac/dkr135. [DOI] [PubMed] [Google Scholar]
- 21.Espinal P, Fugazza G, Lopez Y, Kasma M, Lerman Y, Malhotra-Kumar S, et al. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob Agents Chemother. 2011;55(11):5396–5398. doi: 10.1128/AAC.00679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(2):1087–1089. doi: 10.1128/AAC.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazawi A, Sonnevend A, Bonnin RA, Poirel L, Nordmann P, Hashmey R, et al. NDM-2 carbapenemase-producing Acinetobacter baumannii in the United Arab Emirates. Clin Microbiol Infect. 2012;18(2):E34–E36. doi: 10.1111/j.1469-0691.2011.03726.x. [DOI] [PubMed] [Google Scholar]
- 24.Rogers BA, Sidjabat HE, Silvey A, Anderson TL, Perera S, Li J, et al. Treatment options for New Delhi metallo-beta-lactamase-harboring Enterobacteriaceae. Microb Drug Resist. 2013;19(2):100–103. doi: 10.1089/mdr.2012.0063. [DOI] [PubMed] [Google Scholar]
- 25.Nordmann P, Boulanger AE, Poirel L. NDM-4 metallo-beta-lactamase with increased carbapenemase activity from Escherichia coli. Antimicrob Agents Chemother. 2012;56(4):2184–2186. doi: 10.1128/AAC.05961-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornsey M, Phee L, Wareham DW. A novel variant, NDM-5, of the New Delhi metallo-beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother. 2011;55(12):5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, Woodhouse R, et al. Identification and molecular characterisation of New Delhi metallo-beta-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int J Antimicrob Agents. 2012;39(6):529–533. doi: 10.1016/j.ijantimicag.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Cuzon G, Bonnin RA, Nordmann P. First identification of novel NDM carbapenemase, NDM-7, in Escherichia coli in France. PLoS One. 2013;8(4):e61322. doi: 10.1371/journal.pone.0061322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottig S, Hamprecht AG, Christ S, Kempf VA, Wichelhaus TA. Detection of NDM-7 in Germany, a new variant of the New Delhi metallo-beta-lactamase with increased carbapenemase activity. J Antimicrob Chemother. 2013;68(8):1737–1740. doi: 10.1093/jac/dkt088. [DOI] [PubMed] [Google Scholar]
- 30.Tada T, Miyoshi-Akiyama T, Dahal RK, Sah MK, Ohara H, Kirikae T, et al. NDM-8 Metallo-beta-Lactamase in a Multidrug-Resistant Escherichia coli Strain Isolated in Nepal. Antimicrob Agents Chemother. 2013. [DOI] [PMC free article] [PubMed]
- 31.Jana S, Deb JK. Molecular understanding of aminoglycoside action and resistance. Appl Microbiol Biotechnol. 2006;70(2):140–150. doi: 10.1007/s00253-005-0279-0. [DOI] [PubMed] [Google Scholar]
- 32.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45(1):88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 33.Galimand M, Courvalin P, Lambert T. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother. 2003;47(8):2565–2571. doi: 10.1128/AAC.47.8.2565-2571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamane K, Wachino J, Suzuki S, Shibata N, Kato H, Shibayama K, et al. 16S rRNA methylase-producing, gram-negative pathogens, Japan. Emerg Infect Dis. 2007;13(4):642–646. doi: 10.3201/eid1304.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wachino J, Arakawa Y. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: An update. Drug Resist Updat. 2012;15(3):133–148. doi: 10.1016/j.drup.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing; 25th informational supplement. 7. Wayne: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 37.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 38.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents. 2013;41(1):11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Yang ZL, Wu XM, Wang Y, Liu YJ, Luo H, et al. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J Antimicrob Chemother. 2012;67(12):2825–2832. doi: 10.1093/jac/dks327. [DOI] [PubMed] [Google Scholar]
- 41.Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. Dissemination of 16S rRNA Methylase ArmA-Producing Acinetobacter baumannii and Emergence of OXA-72 Carbapenemase Coproducers in Japan. Antimicrob Agents Chemother. 2014;58(5):2916–2920. doi: 10.1128/AAC.01212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doi Y, Adams JM, Yamane K, Paterson DL. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob Agents Chemother. 2007;51(11):4209–4210. doi: 10.1128/AAC.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CC, Tang CY, Kuo HY, Lu CW, Chang KC, Liou ML. The origin of Acinetobacter baumannii TYTH-1: a comparative genomics study. Int J Antimicrob Agents. 2013;41(4):318–324. doi: 10.1016/j.ijantimicag.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Hamidian M, Hall RM. AbaR4 replaces AbaR3 in a carbapenem-resistant Acinetobacter baumannii isolate belonging to global clone 1 from an Australian hospital. J Antimicrob Chemother. 2011;66(11):2484–2491. doi: 10.1093/jac/dkr356. [DOI] [PubMed] [Google Scholar]
- 45.Isozumi R, Yoshimatsu K, Yamashiro T, Hasebe F, Nguyen BM, Ngo TC, et al. blaNDM-1-positive Klebsiella pneumoniae from environment. Vietnam Emerg Infect Dis. 2012;18(8):1383–1385. doi: 10.3201/eid1808.111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nhu NT, Lan NP, Campbell JI, Parry CM, Thompson C, Tuyen HT, et al. Emergence of carbapenem-resistant Acinetobacter baumannii as the major cause of ventilator-associated pneumonia in intensive care unit patients at an infectious disease hospital in southern Vietnam. J Med Microbiol. 2014;63(Pt 10):1386–1394. doi: 10.1099/jmm.0.076646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran HH, Ehsani S, Shibayama K, Matsui M, Suzuki S, Nguyen MB, et al. Common isolation of New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae in a large surgical hospital in Vietnam. Eur J Clin Microbiol Infect Dis. 2015. [DOI] [PMC free article] [PubMed]
- 48.Revathi G, Siu LK, Lu PL, Huang LY. First report of NDM-1-producing Acinetobacter baumannii in East Africa. Int J Infect Dis. 2013;17(12):e1255–e1258. doi: 10.1016/j.ijid.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Thu TA, Rahman M, Coffin S, Harun-Or-Rashid M, Sakamoto J, Hung NV. Antibiotic use in Vietnamese hospitals: A multicenter point-prevalence study. Am J Infect Control. 2012. [DOI] [PubMed]
- 50.Hughes AJ, Ariffin N, Huat TL, Abdul Molok H, Hashim S, Sarijo J, et al. Prevalence of nosocomial infection and antibiotic use at a university medical center in Malaysia. Infect Control Hosp Epidemiol. 2005;26(1):100–104. doi: 10.1086/502494. [DOI] [PubMed] [Google Scholar]
- 51.Ceyhan M, Yildirim I, Ecevit C, Aydogan A, Ornek A, Salman N, et al. Inappropriate antimicrobial use in Turkish pediatric hospitals: a multicenter point prevalence survey. Int J Infect Dis. 2010;14(1):e55–e61. doi: 10.1016/j.ijid.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Lee MK, Chiu CS, Chow VC, Lam RK, Lai RW. Prevalence of hospital infection and antibiotic use at a university medical center in Hong Kong. J Hosp Infect. 2007;65(4):341–347. doi: 10.1016/j.jhin.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10(3):167–175. doi: 10.1016/S1473-3099(10)70027-X. [DOI] [PubMed] [Google Scholar]
- 54.Kritsotakis EI, Dimitriadis I, Roumbelaki M, Vounou E, Kontou M, Papakyriakou P, et al. Case-mix adjustment approach to benchmarking prevalence rates of nosocomial infection in hospitals in Cyprus and Greece. Infect Control Hosp Epidemiol. 2008;29(8):685–692. doi: 10.1086/588704. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Zhang Z, Hao Q, Wu J, Xiao J, Jing H. Complete Genome Sequence of Acinetobacter baumannii ZW85-1. Genome Announc. 2014 doi: 10.1128/genomeA.01083-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfeifer Y, Wilharm G, Zander E, Wichelhaus TA, Gottig S, Hunfeld KP, et al. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J Antimicrob Chemother. 2011;66(9):1998–2001. doi: 10.1093/jac/dkr256. [DOI] [PubMed] [Google Scholar]
- 57.Christianson SJ, Brand CL, Wilkinson GS. Reduced polymorphism associated with X chromosome meiotic drive in the stalk-eyed fly Teleopsis dalmanni. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doi Y, Hazen TH, Boitano M, Tsai YC, Clark TA, Korlach J, et al. Whole-genome assembly of Klebsiella pneumoniae coproducing NDM-1 and OXA-232 carbapenemases using single-molecule, real-time sequencing. Antimicrob Agents Chemother. 2014;58(10):5947–5953. doi: 10.1128/AAC.03180-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mataseje LF, Boyd DA, Lefebvre B, Bryce E, Embree J, Gravel D, et al. Complete sequences of a novel blaNDM-1-harbouring plasmid from Providencia rettgeri and an FII-type plasmid from Klebsiella pneumoniae identified in Canada. J Antimicrob Chemother. 2014;69(3):637–642. doi: 10.1093/jac/dkt445. [DOI] [PubMed] [Google Scholar]


