Summary
Ordinal outcomes arise frequently in clinical studies when each subject is assigned to a category and the categories have a natural order. Classification rules for ordinal outcomes may be developed with commonly used regression models such as the full continuation ratio (CR) model (fCR), which allows the covariate effects to differ across all continuation ratios, and the CR model with a proportional odds structure (pCR), which assumes the covariate effects to be constant across all continuation ratios. For settings where the covariate effects differ between some continuation ratios but not all, fitting either fCR or pCR may lead to suboptimal prediction performance. In addition, these standard models do not allow for non-linear covariate effects. In this paper, we propose a sparse CR kernel machine (KM) regression method for ordinal outcomes where we use the KM framework to incorporate non-linearity and impose sparsity on the overall differences between the covariate effects of continuation ratios to control for overfitting. In addition, we provide data driven rule to select an optimal kernel to maximize the prediction accuracy. Simulation results show that our proposed procedures perform well under both linear and non-linear settings, especially when the true underlying model is in-between fCR and pCR models. We apply our procedures to develop a prediction model for levels of anti-CCP among rheumatoid arthritis patients and demonstrate the advantage of our method over other commonly used methods.
Keywords: Continuation ratio model, Kernel machine regression, Kernel PCA, Ordinal outcome, Prediction
1. Introduction
Ordinal outcome data, such as pain scales, disease severity, and quality of life scales, arise frequently in medical research. To derive classification rules for an ordinal outcome y with a p × 1 predictor vector x, one may employ regression models relating x to y and classify future subjects into different categories based on their predicted P(y = c | x). Naive analysis strategies, such as dichotomizing y into a binary variable and fitting multinomial regression models, are not efficient as they do not take into account the ordinal property of the outcome. Commonly used traditional methods for modeling ordinal response data include the cumulative proportional odds model, the forward and backward continuation ratio (CR) models and the corresponding proportional odds version of the CR (pCR) model (Ananth and Kleinbaum, 1997). The forward full CR (fCR) model assumes that
| (1) |
where y is assumed to take C ordered categories, {1, …, C}, and β(c) are unknown regression parameters that are allowed to vary across continuation ratios. When the covariate effects β(c) are assumed to be constant across c, (1) reduces to the pCR model
| (2) |
When choosing between these two models, we come across the trade-off between the model complexity and the efficiency in estimating the model parameters. With the fCR model, we might suffer from loss of efficiency due to estimating too many parameters if the true model is a sub-model of (1), especially when the dimension of x is not small. On the other hand, the pCR model might lead to poor prediction performance if the true covariate effects do vary across continuation ratios. However, for many applications, it is reasonable to expect that a compromise between the fCR model and the pCR model might be optimal. That is, β(c) = β(c+1) for some c but not all and thus it is possible to improve the estimation by leveraging the sparsity on β(c) − β(c−1). Regularization methods can be easily adapted to incorporate sparsity for CR models. Under the pCR model, Archer and Williams (2012) imposed L1 penalty to incorporate the sparsity of the elements of β. For the fCR model, to leverage the additional sparsity on β(c) − β(c−1), one may impose a “fused lasso” type of penalty (Tibshirani et al., 2005), which penalizes the L1-norm of both β(c) and β(c) − β(c−1).
In the presence of non-linear covariate effects, these existing methods based on linearity assumptions may lead to classification rules with unsatisfactory performance. On the other hand, fully non-parametric methods are often not feasible due to the curse of dimensionality. Alternatively, one may account for non-linearity by including interaction or non-linear basis functions. However, in practice, there is typically no prior information on which non-linear basis should be used and including a large number of non-informative basis could result in significant overfitting. In recent years, kernel machine (KM) regression has been advocated as a powerful tool to incorporate complex covariate effects (Bishop et al., 2006; Schölkopf and Smola, 2001). The KM regression methods flexibly account for linear/non-linear effects, without necessitating explicit specification of the non-linear basis. For ordinal outcomes, some KM based algorithms have also been proposed. For example, in Cardoso and Da Costa (2007), the problem of classifying ordered classes is reduced to two-class problems and mapped into support vector machines (SVMs) and neural networks. In Chu and Keerthi (2005), SVM is used to optimize multiple thresholds to define parallel discriminant hyperplanes for the ordinal scales. Kernel Discriminant Analysis was extended using a rank constraint to solve the ordinal regression problem in Sun et al. (2010). However, none of these existing methods provide a good solution to leverage the potential similarity between sequential logits or to select optimal kernels.
In this paper, we propose a sparse CR KM (sCRKM ) regression method for ordinal outcomes where we use the KM framework to incorporate non-linear effects and impose sparsity on the differences in the covariate effects between sequential categories to control for overfitting. To improve estimation and computational efficiency, we propose the use of the kernel principal component analysis (PCA) (Mika et al., 1999; Schölkopf et al., 1998) to transform the dual representation of the optimization problem back to the primal form with basis functions estimated from the PCA and reduce the number of parameters by thresholding the estimated eigenvalues. One key challenge in KM regression is the selection of appropriate kernel functions. Here, we propose a data driven rule for selecting an optimal kernel by minimizing a cross-validated prediction error measure. Simulation results suggest that the proposed procedures work well with relatively little price paid for the additional kernel selection. The rest of the paper is organized as the following. We introduce the logistic CR KM model in section 2.1 and detail the estimation procedures under the sparsity assumption in section 2.2. We also describe the model evaluation criteria in section 2.3 and propose a data driven rule for selecting an optimal kernel in section 2.4. Simulation and real data analysis results are given in section 3.1 and 3.2.
2. Continuation Ratio Kernel Machine Regression
Suppose data for analysis consist of n independent and identically distributed random vectors, { , i = 1, …, n}. The forward fCR KM (fCRKM ) model assumes that
| (3) |
where g(x) = ex/(1 + ex), h(c)(·) is an unknown centered smooth function that belongs to a Reproducible Kernel Hilbert Space (RKHS)
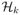 , with the Hilbert space generated by a given positive definite kernel function k(·, ·; ρ), and ρ is some tuning parameter associated with the kernel function (Cristianini and Shawe-Taylor, 2000). The kernel function k(x1,
x2; ρ) measures the similarity between x1 and x2 and different choices of k lead to different RKHS. Some of the popular kernel functions include the gaussian kernel
, which can be used to capture complex smooth non-linear effects; the linear kernel
, which corresponds to h(x) being linear in x; and the quadratic kernel
, which allows for 2-way interactive effects. Here, we use || · ||p to denote the Lp norm and || · ||F to denote Fubini’s norm for matrices. From here onward, for notational ease, we suppress ρ from the kernel function k.
, with the Hilbert space generated by a given positive definite kernel function k(·, ·; ρ), and ρ is some tuning parameter associated with the kernel function (Cristianini and Shawe-Taylor, 2000). The kernel function k(x1,
x2; ρ) measures the similarity between x1 and x2 and different choices of k lead to different RKHS. Some of the popular kernel functions include the gaussian kernel
, which can be used to capture complex smooth non-linear effects; the linear kernel
, which corresponds to h(x) being linear in x; and the quadratic kernel
, which allows for 2-way interactive effects. Here, we use || · ||p to denote the Lp norm and || · ||F to denote Fubini’s norm for matrices. From here onward, for notational ease, we suppress ρ from the kernel function k.
By Mercer’s Theorem (Cristianini and Shawe-Taylor, 2000), any h(x) ∈
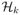 has a primal representation with respect to the eigensystem of k. Specifically, under the probability measure of x, k has eigenvalues {λl, l = 1, …,
has a primal representation with respect to the eigensystem of k. Specifically, under the probability measure of x, k has eigenvalues {λl, l = 1, …,
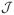 } with λ1 ≥ λ2 ≥ ··· ≥
} with λ1 ≥ λ2 ≥ ··· ≥
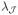 and the corresponding eigenfunctions {ϕl, l = 1, …,
and the corresponding eigenfunctions {ϕl, l = 1, …,
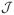 } such that
, where
} such that
, where
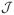 could be infinity and λl > 0 for any l < ∞. The basis functions, {
, l = 1, …,
could be infinity and λl > 0 for any l < ∞. The basis functions, {
, l = 1, …,
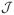 }, span the RKHS
}, span the RKHS
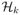 . Hence all h(c) ∈
. Hence all h(c) ∈
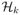 has a primal representation,
, and (3) is equivalent to
has a primal representation,
, and (3) is equivalent to
| (4) |
Assuming for all l, c, c′ leads to a pCR KM (pCRKM ) model. Throughout, we assume that x is bounded and k is smooth, leading to bounded {ψl(x)} on the support of x.
2.1 Inference under the full model
To make inference about the model parameters with observed data, we may maximize the following penalized likelihood with respect to {( , h(c)), c = 1, …, C−1} with penalty accounting for the smoothness of h(c):
| (5) |
where τ2 ≥ 0 is a tuning parameter controlling the amount of penalty,
and . From the primal representation of h(c), maximizing (5) is equivalent to maximizing the following penalized likelihood with respect to {( , β(c)), c = 1, …, C − 1}:
| (6) |
Thus, if the basis functions {ψl} were known, we can directly estimate h(c) in the primal form. Unfortunately, in practice the true basis are typically unknown as they involve the unknown distribution of x. On the other hand, by the representer theorem (Kimeldorf and Wahba, 1970), it is not difficult to show that the maximizer in (5) always takes the dual representation with , where ki = [k(xi, x1), …, k(xi, xn)]T and α(c) is an n × 1 vector of unknown weights to be estimated as model parameters. This representation reduces (6) to an explicit optimization problem in the dual form:
| (7) |
where
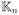 = n−1[k(xi, xj)]n×n. Note that unlike the hinge loss in SVM, the logistic loss function is smooth, and consequently the resulting estimate of α(c) based on (7) is not sparse.
= n−1[k(xi, xj)]n×n. Note that unlike the hinge loss in SVM, the logistic loss function is smooth, and consequently the resulting estimate of α(c) based on (7) is not sparse.
Maximization of (7), however, could be both numerically and statistically unstable due to the large number of (n + 1)(C − 1) parameters to be estimated, especially when the sample size n is not small. On the other hand, if the eigenvalues of k decay quickly, then we may reduce the complexity by approximating k by a truncated kernel
, for some r such that
. The error
can be bounded by
, where
is the kernel matrix constructed from kernel k(r) (Braun et al., 2005, Theorem 3.7). In many practical situations with fast decaying eigenvalues for k, r is typically fairly small and we can effectively approximate
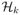 by a finite dimensional space
by a finite dimensional space
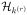 . Although k(r) is generally not attainable directly in practice, we may approximate the space spanned by k(r) through kernel PCA by applying a singular value decomposition to
. Although k(r) is generally not attainable directly in practice, we may approximate the space spanned by k(r) through kernel PCA by applying a singular value decomposition to
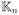 :
:
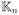 = Φ̃Λ̂Φ̃T, where Φ̃ = (u1, …, un), Λ̂ = diag{a1, …, an}, a1 ≥ … ≥ an ≥ 0 are the eigenvalues of
= Φ̃Λ̂Φ̃T, where Φ̃ = (u1, …, un), Λ̂ = diag{a1, …, an}, a1 ≥ … ≥ an ≥ 0 are the eigenvalues of
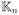 and {u1, …, un} are the corresponding eigenvectors. By the relative-absolute bound for the estimated principal values in kernel PCA, the principal values al converge to the eigenvalues λl and the projection error
can be bounded by
. Thus, with properly chosen rn and sufficiently fast decay rate for {λl},
and {u1, …, un} are the corresponding eigenvectors. By the relative-absolute bound for the estimated principal values in kernel PCA, the principal values al converge to the eigenvalues λl and the projection error
can be bounded by
. Thus, with properly chosen rn and sufficiently fast decay rate for {λl},
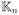 can be approximated well by
, where Φ̃(rn) = [u1, …,
urn], Λ̂(rn) = diag{a1, …, arn}, and
. Replacing
can be approximated well by
, where Φ̃(rn) = [u1, …,
urn], Λ̂(rn) = diag{a1, …, arn}, and
. Replacing
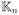 with
and applying a variable transformation
, the maximization of (7) can be approximately solved by maximizing
with
and applying a variable transformation
, the maximization of (7) can be approximately solved by maximizing
| (8) |
with respect to , where ψ̃ is the ith row of Ψ̃(rn). In practice, we may choose for some η close to 1.
Let denote the estimator from the maximization of (8). Then for a future subject with x, the probability π+(c | x) = P(y = c | y ⩾ c, x) can be estimated as
, by the Nystrom method (Rasmussen, 2004). Subsequently, π(c | x) = P(y = c | x) can be estimated as
A future subject with x can then be classified as ỹ(x) = argmaxc π̃(c | x).
2.2 Estimation Under the sCRKM Assumption
When the effects of x may differ across some continuation ratios but not all, one may improve the efficiency in estimating h(c) by imposing sparsity on {h(c+1) −h(c), c = 1, …,C −2} in (3), or equivalently on {β(c+1)–β(c), c = 1, …,C −2} in (4). To leverage the sparsity in estimation under the sCRKM assumption, we propose to modify (8) and instead maximize the penalized likelihood,
| (9) |
where τ1 is another tuning parameter controlling the amount of penalty for the differences between adjacent h(c)’s. The adaptive penalty is imposed here to ensure the consistency in identifying the set of unique h(c)’s.
To carry out the maximization of (9) in practice, we first obtain a quadratic expansion of the log-likelihood
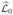 (θ; τ2) around the initial estimator θ̃:
(θ; τ2) around the initial estimator θ̃:
where
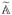 = n−1∂
= n−1∂
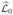 (θ; τ2)/∂θ∂θT|θ=θ̃. Subsequently, we approximate the maximizer of (9) by
(θ; τ2)/∂θ∂θT|θ=θ̃. Subsequently, we approximate the maximizer of (9) by
| (10) |
Such a quadratic approximation has been previously proposed to ease the computation for maximizing LASSO penalized likelihood functions and was shown to perform well in general (Wang and Leng, 2007). Followed by a sequence of variable transformations, we reformulate our optimization problem into a standard group lasso penalized maximization problem (Yuan and Lin, 2006; Wang and Leng, 2008). The detailed algorithm is in Web Appendix A. We tune the 3-tuple parameters (ρ, τ1 and τ2) by varying ρ within a range of values. For any given ρ, we first get τ2(ρ) by GCV criterion in the ridge regression. Subsequently, we select ρ and τ1 by optimizing the AIC. The detailed tuning procedure is in Web Appendix B.
With obtained from (10), h(c)(x) can be estimated as
We also obtain the corresponding π̂+(c |
x) and π̂(c |
x) by replacing θ̃ in π̃+(c |
x) and π̃(c |
x) respectively. Then subjects with x can be classified as ŷ(x) = argmaxc π̂ (c |
x). We expect that the proposed sparse estimator θ̂ and the resulting classification ŷ will outperform the corresponding estimators and classifications derived from the fCRKM model based on θ̃ and the reduced pCRKM model when the underlying model has h(c) = h(c+1) for some c but not all. When
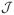 = ∞, the convergence rate of ĥ(c) would depend on the decay rate of the eigenvalues {λl}. On the other hand, for many practical settings,
= ∞, the convergence rate of ĥ(c) would depend on the decay rate of the eigenvalues {λl}. On the other hand, for many practical settings,
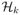 with the optimal ρ can be approximated well with a finite dimensional space
with the optimal ρ can be approximated well with a finite dimensional space
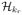 with a fixed r. In Web Appendix C, we show that when
with a fixed r. In Web Appendix C, we show that when
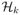 is finite dimensional,
). Furthermore, we establish the model selection consistency in the sense that P(ĥ(c) = ĥ(c+1)) → 1 if h(c) = h(c+1).
is finite dimensional,
). Furthermore, we establish the model selection consistency in the sense that P(ĥ(c) = ĥ(c+1)) → 1 if h(c) = h(c+1).
2.3 Model Evaluation
To evaluate the prediction performances of different methods for future observations (y0,
x0), we consider three prediction error measures: the overall mis-classification error (OME) P(ŷ(x0) ≠ y0), the absolute prediction error (APE) E|ŷ(x0)–y0| and the average size of prediction sets (
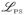 ) to be defined below. The OME puts equal weights to any error as long as ŷ(x0) = y0 and APE weights the error by the absolute distance between ŷ(x0) and y0. When comparing classification rules, one often sees a trade-off between these two error measures as they are capturing slightly different aspects of the prediction performance. In addition to these measures of accuracy based on ŷ(x0), we also examine the performance by taking the uncertainty in the classification into account. Specifically, for a given x0 with predicted probabilities {π̂(c |
x0), c = 1, …,C}, instead of classifying the subject according to ŷ(x0) = argmaxc π̂(c |
x0), we construct a prediction set (PS)
) to be defined below. The OME puts equal weights to any error as long as ŷ(x0) = y0 and APE weights the error by the absolute distance between ŷ(x0) and y0. When comparing classification rules, one often sees a trade-off between these two error measures as they are capturing slightly different aspects of the prediction performance. In addition to these measures of accuracy based on ŷ(x0), we also examine the performance by taking the uncertainty in the classification into account. Specifically, for a given x0 with predicted probabilities {π̂(c |
x0), c = 1, …,C}, instead of classifying the subject according to ŷ(x0) = argmaxc π̂(c |
x0), we construct a prediction set (PS)
 (x0) = {c : π̂(c |
x0) ⩾
(x0) = {c : π̂(c |
x0) ⩾
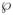 }, consisting of all categories whose predicted probabilities exceed
}, consisting of all categories whose predicted probabilities exceed
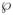 , where
, where
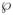 is chosen as the largest value such that these prediction sets of all samples achieve a desired coverage level 100(1 – α0)%, i.e. P{y0
∈
is chosen as the largest value such that these prediction sets of all samples achieve a desired coverage level 100(1 – α0)%, i.e. P{y0
∈
 (x0)} ⩾ 1 – α0(Faulkenberry, 1973; Jeske and Harvallie, 1988; Lawless and Fredette, 2005; Cai et al., 2008). The average size of the PS,
(x0)} ⩾ 1 – α0(Faulkenberry, 1973; Jeske and Harvallie, 1988; Lawless and Fredette, 2005; Cai et al., 2008). The average size of the PS,
can be also used to quantify the prediction performance based on those estimated π̂(c |
x0) derived from each model. The
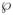 and
and
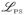 can be calculated from the constructed PS’ for all the samples in the testing set. Using the same argument as given in Cai et al. (2008), we may show that
can be calculated from the constructed PS’ for all the samples in the testing set. Using the same argument as given in Cai et al. (2008), we may show that
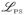 is minimized by the true model and hence is a useful measure as a basis for model selection. The use of
is minimized by the true model and hence is a useful measure as a basis for model selection. The use of
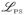 allows us to achieve two goals: (i) to obtain a set of potential classifications
allows us to achieve two goals: (i) to obtain a set of potential classifications
 (x) rather than ŷ(x0) to account for the uncertainty in the classification; and (ii) to provide a more comprehensive evaluation for the prediction performance of π̂(c |
x0) that accounts for the uncertainty.
(x) rather than ŷ(x0) to account for the uncertainty in the classification; and (ii) to provide a more comprehensive evaluation for the prediction performance of π̂(c |
x0) that accounts for the uncertainty.
2.4 Data Driven Rule for Kernel Selection
The choice of kernels is critical in the prediction performance, since different kernels have different features in terms of accounting for the non-linearity properties of the data. For example, the fCRKM with linear kernel is equivalent to the linear CR model in (1). The quadratic kernel is useful for capturing two-way interactions among predictors; while the gaussian kernel performs well in capturing smooth and complex effects. Unfortunately, in practice, with a given dataset, it is typically unclear which kernel would be the most appropriate. Here we propose to select an optimal kernel via K-fold cross-validation to minimize the
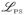 . To carry out the K-fold cross-validation for kernel selection, we randomly split the training data into K disjoint subset of about equal sizes and label them as Sk, k = 1, · · ·, K. For each k, we use all observations which are not in Sk to fit our proposed procedures with several candidate kernels and obtain the corresponding estimate θ̂. Then we use samples in Sk to calculate their predicted probabilities π̂ (c |
x), c = 1, · · ·, C. After obtaining the predicted probabilities for all the samples from cross-validation,
. To carry out the K-fold cross-validation for kernel selection, we randomly split the training data into K disjoint subset of about equal sizes and label them as Sk, k = 1, · · ·, K. For each k, we use all observations which are not in Sk to fit our proposed procedures with several candidate kernels and obtain the corresponding estimate θ̂. Then we use samples in Sk to calculate their predicted probabilities π̂ (c |
x), c = 1, · · ·, C. After obtaining the predicted probabilities for all the samples from cross-validation,
 (x) and
(x) and
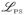 can be computed for each of the kernels. The kernel with the smallest
can be computed for each of the kernels. The kernel with the smallest
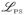 will be selected as the optimal kernel and the corresponding estimate θ̂ would then be used for prediction in the validation set. In regards to the choice of K, it is imperative that the size of the training set is large enough to accurately estimate the sCRKM model parameters. We recommend K = 10 as previously suggested in Breiman and Spector (1992).
will be selected as the optimal kernel and the corresponding estimate θ̂ would then be used for prediction in the validation set. In regards to the choice of K, it is imperative that the size of the training set is large enough to accurately estimate the sCRKM model parameters. We recommend K = 10 as previously suggested in Breiman and Spector (1992).
3. Numerical Studies
3.1 Simulation Study
We conducted extensive simulations to evaluate the finite sample performance of our proposed methods and compared with three existing methods: the “one-against-one” SVM method (Hsu and Lin, 2002), the L1 penalized pCR method (Archer and Williams, 2012), denoted by pCRL1 and the classification tree for ordinal outcomes (CART) (Galimberti et al., 2012). For the “one against-one” SVM, C(C – 1)/2 binary classifiers are trained by SVM, and the appropriate class is found by a voting scheme.
We simulated 5 category ordinal outcome Y with continuous covariates under the CRKM model in (3). The 20×1 predictor vector X was generated from MVN(0, 3.6+6.4
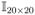 ).We generate Y |
X based on two types of h(c)(X): (i) linear in X; and (ii) linear effects plus two-way interactions between Xj and Xj+1. The regression coefficients β(c), c = 1, · · ·, C – 1 were set to be between 0 and 0.4 and the intercept parameters {
, c = 1, · · ·, C − 1} were selected such that there were approximately the same number of observations in each of the five classes. For each setting, we considered three types of h(c)(X): (a) h(1) ≠ = h(2) ≠ = h(3) ≠ = h(4) representing a fCRKM model; (b) h(1) = h(2) ≠ = h(3) = h(4) representing a model between a fCRKM model and a pCRKM model; and (c) h(1) = h(2) = h(3) = h(4) representing a pCRKM model. For each simulated data, we let n = 500 in the training set to estimate all model parameters including kernel selection. Then to evaluate the performances of different procedures, we generate independent test sets of sample size 5000 to approximate the expected accuracy of the trained models.
).We generate Y |
X based on two types of h(c)(X): (i) linear in X; and (ii) linear effects plus two-way interactions between Xj and Xj+1. The regression coefficients β(c), c = 1, · · ·, C – 1 were set to be between 0 and 0.4 and the intercept parameters {
, c = 1, · · ·, C − 1} were selected such that there were approximately the same number of observations in each of the five classes. For each setting, we considered three types of h(c)(X): (a) h(1) ≠ = h(2) ≠ = h(3) ≠ = h(4) representing a fCRKM model; (b) h(1) = h(2) ≠ = h(3) = h(4) representing a model between a fCRKM model and a pCRKM model; and (c) h(1) = h(2) = h(3) = h(4) representing a pCRKM model. For each simulated data, we let n = 500 in the training set to estimate all model parameters including kernel selection. Then to evaluate the performances of different procedures, we generate independent test sets of sample size 5000 to approximate the expected accuracy of the trained models.
For each scenario, we generate 50 datasets to compare the performance of SVM, pCRL1, CART, and the three models: sCRKM, fCRKM and pCRKM under the different choices of kernels. Three kernels, including linear, quadratic and gaussian, are considered as candidates for kernel selection. Our recommended procedure would be sCRKM with adaptive kernel selection, denoted by . Parameter tuning is performed based on the whole training set, and the selected parameters are fixed and used for the CV as well as building and evaluating the prediction model. We also compare the performance of our proposed procedures with data driven selection of the kernel versus those obtained under the true optimal kernel (linear for setting i and quadratic for setting ii) in each setting to examine the price paid for selecting the kernel.
In Table 1, we present results comparing different procedures when the data are generated from setting i with linear effects. Results for setting ii with interactive effects are given in Table 2. Table 3 shows the percentage of times different kernels being selected as the optimal kernel based on proposed data driven rule for kernel selection. Under both settings, applying our sCRKM method always results in similar performance as the true model when the underlying model is either (a) the fCRKM model; or (c) the pCRKM model. This indicates that little penalty is paid for letting the data determine the underlying sparsity of h(c+1)
– h(c). When the underlying model is in between the two, sCRKM performs the best. When the effects are linear, our proposed procedures with adaptive kernel selection perform similarly to those based on linear kernel. In the settings with interaction effects,
outperforms sCRKM with linear kernel by capturing non-linear effects. For example, when h(1) = h(2) ≠ = h(3) = h(4), the average prediction set size
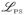 was 2.9 for
and, 4.49 for sCRKM with linear kernel. In this setting,
also outperforms both the fCRKM and the pCRKM models regardless how kernel was selected for these models. The prediction accuracy from
was also similar to sCRKM with quadratic kernel, which is the optimal kernel in this setting, indicating little loss of accuracy for the additional adaptive kernel selection. In general, the kernel selection procedure makes sensible choices of the kernels. When the underlying effects are linear, the linear kernel is selected 100% of the times; when the underlying model involves interactions, either quadratic or gaussian kernels are selected but not the linear kernel. This suggests that the use of cross-validation can overcome the overfitting issue. Under setting ii with interactive effects, the procedures with gaussian kernel appear to perform similarly to those with the quadratic kernel with respect to prediction accuracy. This is not surprising since the gaussian kernel is a universal kernel such that its corresponding RKHS is rich enough to approximate any target function (Steinwart, 2002) and hence could capture quadratic effects reasonably well.
was 2.9 for
and, 4.49 for sCRKM with linear kernel. In this setting,
also outperforms both the fCRKM and the pCRKM models regardless how kernel was selected for these models. The prediction accuracy from
was also similar to sCRKM with quadratic kernel, which is the optimal kernel in this setting, indicating little loss of accuracy for the additional adaptive kernel selection. In general, the kernel selection procedure makes sensible choices of the kernels. When the underlying effects are linear, the linear kernel is selected 100% of the times; when the underlying model involves interactions, either quadratic or gaussian kernels are selected but not the linear kernel. This suggests that the use of cross-validation can overcome the overfitting issue. Under setting ii with interactive effects, the procedures with gaussian kernel appear to perform similarly to those with the quadratic kernel with respect to prediction accuracy. This is not surprising since the gaussian kernel is a universal kernel such that its corresponding RKHS is rich enough to approximate any target function (Steinwart, 2002) and hence could capture quadratic effects reasonably well.
Table 1.
Average prediction performances with respect to average size of the prediction set (
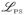 ), the overall mis-classification error (OME), and the absolute prediction error (APE), for setting i with linear effects.
), the overall mis-classification error (OME), and the absolute prediction error (APE), for setting i with linear effects.
| (a) h(1) ≠ h(2) ≠ h(3) ≠ h(4) | ||||
|---|---|---|---|---|
|
| ||||
| kernel choice |
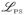
|
OME | APE | |
| Linear | fCRKM | 1.82(0.04) | 0.33(0.01) | 0.44(0.01) |
| sCRKM | 1.83(0.04) | 0.33(0.01) | 0.44(0.01) | |
| pCRKM | 2.29(0.03) | 0.46(0.01) | 0.53(0.01) | |
|
| ||||
| Data Driven | fCRKM | 1.82(0.04) | 0.33(0.01) | 0.44(0.01) |
|
|
1.83(0.04) | 0.33(0.01) | 0.44(0.01) | |
| pCRKM | 2.29(0.03) | 0.46(0.01) | 0.53(0.01) | |
|
| ||||
| - | SVM | 2.00(0.04) | 0.34(0.01) | 0.46(0.01) |
| CART | - | 0.56(0.02) | 0.80(0.02) | |
| pCRL1 | 2.33(0.04) | 0.47(0.01) | 0.55(0.01) | |
|
| ||||
| (b) h(1) = h(2) ≠ h(3) = h(4) | ||||
|
| ||||
| Linear | fCRKM | 1.90(0.05) | 0.36(0.01) | 0.44(0.02) |
| sCRKM | 1.85(0.04) | 0.35(0.01) | 0.42(0.02) | |
| pCRKM | 2.21(0.03) | 0.44(0.01) | 0.51(0.01) | |
|
| ||||
| Data Driven | fCRKM | 1.90(0.05) | 0.36(0.01) | 0.44(0.02) |
|
|
1.85(0.04) | 0.35(0.01) | 0.42(0.02) | |
| pCRKM | 2.21(0.03) | 0.44(0.01) | 0.51(0.01) | |
|
| ||||
| - | SVM | 2.04(0.04) | 0.38(0.01) | 0.47(0.01) |
| CART | - | 0.55(0.02) | 0.76(0.03) | |
| pCRL1 | 2.19(0.03) | 0.44(0.01) | 0.51(0.01) | |
|
| ||||
| (c) h(1) = h(2) = h(3) = h(4) | ||||
|
| ||||
| Linear | fCRKM | 1.93(0.03) | 0.39(0.01) | 0.43(0.01) |
| sCRKM | 1.86(0.03) | 0.37(0.01) | 0.39(0.01) | |
| pCRKM | 1.85(0.03) | 0.36(0.01) | 0.39(0.01) | |
|
| ||||
| Data Driven | fCRKM | 1.93(0.03) | 0.39(0.01) | 0.43(0.01) |
|
|
1.86(0.03) | 0.37(0.01) | 0.39(0.01) | |
| pCRKM | 1.85(0.03) | 0.36(0.01) | 0.39(0.01) | |
|
| ||||
| - | SVM | 2.03(0.04) | 0.42(0.01) | 0.46(0.01) |
| CART | - | 0.55(0.01) | 0.68(0.02) | |
| pCRL1 | 1.82(0.03) | 0.36(0.01) | 0.38(0.01) | |
Table 2.
Average prediction performances with respect to average size of the prediction set (
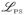 ), the overall mis-classification error (OME), and the absolute prediction error (APE), for setting ii with interactive effects.
), the overall mis-classification error (OME), and the absolute prediction error (APE), for setting ii with interactive effects.
| (a) h(1) ≠ h(2) ≠ h(3) ≠ h(4) | ||||
|---|---|---|---|---|
|
| ||||
| kernel choice |
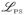
|
OME | APE | |
| Linear | fCRKM | 4.47(0.10) | 0.75(0.03) | 1.53(0.15) |
| sCRKM | 4.45(0.11) | 0.75(0.03) | 1.55(0.16) | |
| pCRKM | 4.44(0.05) | 0.76(0.02) | 1.65(0.17) | |
|
| ||||
| Data Driven | fCRKM | 1.99(0.07) | 0.36(0.02) | 0.52(0.02) |
|
|
2.03(0.09) | 0.37(0.02) | 0.52(0.02) | |
| pCRKM | 2.70(0.14) | 0.53(0.02) | 0.67(0.05) | |
|
| ||||
| Quadratic | fCRKM | 1.93(0.05) | 0.35(0.01) | 0.51(0.02) |
| sCRKM | 1.95(0.05) | 0.35(0.01) | 0.51(0.02) | |
| pCRKM | 2.84(0.06) | 0.55(0.01) | 0.72(0.02) | |
|
| ||||
| - | SVM | 2.32(0.06) | 0.44(0.01) | 0.63(0.02) |
| CART | - | 0.66(0.03) | 1.02(0.04) | |
| pCR L1 | 4.05(0.14) | 0.79(0.02) | 1.72(0.30) | |
|
| ||||
| (b) h(1) = h(2) ≠ h(3) = h(4) | ||||
|
| ||||
| Linear | fCRKM | 4.50(0.10) | 0.75(0.03) | 1.42(0.16) |
| sCRKM | 4.49(0.10) | 0.75(0.03) | 1.44(0.18) | |
| pCRKM | 4.46(0.04) | 0.75(0.03) | 1.53(0.19) | |
|
| ||||
| Data Driven | fCRKM | 2.19(0.07) | 0.43(0.02) | 0.55(0.02) |
|
|
2.09(0.10) | 0.41(0.02) | 0.52(0.02) | |
| pCRKM | 2.44(0.07) | 0.50(0.01) | 0.59(0.03) | |
|
| ||||
| Quadratic | fCRKM | 2.14(0.04) | 0.42(0.01) | 0.54(0.02) |
| sCRKM | 2.00(0.05) | 0.39(0.01) | 0.50(0.02) | |
| pCRKM | 2.50(0.06) | 0.51(0.01) | 0.61(0.02) | |
|
| ||||
| - | SVM | 2.48(0.05) | 0.51(0.01) | 0.68(0.02) |
| CART | - | 0.64(0.02) | 0.92(0.04) | |
| pCR L1 | 4.06(0.18) | 0.79(0.02) | 1.54(0.34) | |
|
| ||||
| (c) h(1) = h(2) = h(3) = h(4) | ||||
|
| ||||
| Linear | fCRKM | 4.45(0.09) | 0.77(0.02) | 1.52(0.13) |
| sCRKM | 4.44(0.09) | 0.77(0.02) | 1.55(0.15) | |
| pCRKM | 4.44(0.05) | 0.77(0.02) | 1.63(0.18) | |
|
| ||||
| Data Driven | fCRKM | 2.53(0.07) | 0.49(0.01) | 0.62(0.03) |
|
|
2.34(0.10) | 0.46(0.02) | 0.57(0.04) | |
| pCRKM | 2.05(0.08) | 0.40(0.02) | 0.46(0.02) | |
|
| ||||
| Quadratic | fCRKM | 2.48(0.05) | 0.48(0.01) | 0.60(0.02) |
| sCRKM | 2.26(0.06) | 0.45(0.01) | 0.55(0.02) | |
| pCRKM | 2.03(0.08) | 0.40(0.02) | 0.46(0.03) | |
|
| ||||
| - | SVM | 2.76(0.05) | 0.56(0.01) | 0.80(0.03) |
| CART | - | 0.64(0.04) | 0.87(0.04) | |
| pCR L1 | 4.09(0.18) | 0.79(0.02) | 1.64(0.31) | |
Table 3.
% of times different kernels being selected as the optimal kernel based on proposed data driven rule for kernel selection.
| setting i with linear effects | setting ii with interaction effects | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Linear | Guassian | Quadratic | Linear | Guassian | Quadratic | |
| h(1) ≠ h(2) ≠ h(3) ≠ h(4) | 100 | 0 | 0 | 0 | 54 | 46 |
| h(1) = h(2) ≠ h(3) = h(4) | 100 | 0 | 0 | 0 | 50 | 50 |
| h(1) = h(2) = h(3) = h(4) | 100 | 0 | 0 | 0 | 40 | 60 |
Comparing to the three existing methods, our proposed procedures also show great advantage. Across all settings, our proposed
method outperforms the SVM. For example, in setting ii, when h(1) ≠ h(2) ≠ h(3) ≠ h(4), SVM has an average
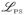 of 2.32 vs 2.03 from
; when h(1) = h(2) = h(3) = h(4), SVM has an average
of 2.32 vs 2.03 from
; when h(1) = h(2) = h(3) = h(4), SVM has an average
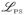 of 2.76 vs 2.34 from
. This is in part due to the fact that SVM doesn’t consider the ordinal property of the outcome or the underlying sparsity of h(c+1)
− h(c). The pCRL1 performs similarly to pCRKM with linear kernel because it only considers linear effect of the predictors. However, when the true underlying effects are non-linear as in setting ii, the pCRL1 performs poorly as expected. The CART method generally provides less accurate prediction compared to both SVM and sCRKM in both linear and non-linear settings. These comparisons imply the precision gain of our method due to imposing sparsity on the differences in the covariate effects of the sequential categories and incorporating potential non-linear effects via kernel selection.
of 2.76 vs 2.34 from
. This is in part due to the fact that SVM doesn’t consider the ordinal property of the outcome or the underlying sparsity of h(c+1)
− h(c). The pCRL1 performs similarly to pCRKM with linear kernel because it only considers linear effect of the predictors. However, when the true underlying effects are non-linear as in setting ii, the pCRL1 performs poorly as expected. The CART method generally provides less accurate prediction compared to both SVM and sCRKM in both linear and non-linear settings. These comparisons imply the precision gain of our method due to imposing sparsity on the differences in the covariate effects of the sequential categories and incorporating potential non-linear effects via kernel selection.
3.2 Data Example: Genetic Risk Prediction of Shared Autoimmunity
Autoimmune diseases (ADs), roughly defined as conditions where the immune system attacks self tissues and organs, affect 1 out of 31 individuals in the United States (Jacobson et al., 1997). Although ADs encompass a broad range of clinical manifestations, e.g. joint swelling, skin rash, and vasculitis. Recent studies have uncovered shared genetic risk factors across different ADs (Criswell et al., 2005). Epidemiologic studies corroborate with findings from genetic studies demonstrating that autoimmune diseases co-occur within individuals and families (Somers et al., 2006).
The presence of autoantibodies defines the majority of autoimmune diseases. Cyclic Citrullinated Peptide (CCP) antibodies are associated with rheumatoid arthritis (RA), and assays employing CCP to measure antibodies recognizing citrullinated antigens are used as a diagnostic test for RA (Lee and Schur, 2003). Among RA patients, different levels of CCP also indicate different subtypes of RA and are associated with different disease progressions (Kroot et al., 2000). Positive CCP indicates increased likelihood of erosive disease in RA, and high level of CCP may be useful to identify patients with aggressive disease. Given the shared genetic risk factors across autoimmune diseases, we would expect that subjects with one autoimmune disease, would be at higher risk for other autoimmune diseases. For example, CCP may be positive in patients with other autoimmune diseases such as systemic lupus erythematosus (SLE) (Harel and Shoenfeld, 2006). So patients with other autoimmune diseases or with genetic profiles that are indicative of elevated risk of other autoimmune diseases may have worse RA disease progression, which is partially reflected in the CCP levels.
To study the relationship between CCP levels and measurements of autoimmunity, we applied our methods to a dataset of 1265 rheumatoid arthritis (RA) patients of European decsent nested in an EMR cohort at Partner’s Healthcare (Liao et al., 2010). In this RA cohort, all subjects were genotyped for 67 single nucleotide polymorphisms (SNPs) with published associations with RA, Systemic lupus erythematosus (SLE), and celiac disease, and an aggregate genetic risk score (GRS) is calculated for each of the three diseases based on the number of SNPs for the particular diseases (Liao et al., 2013). These three GRSs represent genetic markers of autoimmunity. In addition to genetic information, billing codes of four ADs, RA, JRA and Psoriatic arthritis (PsA), as well as radiology findings of erosions are also available as predictors. For the CCP levels, we categorized into 4 ordinal categories with the total numbers of patients being 353, 266, 312 and 334, in categories 1, 2, 3 and 4, respectively. To construct and evaluate various prediction methods, we randomly split the data into two independent sets (evenly split within each category) with 633 subjects in the training set and 632 subjects in the validation set.
Applying our proposed procedure to the training data, our adaptive kernel selection rule selected the RBF kernel out of the linear, quadratic and RBF kernels, suggesting the presence of non-linear effects. Prediction models using SVM, pCRL1 and CART were also developed for comparison, and the results are shown in Table 4. When applying the proposed prediction rules to the validation set, the
method results in the smallest
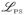 (2.58), comparing to the SVM model (3.44) and the pCRL1 model (3.43). As expected, if we enforce the sCRKM with quadratic kernel or linear kernel, the procedure yields a larger
(2.58), comparing to the SVM model (3.44) and the pCRL1 model (3.43). As expected, if we enforce the sCRKM with quadratic kernel or linear kernel, the procedure yields a larger
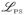 (3 and 2.9), highlighting the advantage of kernel selection. With respect to the OME and APE, the
model also outperforms the three existing methods. The
has OME of 0.59, versus 0.67 from SVM, 0.66 from pCRL1 and 0.67 from CART; has APE of 1.02, versus 1.18 from SVM, 1.27 from pCRL1 and 1.22 from CART. In order to evaluate whether the differences in the prediction is significant, we applied the bootstrap procedure to the validation data to estimate the standard errors for the estimated
(3 and 2.9), highlighting the advantage of kernel selection. With respect to the OME and APE, the
model also outperforms the three existing methods. The
has OME of 0.59, versus 0.67 from SVM, 0.66 from pCRL1 and 0.67 from CART; has APE of 1.02, versus 1.18 from SVM, 1.27 from pCRL1 and 1.22 from CART. In order to evaluate whether the differences in the prediction is significant, we applied the bootstrap procedure to the validation data to estimate the standard errors for the estimated
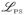 , OME, and APE, as well as the differences between
and other methods with respect to these prediction errors. As shown in Table 4,
leads to significantly lower prediction errors with p-values < 0.001. Therefore, our
method leads to a more accurate model for predicting anti-CCP levels compared with existing methods.
, OME, and APE, as well as the differences between
and other methods with respect to these prediction errors. As shown in Table 4,
leads to significantly lower prediction errors with p-values < 0.001. Therefore, our
method leads to a more accurate model for predicting anti-CCP levels compared with existing methods.
Table 4.
Prediction performances with respect to average size of the prediction set (
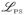 ), the overall mis-classification error (OME), and the absolute prediction error (APE); differences in performances between sCRKM and existing methods with standard deviations (SD)
), the overall mis-classification error (OME), and the absolute prediction error (APE); differences in performances between sCRKM and existing methods with standard deviations (SD)
| Methods for comparison | Prediction measurements | Differences between sCRKM and other(SD) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
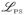
|
OME | APE |
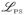
|
OME | APE | |
| sCRKM | 2.58(0.09) | 0.59(0.02) | 1.02(0.04) | - | - | - |
| SVM | 3.5(0.05) | 0.67(0.02) | 1.18(0.04) | −0.92(0.09) | −0.09(0.02) | −0.16(0.04) |
| pCR L1 | 3.43(0.05) | 0.66(0.02) | 1.27(0.04) | −0.85(0.10) | −0.07(0.02) | −0.25(0.04) |
| CART | - | 0.67(0.02) | 1.22(0.04) | - | −0.08(0.02) | −0.20(0.04) |
4. Discussion
In this paper, we proposed the sCRKM procedure to construct optimal classification rules for ordinal outcomes. Our proposed method has advantage over existing methods by incorporating potentially non-linear effects while allowing for adaptive selection of optimal kernels. When there is sparsity for the differences in the covariate effects between two sequential categories, our method will also automatically assign the same coefficients to the sequential categories to achieve an optimal balance between model complexity and prediction accuracy. Our numerical studies suggest that when the underlying model is either the fCRKM model or the reduced pCRKM model, our proposed sCRKM method performs similarly to fitting the corresponding model. In the case that the underlying model is in between the full and reduced model, the sCRKM method performs better than fitting either the fCRKM or the pCRKM model. The proposed data driven rule for kernel selection also enables us to choose an optimal kernel for a given dataset. When we select how many components to use in the singular value decomposition of
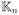 , we choose rn as the largest r such that the estimated proportion of variation explained by the first rn eigenfunctions, defined as
, is at least η ∈ (0, 1]. This selection rule is similar to those considered in the standard PCA literature (Park, 1981). Alternatively, one may treat rn as an additional tuning parameter and select the appropriate rn from a certain range to make sure the final AIC reaches its optimal.
, we choose rn as the largest r such that the estimated proportion of variation explained by the first rn eigenfunctions, defined as
, is at least η ∈ (0, 1]. This selection rule is similar to those considered in the standard PCA literature (Park, 1981). Alternatively, one may treat rn as an additional tuning parameter and select the appropriate rn from a certain range to make sure the final AIC reaches its optimal.
Supplementary Material
Acknowledgments
This research was partially supported by NIH grants R01 GM079330, U54 LM008748, P01CA134294, R01 HL089778 and R01 AI052817.
Footnotes
Web Appendices referenced in Section 2.2 and the source code for computation are available with this paper at the Biometrics website on Wiley Online Library.
References
- Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. International journal of epidemiology. 1997;26:1323–1333. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- Archer K, Williams A. L1 penalized continuation ratio models for ordinal response prediction using high-dimensional datasets. Statistics in Medicine. 2012;31:1464–1474. doi: 10.1002/sim.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CM, et al. Pattern recognition and machine learning. Vol. 1. springer; New York: 2006. [Google Scholar]
- Braun ML, et al. PhD thesis. Friedrich-Wilhelms-Universität Bonn; 2005. Spectral properties of the kernel matrix and their relation to kernel methods in machine learning. [Google Scholar]
- Breiman L, Spector P. Submodel selection and evaluation in regression: the x-random case. International Statistical Review/Revue Internationale de Statistique. 1992;60:291–319. [Google Scholar]
- Cai T, Tian L, Solomon SD, Wei L. Predicting future responses based on possibly mis-specified working models. Biometrika. 2008;95:75–92. [Google Scholar]
- Cardoso JS, Da Costa JFP. Learning to classify ordinal data: The data replication method. Journal of Machine Learning Research. 2007;8:6. [Google Scholar]
- Chu W, Keerthi SS. New approaches to support vector ordinal regression. Proceedings of the 22nd international conference on Machine learning; ACM; 2005. pp. 145–152. [Google Scholar]
- Cristianini N, Shawe-Taylor J. An introduction to support vector machines and other kernel-based learning methods. Cambridge university press; 2000. [Google Scholar]
- Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VE, Li W, et al. Analysis of families in the multiple autoimmune disease genetics consortium (madgc) collection: the ptpn22 620w allele associates with multiple autoimmune phenotypes. The American Journal of Human Genetics. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkenberry GD. A method of obtaining prediction intervals. Journal of the American Statistical Association. 1973;68:433–435. [Google Scholar]
- Galimberti G, Soffritti G, Di Maso M. Classification trees for ordinal responses in r: The rpartscore package. Journal of Statistical Software. 2012;47:1–25. [Google Scholar]
- Harel M, Shoenfeld Y. Predicting and preventing autoimmunity, myth or reality? Annals of the New York Academy of Sciences. 2006;1069:322–345. doi: 10.1196/annals.1351.031. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Lin CJ. A comparison of methods for multiclass support vector machines. Neural Networks, IEEE Transactions on. 2002;13:415–425. doi: 10.1109/72.991427. [DOI] [PubMed] [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the united states. Clinical immunology and immunopathology. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Jeske DR, Harvallie DA. Prediction-interval procedures and (fixed-effects) confidence-interval procedures for mixed linear models. Communications in Statistics-Theory and Methods. 1988;17:1053–1087. [Google Scholar]
- Kimeldorf GS, Wahba G. A correspondence between bayesian estimation on stochastic processes and smoothing by splines. The Annals of Mathematical Statistics. 1970;41:495–502. [Google Scholar]
- Kroot EJJ, De Jong BA, Van Leeuwen MA, Swinkels H, Van Den Hoogen FH, Van’t Hof M, Van De Putte L, Van Rijswijk MH, Van Venrooij WJ, Van Riel PL. The prognostic value of anti–cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis & Rheumatism. 2000;43:1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lawless J, Fredette M. Frequentist prediction intervals and predictive distributions. Biometrika. 2005;92:529–542. [Google Scholar]
- Lee D, Schur P. Clinical utility of the anti-ccp assay in patients with rheumatic diseases. Annals of the rheumatic diseases. 2003;62:870–874. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao KP, Cai T, Gainer V, Goryachev S, Zeng-treitler Q, Raychaudhuri S, Szolovits P, Churchill S, Murphy S, Kohane I, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis care & research. 2010;62:1120–1127. doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao KP, Kurreeman F, Li G, Duclos G, Murphy S, Guzman R, Cai T, Gupta N, Gainer V, Schur P, et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non–rheumatoid arthritis controls. Arthritis & Rheumatism. 2013;65:571–581. doi: 10.1002/art.37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika S, Schölkopf B, Smola A, Müller KR, Scholz M, Rätsch G. Kernel PCA and de-noising in feature spaces. Advances in neural information processing systems. 1999;11:536–542. [Google Scholar]
- Park SH. Collinearity and optimal restrictions on regression parameters for estimating responses. Technometrics. 1981;23:289–295. [Google Scholar]
- Rasmussen CE. Advanced Lectures on Machine Learning. Springer; 2004. Gaussian processes in machine learning; pp. 63–71. [Google Scholar]
- Schölkopf B, Mika S, Smola A, Rätsch G, Müller K-R. ICANN 98. Springer; 1998. Kernel PCA pattern reconstruction via approximate pre-images; pp. 147–152. [Google Scholar]
- Schölkopf B, Smola AJ. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond (Adaptive Computation and Machine Learning) The MIT Press; 2001. [Google Scholar]
- Somers EC, Thomas SL, Smeeth L, Hall AJ. Autoimmune diseases co-occurring within individuals and within families: a systematic review. Epidemiology. 2006;17:202–217. doi: 10.1097/01.ede.0000193605.93416.df. [DOI] [PubMed] [Google Scholar]
- Steinwart I. On the influence of the kernel on the consistency of support vector machines. The Journal of Machine Learning Research. 2002;2:67–93. [Google Scholar]
- Sun BY, Li J, Wu DD, Zhang XM, Li WB. Kernel discriminant learning for ordinal regression. Knowledge and Data Engineering, IEEE Transactions on. 2010;22:906–910. [Google Scholar]
- Tibshirani R, Saunders M, Rosset S, Zhu J, Knight K. Sparsity and smoothness via the fused lasso. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2005;67:91–108. [Google Scholar]
- Wang H, Leng C. Unified lasso estimation by least squares approximation. Journal of the American Statistical Association. 2007:102. [Google Scholar]
- Wang H, Leng C. A note on adaptive group lasso. Computational Statistics & Data Analysis. 2008;52:5277–5286. [Google Scholar]
- Yuan M, Lin Y. Model selection and estimation in regression with grouped variables. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2006;68:49–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


