Abstract
Phosphorus (P) is a major macronutrient for plant health and development. The available form of P is generally low in the rhizosphere even in fertile soils. A major proportion of applied phosphate (Pi) fertilizers in the soil become fixed into insoluble, unavailable forms, which restricts crop production throughout the world. Roots possess two distinct modes of P uptake from the soil, direct and indirect uptake. The direct uptake of P is facilitated by the plant’s own Pi transporters while indirect uptake occurs via mycorrhizal symbiosis, where the host plant obtains P primarily from the fungal partner, while the fungus benefits from plant-derived reduced carbon. So far, only one Pi transporter has been characterized from the mycorrhizal fungus Glomus versiforme. As arbuscular mycorrhizal fungi cannot be cultured axenically, their Pi transporter network is difficult to exploite for large scale sustainable agriculture. Alternatively, the root-colonizing endophytic fungus Piriformospora indica can grow axenically and provides strong growth-promoting activity during its symbiosis with a broad spectrum of plants. P. indica contains a high affinity Pi transporter (PiPT) involved in improving Pi nutrition levels in the host plant under P limiting conditions. As P. indica can be manipulated genetically, it opens new vistas to be used in P deficient fields.
Keywords: phosphates, transporters, rhizosphere, phosphate transport proteins, phsophate uptake
Introduction
Nutrients play essential roles in cell metabolism since limitations restrict cell growth, development and productivity. One of the major nutrients is Pi but in most soils Pi is not available as it is immobilized by adsorption, P mineralization and fixation into organic molecules (Marschner, 1995). An available form of P for plants is soluble orthophosphate (Pi), but its concentration in the soil solution hardly reaches 10 μM, and Pi is often present in sub-micromolar levels at the root-soil interface. The area around the root surface, where active Pi acquisition takes place, is called the Pi depletion zone (Lewis and Quirk, 1976). This zone is persistent due to slow diffusion of Pi from other unexplored areas. When plants are unable to acquire sufficient amounts of Pi, they perform morphological and physiological changes in the roots to increase soil exploration and the total absorptive surface area. These changes include extensive root branching, increase in length of root hairs (Raghothama, 1999), and the activation of an advanced bio-molecular system, which results in enhanced P absorption. Furthermore, enhanced acid and acid phosphatase (rAPase) secretion and expression of a new kind of Pi transporter in the root cell that is highly efficient in Pi acquisition under P-limiting conditions are typical features of roots under Pi limitation.
P deficiency shifts the priority of energy expenditure from growth, development and reproduction to P acquisition, a situation that adversely affects the plant productivity. To overcome or minimize the effect of P shortage, plants associate with AMF as obligate biotrophs (Parniske, 2008; Bonfante and Genre, 2010), but also other beneficial root-colonizing fungi such as Piriformospora indica.
P Fertility in Soil and Accessibility for the Plants
P is one of the 17 essential elements required for plant growth and development (Bieleski, 1973; Raghothama, 1999). The P concentration in plants ranges from 0.05 to 0.5% of the dry weight. The Pi concentration gradient from the soil to plant cells increases more than 2,000-fold, with an average physiological concentration of 10 μM in the soil (Schachtman et al., 1998). P is a highly reactive element and thus does not exist in the elemental form in nature. Although P is quite abundant it is mainly present as Pi in insoluble complexes with cations, particularly aluminum and iron under acidic conditions. Only 10–15% of the total P is present as soluble Pi (Hinsinger, 2001). Because of this, the crop yield on 30–40% of the world’s arable land is limited by the P availability (Runge-Metzger, 1995; Von Uexkull and Mutert, 1995). The continuous depletion of terrestrial P in the soil is counteracted by the use of fertilizers (Raghothama, 1999; Gilbert, 2009), which contain P mainly as Pi. However, up to 80% of supplied Pi is again fixed in insoluble complexes leading farmers to use up to four times more fertilizer than required for optimal crop production (Barrow, 1980; Goldstein, 1992; Holford, 1997). Furthermore, in the soil, P exists as also organic Pi and phytates (Box 1), and the solubility of these unaccessible P from is largely dependent on the pH of the rhizosphere, which changes locally and depends on the microbial community in the root environment (Haynes, 1982; Robinson, 1994; Marschner, 1995; Rengel and Marschner, 2005).
BOX 1. Availability of total Pi in the soil. Out of three forms present in the soil, plant utilizes only inorganic Pi.

P is taken up by the roots as either monovalent H2PO4- or to a lesser extent as secondary HPO42- (Ullrich-Eberius et al., 1984; Furihata et al., 1992). H2PO4- is dominant in acid soils and taken up about 10 times more efficiently than HPO42-. At a soil pH of 7 approximately equal amounts of the two Pi forms are present, and the secondary ortho-Pi ion becomes the dominant form above pH 7. In extremely acidic or alkaline soils the solubility of Pi is decreased, with the dominant forms being H3PO4 or PO43-, respectively, (Brady, 1990). Inositol Pi in soil is represented by its hexa-Pi esters, generally called phytates, which constitute up to 60% of soil organic P and often form salts with different ions (Stevensons, 1994; Jennings, 1995). Because phytates are less soluble, they cannot be utilized by plants (Pearson et al., 1941; Jennings, 1995).
P Acquisition by Plants: Secret Hidden Below the Ground
A balanced Pi metabolism requires Pi acquisition, translocation from roots to shoots, as well as distribution and remobilization within the plant (Lauer et al., 1989; Liu et al., 2010). Generally, plants maintain a threshold cytoplasmic Pi concentration regardless of the variation in external concentration, which is necessary for safeguarding the primary and secondary metabolism. For short term P security, plants store P as poly Pi in the cytoplasm or vacuoles (Bieleski, 1973; Mimura et al., 1990, 1996; Tu et al., 1990; Sakano et al., 1992).
Pi is supplied to the roots by diffusion rather than mass flow, and the rate of diffusion of Pi is slow in soil. To meet the plant’s demand for soluble P from the rhizosphere, the soil solution should be replaced 20 to 50 times per day (Marschner, 1995). The ion absorption occurs primarily at the young root tip, before uptake into the epidermal cells of the root hairs and apoplastic absorption through the outer layers of the cortical cells. The apoplastic uptake/capture of Pi from the soil is the critical step before the transport into the cells by an energy-driven mechanism (Mikkelsen, 2005). The interlaced fibers of the cell wall form an open latticework in roots that serves as a sieve for the soil solution. The apoplast allows the soil solution to move into the tissue until it reaches the Casparian strips. Because the cell wall fibers have a net negative charge, they repel anions such as Pi and nitrate in solution and confine their transport to larger pores within the apoplastic space. Some root secretions, such as mucilage (an organic compound secreted by the root cells) also bear an overall negative charge that can work as an additional anion repellant from the root (Mikkelsen, 2005). The symplastic route of ion transport is rather complex and connected to all living cells through plasmodesmata. The epidermis and the outer cortical layer pass the ion to the inner adjacent cortical cells.
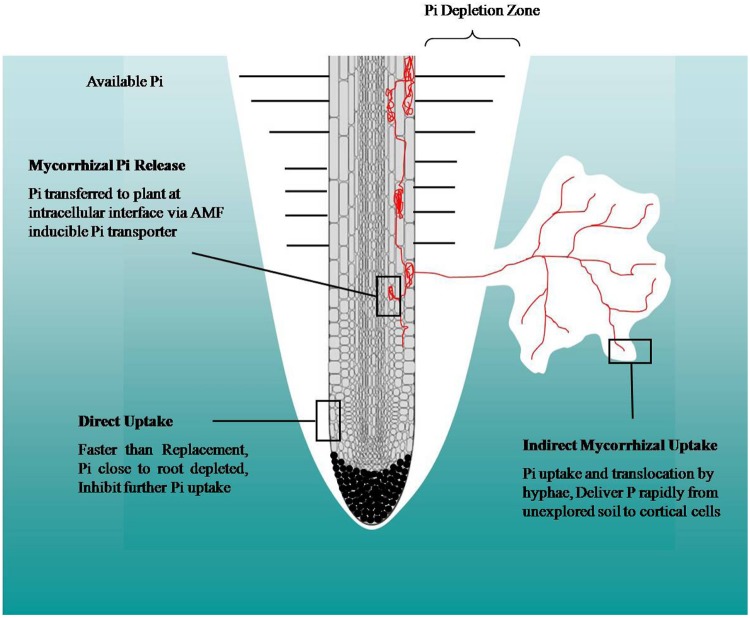
AMF increase the absorptive area (Bolan, 1991; Smith and Read, 1997), because the fine and thinner structure of the fungal hyphae have better access to soil pores and can explore larger soil volumes, which results in more efficient mining for Pi sources (Rosewarne et al., 1999; Drew et al., 2003; Smith and Read, 2008; Schnepf et al., 2011; Figure 1).
FIGURE 1.
Uptake of phosphorus by plants from soil. Two modes of P uptake are present in plants. (A) Direct uptake – between soil and plant root and (B) indirect uptake – through mycorrhizal symbiosis, where AM fungus extra-radical mycelium acquire Pi beyond rhizosphere and Pi transported to intra-radical mycelium as poly-phosphate (Rosewarne et al., 1999).
Root hairs are the primary site for the Pi acquisition and in response to Pi scarcity both the density and length of root hairs increase to explore a larger volume of soil. P-deficient plants are characterized by increases in root/shoot ratio, root branching, root elongation, and root top soil exploration. The root hairs are commonly longer, while primary root growth is reduced (Lynch, 1995; Ma et al., 2001a,b; Lopez-Bucio et al., 2003; Lynch and Brown, 2008; Vance, 2010). The development of dense lateral root clusters with large numbers of root hairs has been observed in white lupine (Lupinus albus; Wang et al., 2010).
Besides acidification of the rhizosphere, exudation of citrate, malate, or oxalate greatly enhances mobilization of Pi by chelation or ligand exchange (such as Ca, Fe, or Al). Root-induced acidification can decrease the rhizosphere pH by 2–3 units relative to the bulk soil (Marschner, 1995). Secretion of phosphatases or phytases mobilize organic Pi through hydrolysis (Vance et al., 2003; Hinsinger et al., 2005; Popova et al., 2010; Zhang et al., 2010). A major problem in P acquisition is the uptake of Pi against a concentration gradient, and this requires specific high-affinity Pi transporters and energy. The symport requires a proton gradient and the electrochemical gradient across the plasma membrane is generated by P-type H+ ATPases at the expense of ATP. The changes of the root architecture under P limitation have a strong influence on the carbohydrate metabolism and their distribution between roots and shoots, and these changes often involve plant hormone (Nacry et al., 2005; Rohmeld and Newmann, 2006; Wang et al., 2010) and sugar signaling (Karthikeyan et al., 2007; Vance, 2010). The formation of a highly branched root system in response to Pi starvation is a consequence of the canalization of carbon and energy resources to the root surface (Stitt and Rudige-Scheible, 1998). Root exudation of organic acids and enzymes ultimately causes a loss of carbon that results in loss of crop yield under Pi limitation.
Molecular Mechanism of P Uptake
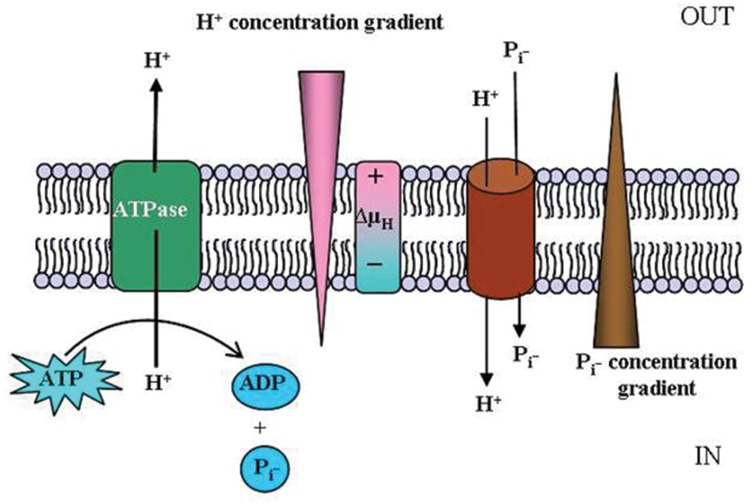
The existence of high- and low-affinity Pi transport systems have been reported in plants, bacteria, yeast, AMF, endophytic fungi and animals (Burns and Beever, 1977; Thomson et al., 1990; Olah et al., 1994; Zvyagilskaya et al., 2001; Yadav et al., 2010; Pedersen et al., 2013). To overcome the steep concentration gradient (Bieleski and Ferguson, 1983) and negative membrane potential, the cells require an energized transport of Pi across the plasma membrane (Bieleski and Ferguson, 1983). Pi transport transiently depolarizes the plasma membrane, which indicates that Pi does not simply enter the cell as H2PO4- or HPO42-, both of which would lead to a membrane hyperpolarization if Pi is transported alone (Ullrich-Eberius et al., 1984). An energy-dependent Pi uptake via H+/Pi co-transport has been first demonstrated for Lemna gibba which depends on the electrochemical proton gradient and therefore on the activity of an H+-extrusion pump such as the P-type H+-ATPase (Ullrich-Eberius et al., 1981, 1984; Figure 2). The large membrane potential difference with a negative potential on the cytoplasmic site (-150 to -200 mV) provides the driving force for co-transport of Pi and other ions with protons (Ullrich-Eberius et al., 1984; Daram et al., 1998; Sze et al., 1999; Karandashov and Bucher, 2005). Hyphae of the ectomycorrhizal Hebeloma cylindrosporum have at least two high-affinity Pi transporters (HcPT1 and HcPT2) that are differentially expressed depending on the P availability and mycorrhizal status (Tatry et al., 2009). Further, functional studies of these transporters heterologously expressed in yeast suggest that P uptake into yeast cells is pH sensitive. Disruption of the pH gradient by uncouplers drastically reduced the Pi uptake, which confirmed that these transporters are proton-coupled and indirect energy-dependent symporters (Tatry et al., 2009). In contrast, at higher pH values (9.5–10) H+ cannot influence the Pi uptake. Under these conditions, uptake occurs by several Na+-dependent transport systems that are kinetically discrete from the H+-dependent transport, specifically activated by Na+ ions and thus insensitive to the protonophore CCCP. The H+-coupled P transport systems provide most, if not all, of the P uptake at pH values of 4.5 and 6.0. The contribution of the Na+/Pi co-transport systems to the total cellular P uptake activity progressively increases with increasing pH and reaches its maximum at pH 9 and higher, i.e., conditions where P accumulation was preferentially, if not exclusively, maintained through the Na+/Pi co-transport systems. H+/Pi co-transport occurred even at pH 8.0, presumably as a consequence of local pH gradients in the vicinity of the carriers in the plasma membrane. At pH 7.0, both H+/Pi and Na+/Pi co-transport systems are equally responsible for P uptake. The H+- and Na+-coupled P transport systems thus possess overlapping but distinct biological roles in the acquisition of P under different growth conditions (Zvyagilskaya et al., 2001).
FIGURE 2.
Pi uptake mechanism across the plasma membrane. A membrane-integral proton ATPase uni-directionally extrudes protons (H+) at the expense of ATP (primary transport). The generated proton concentration gradient and membrane potential constitute a proton electrochemical potential (ΔμH) across the membrane. Proton movement along concentration and electrical gradients facilitates Pi (Pi-) allocation through Pi transporters against a steep concentration gradient (secondary transport; Karandashov and Bucher, 2005).
P Transporters: Entrance of Pi into Cell
When the external P level drops to micro-molar concentrations, the transcript levels for high affinity transporters in roots increase, preferentially in cells with close contact to the soil solution. The low-affinity transporters are mainly active in vascular tissues and involved in the internal distribution and re-mobilization of P (Smith, 2001).
High-affinity Pi transporters have been identified and characterized in several plant and fungal species, including Arabidopsis thaliana, Medicago truncatula, Lycopersicon esculentum, Solanum tuberosum, Saccharomyces cerevisiae and Neurospora crassa (Bun-Ya et al., 1991; Olah et al., 1994; Versaw, 1995; Munchhal et al., 1996; Leggewie et al., 1997; Daram et al., 1998; Liu et al., 1998a,b; Rausch et al., 2001). Plant Pi transporters are grouped into three families: the Pht1 family which contains high-affinity transporters, the Pht2 family which contains transporters responsible for Pi translocation, and the Pht3 family for plastid and mitochondrial P transporters. The fungal Pi transporters are an extension of the Pht1 family (Karandashov et al., 2004; Karandashov and Bucher, 2005). Phylogenetically, they are closely related proteins, although the similarity between the plant transporters is higher than between plant and fungal transporters (Munchhal et al., 1996). The transporters of this family are 500–600 amino acids long and contain 12 predicted membrane-spanning hydrophobic regions of 17–25 amino acid residues which are arranged in a helix. The membrane spanning regions are arranged in two groups of six well defined configurations with a large central hydrophilic, charged loop. This topology is shared by fungal, yeast, plant, and animal Pht1 family members as well as several members of other transporter families (Saier and Reizer, 1991; Pao et al., 1998).
Recently, Yadav et al. (2010) demonstrated that the high affinity P. indica Pi transporter PiPT improves the Pi nutrition levels in the host plant under P limitation. Furthermore, the crystal structure of PiPT confirms that the Major Facilitator Superfamily (MFS)-fold found in bacteria is conserved in eukaryotes. PiPT has 12 transmembrane helices divided into two homologous N- and C-terminal domains (Pedersen et al., 2013; Figure 3). Overall, the conformation of PiPT is similar to structures of transporters of the MFS in their occluded state. The Pi is coordinated by Tyr, Gln, Trp, Asp, and Asn side chains. All these residues are fully conserved in the family of Pi:H+ symporters. Asp coordinates the Pi with both carboxyl oxygens (Figure 3). The structure of PiPT (Pedersen et al., 2013) explains the structural/functional relationships of Pi/H+ symport by providing structural confirmation for Pi affinity and specificity and relating the proton motive force to Pi translocation.
FIGURE 3.
Structure of the high-affinity phosphate transporter, PiPT. The structure represents an inward facing occluded state of the phosphate transporter in complex with phosphate. (A) Phosphate (shown as spheres) is buried in the membrane at the interface between the N domain (pale green) and C domain (blue). Selected residues are shown as sticks. Black bars depict the approximate location of the membrane. (B) The phosphate-binding site with yellow dashes indicating possible hydrogen bonds (2.2–3.8Å distances) to phosphate. Top: the omit 2mFo-DFc density for phosphate is contoured in orange (4s). Bottom: the 2mFo-DFc density for phosphate and selected M7 residues is contoured in red (2s). Reproduced with permission from Macmillan Scientific Publishers Ltd. (London). For more detailed information on PiPT structure please see reference Pedersen et al. (2013).
While the Na+-dependent P transport system is found in animal cells; the H+-coupled Pi transport occurs mainly in plants (Escoubet et al., 1989; Werner and Kinne, 2001). A Na+-dependent Pi-uptake system has not yet found in vascular plants, but its existence cannot be excluded especially in halophytes, which have developed mechanisms for salt resistance, or in plants living in alkaline soils. Based on transport measurements, it appears that both types of transporter systems are present in fungal species (Versaw and Metzenberg, 1995; Zvyagilskaya et al., 2001; Pedersen et al., 2013). H+-driven P transport in yeast and N. crassa was reported to exhibit a pH optimum between 4.5 and 6.0, which is in the range of apoplastic pH values in plants, while Na+-coupled co-transport had a pH optimum of 8.0 and higher (up to 10; Versaw and Metzenberg, 1995; Zvyagilskaya et al., 2001). This suggests a high flexibility of these organisms for changing environmental conditions.
Functional Analysis of P Transporters
The yeast S. cerevisiae provides a useful model system for heterologous expression and functional analyses of plant and fungal P transporters. The pho84 yeast mutant, defective in a gene encoding one of the yeast P transporters (Bun-Ya et al., 1991), has been used for complementation studies. Michaelis constant (Km) values for plant P transporters derived by heterologous expression studies in this mutant are generally higher than expected making it difficult to obtain reliable kinetic data (Leggewie et al., 1997; Liu et al., 1998b). Recently, a S. cerevisiae double mutant, PAM2, became available. This mutant has disruptions in the genes encoding both the H+-coupled Pho84 and the Na+-coupled Pho89 high-affinity Pi transporters (Martinez and Persson, 1998). The Pho84 transporter is involved in sensing extracellular Pi levels and cytosolic signaling due to its dual function as a transceptor (Popova et al., 2010; Samyn et al., 2012). The activity of the Pho84 transceptor mediates signaling via the PHO pathway and is also involved in the activation of the protein kinase A pathway. Mutational analysis of Pho84 has shown that the residues Asp-358 and Lys-492 are critical for the Pi transport function suggesting that they are part of the substrate-binding pocket. Mutants of Asp-358 in the putative H+-binding site are still capable of activating protein kinase A targets, despite a severely hampered transport activity (Samyn et al., 2012). Translocation of H+ and Pi relies on an asymmetric ‘rocker-switch’ mechanism compatible with the mechanism of other MFS transporters.
Typical Km values of high-affinity systems in plants and fungi are in the range of 5–50 μM for Pi. The values for AMF transporters are likely within the same range, although substantial variations have been shown (Burns and Beever, 1977; Mimura, 1999). For instance, the Km values of the high-affinity and low-affinity systems for germ tubes of Gigaspora marginata were 1.8–3.1 μM and 10.2–11.3 mM, respectively, (Thomson et al., 1990), while that of Glomus versiforme (GvPT) for a high-affinity Pi transporter expressed in the external mycelium of the AM fungus GvPT was 18 μM (Harrison and van Buuren, 1995). PiPT is expressed in extra-radical hyphae of P. indica. When heterologously expressed in yeast PiPT exhibited an apparent Km of 25 mM (Yadav et al., 2010). An apparent Km of 31 μM was measured for the tomato LePT1 Pi transporter using the yeast PAM mutants (Daram et al., 1998). While this may still be higher than expected for a high-affinity Pi transporter, it is an order of magnitude lower than the values measured for plant Pi transporters expressed in the pho84 mutant. Expression of a cDNA encoding a plant Pi transporter in cultured tobacco cells has yielded the most reliable functional analysis. Mitsukawa et al. (1997) proved that the PHT1 transporter from Arabidopsis is a high-affinity P transporter with an apparent Km of 31 μM. The sequences and expression patterns of some of the P transporters from Arabidopsis and barley are almost identical suggesting that they have similar functions (Smith and Read, 1997; Smith et al., 1999). It seems that at least in some diploid plant genomes, there is a redundancy of genes encoding P transporters that are critical for the P uptake and hence plant performance. The high degree of sequence similarities suggests that the genes are the result of recent gene duplications.
“The Helping Hands” – P Uptake Through Mycorrhizal Association
In AM associations, plants acquire Pi from the extensive network of extra-radical hyphae of fungi that extend beyond root depletion zones to mine new regions of the soil (Harrison and van Buuren, 1995). The indirect uptake of Pi in mycorrhizal plants results in higher Pi uptake rates than in non-mycorrhizal plants under P limited conditions (Haynes, 1982; Kumar et al., 2011). The role of AMF in nutrient acquisition of their host seems to be inversely related to the development of the root system (Newsham et al., 1995; Schweiger et al., 1995). In nature, there is, however, a variation in P uptake in relation to colonization by different AMF, since isolates differ in P transfer efficiency and also in P supply to the plant (Pearson and Jakobsen, 1993; Joner and Jakobsen, 1995). For instance, G. aggregatum provides less and G. intraradices more Pi to the same host (Kiers et al., 2011). Interestingly, plants also regulate the fungal growth in the root depending upon the external P status, and at high P supply root colonization seems to be reduced (Thomson et al., 1990).
In case of GvPT, G. intraradices, and G. mosseae, the function of Pi transporters have been studied in heterologous systems but their role in Pi transportation in AMF could not be verified due to the lack of a stable transformation systems (Maldonado-Mendoza et al., 2001; Harrison et al., 2002; Benedetto et al., 2005). Since the difference in electrochemical potential between soil and extra-radical mycelium is large, being negative in the mycelium, and since the P concentration inside the mycelium is high compared with the soil solution, the absorption of P by AMF must be an active process (Smith and Read, 1997). The first fungal P transporter that is involved in the uptake of P from soil has been identified in GvPT. The GvPT encodes a high-affinity fungal P transporter that is similar in both structure and function to high affinity transporters of plants (Harrison and van Buuren, 1995). Maldonado-Mendoza et al. (2001) have identified a P transporter gene in the extra-radical mycelium of G. intraradices named GiPT, which is expressed at low P concentration in the growth substrate. Thus, extra-radical hyphae in a mycorrhiza are involved in the absorption of Pi and other nutrients from the soil, its translocation from the surrounding soil to the hyphae and through the mycelium into the fungal structures within the root (Smith and Read, 1997). Other reports have shown that plant P transporters, inducible by P starvation, are down-regulated in mycorrhizal roots, indicating that these transporters are not involved in symbiotic P transfer (Chiou et al., 2001). It is believed that the initial Pi uptake under Pi limitation into the fungus-plant community is almost entirely due to uptake by the extra-radical fungal hyphae (Pearson and Jakobsen, 1993).
Mycorrhizal associations are not always beneficial for Pi nutrition. It can be costly for the plant and the fungal partners may alter their mutualistic responsibility from beneficial to neutral (Kiers and Van der Heijden, 2006; Douglas, 2008). Consequently, AMF colonization can have no or negative effects on the plant’s growth and performance (Johnson et al., 1997). Depression of plant growth can be due to high C-demand of the fungal partners leading to lack of P benefit for the plant. Poor growth can already occur with low fungal biomass. Furthermore, growth depression can also be the result of reduction in P acquisition through the direct P-uptake pathway of the plant that is not compensated for by the fungal P delivery (Smith et al., 2009, 2011). However, in the plant-mycorrhizal mutualism both symbionts are able to detect variations in the resources supplied by their partners, allowing them to adjust their own resource allocation accordingly. Host plants discriminate between cooperative and less cooperative AMF partners. They may supply them with more or less amounts of carbon, depending on the amount of P they receive from the fungal partner. However, this reciprocal reward between the plant and more cooperative partners depends on carbon supply. The P transfer to the plant is proportional to the C supply (Kiers et al., 2011). Reprogramming of root development under Pi limitation shares similarities with processes known from root colonization by AMF (Rubio et al., 2009), or other beneficial root-colonizing fungi. This offers a great potential for cross-talk studies.
Mechanism of Indirect P Uptake
P uptake into the mycorrhizal plants cells takes place at a specialized interface between AMF and host cells called arbuscules. When a fungal hypha penetrates a cortical cell and differentiates into an arbuscule, the plant cell plasma membrane extends to surround it with the so-called periarbuscular membrane, localizing the arbuscule essentially to the plant apoplast. The extended periarbuscular membrane including the residual plant cell wall is separated from the fungal wall and the underlying membrane of the AMF by a narrow compartment, the interface matrix (Harrison, 1999).
The indirect pathway of Pi acquisition through AM associations involves (a) uptake of P from the soil across the membrane of the fungal hyphae, (b) movement of P along the hyphae to the arbuscules, (c) unloading the P from the fungal arbuscules at the arbuscule-cortical cell interface, and (d) uptake of that P by the plant cortical cells. The suggested mechanisms of P translocation within the AM fungal hyphae from the place of uptake to the fungus–root interfaces involve processes based on cytoplasmic streaming and to a lesser extent on mass flow (Cooper and Tinker, 1981). The molecular components involved in the efflux of P across the arbuscular membrane are not known. The efflux of Pi from arbuscules might be connected to the degree of arbuscule formation and/or vacuolar poly Pi hydrolysis in the fungal cells (Figure 4). Phosphatases are responsible for the conversion of Pi-esters into Pi in the fungal vacuoles prior to their export. Non-specific alkaline and acid phosphatases (ALPase and ACPase, respectively) have been identified in the vacuole of AMF (Gianinazzi-Pearson and Gianinazzi, 1978; Tisserant et al., 1993; Ezawa et al., 1995, 1999). Most of their activities were insoluble in aequous media, possibly due to the association of the enzymes with the tonoplast, as also shown for yeast (Gianinazzi et al., 1979; Klionsky et al., 1990; Ezawa et al., 1999). Immunolabeling studies indicated that the periarbuscular membrane of the plant contains high H+-ATPase activity (Gianinazzi-Pearson et al., 2000). This supports the idea of a plant Pi transporter being active at the periarbuscular membrane surrounding the arbuscule.
FIGURE 4.
Translocation of phosphorus (P) and carbon (C) at fungus – plant interface. Inorganic P is taken up by specialized transporters located on the fungal membrane in the extra-radical mycelium. Pi (originated in AM fungi from the hydrolysis of polyphosphate) is imported from the symbiotic interface to the plant cells through AM inducible Pi transporters (AMiPT). Hexose transporters (HT) import plant-derived carbon to the fungus, whereas transporter proteins involved in the export of nutrients from plant have not been identified yet.
Several plant P transporters appear to be responsible for the uptake of P released by mycorrhizal fungi (Daram et al., 1998; Rosewarne et al., 1999; Liu et al., 2002). Characterization of the Pi transporters StPT3 from potato and MtPT4 from M. truncatula provides molecular and biochemical evidence for plant Pi uptake at the AM fungus–root interface in AM (Rausch et al., 2001; Harrison et al., 2002). In accordance with Daram et al. (1998) StPT3 functions as a high-affinity Pi transporter, and StPT3 expression is locally induced upon colonization by the AM fungus G. intraradices. The mRNA levels correlated with arbuscule formation in the roots. MtPT4 is exclusively present on the periarbuscular membranes. In contrast, the potato P transporter StPT1 showed high expression in non-mycorrhizal roots, independently of Pi fertilization, while the amount of StPT2 transcripts was reduced under high-Pi conditions. In mycorrhizal roots, StPT1 and StPT2 mRNA concentrations were reduced independently of the Pi treatment. A reduction in Pi transporter mRNA levels upon AMF colonization has also been reported for M. truncatula MtPT1 and MtPT2 (Liu et al., 1998b; Chiou et al., 2001; Javot et al., 2007). Therefore, it seems unlikely that these transporters are involved in Pi transfer at the arbuscular interface. The reduced StPT2 and MtPT mRNA levels could either be a local response after colonization, or caused by the increased Pi status of the root or shoot (systemic response; Rausch et al., 2001). The mycorrhiza-responsive plant P transporters are localized on the periarbuscular membrane at the symbiotic interface and not at the arbuscular stalk interface. The localization of these P transporters is regulated by a polarization of the bulk secretary pathway favoring vesicle fusion with the developing periarbuscular membrane rather than with the plasma membrane, which might ensure the proper direction of Pi and other (e.g., sugar) transporters to the peri-arbuscular membrane during arbuscules development (Popova et al., 2010).
Recent advances in genomics and transcriptomics of AMF and other plant beneficial fungi provides a better picture of the fungi-induced biochemical and physiological re-programming of P acquisition mechanisms. G. intraradices has a combination of low affinity as well as high affinity Pi transporter genes similar to Pho91, and the Na+/Pi symporter genes Pho89 and GintPT, respectively, and they are expressed in spores, extra-radical and intra-radical mycelia (Tisserant et al., 2012). The expression pattern of GintPT in combination with the arbuscular localization of the GmosPT protein in G. mosseae confirmed the involvement, site and role of these P transporters in P delivery to the plant (Benedetto et al., 2005; Balestrini et al., 2007; Gómez-Ariza et al., 2009). The P transporters GintPT, Pho91, and Pho89 are functional at a broad pH range from 4 to 9 and thus active in a variety of acidic and alkaline soils.
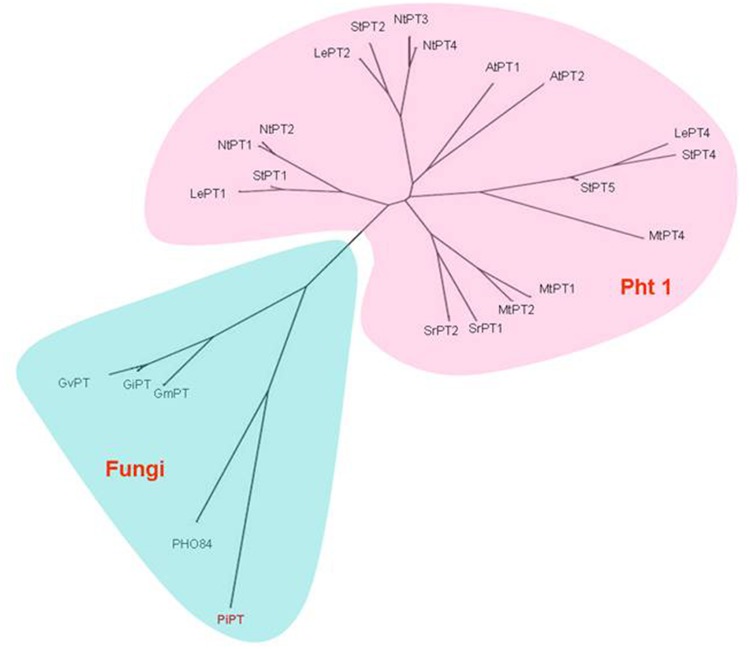
Analysis of the genome sequence of P. indica revealed the presence of three putative P transporter genes but only PiPT has been studied yet (Yadav et al., 2010; Zuccaro et al., 2011). PiPT falls in the category of high affinity fungal phosphate transporters and very distinct from phosphate transporters of plants (Figure 5). PiPT is expressed in extra-radical hyphae under P deprived conditions, similar to transporters from MF (Harrison and van Buuren, 1995; Tatry et al., 2009; Yadav et al., 2010). However, the P. indica-mediated mechanism for P delivery is different from that of AMF because P. indica does not form arbuscule-like structures (Kumar et al., 2011). Nevertheless, P. indica might be an alternative to mycorrhizal fungi and extensive fertilization for crop management strategies. PiPT is regulated by the amount of Pi present on the outside of the root, and not by the intracellular Pi pool. The degree of colonization by P. indica in maize was similar under low and high Pi concentrations indicating that the colonization is independent of Pi availability (Yadav et al., 2010). Thus, P. indica would allow a more efficient Pi uptake independently of the degree of root colonization and other similar responses as in case of AMF colonization (Table 1).
FIGURE 5.
Unrooted phylogenetic relationship of PiPT to other high affinity Pi transporters from plants and fungi (Yadav et al., 2010).
Table 1.
Multiple responses of plants under Pi deficient conditions (Raghothama, 1999).
| Kind of Responses | Plant Responses |
|---|---|
| Morphological responses | Increased root:shoot ratio, changes in root morphology and architecture, increased root hair proliferation, root hair elongation, accumulation of pigments, proteoid roots, increased association with mycorrhizal fungi |
| Physiological responses | Enhanced Pi uptake, reduced Pi efflux, increased Pi use efficiency, mobilization of Pi from the vacuole to cytoplasm, increased translocation of Pi within plants, retention of more Pi in roots, secretion of organic acids, protons and chelators, secretion of phosphatases and RNases, altered respiration, carbon metabolism, photosynthesis, nitrogen fixation, and aromatic enzyme pathways |
| Biochemical responses | Activation of enzymes, enhanced production of phosphatases, RNases and organic acids, changes in protein phosphorylation, activation of glycolytic bypass pathway |
| Molecular responses | Activation of genes (RNases, phosphatases, phosphate transporters, Ca-ATPase, vegetative storage proteins, β-glucosidase). |
Pi Mobilization and P. indica: Future Prospects for Agricultural Application?
P. indica (Box 2) produces significant amounts of acid phosphatases for the mobilization of a broad range of insoluble forms of Pi, enabling the host plant to access adequate P from immobilized reserves in the soil (Malla, 2005). The potential of this root-endophytic fungus in improving plant performance and its nutritional status has been demonstrated by Yadav et al. (2010) for maize, by characterizing and analyzing the role of PiPT in P. indica-colonized roots. Similar findings were described by Shahollari et al. (2005) who showed that P. indica increases the Pi uptake two–threefold in Arabidopsis seedlings. Whether this can be generalized, and is valid for all crop species, need to be investigated. Barazani et al. (2005) and Achatz et al. (2010) reported that P. indica is not involved in P acquisition and thus increased biomass production in barley and Nicotiana attenuate, respectively.
BOX 2. Piriformospora indica: Magic without a Wand.
An axenically cultivable AMF like-fungus named P. indica has been discovered in the Indian Thar desert (Verma et al., 1998). P. indica colonizes dicot as well as monocot plants including members of the Brassicaceae, like Arabidopsis, which are not colonized by AMF (Shahollari et al., 2005). Colonization of P. indica with the medicinal and other economically important plants results in the increased growth yield, high salt tolerance, disease resistance, and nutrition capabilities of the host plant. Because of its beneficial nature this fungus has been termed as plant probiotic (Peškan-Berghöfer et al., 2004; Aschheim et al., 2005; Waller et al., 2005; Kumar et al., 2009).
Efficient transformation systems for P. indica has been established by two groups (Yadav et al., 2010; Lahrmann et al., 2013) which allows now functional analyses and the identification of new regulatory genes/proteins controlling Pi acquisition and transfer from the fungus to the host. The availability of the genome sequence of P. indica further helps in the identification of novel players in this scenario. As far as we know, P. indica can associate with the roots of all plant species tested so far. Furthermore, Sebacinales have been identified around the globe, and P. indica transfers benefits to plants under quite different climate, temperature and growth conditions. The fungus can grow axenically and does not need a host for growth and large-scale propagation. An increasing body of studies on this fungus also provides a nice scientific basement for agricultural application. Pi limitation results in major reprogramming of plant developmental processes and they are linked to many signaling pathways influencing plant development in symbiotic interactions. Besides stimulation of biomass, P. indica confers tolerance to biotic and abiotic stresses, and can be used as biocontrol agent (Sun et al., 2014). These examples suggest a high potential of the fungus for biotechnological and agricultural applications, in particular under Pi limitation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AJ and MD are thankful to Jawaharlal Nehru University for providing the DST-PURSE fund. AJ and NT are also thankful to the Department of Science and Technology, Govt. of India for providing funds. VY and MK are thankful to Council of Scientific and Industrial Research, and Indian Council of Medical Research, Govt. of India, respectively for providing fellowships. AJ is also grateful to American Society for Microbiology for providing Research Assistant Professorship to work in the laboratory of Prof. RS, at University of California, San Francisco, USA, who is supported by the NIH [GM24485].
References
- Achatz B., Rüden S., Andrade D., Neumann E., Pons-Kühnemann J., Kogel K. H., et al. (2010). Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil. 333 59–70. 10.1007/s11104-010-0319-0 [DOI] [Google Scholar]
- Aschheim K., Cervoni N., DeFrancesco L., Hare P., Taroncher-Oldenburg G. (2005). Plant probiotic (News & Views). Nat. Biotech. 23 1241. [Google Scholar]
- Balestrini R., Gómez-Ariza J., Lanfranco L., Bonfante P. (2007). Laser microdissection reveals that transcripts for five plant and one fungal Pi transporter genes are contemporaneously present in arbusculated cells. Mol. Plant Microbe Interact. 20 1055–1062. [DOI] [PubMed] [Google Scholar]
- Barazani O., Benderoth M., Groten K., Kuhlemeier C., Baldwin I. T. (2005). Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146 234–243. 10.1007/s00442-005-0193-2 [DOI] [PubMed] [Google Scholar]
- Barrow N. J. (1980). “Evaluation and utilization of residual phosphorus in soils,” in The Role of Phosphorus in Soils eds Khasawneh F. E., Sample E. C., Kamprath E. J., Madison W. I. (Madison, WI: American Society Agronomy; ) 335–355 [Google Scholar]
- Benedetto A., Magurno F., Bonfante P., Lanfranco L. (2005). Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15 620–627. 10.1007/s00572-005-0006-9 [DOI] [PubMed] [Google Scholar]
- Bieleski R. L. (1973). Phosphate pools, phosphate transport and phosphate availability. Annu. Rev. Plant Physiol. 24 225–252. 10.1146/annurev.pp.24.060173.001301 [DOI] [Google Scholar]
- Bieleski R. L., Ferguson I. B. (1983). “Physiology and metabolism of phosphate and its compound,” in Encyclopedia of Plant Physiology; Inorganic Plant Nutrient eds Lauchi A., Bieleski R. L. (Berlin: Springer-Verlag; ) 422–429. [Google Scholar]
- Bolan N. S. (1991). A critical-review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 134 189–207. [Google Scholar]
- Bonfante P., Genre A. (2010). Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 1 1–11. 10.1038/ncomms1046 [DOI] [PubMed] [Google Scholar]
- Brady N. C. (1990). The Nature and Properties of the Soils. New York, NY: Macmillan Publishing Co. [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y. (1991). The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell Biol. 11 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. J. W., Beever R. E. (1977). Kinetic characterization of the two phosphate uptake systems in the fungus Neurospora crassa. J. Bacteriol. 132 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou T. J., Liu H., Harrison M. J. (2001). The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J. 25 281–293. 10.1046/j.1365-313x.2001.00963.x [DOI] [PubMed] [Google Scholar]
- Cooper K. M., Tinker P. B. (1981). Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. New Phytol. 88 327–339. 10.1111/j.1469-8137.1981.tb01728.x [DOI] [Google Scholar]
- Daram P., Brunner S., Persson B. L., Amrhein N., Bucher M. (1998). Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta 206 225–233. 10.1007/s004250050394 [DOI] [PubMed] [Google Scholar]
- Douglas A. E. (2008). Conflict, cheats and persistence of symbioses. New Phytol. 177 849–858. 10.1111/j.1469-8137.2007.02326.x [DOI] [PubMed] [Google Scholar]
- Drew E. A., Murray R. S., Smith S. E., Jakobsen I. (2003). Beyond the rhizosphere: growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 251 105–114. [Google Scholar]
- Escoubet B., Djabali K., Amiel C. (1989). Adaptation to Pi deprivation of cell Na+ dependent Pi uptake-a widespread process. Am. J. Physiol. 256 322–328. [DOI] [PubMed] [Google Scholar]
- Ezawa T., Kuwahara S., Sakamoto K., Yoshida T., Saito M. (1999). Specific inhibitor and substrate specificity of alkaline phosphatase expressed in the symbiotic phase of the arbuscular mycorrhizal fungus, Glomus etunicatum. Mycologia 91 636–641. 10.2307/3761249 [DOI] [Google Scholar]
- Ezawa T., Saito M., Yoshida T. (1995). Comparison of phosphatase localization in the intraradical hyphae of arbuscular mycorrhizal fungi, Glomus spp. and Gigaspora spp. Plant Soil 176 57–63. 10.1007/BF00017675 [DOI] [Google Scholar]
- Furihata T., Suzuki M., Sakurai H. (1992). Kinetic characterization of two phosphate uptake systems with different affinities in suspension-cultured Catharanthus roseus protoplasts. Plant Cell Physiol. 33 1151–1157. [Google Scholar]
- Gianinazzi S., Gianinazzi P. V., Dexheimer J. (1979). Enzymatic studies on the metabolism of vasicular arbuscular mycorrhiza. New Phytol. 82 127–132. 10.1111/j.1469-8137.1979.tb07566.x [DOI] [Google Scholar]
- Gianinazzi-Pearson V., Arnould C., Oufattole M., Arango M., Gianinazzi S. (2000). Differential activation of H+-ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco. Planta 211 609–613. 10.1007/s004250000323 [DOI] [PubMed] [Google Scholar]
- Gianinazzi-Pearson V., Gianinazzi S. (1978). Enzymatic studies on the metabolism of vesicular- arbuscular mycorrhizas. Physiol. Plant Pathol. 12 45–53. 10.1016/0048-4059(78)90017-6 [DOI] [Google Scholar]
- Gilbert N. (2009). The disappearing nutrient (news feature). Nature 461 716–718. 10.1038/461716a [DOI] [PubMed] [Google Scholar]
- Goldstein A. H. (1992). “Phosphate starvation inducible enzymes and proteins in higher plants,” in Inducible Plant Proteins. Seminar Series 49. Society for Experimental Biology ed. Wray J. L. (Cambridge: Cambridge University Press; ) 25–44 [Google Scholar]
- Gómez-Ariza J., Balestrini R., Novero M., Bonfante P. (2009). Cell-specific gene expression of phosphate transporters in mycorrhizal tomato roots. Biol. Fertil. Soils 45 845–853. 10.1007/s00374-009-0399-2 [DOI] [Google Scholar]
- Harrison M. J. (1999). Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 361–389. [DOI] [PubMed] [Google Scholar]
- Harrison M. J., Dewbre G. R., Liu J. (2002). A phosphate transporter from medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14 2413–2429. 10.1105/tpc.004861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. J., van Buuren M. L. (1995). A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378 626–629. 10.1038/378626a0 [DOI] [PubMed] [Google Scholar]
- Haynes R. J. (1982). Effects of liming on phosphate availability in acid soils. Plant Soil 68 289–308. 10.1007/BF02197935 [DOI] [Google Scholar]
- Hinsinger P. (2001). Biodiversity of soil inorganic P in the rhizosphere as affected root induced chemical changes. Plant Soil 237 173–195. 10.1023/A:1013351617532 [DOI] [Google Scholar]
- Hinsinger P., Gobran G. R., Gregory P. J., Wenzel W. W. (2005). Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 168 293–303. 10.1111/j.1469-8137.2005.01512.x [DOI] [PubMed] [Google Scholar]
- Holford I. C. R. (1997). Soil phosphorus: its measurement and its uptake by plants. Aust. J. Soil Res. 35 227–239. 10.1071/S96047 [DOI] [Google Scholar]
- Javot H., Pumplin N., Harrison M. J. (2007). Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ. 30 310–322. 10.1111/j.1365-3040.2006.01617.x [DOI] [PubMed] [Google Scholar]
- Jennings D. H. (1995). The Physiology of Fungal Nutrition. Cambridge: Cambridge University Press. [Google Scholar]
- Johnson N. C., Graham J. H., Smith F. A. (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 135 575–586. [Google Scholar]
- Joner E. J., Jakobsen I. (1995). Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol. Biochem. 27 1153–1159. 10.1016/0038-0717(95)00047-I [DOI] [Google Scholar]
- Karandashov V., Bucher M. (2005). Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 10 22–29. 10.1016/j.tplants.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Karandashov V., Nagy R., Wegmüller S., Amrhein N., Bucher M. (2004). Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U.S.A. 101 6285–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan A. S., Varadarajan D. K., Jain A, Held M. A., Carpita N. C., Raghothama K. G., et al. (2007). Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225 907–918. [DOI] [PubMed] [Google Scholar]
- Kiers E. T., Duhamel M., Beesetty Y., Mensah J. A., Franken O., Verbruggen E., et al. (2011). Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333 880–882. [DOI] [PubMed] [Google Scholar]
- Kiers E. T., Van der Heijden M. G. A. (2006). Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology 87 1627–1163. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. (1990). The fungal vacuole, composition, function, and biogenesis. Microbiol. Rev. 54 266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Yadav V., Kumar H., Sharma R., Singh A., Tuteja N., et al. (2011). Piriformospora indica enhances plant growth by transferring Phosphate. Plant Signal. Behav. 6723–725. 10.4161/psb.6.5.15106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Yadav V., Tuteja N., Johri A. K. (2009). Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 155 780–790. [DOI] [PubMed] [Google Scholar]
- Lahrmann U., Ding Y., Banhara A., Rath R., Hajirezaei M. R., Döhlemann S., et al. (2013). Host-related metabolic cues affect colonization strategies of a root endophyte. Proc. Natl. Sci. Acad. Biol. Sci. U.S.A. 110 13965–13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer M. J., Blevins D. G., Gracz S. H. (1989). 32P-nuclear magnetic resonance determination of phosphate compartmentation in leaves of reproductive soybeans (Glycine max L.) as affected by phosphate nutrition. Plant Physiol. 89 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggewie G., Willmitzer L., Reismeier J. W. (1997). Two cDNAs from Potato are able to complement a phosphate uptake deficient yeast mutant: identification of phosphate transporters from higher plants. Plant Cell 9 381–392. 10.1105/tpc.9.3.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. G., Quirk J. P. (1976). Phosphorus deficiency in soil and uptake by plants. III. P31 movement and uptake in plants as indicated by P32 autoradiography. Plant Soil 27 445–453. [Google Scholar]
- Liu C., Munchhal U. S., Uthappa M., Kononowicz A. K., Raghothama K. G. (1998a). Tomato phosphate transporter genes are differentially regulated in plants tissues by phosphorous. Plant Physiol. 116 91–96. 10.1104/pp.116.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Trieu A. T., Blaylock L. A., Harrison M. J. (1998b). Cloning and characterization of two phosphate transporters from Medicago truncatula roots: regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol. Plant Microbe Interact. 11 14–22. [DOI] [PubMed] [Google Scholar]
- Liu F., Wang Z., Ren H., Shen C., Li Y., Ling H. Q., et al. (2010). OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J. 62 508–517. [DOI] [PubMed] [Google Scholar]
- Liu H., Cottrell T. R., Pierini L. M., Goldman W., Doering T. L. (2002). RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics 160 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J., Cruz-Ramírez A., Herrera-Estrella L. (2003). The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6 280–287. [DOI] [PubMed] [Google Scholar]
- Lynch J. P. (1995). Root architecture and plant productivity. Plant Physiol. 109 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. P., Brown K. M. (2008). “Root strategies for phosphorus acquisition,” in The Ecophysiology of Plant-Phosphorus Interactions eds White P. J., Hammond J. P. (Dordrecht: Springer; ) 83–116. [Google Scholar]
- Ma L. Q., Komar K. M., Tu C., Zhang W., Cai Y., Kennelley E. D. (2001a). A fern that hyper accumulates arsenic. Nature 409 579. [DOI] [PubMed] [Google Scholar]
- Ma Z., Bielenberg D. G., Brown K. M., Lynch J. P. (2001b). Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 24 459–467. [Google Scholar]
- Maldonado-Mendoza I. E., Dewbre G. R., Harrison M. J. (2001). A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol. Plant Microbe Interact. 14 1140–1148. [DOI] [PubMed] [Google Scholar]
- Malla R. (2005). Characterization of Piriformospora indica-Phosphatases. Ph.D. thesis, School of Life Sciences, Jawaharlal Nehru University; New Delhi. [Google Scholar]
- Marschner H. (1995). Nutrient Availability in Soils: Mineral Nutrition of Higher Plants 2nd Edn London: Acadmic Press; 483–507. [Google Scholar]
- Martinez P., Persson B. L. (1998). Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258 628–638. 10.1007/s004380050776 [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. L. (2005) A Closer Look at Phosphorus Uptake by Plants. News and Views. Norcross, GA: Potash and Phosphate Institute. [Google Scholar]
- Mimura T. (1999). Regulation of phosphate transport and homeostasis in plant cells. Int. Rev. Cytol. 191 149–200. 10.1016/S0074-7696(08)60159-X [DOI] [Google Scholar]
- Mimura T., Dietz K. J., Kaiser W, Schramm M. J., Kaiser G., Heber U. (1990). Phosphate transport across biomembranes and cytosolic phosphate homeostasis in barley leaves. Planta 180 139–146. [DOI] [PubMed] [Google Scholar]
- Mimura T., Sakano K., Shimmen T. (1996). Studies on the distribution, retranslocation and homeostasis of inorganic phosphate in barley leaves. Plant Cell Environ. 19 311–320. 10.1111/j.1365-3040.1996.tb00253.x [DOI] [Google Scholar]
- Mitsukawa N., Okumura S., Shirano Y., Sato S., Kato T., Harashima S., et al. (1997). Overexpression of an Arabidopsis thaliana high affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate limited conditions. Proc. Natl. Acad. Sci. 93 10519–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munchhal U. S., Pardo J. M., Raghothama K. G. (1996). Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. 93 10519–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P., Canivenc G., Muller B., Azmi A., Van Onckelen H., Rossignol M., et al. (2005). A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 138 2061–2074. 10.1104/pp.105.060061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham K. K., Fitter A. H., Watkinson A. R. (1995). Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10 407–411. [DOI] [PubMed] [Google Scholar]
- Olah Z., Lehel C., Anderson W., Eiden M., Wilson C. (1994). The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 269 25426–25431. [PubMed] [Google Scholar]
- Pao S. S., Paulsen I. T., Saier M. H. (1998). Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Rev. Microbiol. 6 763–775. 10.1038/nrmicro1987 [DOI] [PubMed] [Google Scholar]
- Pearson J. N., Jakobsen I. (1993). Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 124 481–488. [Google Scholar]
- Pearson R. W., Naorman A. G., Ho C. (1941). Mineralization of organic phosphorus of various compounds in soil. Proc. Soil Sci. Soc. Am. 6 168–175. [Google Scholar]
- Pedersen B. P., Kumar H., Waight A. B., Risenmay A. J., Roe-Zurz Z., Chau B. H., et al. (2013). Crystal structure of a eukaryotic phosphate transporter. Nature 25 496 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peškan-Berghöfer T., Shahollari B., Pham H. G., Hehl S., Markent C., Blank V., et al. (2004). Association of Piriformospora indica with Arabidopsis thaliana roots represent a novel system to study beneficial plant-microbe interactions and involve in early plant protein modifications in the endocytoplasmic reticulum and in the plasma membrane. Physiol. Plant 122 465–471. 10.1111/j.1399-3054.2004.00424.x [DOI] [Google Scholar]
- Popova Y., Thayumanavan P., Lonati E., Agrochao M., Thevelein J. M. (2010). Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. 107 2890–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama K. G. (1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 665–693. 10.1146/annurev.arplant.50.1.665 [DOI] [PubMed] [Google Scholar]
- Rausch C., Daram P., Brunner S., Jansa J., Laloi M., Leggewie G., et al. (2001). A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 414 462–470. 10.1038/35106601 [DOI] [PubMed] [Google Scholar]
- Rengel Z., Marschner P. (2005). Nutrient availability and management in the rhizosphere of plant genotypes. New Phytol. 168 305–312. 10.1111/j.1469-8137.2005.01558.x [DOI] [PubMed] [Google Scholar]
- Robinson D. (1994). The responses of plants to non-uniform supplies of nutrients. New Phytol. 127 635–674. 10.1111/j.1469-8137.1994.tb02969.x [DOI] [PubMed] [Google Scholar]
- Rohmeld V., Newmann G. (2006). “The rhizosphere: contributions of soil-root interface to sustainable soil systems,” in Biological Approaches to Sustainable Soil Systems eds Uphoff N., Ball N. A. S., Fernandes E., Herren H., Husson O., Laing M., et al. (Boca Raton, FL: CRC Press; ) 92–107. [Google Scholar]
- Rosewarne G. M., Barker S. J., Smith S. E., Smith A. F., Schachtman D. P. A., Schachtman D. P. (1999). A Lycopersicon esculentum phosphate transporter (LePT1) involved in phosphorous uptake from a vesicular-arbuscular mycorrhizal fungus. New Phytol. 144 507–516. 10.1046/j.1469-8137.1999.00536.x [DOI] [PubMed] [Google Scholar]
- Rubio V., Bustos R., Irigoyen M. L., Cardona-Lopez X., Rojas-Triana M., Paz-Ares J. (2009). Plant hormones and nutrient signaling. Plant Mol. Biol. 69 361–373. 10.1007/s11103-008-9380-y [DOI] [PubMed] [Google Scholar]
- Runge-Metzger A. (1995). “Closing the cycle: obstacles to efficient P management for improved global food security,” in Phosphorus in the Global Environment: Transfers, Cycles and Management ed. Tiessen H. (New York, NY: Wiley; ) 27–42. [Google Scholar]
- Saier M. H., Reizer J. (1991). Families and superfamilies of transport proteins common to prokaryotes and eukaryotes. Curr. Opin. Struct. Biol. 1 362–368. 10.1016/0959-440X(91)90034-Q [DOI] [Google Scholar]
- Sakano K., Yazaki Y., Mimura T. (1992). Cytoplasmic acidification induced by inorganic phosphate uptake in suspension cultured Catharanthus roseus cells. Plant Physiol. 99 672–680. 10.1104/pp.99.2.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samyn D. R., Ruiz-Pávon L., Andersson M. R., Popova Y., Thevelein J. M., Persson B. L. (2012). Mutational analysis of putative phosphate- and proton-binding sites in the Saccharomyces cerevisiae Pho84 phosphate:H+ transceptor and its effect on signalling to the PKA and PHO pathways. Biochem. J. 445 413–422. 10.1042/BJ20112086 [DOI] [PubMed] [Google Scholar]
- Schachtman D. P., Reid R. J., Ayling S. M. (1998). Phosphorus uptake by plants from soil to cell. Plant Physiol. 116 447–453. 10.1104/pp.116.2.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf A., Leitner D., Klepsch S., Pellerin S., Mollier A. (2011). “Modelling phosphorus dynamics in the soil-plant system,” in Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling eds Bünemann E. K., Obserson A., Frossard E. (Heidelberg: Springer; ) 113–133. [Google Scholar]
- Schweiger P. F., Robson A. D., Barrow N. J. (1995). Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytol. 131 247–254. [Google Scholar]
- Shahollari B., Varma A., Oelmüller R. (2005). Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J. Plant Physiol. 162 945–958. [DOI] [PubMed] [Google Scholar]
- Smith F. A., Grace E. J., Smith S. E. (2009). More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 182 347–358. 10.1111/j.1469-8137.2008.02753.x [DOI] [PubMed] [Google Scholar]
- Smith F. W. (2001). “Plant responses to nutritional stress,” in Molecular Analysis of Plant Adaptation to the Environment eds Hawkesford M. J., Buchner P. (Dordrecht: Kluwer Academic Publishers; ) 249–269. [Google Scholar]
- Smith F. W., Cybinski D., Rae A. L. (1999). “Regulation of expression of genes encoding phosphate transporters in barley roots,” in Plant Nutrition – Molecular Biology and Genetics eds Gissel-Niuelsen G., Jensen A. (Dordrecht: Kluwer Academic Publishers; ) 145–150. [Google Scholar]
- Smith S. E., Jakobsen I., Grønlund M., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant phosphorus (P) nutrition: interactions between pathways of P uptake in arbuscular mycorrhizal (AM) roots have important implications for understanding and manipulating plant P acquisition. Plant Physiol. 156 1050–1057. 10.1104/pp.111.174581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E., Read D. J. (1997). Mycorrhizal Symbiosis 2nd Edn London: Academic Press. [Google Scholar]
- Smith S. E., Read D. J. (2008). Mycorrhizal Symbiosis 3rd Edn New York, NY: Academic Press. [Google Scholar]
- Stevensons F. J. (1994). Humun Chemistry: Genesis. Composition, Reactions. New York, NY: John Wiley and Sons. [Google Scholar]
- Stitt M., Rudige-Scheible W. (1998). Understanding allocation to shoot and root growth will require molecular information about which compounds act as signals for the plant nutrient status, and how meristem activity and cellular growth are regulated: opinion. Plant Soil 201 259–263. 10.1023/A:1004305106426 [DOI] [Google Scholar]
- Sun C., Shao Y., Vahabi K., Lu J., Bhattacharya S., Dong S., et al. (2014). The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC Plant Biol. 14:268 10.1186/s12870-014-0268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Li X., Palmgren M. J. (1999). Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11 677–689. 10.2307/3870892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatry M. V., El Kassis E., Lambilliotte R., Corratgé C., van Aarle I., Amenc L. K., et al. (2009). Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J. 57 1092–1102. [DOI] [PubMed] [Google Scholar]
- Thomson B. D., Clarkson D. T., Brain P. (1990). Kinetics of phosphorous uptake by the germ tube of the vesicular-arbuscular mycorrhizal fungus Gigaspora marginata. New Phytol. 116 647–653. [Google Scholar]
- Tisserant B., Gianinazzi-Pearson V., Gianinazzi S., Gollotte A. (1993). In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol. Res. 97 245–250. 10.1016/S0953-7562(09)80248-7 [DOI] [Google Scholar]
- Tisserant E., Kohler A., Dozolme-Seddas P., Balestrini R., Benabdellah K., Colard A., et al. (2012). The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 193 755–769. 10.1111/j.1469-8137.2011.03948.x [DOI] [PubMed] [Google Scholar]
- Tu S. I., Cananaugh J. R., Boswell R. T. (1990). Phosphate uptake by excised maize root tips studied by in vivo 31P nuclear magnetic resonance spectroscopy. Plant Physiol. 93 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Fischer E., Lüttge U. (1981). Relationship between energy-dependent phosphate uptake and the electrical membrane potential in Lemna gibba G1. Plant Physiol. 67 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich-Eberius C. I., Novacky A., Van Bel A. J. E. (1984). Phosphate uptake in Lemna gibba G1: energetics and kinetics. Planta 161 46–52. [DOI] [PubMed] [Google Scholar]
- Vance C. P. (2010). Quantitative trait loci, epigenetics, sugars, and microRNAs: quaternaries in phosphate acquisition and use. Plant Physiol. 154 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Uhde-Stone C., Allan D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 157 423–447. 10.1046/j.1469-8137.2003.00695.x [DOI] [PubMed] [Google Scholar]
- Verma S., Varma A., Rexer K. H., Hassel A., Kost G., Sarbhoy A., et al. (1998). Piriformospora indica gen. nov., A new root –colonizing fungus. Mycologia 90 896–903. [Google Scholar]
- Versaw W. K. (1995). A phosphate-repressible, high-affinity phosphate permease is encoded by the pho-5+ gene of Neurospora crassa. Gene 153 135–139. 10.1016/0378-1119(94)00814-9 [DOI] [PubMed] [Google Scholar]
- Versaw W., Metzenberg R. (1995). Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. U.S.A. 92 3884–3887. 10.1073/pnas.92.9.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Uexkull H. R., Mutert E. (1995). Global extent development and economic importance of acid soils. Plant Soil. 171 1–15. [Google Scholar]
- Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., et al. (2005). The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci.U.S.A. 102 13386–13391. 10.1073/pnas.0504423102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. L., Tang X. Y., Cheng L. Y., Zhang A. Z., Zhang W. H., Zhang F. S., et al. (2010). Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 187 1112–1123. [DOI] [PubMed] [Google Scholar]
- Werner A., Kinne R. K. (2001). Evolution of the Na-Pi co transport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280 R301–R312. [DOI] [PubMed] [Google Scholar]
- Yadav V., Kumar M., Deep D. K., Kumar H., Sharma R., Tripathi T., et al. (2010). A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in the phosphate transport to the host plant. J. Biol. Chem. 285 26532–26544. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang F., Shen J., Zhang J., Zuo Y., Li L., Chen X. (2010). Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China. Adv. Agron. 107 1–32. 10.2134/agronj14.0122 [DOI] [Google Scholar]
- Zuccaro A., Lahrmann U., Güldener U., Langen G., Pfiffi S., Biedenkopf D., et al. (2011). Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 7:e1002290 10.1371/journal.ppat.1002290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvyagilskaya R., Parchomenko O., Abramova N., Allard P., Panaretakis T., Pattison-Granberg J., et al. (2001). Proton– and sodium-coupled phosphate transport systems and energy status of Yarrowia lipolytica cells grown in acidic and alkaline conditions. J. Membr. Biol. 183 39–50. 10.1007/s00232-001-0054-9 [DOI] [PubMed] [Google Scholar]