Abstract
Background
Metformin is effective for the treatment of polycystic ovary syndrome, but conflicting results regarding its effect on adipocytokine levels (adiponectin, resistin, visfatin, and leptin) in patients with polycystic ovary syndrome receiving metformin treatment have been reported. To provide high-quality evidence about the effect of metformin treatment on adipocytokines in patients with polycystic ovary syndrome, relevant studies that assessed the levels of adipocytokines (adiponectin, resistin, visfatin, and leptin) in patients with polycystic ovary syndrome receiving treatment with metformin administration were reviewed and analyzed.
Methods
A literature search was conducted in the SCI, PUBMED, EMBASE, and Elsevier databases, and personal contact was made with the authors. Standard mean differences and 95% confidence intervals were calculated and combined appropriately. To ensure synthesis of the best available evidence, sensitivity analyses were performed.
Results
A total of 34 data sets were included in 4 different outcomes, involving 744 women with polycystic ovary syndrome and adipocytokine levels measured both before and after metformin administration. Metformin treatment was associated with significantly elevated serum adiponectin concentrations (standard mean differences [95% confidence interval], −0.43 [−0.75 to −0.11]) and decreased serum leptin concentrations (0.65 [0.26 to 1.04]), whereas no significant difference in resistin level (−0.01 [−0.49 to 0.45]) or visfatin level (−0.04 [−1.55 to 1.46]) was found.
Conclusions
Metformin administration was associated with increased serum adiponectin concentrations and decreased serum leptin levels. Further study is needed to elucidate whether this apparent effect decreases the incidence of type 2 diabetes and other metabolic diseases in patients with polycystic ovary syndrome later in life.
Introduction
The number of women with polycystic ovary syndrome (PCOS) has been continuously and rapidly increasing. Recent reports indicated that the prevalence of PCOS in women of reproductive age is 5–10% [1, 2]. About 50–70% of patients with PCOS have been confirmed to have insulin resistance and central obesity [3–5]. Both insulin resistance and central obesity predict the development of metabolic syndrome, which increases the risks of type 2 diabetes, hyperlipidemia, and cardiovascular disease [6–8]. Adipose tissue, especially visceral adipose tissue, is not only a fat storage depot but also an important endocrine organ, as it produces, synthesizes, and releases a number of bioactive proteins referred to as adipocytokines [9]. Recently, studies have demonstrated that adipocytokines are extensively implicated in the pathophysiological processes of metabolic disorders such as PCOS, insulin resistance, obesity, type 2 diabetes, and cardiovascular diseases [10]. Adiponectin is an adipocytokine synthesized in white adipose tissue. Its level is negatively correlated with obesity, insulin resistance, type 2 diabetes, coronary vascular diseases, and metabolic syndrome [11–13]. High adiponectin levels may reduce the risk of insulin resistance and type 2 diabetes [14]. Resistin, which contributes to insulin sensitivity, is another peptide secreted by the adipose tissue. Circulating resistin levels are increased in diet-induced and genetic forms of obesity animal models such as ob/ob and db/db mice. Serum resistin levels are decreased by using anti-diabetic drugs such as rosiglitazone [15]. Recent studies report a new adipocytokine, visfatin, also known as pre-B cell colony-enhancing factor, which is expressed in visceral lipid tissue and linked to metabolic syndrome. Studies have shown that visfatin expression is increased in individuals with abdominal obesity and type 2 diabetes [16]. Furthermore, leptin is a circulating hormone produced by adipocytes and is associated with insulin resistance in patients with PCOS [17, 18].
Metformin, a widely used insulin sensitizer, is an effective treatment of PCOS. It may improve ovulatory function in patients with PCOS, partly via weight loss or changes in body composition [19]. However, this hypothesis could not be confirmed with short-term treatment.
To understand how metformin improves the metabolic and hormonal disturbances in patients with PCOS, numerous studies have been conducted to determine the effect of metformin on adipocytokines in patients with PCOS, but their conclusions are far from uniform. Considering the large body of published evidence and amount of conflicting results, the primary purpose of the present study was to systematically review literature on the association between adipocytokines and metformin treatment in patients with PCOS in order to meta-analyze the best evidence available to provide high-quality data on the link between metformin treatment and adipocytokine levels in patients with PCOS.
Materials and Methods
Search strategy
We searched for English articles included in the SCI, PUBMED, EMBASE, and Elsevier databases. The following search terms were included:
(visfatin AND metformin) AND (PCOS OR polycystic ovary syndrome)
(adiponectin AND metformin) AND (PCOS OR polycystic ovary syndrome)
(resistin AND metformin) AND (PCOS OR polycystic ovary syndrome)
(leptin AND metformin) AND (PCOS OR polycystic ovary syndrome)
The computerized search was supplemented with a manual search of the bibliographies of all articles retrieved. Potentially relevant articles were assessed for inclusion based on prespecified inclusion and exclusion criteria.
Eligibility of relevant studies
Studies that met the following criteria were included:
All study subjects were limited to adults with PCOS diagnosed consistently by using either the Rotterdam [20] or National Institute of Health (NIH) criteria [21].
The study reported adiponectin, leptin, resistin, or visfatin values obtained both before- and after metformin therapy.
Studies were excluded if they met the following criteria:
The information available was not adequate for data extraction.
Abstracts, letters to the editor, and case reports.
Data extraction
Information from each study was extracted independently by 2 reviewers by using a standardized data extraction form. The general characteristics, methodology (PCOS definition and adiponectin/resistin/visfatin/leptin measurement method), and outcomes (both the total adiponectin/resistin/visfatin/leptin means and SDs before and after metformin administration) of each study were recorded, where available, and double-checked. Where appropriate, the data set was completed through communication with the authors. Disagreements were resolved by consensus.
Statistical analysis
The meta-analysis was conducted by using STATA version 12.0 and Review manager 5.2. Standard mean differences (SMD) and 95% confidence intervals (CIs) of the adipocytokine levels were calculated for all eligible studies and combined by using appropriate fixed or random effects models. The Mantel-Haenszel analysis method was used for dichotomous variables, and the inverse variance method was used for continuous variables [22]. A P value < 0.05 was considered statistically significant.
Statistical heterogeneity was analyzed based on the I 2 value. A P value < 0.10 was considered statistically significant. An I 2 value < 25% was considered homogeneous; an I 2 value > 75%, as high heterogeneity; an I 2 value between 50% and 75%, as moderate heterogeneity; and an I 2 value between 25% and 50%, as low heterogeneity [22, 23]. If the I 2 value was >50%, the studies were believed to be moderately or highly heterogeneous, and the random effects model was used to combine the effect size. If the I 2 value was <50%, the studies were believed to be homogeneous or have low heterogeneity, and the fix effects model was used to combine the effect size [24, 25].
A sensitivity analysis was performed to evaluate the stability of the meta-analysis results. Forest plots were synthesized. Potential publication bias was investigated by using the funnel plot, Begg test, and Egger test.
A special meta-analysis was performed on the effect of metformin on body mass index (BMI) in patients with PCOS.
Results
Search results
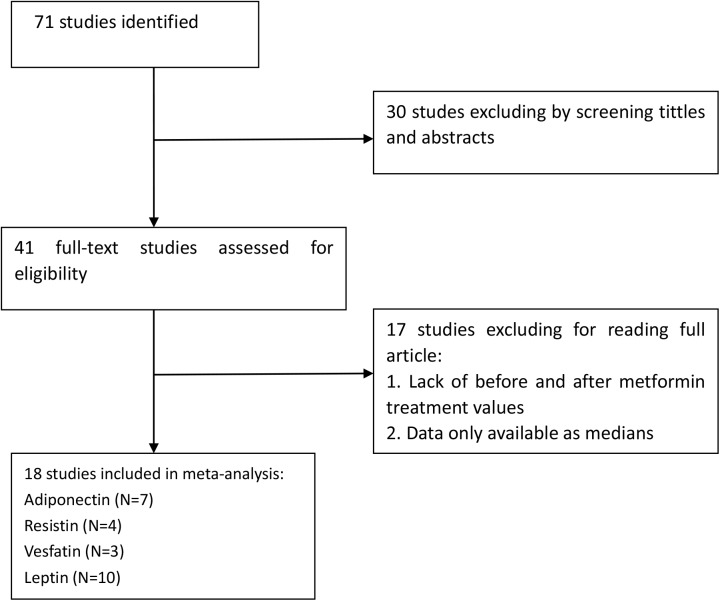
The initial search was independently executed by 2 reviewers. The details of the steps of the literature search are shown in Fig 1.
Fig 1. Flow diagram of the literature search.
Characteristics of the Eligible Studies
Eighteen studies [26–43], with data from 744 participants, were included in this review. A total of 7 studies with 11 data sets including 280 subjects were pooled for adiponectin, 4 studies with 6 data sets including 184 subjects were pooled for resistin, 3 studies with 78 subjects were pooled for visfatin, and 10 studies with 14 data sets including 202 subjects were pooled for leptin. The characteristics of the included studies for the different adipocytokines are provided in Tables 1–4, respectively. All the self-control trials included were defined as level 2, and the randomized controlled trials included were defined as level 1, according to study design [44, 45].
Table 1. Characteristics of the 7 included studies on adiponectin.
| BMI (mean ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Sample size | Age (mean ± SD) | Pre-Met | Post-Met | Inclusion/exclusio n Criteria | Study design | LOE | Therapy duration (months) | Daily dose |
| Karen EH [27] | 2008 | America | 15 | 27.7±1.3 | 43.3±2 | 42.3 ± 2 | H | RCT | 1 | 3 | 2000 |
| Hossam O.H [28] | 2013 | Egypt | 62 | 29.3±4.2 | 30.2±4.8 | 29.3±2.7 | H | SCT | 2 | 6 | 1500 |
| Joanna J [29] | 2008 | Poland | 29 | 28.24±6.27 | 35.32±5.07 | 33.49±.80 | H | SCT | 2 | 6 | 1000 |
| Aleksandra [31]A | 2014 | Poland | 32 | NR | 26.26±5.95 | 25.6±5.54 | H | SCT | 2 | 3 | 1000 |
| Aleksandra [31]B | 2014 | Poland | 32 | NR | 26.26±5.95 | 25.5±5.43 | H | SCT | 2 | 6 | 1000 |
| Aleksandra [31]C | 2014 | Poland | 16 | NR | 31.19±4.44 | 30.2±4.06 | H | SCT | 2 | 3 | 1000 |
| leksandra [31]D | 2014 | Poland | 16 | NR | 31.19±4.44 | 30.0±4.00 | H | SCT | 2 | 6 | 1000 |
| Luque RM [32]A | 2008 | Spain | 10 | 23±5 | 25.0±2.8 | NR | H | RCT | 1 | 3 | 850 |
| Luque RM [32]B | 2008 | Spain | 9 | 27±8 | 36.6±4.4 | NR | H | RCT | 1 | 3 | 850 |
| Charles AS [42] | 2007 | Germany | 35 | 24.7±4.8 | 29.3±6.5 | 28.6±7.2 | H | RCT | 1 | 6 | 1700 |
| İlhan T [43] | 2010 | Turkey | 24 | 25.21±5.99 | 31.69±6.52 | 30.7±6.65 | H | SCT | 2 | 6 | 1700 |
Abbreviations: SD, standard deviation; BMI, body mass index; NR, not reported; H, Have; LOE, level of evidence; SCT, self-control trials; RCT, randomized controlled trials.
Table 4. Characteristics of the 10 included studies on leptin.
| BMI (mean ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Sample size | Age (mean ± SD) | Pre-Met | Post-Met | Inclusion/exclusion criteria | Study design | LOE | Therapy duration (month) | Daily dose (mg) |
| Kowalska I [30] | 2001 | Poland | 11 | 27.7 ± 1.3 | 34.9 ± 5.6 | 31.4±4.8 | H | SCT | 2 | 4–5 | 1500 |
| Luque RM [32]A | 2008 | Spain | 10 | 23 ±5 | 25.0 ± 2.8 | NR | H | SCT | 2 | 3 | 850 |
| Luque RM [32]B | 2008 | Spain | 9 | 27 ± 8 | 36.6 ± 4.4 | NR | H | RCT | 1 | 3 | 850 |
| Maciel GA [33] | 2003 | Brazil | 7 | 22.5 ± 1.9 | 25.3 ± 2.1 | 24.9±2.7 | H | RCT | 1 | 6 | 1500 |
| Marciniak A [34] A | 2009 | Poland | 23 | NR | 30.86 ± 5.35 | 30.39±6.1 | H | SCT | 2 | 3 | 1700 |
| Marciniak A [34] B | 2009 | Poland | 12 | NR | 20.99 ± 1.87 | 20.75±1.79 | H | SCT | 2 | 3 | 1700 |
| Moran L J [35] | 2010 | Australia | 29 | 33.5±6.7 | 36.1 ± 7.2 | NR | H | SCT | 2 | 6 | 2000 |
| Morin PL [36] A | 2003 | Finland | 8 | 28.2±1.4 | 22.5 ± 0.8 | 21.7±0.7 | H | RCT | 1 | 3 | 1000 |
| Morin PL [36] B | 2003 | Finland | 8 | 28.2 ±1.4 | 22.5 ± 0.8 | 22.1 ± 0.8 | H | RCT | 1 | 6 | 2000 |
| Morin PL [37] A | 1998 | Finland | 26 | 30 ± 6.8 | 31 ± 4.6 | 30.66± 4.7 | H | SCT | 2 | 2 | 1500 |
| Morin PL [37] B | 1998 | Finland | 12 | NR | 31 ± 4.6 | 31.7±6 5.4 | H | SCT | 2 | 4–6 | 1500 |
| Ng E H [38] | 2001 | China | 8 | 30.5 | 24.1 | NR | H | RCT | 2 | 3 | 1500 |
| Maria M [39] | 2011 | Bulgaria | 32 | 23.58±4.17 | 28.45±4.38 | 27.45±3.73 | H | SCT | 2 | 3 | 1275 |
| Romualdi D [41] | 2008 | Italy | 7 | 23.57±6.97 | 32.64±4.35 | 31.64±3.15 | H | SCT | 2 | 4 | 1500 |
Abbreviations: SD, standard deviation; BMI, body mass index; NR, not reported; H, have; LOE, level of evidence; SCT, self-control trials; RCT, randomized controlled trials.
Table 2. Characteristics of the 4 included studies on resistin.
| BMI (mean ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Sample size | Age (mean ± SD) | Pre-Met | Post-Met | Inclusion/exclusion criteria | Study design | LOE | Therapy duration (month) | Daily dose (mg) |
| Basios G [26]A | 2014 | Greece | 31 | 25.7 ± 6.7 | <25 | NR | H | SCT | 2 | 6 | 1275 |
| Basios G [26]B | 2014 | Greece | 31 | NR | 25–30 | NR | H | SCT | 2 | 6 | 1275 |
| Basios G [26]C | 2014 | Greece | 31 | NR | >30 | NR | H | SCT | 2 | 6 | 1275 |
| Maria M [39] | 2011 | Bulggaria | 32 | 23.58±4.17 | 28.45± 4.38 | 27.45±3.73 | H | SCT | 2 | 3 | 1275 |
| Charles AS [42] | 2007 | Germany | 35 | 24.7±4.8 | 29.3 ± 6.5 | 28.6 ± 7.2 | H | RCT | 1 | 6 | 1700 |
| İlhan T [43] | 2010 | Turkey | 24 | 25.21±5.99 | 31.69 ± 6.52 | 30.7 ± 6.65 | H | SCT | 2 | 6 | 1700 |
Abbreviations: SD, standard deviation; BMI, body mass index; H, Have; NR, not reported; LOE, level of evidence; SCT, self-control trials; RCT, randomized controlled trials.
Table 3. Characteristics of the 3 included studies on visfatin.
| BMI (mean ± SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Sample size | Age (mean ± SD) | Pre-Met | Post-Met | Inclusion/exclusion criteria | Study design | LOE | Therapy duration (month) | Daily dose (mg) |
| Ozkaya M [40] | 2008 | Turkey | 19 | 25.1 ± 3.8 | 27.1 ± 1.7 | 26.1±1.6 | H | SCT | 2 | 3 | 1700 |
| Charles AS [42] | 2007 | Germany | 35 | 24.7 ± 4.8 | 29.3 ± 6.5 | 28.6±7.2 | H | RCT | 1 | 6 | 1700 |
| İlhan T [43] | 2010 | Turkey | 24 | 25.21±5.99 | 31.69 ±6.52 | 30.7±6.65 | H | SCT | 2 | 6 | 1700 |
Abbreviations: SD, standard deviation; BMI, body mass index; H, have; LOE, level of evidence; SCT, self-control trials; RCT, randomized controlled trials.
Adiponectin
Selection of studies
There were 23 potentially eligible studies identified. Three were excluded because they did not fulfill the standard quality requirements. Thirteen studies were excluded because only median data were available. Finally, 7 studies [27–29, 31, 32, 42, 43] were considered as having the best available evidence.
Main analysis
Seven studies, including 280 women, were eligible for the meta-analysis [27–29, 31, 32, 42, 43]. The I 2 value was 69%, indicating moderate heterogeneity among the included studies. Therefore, we used the random effects model to combine effect size. The meta-analysis revealed that metformin treatment was associated with a significant increases in serum adiponectin concentrations in the patients with PCOS, with a corresponding SMD of -0.43 (95% CI: -0.75 to -0.11, P<0.05) (Fig 2).
Fig 2. Adiponectin level.
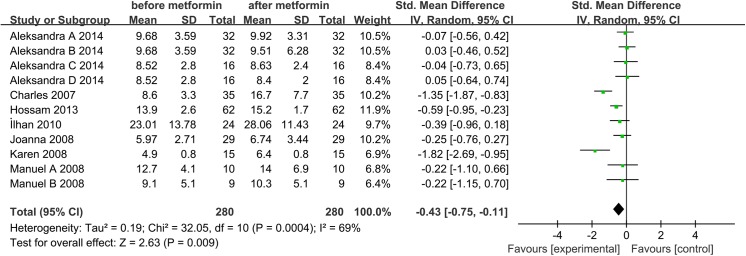
Adiponectin serum concentrations in the women with polycystic ovary syndrome before and after metformin treatment.
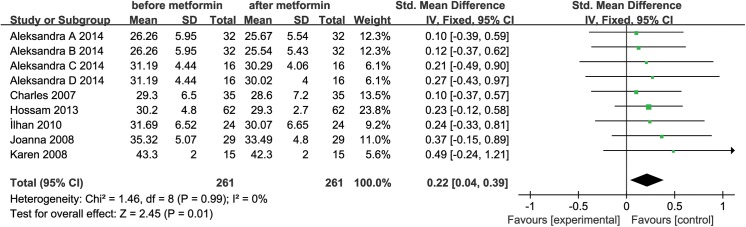
We also performed a special meta-analysis regarding the effect of metformin administration on BMI in patients with PCOS. The results showed that the total SMD for BMI was 0.22 units (95% CI, 0.04 to 0.39, P = 0.01) (Fig 3). The result suggests that metformin therapy was associated with a significant decrease in BMI in the women with PCOS.
Fig 3. Comparison of body mass index (BMI) before and after metformin treatment in the adiponectin-related studies.
Sensitivity Analysis
Sensitivity analysis revealed that removal of any study from the analysis did not subvert the result of the present pooled analysis (data not shown). Pooled analysis using the random effects model revealed that adiponectin levels significantly increased after metformin therapy (SMD: -0.43, 95% CI: -0.75 to -0.11, P<0.05). The fixed-effects model produced a similar result (SMD: -0.43, 95% CI: -0.60 to -0.26, P<0.05). Therefore, this pooled analysis outcome could be regarded with a higher degree of certainty.
Publication bias
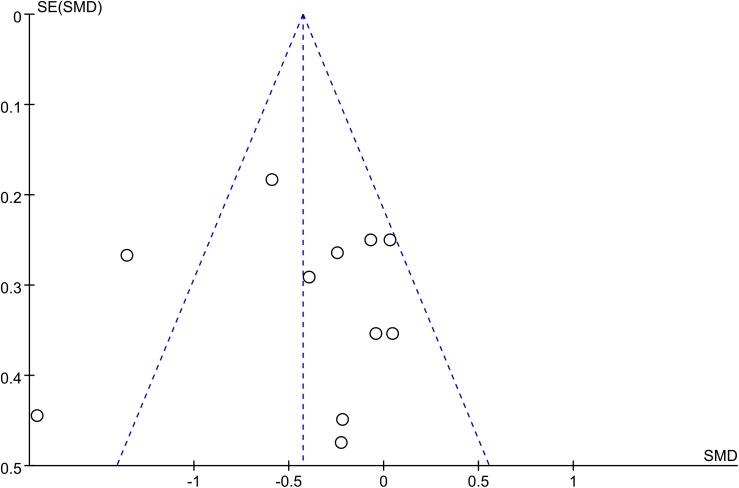
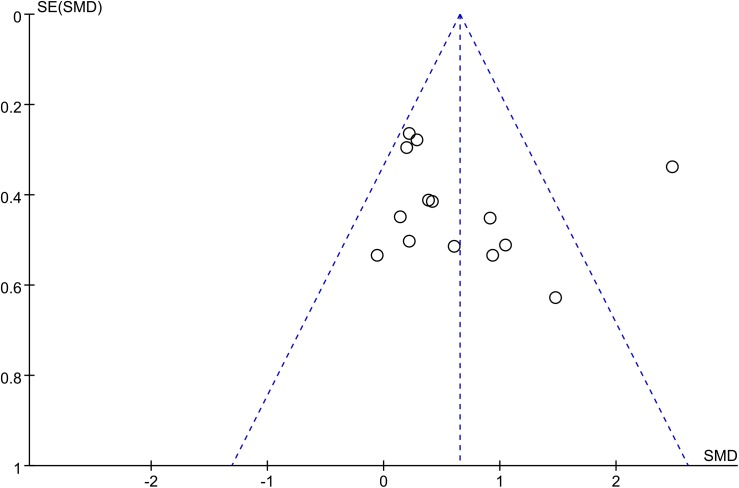
The funnel plot was not perfectly symmetrical (Fig 4), suggesting possible slight publication bias. However, Begg tests (P = 0.35) and Egger tests (P = 0.09) revealed no evidence to support publication bias in this pooled analysis. In addition, in the trim and fill method, none of the studies needed to be statistically corrected for funnel plot asymmetry.
Fig 4. Adiponectin funnel plot.
The funnel plot shows the possibility of a small publication bias. SE, standard error; SMD, standardized mean difference.
Resistin
Selection of studies
Seven potentially eligible studies were identified. One study was excluded, as data were not available in an extractable format. The 6 articles were then roughly screened based on their abstracts and titles, according to the inclusion/exclusion criteria. After careful discussion between the 2 reviewers, articles that fulfilled the inclusion criteria of our study were identified [26, 39, 42, 43].
Main analysis
Four studies, involving 184 women, were eligible for the meta-analysis [26, 39, 42, 43]. An I 2 value of 84% indicated that high heterogeneity between the included studies. Therefore, the random effects model was performed to combine effect size. The meta-analysis revealed that circulating resistin concentration was not significantly changed after metformin treatment in the patients with PCOS, with a corresponding SMD of -0.01 (95% CI: -0.49 to 0.45, P>0.05) (Fig 5).
Fig 5. Resistin level.
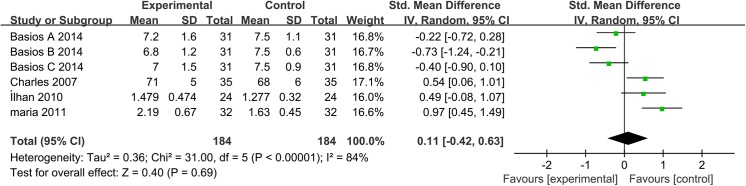
Resistin serum concentration in the patients with polycystic ovary syndrome before and after metformin treatment.
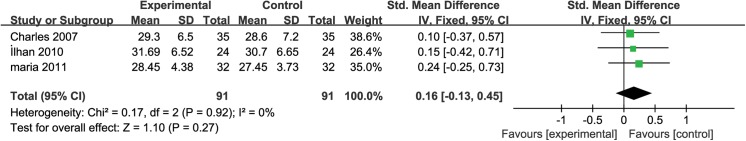
We also performed a special meta-analysis regarding the effect of metformin administration on BMI in the patients with PCOS. The results showed that the total SMD for BMI was 0.16 units (95% CI, -0.13 to 0.45, P = 0.27) (Fig 6). The result suggests that metformin therapy exerted no significant effect on BMI in the women with PCOS.
Fig 6. Comparison of body mass index (BMI) before and after metformin treatment in the resistin-related studies.
Sensitivity Analysis
Pooled analysis using the random effects model revealed that circulating resistin concentration was not significantly changed after metformin treatment in the patients with PCOS (SMD: -0.01, 95% CI: -0.49 to 0.45, P>0.05). The same conclusion was reached with the fixed-effects model (SMD: -0.10, 95% CI: -0.11 to 0.31, P>0.05). Meanwhile, sensitivity analysis revealed that removal of any study from the analysis did not subvert the result of the present pooled analysis (data not shown). Therefore, this pool analysis outcome could be regarded with a higher degree of certainty.
Publication bias
The funnel plot was not perfectly symmetrical (Fig 7). This seems to demonstrate slight publication bias. However, Begg tests (P = 0.27) and Egger tests (P = 0.13) revealed no evidence to support publication bias in this pooled analysis. In addition, in the trim and fill method, none of the studies needed to be statistically corrected for funnel plot asymmetry.
Fig 7. Resistin funnel plot.
The funnel plot shows the possibility of a small publication bias. SE, standard error; SMD, standardized mean difference.
Visfatin
Selection of studies
Six potentially eligible studies were identified. Three studies were excluded because they did not fulfill the standard quality requirements. The remaining 3 studies were included in the meta-analysis [40, 42, 43].
Main analysis
Meta-analysis included 3 studies, involving 184 women [40, 42, 43]. An I 2 value of 95% indicated high heterogeneity between the included studies. Therefore, the random effects model was used to combine effect size. The results showed that metformin administration did not change serum visfatin levels in the patients with PCOS, with a corresponding SMD of -0.04 (95% CI: -1.55 to 1.46, P>0.05) (Fig 8).
Fig 8. Visfatin level.
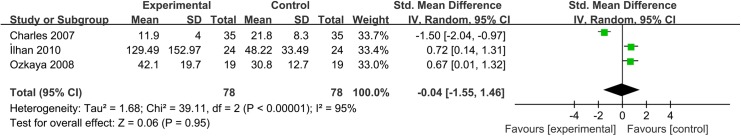
Visfatin serum concentration in the women with polycystic ovary syndrome before and after metformin treatment.
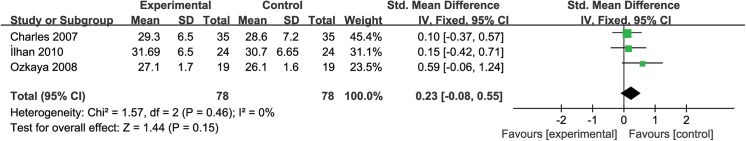
We also performed a special meta-analysis regarding the effect of metformin administration on BMI in patients with PCOS. The results showed that the total SMD for BMI was 0.23 units (95% CI, -0.08 to 0.55, P = 0.15) (Fig 9). The result suggests that metformin therapy exerted no significant effect on BMI in the women with PCOS.
Fig 9. Comparison of body mass index (BMI) before and after metformin treatment in the visfatin-related studies.
Sensitivity Analysis
Pooled analysis using the random effects model revealed that metformin administration did not change serum visfatin levels in the patients with PCOS (SMD: -0.04, 95% CI: -1.55 to 1.46, P>0.05). The fixed-effects model produced the same conclusion (SMD: -0.18, 95% CI: -0.52 to 0.15, P>0.05). Conversely, sensitivity analysis showed that removal of any study from the analysis could subvert the result of the present pooled analysis (data not shown). Therefore, this pooled analysis outcome may not be regarded with a higher degree of certainty.
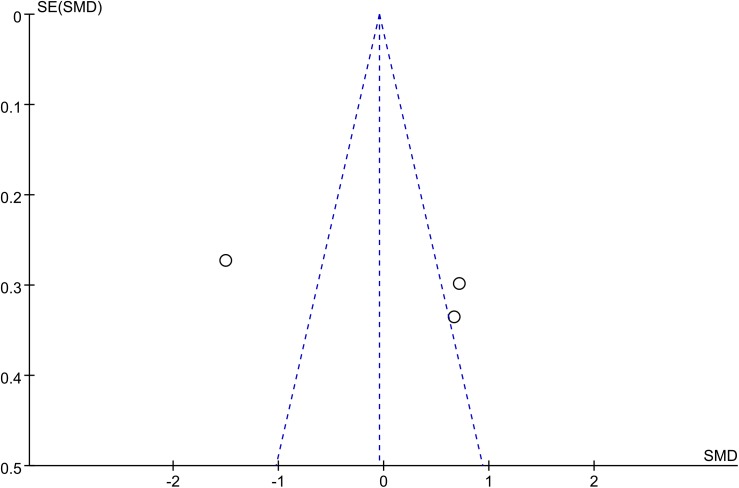
Publication bias
The funnel plot was highly asymmetrical (Fig 10). This indicated a clear publication bias. To better understand the effect of metformin on serum visfatin concentration in patients with PCOS, a well-designed, large-scale, high-quality, multicenter randomized controlled trial is needed.
Fig 10. Visfatin funnel plot.
The funnel plot shows the possibility of a small publication bias. SE, standard error; SMD, standardized mean difference.
Leptin
Selection of studies
We identified 35 potentially eligible studies. These studies were roughly screened based on their abstracts and titles, according to the inclusion/exclusion criteria. Twenty-two studies were excluded because they did not fulfill the standard quality requirements. Three studies were excluded because relevant data were not extractable, being available only as medians or in abstracts. Finally, 10 studies were considered as having the best available evidence [30, 32–39, 41].
Main analysis
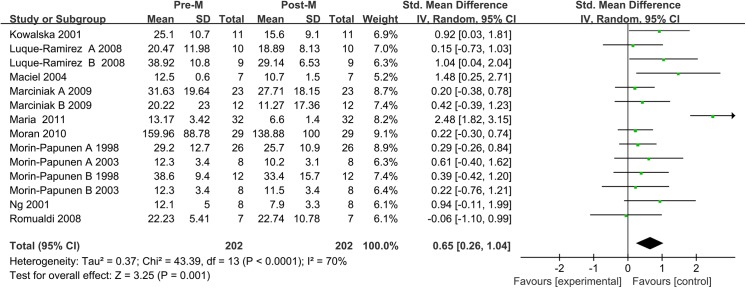
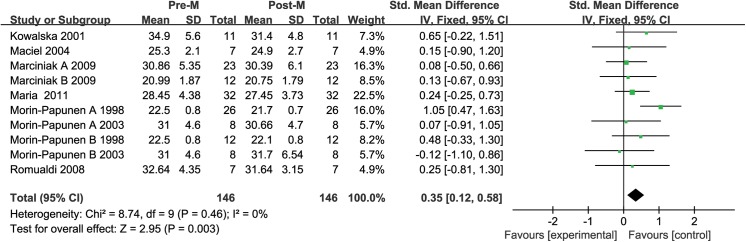
Meta-analysis included 10 studies, involving 202 women. An I 2 value of 70% indicated moderate heterogeneity between the included studies. Therefore, the random effects model was used to combine effect size. The meta-analysis revealed that metformin administration demonstrated an association with significantly lower leptin concentrations after treatment than before treatment in the patients with PCOS, with a corresponding SMD of 0.65 (95% CI: 0.26 to1.04, P<0.05) (Fig 11).
Fig 11. Leptin level.
Leptin serum concentration in the patients with polycystic ovary syndrome before and after metformin treatment.
We also performed a special meta-analysis regarding the effect of metformin administration on BMI in patients with PCOS. The results showed that the total SMD for BMI was 0.35 units (95% CI, 0.12 to 0.58, P = 0.01) (Fig 12). The result suggests that metformin therapy was associated with a significant decrease in BMI in the women with PCOS.
Fig 12. Comparison of body mass index (BMI) before and after metformin treatment in the leptin-related studies.
Sensitivity Analysis
Sensitivity analysis showed that removal of any study from the analysis did not subvert the result of the present pooled analysis (data not shown). Pooled analysis using the random effects model revealed that leptin levels significantly increased after metformin therapy (SMD: 0.65, 95% CI: 0.26 to 1.04, P<0.05). The fixed-effects model produced the same result (SMD: 0.61, 95% CI: 0.40 to 0.82, P<0.05). Therefore, the outcome of this pool analysis could be regarded with a higher degree of certainty.
Publication bias
The funnel plot was not perfectly symmetrical (Fig 13). This seemed to indicate slight publication bias. However, Begg tests (P = 0.53) and Egger tests (P = 0.29) revealed no evidence to support publication bias in this pooled analysis. In addition, in the trim and fill method, none of the studied needed to be statistically corrected for funnel plot asymmetry.
Fig 13. Leptin funnel plot.
The funnel plot shows the possibility of a small publication bias. SE, standard error; SMD, standardized mean difference.
Discussion
PCOS has been repeatedly noted to be an independent risk factor of insulin resistance [46–50]. Thus, metformin, as the longest established oral antidiabetic agent, has been widely used in patients with PCOS to improve insulin sensitivity and induce ovulation. Adipocytokines, which are secreted by adipose tissue, may play a significant role in the pathogenesis of insulin resistance [51]. However, the effect of metformin on adipocytokine levels remains controversial. Some studies reported positive results [27–30, 34–40], whereas others found no significant changes in adipocytokine levels after metformin administration [26, 31].
The aim of the present study was to systematically review the literature for randomized, controlled, self-controlled trials that studied changes in the levels of adipocytokines (adiponectin, resistin, visfatin, and leptin) in patients with PCOS treated with metformin and to meta-analyze the best evidence available in order to provide high-quality data on the effect of metformin on adipocytokine levels in patients with PCOS. For this study, 34 data sets were used, including 744 patients with PCOS and adipocytokine levels measured before and after metformin administration. Thus, according to these results, metformin treatment in the patients with PCOS was associated with a significant increase in adiponectin level and decrease in leptin level, but not with the resistin and visfatin levels.
Similar to the findings of our meta-analysis, increased adiponectin levels have previously been observed in patients with PCOS who were receiving 850-mg metformin twice daily for 6 months [42, 43]. Insulin and glucose levels induced by activation of the AMP-kinase pathways have been shown to directly affect adiponectin levels [52, 53]. Increased adiponectin levels observed in patients with PCOS after metformin administration may be the result of reduced insulin resistance and insulin levels. However, inconsistent with our results, the results of another study [31] indicated no change in adiponectin concentration after treatment with 1000 mg of metformin daily for 6 months. These conflicting results could be explained by the relatively small sample size and difference in study populations, including differences in race, BMI, and severity of insulin resistance. Because of the complexity of the factors that influence the effect of metformin on adiponectin levels, we could not identify through our meta-analysis the precise dose or duration of metformin therapy needed to maximize the increase in adiponectin concentration in patients with PCOS. Nevertheless, based on the limited data available, our meta-analysis revealed that metformin treatment was associated with elevated serum adiponectin levels.
The role of leptin, as a marker of overall fat depots, in patients with PCOS is not well understood [54, 55]. Most studies reported higher leptin concentrations in patients with PCOS [56, 57], whereas others did not [58]. Various confounding factors (e.g., age and body weight) could have led to these conflicting results. Assessing the effects of the disease-specific treatment (metformin in this study) may be a way to address this issue. Based on the decreasing leptin concentrations after metformin administration in the patients with PCOS, our data seem to support the occurrence of hyperlipidemia, which is a state of leptin resistance or tolerance, in the patients with PCOS. Furthermore, leptin has been demonstrated to be associated with the development of insulin resistance and diabetes [17]. The result of our meta-analysis suggested that metformin administration was significantly associated with the reduction in circulation leptin concentration and that delaying or preventing the occurrence of insulin resistance and diabetes might be beneficial in patients with PCOS.
In addition, we found no significant changes in resistin or visfatin level during metformin treatment in the present study. A recent meta-analysis revealed no statistically significant differences in serum resistin and visfatin levels between patients with PCOS and control women, although serum resistin and visfatin levels were higher among both obese and normal-weight patients with PCOS than in the controls [59]. No correlation was observed between resistin level, BMI, homeostasis model assessment-estimated insulin resistance, area under the curve of blood insulin, insulin, lipid parameters, and serum androgen levels [60]. Meanwhile, Pagano and colleagues reported that insulin resistance did not lead to changes in the circulating plasma visfatin concentrations in human subjects [61]. These results might suggest that both resistin and visfatin levels were independently associated with insulin resistance in patients with PCOS.
Considering that more than half of the patients with PCOS had central obesity and many investigators found that metformin treatment induced a significant reduction in BMI [62–64], we performed a special meta-analysis regarding the effect of metformin administration on BMI in patients with PCOS for each adipocytokine. We found that metformin therapy could decrease BMI and blood concentrations of adiponectin and leptin. Moreover, we found no significant changes in BMI in the women with PCOS whose resistin and visfatin levels were unchanged. Thus, this may imply a significant correlation between the both adiponectin and leptin levels and BMI in patients with PCOS. Such results were also reported by many other authors. Orio’s and Panidis’s groups demonstrated a negative correlation between plasma adiponectin level and BMI in patients with PCOS [65, 66]. Similarly, the authors reported a positive correlation between plasma leptin level and BMI [67]. Nevertheless, the reasons for the conflicting BMI results are not clear. A possible explanation is the limited numbers and sample sizes of the studies included in the analysis of the effect of metformin on BMI in terms of resistin and visfatin levels. Meanwhile, another study reported that only about 50% of women with PCOS responded to metformin [68].
Although our findings are important, the limitations of our study should not be ignored. Presence of confounding variables (e.g., age, BMI, and concomitant subclinical inflammatory diseases), lack of uniformity (e.g., PCOS diagnostic criteria and assay methodology for index markers), and exclusion of studies not written in English are limitations that are difficult to avoid. Moreover, various PCOS phenotypes could not be considered. Furthermore, more studies with a large sample size would allow for precise estimation of the effect, whereas the limited numbers and sample sizes of the studies included in this analysis led to a high degree of heterogeneity in the reported resistin and visfatin levels, increasing the difficulty of interpreting the results and limiting the strength of the evidence provided by the present meta-analysis. The observed lack of effect should be interpreted as a failure to document any potential existing effect of metformin on resistin and visfatin levels in patients with PCOS rather than as evidence of no effect [69].
Conclusion
The present meta-analysis demonstrates that metformin administration was associated with increased serum adiponectin concentration and decreased serum leptin levels. Further study is needed to elucidate whether this apparent effect decreases the incidence of type 2 diabetes and other metabolic diseases in patients with PCOS later in life.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Franks S. Polycystic ovary syndrome. The New England journal of medicine. 1995;333(13):853–61. Epub 1995/09/28. 10.1056/nejm199509283331307 . [DOI] [PubMed] [Google Scholar]
- 2. Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2010;26(4):281–96. Epub 2010/02/10. 10.3109/09513590903247873 . [DOI] [PubMed] [Google Scholar]
- 3. Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reproductive biomedicine online. 2009;19(4):552–63. Epub 2009/11/17. . [DOI] [PubMed] [Google Scholar]
- 4. Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Human reproduction (Oxford, England). 2001;16(6):1255–60. Epub 2001/06/02. . [DOI] [PubMed] [Google Scholar]
- 5. Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. The Journal of clinical endocrinology and metabolism. 2007;92(12):4546–56. Epub 2007/12/07. 10.1210/jc.2007-1549 . [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. Epub 2005/04/20. 10.1016/s0140-6736(05)66378-7 . [DOI] [PubMed] [Google Scholar]
- 7. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288(21):2709–16. Epub 2002/12/04. . [DOI] [PubMed] [Google Scholar]
- 8. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. Epub 2002/03/06. . [DOI] [PubMed] [Google Scholar]
- 9. Samad F, Badeanlou L, Shah C, Yang G. Adipose tissue and ceramide biosynthesis in the pathogenesis of obesity. Advances in experimental medicine and biology. 2011;721:67–86. Epub 2011/09/13. 10.1007/978-1-4614-0650-1_5 . [DOI] [PubMed] [Google Scholar]
- 10. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews Immunology. 2011;11(2):85–97. Epub 2011/01/22. 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. Inflammation, adiponectin, obesity and cardiovascular risk. Current medicinal chemistry. 2010;17(36):4511–20. Epub 2010/11/11. . [DOI] [PubMed] [Google Scholar]
- 12. Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Human reproduction update. 2009;15(3):297–307. 10.1093/humupd/dmp006 . [DOI] [PubMed] [Google Scholar]
- 13. Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Human reproduction update. 2011;17(6):741–60. Epub 2011/06/02. 10.1093/humupd/dmr025 . [DOI] [PubMed] [Google Scholar]
- 14. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302(2):179–88. Epub 2009/07/09. 10.1001/jama.2009.976 . [DOI] [PubMed] [Google Scholar]
- 15. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. Epub 2001/02/24. 10.1038/35053000 . [DOI] [PubMed] [Google Scholar]
- 16. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science (New York, NY). 2005;307(5708):426–30. Epub 2004/12/18. 10.1126/science.1097243 . [DOI] [PubMed] [Google Scholar]
- 17. Chakrabarti J. Serum leptin level in women with polycystic ovary syndrome: correlation with adiposity, insulin, and circulating testosterone. Annals of medical and health sciences research. 2013;3(2):191–6. Epub 2013/08/07. 10.4103/2141-9248.113660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yildizhan R, Ilhan GA, Yildizhan B, Kolusari A, Adali E, Bugdayci G. Serum retinol-binding protein 4, leptin, and plasma asymmetric dimethylarginine levels in obese and nonobese young women with polycystic ovary syndrome. Fertility and sterility. 2011;96(1):246–50. Epub 2011/05/24. 10.1016/j.fertnstert.2011.04.073 . [DOI] [PubMed] [Google Scholar]
- 19. Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. The Cochrane database of systematic reviews. 2007;(1):CD005552 Epub 2007/01/27. 10.1002/14651858.CD005552.pub2 . [DOI] [PubMed] [Google Scholar]
- 20. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and sterility. 2004;81(1):19–25. Epub 2004/01/09. . [DOI] [PubMed] [Google Scholar]
- 21. Carmina E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva ginecologica. 2004;56(1):1–6. Epub 2004/02/20. . [PubMed] [Google Scholar]
- 22. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–48. Epub 1959/04/01. . [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. Epub 2002/07/12. 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. . [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basios G, Trakakis E, Chrelias C, Panagopoulos P, Vaggopoulos V, Skarpas P, et al. The impact of metformin treatment on adiponectin and resistin levels in women with polycystic ovary syndrome: a prospective clinical study. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2015;31(2):136–40. 10.3109/09513590.2014.975684 . [DOI] [PubMed] [Google Scholar]
- 27. Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2008;93(7):2670–8. Epub 2008/05/08. 10.1210/jc.2008-0115 . [DOI] [PubMed] [Google Scholar]
- 28. Hamed HO. Role of adiponectin and its receptor in prediction of reproductive outcome of metformin treatment in patients with polycystic ovarian syndrome. The journal of obstetrics and gynaecology research. 2013;39(12):1596–603. Epub 2013/07/24. 10.1111/jog.12101 . [DOI] [PubMed] [Google Scholar]
- 29. Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, Demissie M. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2008;24(7):378–84. Epub 2008/07/23. 10.1080/09513590802128968 . [DOI] [PubMed] [Google Scholar]
- 30. Kowalska I, Kinalski M, Straczkowski M, Wolczyski S, Kinalska I. Insulin, leptin, IGF-I and insulin-dependent protein concentrations after insulin-sensitizing therapy in obese women with polycystic ovary syndrome. European journal of endocrinology / European Federation of Endocrine Societies. 2001;144(5):509–15. Epub 2001/05/02. . [DOI] [PubMed] [Google Scholar]
- 31. Kruszynska A, Slowinska-Srzednicka J, Jeske W, Zgliczynski W. Proinsulin, adiponectin and hsCRP in reproductive age women with polycystic ovary syndrome (PCOS)—the effect of metformin treatment. Endokrynologia Polska. 2014;65(1):2–10. Epub 2014/02/20. 10.5603/ep.2014.0001 . [DOI] [PubMed] [Google Scholar]
- 32. Luque-Ramirez M, Alvarez-Blasco F, Escobar-Morreale HF. Antiandrogenic contraceptives increase serum adiponectin in obese polycystic ovary syndrome patients. Obesity (Silver Spring, Md). 2009;17(1):3–9. Epub 2008/11/11. 10.1038/oby.2008.491 . [DOI] [PubMed] [Google Scholar]
- 33. Maciel GA, Soares Junior JM, Alves da Motta EL, Abi Haidar M, de Lima GR, Baracat EC. Nonobese women with polycystic ovary syndrome respond better than obese women to treatment with metformin. Fertility and sterility. 2004;81(2):355–60. Epub 2004/02/18. 10.1016/j.fertnstert.2003.08.012 . [DOI] [PubMed] [Google Scholar]
- 34. Marciniak A, Nawrocka-Rutkowska J, Brodowska A, Sienkiewicz R, Szydlowska I, Starczewski A. Leptin concentrations in patients with polycystic ovary syndrome before and after met-formin treatment depending on insulin resistance, body mass index and androgen con-centrations—introductory report. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2009;47(2):323–8. Epub 2009/12/10. 10.2478/v10042-009-0032-0 . [DOI] [PubMed] [Google Scholar]
- 35. Moran LJ, Meyer C, Hutchison SK, Zoungas S, Teede HJ. Novel inflammatory markers in overweight women with and without polycystic ovary syndrome and following pharmacological intervention. Journal of endocrinological investigation. 2010;33(4):258–65. Epub 2009/10/17. 10.3275/6563 . [DOI] [PubMed] [Google Scholar]
- 36. Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, Tapanainen JS. Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: a randomized study. The Journal of clinical endocrinology and metabolism. 2003;88(1):148–56. Epub 2003/01/10. 10.1210/jc.2002-020997 . [DOI] [PubMed] [Google Scholar]
- 37. Morin-Papunen LC, Koivunen RM, Tomas C, Ruokonen A, Martikainen HK. Decreased serum leptin concentrations during metformin therapy in obese women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 1998;83(7):2566–8. Epub 1998/07/14. 10.1210/jcem.83.7.4944 . [DOI] [PubMed] [Google Scholar]
- 38. Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: a randomized, double-blinded placebo-controlled trial. Human reproduction (Oxford, England). 2001;16(8):1625–31. Epub 2001/07/28. . [DOI] [PubMed] [Google Scholar]
- 39. Orbetzova MM, Pehlivanov BK, Mitkov MM, Atanassova IB, Kamenov ZA, Kolarov GB, et al. Effect of short-term standard therapeutic regimens on neuropeptide Y and adipose tissue hormones in overweight insulin-resistant women with polycystic ovary syndrome. Folia medica. 2011;53(3):15–24. Epub 2012/03/01. . [DOI] [PubMed] [Google Scholar]
- 40. Ozkaya M, Cakal E, Ustun Y, Engin-Ustun Y. Effect of metformin on serum visfatin levels in patients with polycystic ovary syndrome. Fertility and sterility. 2010;93(3):880–4. 10.1016/j.fertnstert.2008.10.058 . [DOI] [PubMed] [Google Scholar]
- 41. Romualdi D, Campagna G, Selvaggi L Jr., Cento R, Proto C, Lanzone A, et al. Metformin treatment does not affect total leptin levels and free leptin index in obese patients with polycystic ovary syndrome. Fertility and sterility. 2008;89(5):1273–6. Epub 2007/07/31. 10.1016/j.fertnstert.2007.05.004 . [DOI] [PubMed] [Google Scholar]
- 42. Steiner CA, Janez A, Jensterle M, Reisinger K, Forst T, Pfutzner A. Impact of treatment with rosiglitazone or metformin on biomarkers for insulin resistance and metabolic syndrome in patients with polycystic ovary syndrome. Journal of diabetes science and technology. 2007;1(2):211–7. Epub 2007/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tarkun I, Dikmen E, Cetinarslan B, Canturk Z. Impact of treatment with metformin on adipokines in patients with polycystic ovary syndrome. European cytokine network. 2010;21(4):272–7. Epub 2010/12/04. 10.1684/ecn.2010.0217 . [DOI] [PubMed] [Google Scholar]
- 44. Petrisor BA, Keating J, Schemitsch E. Grading the evidence: levels of evidence and grades of recommendation. Injury. 2006;37(4):321–7. Epub 2006/02/21. 10.1016/j.injury.2006.02.001 . [DOI] [PubMed] [Google Scholar]
- 45. Niu X, Chen X, Xiao Y, Dong J, Zhang R, Lu M, et al. The differences in homocysteine level between obstructive sleep apnea patients and controls: a meta-analysis. PloS one. 2014;9(4):e95794 Epub 2014/04/29. 10.1371/journal.pone.0095794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165–74. Epub 1989/09/01. . [DOI] [PubMed] [Google Scholar]
- 47. Puurunen J, Piltonen T, Morin-Papunen L, Perheentupa A, Jarvela I, Ruokonen A, et al. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with PCOS. The Journal of clinical endocrinology and metabolism. 2011;96(6):1827–34. Epub 2011/04/01. 10.1210/jc.2011-0039 . [DOI] [PubMed] [Google Scholar]
- 48. Taponen S, Martikainen H, Jarvelin MR, Sovio U, Laitinen J, Pouta A, et al. Metabolic cardiovascular disease risk factors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. The Journal of clinical endocrinology and metabolism. 2004;89(5):2114–8. Epub 2004/05/06. 10.1210/jc.2003-031720 . [DOI] [PubMed] [Google Scholar]
- 49. Wang D, Zhu W, Li J, An C, Wang Z. Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PloS one. 2013;8(11):e81190 Epub 2013/11/22. 10.1371/journal.pone.0081190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manco M, Castagneto-Gissey L, Arrighi E, Carnicelli A, Brufani C, Luciano R, et al. Insulin dynamics in young women with polycystic ovary syndrome and normal glucose tolerance across categories of body mass index. PloS one. 2014;9(4):e92995 Epub 2014/04/08. 10.1371/journal.pone.0092995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arner P. Insulin resistance in type 2 diabetes—role of the adipokines. Current molecular medicine. 2005;5(3):333–9. Epub 2005/05/17. . [DOI] [PubMed] [Google Scholar]
- 52. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496–505. Epub 2006/01/31. . [DOI] [PubMed] [Google Scholar]
- 53. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006;116(7):1784–92. Epub 2006/07/11. 10.1172/jci29126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrom V, Flyvbjerg A, et al. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin and body composition in hirsute PCOS patients and controls. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155(2):337–45. Epub 2006/07/27. 10.1530/eje.1.02207 . [DOI] [PubMed] [Google Scholar]
- 55. Marino JS, Iler J, Dowling AR, Chua S, Bruning JC, Coppari R, et al. Adipocyte dysfunction in a mouse model of polycystic ovary syndrome (PCOS): evidence of adipocyte hypertrophy and tissue-specific inflammation. PloS one. 2012;7(10):e48643 Epub 2012/11/03. 10.1371/journal.pone.0048643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecologic and obstetric investigation. 2013;75(4):268–74. Epub 2013/04/11. 10.1159/000350217 . [DOI] [PubMed] [Google Scholar]
- 57. Lecke SB, Mattei F, Morsch DM, Spritzer PM. Abdominal subcutaneous fat gene expression and circulating levels of leptin and adiponectin in polycystic ovary syndrome. Fertility and sterility. 2011;95(6):2044–9. Epub 2011/03/23. 10.1016/j.fertnstert.2011.02.041 . [DOI] [PubMed] [Google Scholar]
- 58. Kale-Gurbuz T, Akhan SE, Bastu E, Telci A, Iyibozkurt AC, Topuz S. Adiponectin, leptin and ghrelin levels in obese adolescent girls with polycystic ovary syndrome. Journal of pediatric and adolescent gynecology. 2013;26(1):27–30. Epub 2012/11/20. 10.1016/j.jpag.2012.09.002 . [DOI] [PubMed] [Google Scholar]
- 59. Farshchian F, Ramezani Tehrani F, Amirrasouli H, Rahimi Pour H, Hedayati M, Kazerouni F, et al. Visfatin and resistin serum levels in normal-weight and obese women with polycystic ovary syndrome. International journal of endocrinology and metabolism. 2014;12(3):e15503 Epub 2014/09/23. 10.5812/ijem.15503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yilmaz M, Bukan N, Demirci H, Ozturk C, Kan E, Ayvaz G, et al. Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2009;25(4):246–52. 10.1080/09513590802653833 . [DOI] [PubMed] [Google Scholar]
- 61. Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. The Journal of clinical endocrinology and metabolism. 2006;91(8):3165–70. Epub 2006/05/25. 10.1210/jc.2006-0361 . [DOI] [PubMed] [Google Scholar]
- 62. Harborne LR, Sattar N, Norman JE, Fleming R. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. The Journal of clinical endocrinology and metabolism. 2005;90(8):4593–8. 10.1210/jc.2004-2283 . [DOI] [PubMed] [Google Scholar]
- 63. Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. European journal of endocrinology / European Federation of Endocrine Societies. 2007;157(5):669–76. 10.1530/EJE-07-0294 . [DOI] [PubMed] [Google Scholar]
- 64. Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. The New England journal of medicine. 2007;356(6):551–66. 10.1056/NEJMoa063971 . [DOI] [PubMed] [Google Scholar]
- 65. Orio F Jr., Palomba S, Zullo F, Colao A, Lombardi G. Are serum adiponectin levels really reduced in obese women with polycystic ovary syndrome? Human reproduction. 2004;19(1):215; author reply -6. . [DOI] [PubMed] [Google Scholar]
- 66. Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Human reproduction. 2003;18(9):1790–6. . [DOI] [PubMed] [Google Scholar]
- 67. McGarvey ST, Forrest W, Weeks DE, Sun G, Smelser D, Tufa J, et al. Human leptin locus (LEP) alleles and BMI in Samoans. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(6):783–8. 10.1038/sj.ijo.0801996 . [DOI] [PubMed] [Google Scholar]
- 68. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–72. . [DOI] [PubMed] [Google Scholar]
- 69. Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertility and sterility. 2009;92(2):667–77. Epub 2008/08/20. 10.1016/j.fertnstert.2008.06.045 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.