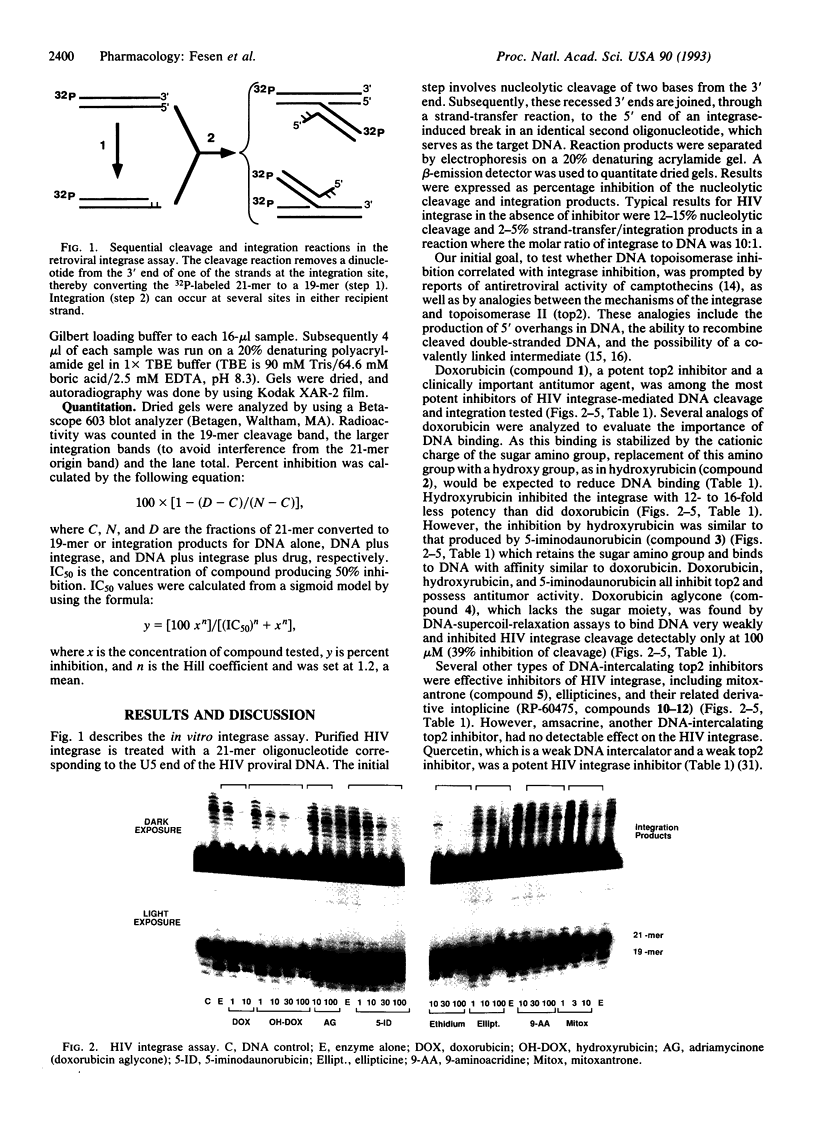
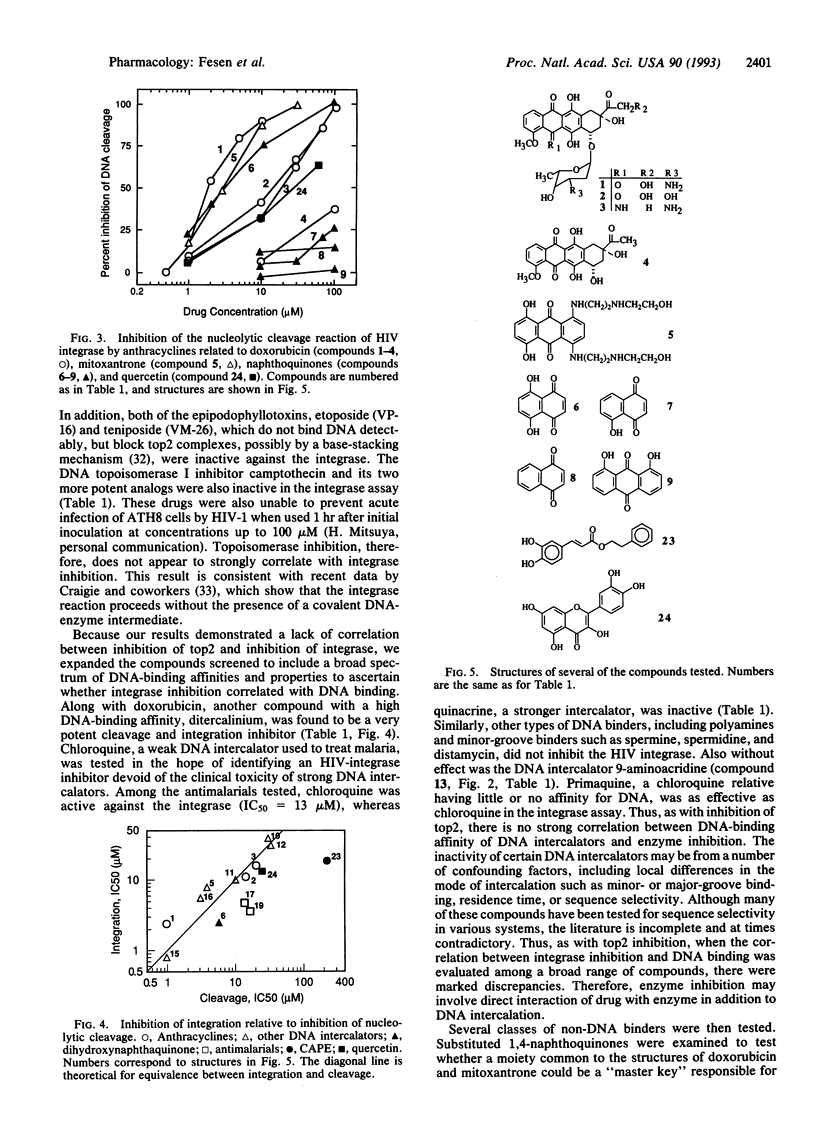
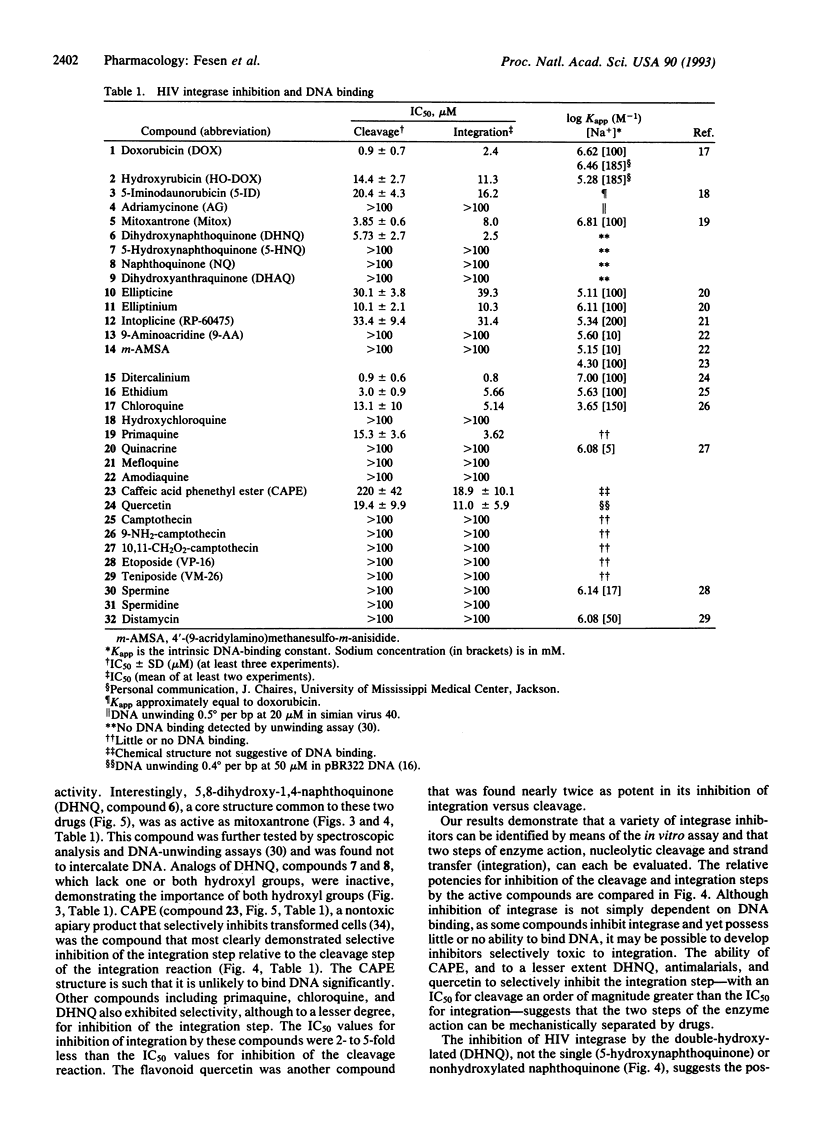
Abstract
In an effort to further extend the number of targets for development of antiretroviral agents, we have used an in vitro integrase assay to investigate a variety of chemicals, including topoisomerase inhibitors, antimalarial agents, DNA binders, naphthoquinones, the flavone quercetin, and caffeic acid phenethyl ester as potential human immunodeficiency virus type 1 integrase inhibitors. Our results show that although several topoisomerase inhibitors--including doxorubicin, mitoxantrone, ellipticines, and quercetin--are potent integrase inhibitors, other topoisomerase inhibitors--such as amsacrine, etoposide, teniposide, and camptothecin--are inactive. Other intercalators, such as chloroquine and the bifunctional intercalator ditercalinium, are also active. However, DNA binding does not correlate closely with integrase inhibition. The intercalator 9-aminoacridine and the polyamine DNA minor-groove binders spermine, spermidine, and distamycin have no effect, whereas the non-DNA binders primaquine, 5,8-dihydroxy-1,4-naphthoquinone, and caffeic acid phenethyl ester inhibit the integrase. Caffeic acid phenethyl ester was the only compound that inhibited the integration step to a substantially greater degree than the initial cleavage step of the enzyme. A model of 5,8-dihydroxy-1,4-naphthoquinone interaction with the zinc finger region of the retroviral integrase protein is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Denny W. A., Atwell G. J., Cain B. F. Potential antitumor agents. 34. Quantitative relationships between DNA binding and molecular structure for 9-anilinoacridines substituted in the anilino ring. J Med Chem. 1981 Feb;24(2):170–177. doi: 10.1021/jm00134a009. [DOI] [PubMed] [Google Scholar]
- Brown P. O. Integration of retroviral DNA. Curr Top Microbiol Immunol. 1990;157:19–48. doi: 10.1007/978-3-642-75218-6_2. [DOI] [PubMed] [Google Scholar]
- Burke C. J., Sanyal G., Bruner M. W., Ryan J. A., LaFemina R. L., Robbins H. L., Zeft A. S., Middaugh C. R., Cordingley M. G. Structural implications of spectroscopic characterization of a putative zinc finger peptide from HIV-1 integrase. J Biol Chem. 1992 May 15;267(14):9639–9644. [PubMed] [Google Scholar]
- Bushman F. D., Fujiwara T., Craigie R. Retroviral DNA integration directed by HIV integration protein in vitro. Science. 1990 Sep 28;249(4976):1555–1558. doi: 10.1126/science.2171144. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K., Bushman F. D., Engelman A. A rapid in vitro assay for HIV DNA integration. Nucleic Acids Res. 1991 May 25;19(10):2729–2734. doi: 10.1093/nar/19.10.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M., Sherman P. Inhibition of HIV-1 integration protein by aurintricarboxylic acid monomers, monomer analogs, and polymer fractions. Biochem Biophys Res Commun. 1992 May 29;185(1):85–90. doi: 10.1016/s0006-291x(05)80958-1. [DOI] [PubMed] [Google Scholar]
- Engelman A., Craigie R. Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol. 1992 Nov;66(11):6361–6369. doi: 10.1128/jvi.66.11.6361-6369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A., Mizuuchi K., Craigie R. HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell. 1991 Dec 20;67(6):1211–1221. doi: 10.1016/0092-8674(91)90297-c. [DOI] [PubMed] [Google Scholar]
- Glazer R. I., Hartman K. D., Richardson C. L. Cytokinetic and biochemical effects of 5-iminodaunorubicin in human colon carcinoma in culture. Cancer Res. 1982 Jan;42(1):117–121. [PubMed] [Google Scholar]
- Grunberger D., Banerjee R., Eisinger K., Oltz E. M., Efros L., Caldwell M., Estevez V., Nakanishi K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988 Mar 15;44(3):230–232. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- Katzman M., Katz R. A., Skalka A. M., Leis J. The avian retroviral integration protein cleaves the terminal sequences of linear viral DNA at the in vivo sites of integration. J Virol. 1989 Dec;63(12):5319–5327. doi: 10.1128/jvi.63.12.5319-5327.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman M., Mack J. P., Skalka A. M., Leis J. A covalent complex between retroviral integrase and nicked substrate DNA. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4695–4699. doi: 10.1073/pnas.88.11.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J., Jones K. S., Katz R. A., Mack J. P., Skalka A. M. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol Cell Biol. 1992 May;12(5):2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFemina R. L., Callahan P. L., Cordingley M. G. Substrate specificity of recombinant human immunodeficiency virus integrase protein. J Virol. 1991 Oct;65(10):5624–5630. doi: 10.1128/jvi.65.10.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Leavitt A. D., Rose R. B., Varmus H. E. Both substrate and target oligonucleotide sequences affect in vitro integration mediated by human immunodeficiency virus type 1 integrase protein produced in Saccharomyces cerevisiae. J Virol. 1992 Apr;66(4):2359–2368. doi: 10.1128/jvi.66.4.2359-2368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown J. W., Morgan A. R., Yen S. F., Wang Y. H., Wilson W. D. Characteristics of the binding of the anticancer agents mitoxantrone and ametantrone and related structures to deoxyribonucleic acids. Biochemistry. 1985 Jul 16;24(15):4028–4035. doi: 10.1021/bi00336a034. [DOI] [PubMed] [Google Scholar]
- Luck G., Triebel H., Waring M., Zimmer C. Conformation dependent binding of netropsin and distamycin to DNA and DNA model polymers. Nucleic Acids Res. 1974 Mar;1(3):503–530. doi: 10.1093/nar/1.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti C., Le Pecq J. B., Dat-Xuong N., Juret P., Garnier H., Amiel J. L., Rouesse J. Antitumor activity, pharmacology, and toxicity of ellipticines, ellipticinium, and 9-hydroxy derivatives: preliminary clinical trials of 2-methyl-9-hydroxy ellipticinium (NSC 264-137). Recent Results Cancer Res. 1980;74:107–123. doi: 10.1007/978-3-642-81488-4_15. [DOI] [PubMed] [Google Scholar]
- Pelaprat D., Delbarre A., Le Guen I., Roques B. P., Le Pecq J. B. DNA intercalating compounds as potential antitumor agents. 2. Preparation and properties of 7H-pyridocarbazole dimers. J Med Chem. 1980 Dec;23(12):1336–1343. doi: 10.1021/jm00186a010. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Capranico G., Orr A., Kohn K. W. Local base sequence preferences for DNA cleavage by mammalian topoisomerase II in the presence of amsacrine or teniposide. Nucleic Acids Res. 1991 Nov 11;19(21):5973–5980. doi: 10.1093/nar/19.21.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Covey J. M., Kerrigan D., Markovits J., Pham R. DNA unwinding and inhibition of mouse leukemia L1210 DNA topoisomerase I by intercalators. Nucleic Acids Res. 1987 Aug 25;15(16):6713–6731. doi: 10.1093/nar/15.16.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porschke D. Dynamics of DNA condensation. Biochemistry. 1984 Oct 9;23(21):4821–4828. doi: 10.1021/bi00316a002. [DOI] [PubMed] [Google Scholar]
- Priel E., Showalter S. D., Blair D. G. Inhibition of human immunodeficiency virus (HIV-1) replication in vitro by noncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res Hum Retroviruses. 1991 Jan;7(1):65–72. doi: 10.1089/aid.1991.7.65. [DOI] [PubMed] [Google Scholar]
- STOLLAR D., LEVINE L. Antibodies to denatured deoxyribonucleic acid in lupus erythematosus serum. V. Mechanism of DNA-anti-DNA inhibition by chloroquine. Arch Biochem Biophys. 1963 May;101:335–341. doi: 10.1016/s0003-9861(63)80021-1. [DOI] [PubMed] [Google Scholar]
- Sherman P. A., Fyfe J. A. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5119–5123. doi: 10.1073/pnas.87.13.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink C., Yeheskiely E., van der Marel G. A., van Boom J. H., Plasterk R. H. Site-specific hydrolysis and alcoholysis of human immunodeficiency virus DNA termini mediated by the viral integrase protein. Nucleic Acids Res. 1991 Dec 25;19(24):6691–6698. doi: 10.1093/nar/19.24.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink C., van Gent D. C., Elgersma Y., Plasterk R. H. Human immunodeficiency virus integrase protein requires a subterminal position of its viral DNA recognition sequence for efficient cleavage. J Virol. 1991 Sep;65(9):4636–4644. doi: 10.1128/jvi.65.9.4636-4644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadkins R. M., Graves D. E. Thermodynamics of the interactions of m-AMSA and o-AMSA with nucleic acids: influence of ionic strength and DNA base composition. Nucleic Acids Res. 1989 Dec 11;17(23):9933–9946. doi: 10.1093/nar/17.23.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Kawada S., Nakano H. Induction of mammalian topoisomerase II dependent DNA cleavage by nonintercalative flavonoids, genistein and orobol. Biochem Pharmacol. 1990 Feb 15;39(4):737–744. doi: 10.1016/0006-2952(90)90153-c. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Pluda J. M., Perno C. F., Mitsuya H., Broder S. Anti-retroviral therapy of human immunodeficiency virus infection: current strategies and challenges for the future. Blood. 1991 Aug 15;78(4):859–884. [PubMed] [Google Scholar]
- Zunino F., Di Marco A., Zaccara A. Molecular structural effects involved in the interaction of anthracyclines with DNA. Chem Biol Interact. 1979 Feb;24(2):217–225. doi: 10.1016/0009-2797(79)90010-3. [DOI] [PubMed] [Google Scholar]
- van Gent D. C., Elgersma Y., Bolk M. W., Vink C., Plasterk R. H. DNA binding properties of the integrase proteins of human immunodeficiency viruses types 1 and 2. Nucleic Acids Res. 1991 Jul 25;19(14):3821–3827. doi: 10.1093/nar/19.14.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent D. C., Groeneger A. A., Plasterk R. H. Mutational analysis of the integrase protein of human immunodeficiency virus type 2. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9598–9602. doi: 10.1073/pnas.89.20.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]



