Abstract
Both cerebral amyloid angiopathy and Alzheimer’s disease pathology involve abnormal β-amyloid processing. We aim to elucidate the relationship of the apolipoprotein E (APOE) genotypes with amyloid angiopathy in the presence of variable amounts of Alzheimer’s pathology. Data came from 1,062 autopsied subjects from two community-based studies of aging. Common neuropathologies including Alzheimer’s disease and amyloid angiopathy were assessed using uniform methods. APOE was genotyped by sequencing the two polymorphisms in codons 112 and 158 of exon 4. We examined the associations of APOE with amyloid angiopathy using ordinal logistic regression analyses, controlling for demographics and subsequently Alzheimer’s and other common pathologies. Moderate to severe amyloid angiopathy was identified in 35.2% (n=374) of the subjects. 15.3% (n=162) of the subjects were APOE ε2 carriers and 26.1% (n=277) ε4 carriers. Adjusting for demographics, the presence of ε4 allele, but not ε2, was associated with more severe amyloid angiopathy. After further adjustment for Alzheimer’s pathology, both ε2 (odds ratio 1.707, 95% confidence interval 1.236–2.358, p=0.001) and ε4 (odds ratio 2.284, 95% confidence interval 1.730–3.014, p<0.001) were independently associated with amyloid angiopathy. The results were confirmed by path analysis. Further, APOE ε4 carriers, but not ε2 carriers, were more likely to have capillary amyloid angiopathy. Accounting for capillary involvement did not alter the APOE associations with amyloid angiopathy. We conclude that both APOE ε2 and ε4 alleles are associated with more severe cerebral amyloid angiopathy, and the direct effect of ε2 is masked by the allele’s negative association with comorbid Alzheimer’s pathology. APOE ε4, but not ε2, is associated with capillary amyloid angiopathy.
Keywords: APOE, cerebral amyloid angiopathy, capillaries, Alzheimer’s disease
Introduction
A prominent feature of cerebral amyloid angiopathy (CAA) is the mural deposition of β-amyloid in the walls of cerebral arteries and arterioles (Greenberg and Vonsattel, 1997). The prevalence of CAA increases with age and is commonly present in persons with Alzheimer’s disease (AD) pathology (Love, et al., 2009). CAA is associated with a spectrum of neurological disorders including lobar intracerebral hemorrhage (ICH) and more debatably, ischemic infarction (Arvanitakis, et al., 2011b, Auriel and Greenberg, 2012, Cadavid, et al., 2000, Samarasekera, et al., 2012).
The genetics of sporadic CAA is largely unexplored; however, its pathophysiology suggests that mutations implicated in dysfunctional β-amyloid processing may play a role (Obici, et al., 2005). The apolipoprotein E (APOE) gene is a known risk gene for late-onset AD (Roses and Saunders, 1994). The APOE isoforms differentially regulate the concentration and clearance of β-amyloid, such that brain amyloid deposition follows an isoform-dependent pattern (APOE4>APOE3>APOE2) (Arold, et al., 2012, Castellano, et al., 2011, Holtzman, et al., 2000). As a result, the APOE ε4 allele markedly increases the risk of AD while the ε2 allele is protective against the disease relative to the reference ε3 allele. A recent heritability study estimates that the APOE locus contributes to 15% of ICH risk variance, and the heritability is driven by lobar ICH predominantly attributable to CAA (Devan, et al., 2013). Indeed, prior literature shows that the APOE ε4 allele is associated with a higher risk of CAA (Greenberg, et al., 1995, Premkumar, et al., 1996), suggesting that CAA and AD may share a common biological mechanism such that APOE ε4 increases β-amyloid deposition in both brain and cerebral blood vessels. However, the association of APOE ε2 with CAA- related ICH is discordant with that of AD. The ε2 allele increases the risk for lobar ICH and the association is stronger in cases with probable or definite CAA (Biffi, et al., 2010, Tzourio, et al., 2008, Valant, et al., 2012). APOE ε2 carriers with lobar ICH also tend to have larger haematoma volume (Biffi, et al., 2011) and an increased risk for haematoma expansion (Brouwers, et al., 2012). These findings on ε2 suggest that distinct from ε4 which promotes the vascular amyloid deposition, ε2 is implicated in other vasculopathic changes leading to vessel rupture (Greenberg, et al., 1998). Interestingly, an investigation on CAA burden by APOE genotypes shows that, compared with ε3/ε3, ε2 carriers have more severe CAA, and parenchymal CAA in particular (Nelson, et al., 2013). However, the relationship of ε2 with CAA is not clear as the association was not replicated in a more recent study using data from the National Alzheimer’s Coordinating Center (NACC) autopsied subjects (Serrano-Pozo, et al., 2015). Notably, the latter is based on the clinical cohorts for AD, which may result in a disproportional distribution of APOE genotypes such as over-representation of ε4.
In this study, utilizing data from more than 1,000 autopsied subjects from the community, we investigated the relationship of the APOE genotypes and CAA in the presence of variable amounts of AD pathology. First, we examined the association of the APOE genotypes with a semi-quantitative measure of CAA, adjusted for demographics and subsequently for AD and other pathologies. Since earlier literature suggests that amyloid deposition into capillary walls may represent a distinct morphological type (Thal, et al., 2002), we also examined the association of APOE and CAA by capillary involvement. Next, we tested the hypothesis that there are two pathways that link the APOE genotypes to CAA; a direct pathway and an indirect pathway through AD pathology. The hypothesis for the indirect pathway is supported by findings from transgenic mice models that perivascular drainage pathways, rather than local production or blood uptake, are the mechanism underlying amyloid deposition in CAA, and that β-amyloid of neuronal origin is sufficient to cause cerebral neurodegeneration including CAA (Calhoun, et al., 1999, Carare, et al., 2008, Herzig, et al., 2004, Van Dorpe, et al., 2000).
Material and methods
Study cases
Brains are donated by the individuals from two ongoing clinical pathologic studies of aging, the Religious Orders Study and the Rush Memory and Aging Project (Bennett, et al., 2012a, Bennett, et al., 2012b). Participants enroll without known dementia, and agree to annual clinical evaluations and brain donation after death. Both studies are approved by the Institutional Review Board of the Rush University Medical Center. An informed consent and an anatomical gift act are obtained from each participant.
At the time of the analysis, 3,069 participants had enrolled and 1,429 had died. Of the deceased, 1,232 had undergone autopsy (86%). Neuropathologic diagnosis had been completed for 1,188 autopsied subjects. We restricted to those with complete clinical, genotypic and pathologic information (N=1,083) and further excluded 21 with non-AD dementia. This leaves a total of 1,062 subjects included in the primary analysis. The average age at death was 88.7 years (standard deviation (SD) =6.6, range=65.9–108.3) and the average years of education was 16.3 (SD=3.6, range=3–30). 687 (64.7%) were females.
Neuropathologic assessment
Brain autopsy follows a standard protocol, as previously described (Bennett, et al., 2006b). The brain was removed, weighed, and cut into 1cm slabs. One hemisphere was frozen and the other was fixed in paraformaldehyde for further dissection.
Blocks of tissue from pre-determined brain regions were embedded in paraffin, and then cut and stained for diagnostic assessment by the neuropathologist, blinded to all clinical data. CAA was assessed in four neocortical regions: midfrontal, midtemporal, angular and calcarine cortices. Three monoclonal anti-human β-amyloid antibodies with the following dilutions were used: 1) 6F/3D (1:50, Dako North America Inc., Carpinteria, CA), 2) 10D5 (1:600, Elan Pharmaceuticals, San Francisco, CA) and 3) 4G8 (1:9000, Covance Labs, Madison, WI). We extended a previously published methodology for CAA assessment (Deramecourt, et al., 2012), and a similar protocol is proposed in a recent publication (Love, et al., 2014). Specifically, for each region, meningeal and parenchymal vessels were assessed separately and scored for β-amyloid deposition. Each score was from 0 to 4, where 0=no deposition, 1= scattered segmental but no circumferential deposition, 2=circumferential deposition in up to 10 vessels, 3= circumferential deposition in up to 75% of vessels in the region, and 4=circumferential deposition in over 75% of vessels in the total region. The maximum between the meningeal and parenchymal CAA scores defined the CAA score for that region. If over 50% of the meninges were missing from the section then the cortical region was used to define the score for that region. The scores were averaged across the 4 regions for each case. At least 2 regions must be available for a score to be calculated. The average score was further classified into a 4-level semi-quantitative measure, rated as none, mild, moderate and severe, using consistent cut-offs selected by the neuropathologist. A separate binary measure (present versus absent) was used to assess capillary CAA in any of the four cortical regions. Both CAA measures show moderate to substantial inter-rater agreement. The weighted κ coefficient for the primary CAA measure was 0.47 and the κ coefficient for the binary capillary CAA measure was 0.65.
Counts of neuritic plaque, diffuse plaque and neurofibrillary tangles were measured in midfrontal, superior temporal, inferior parietal, entorhinal cortices, and hippocampus using the Bielschowsky silver stain (Bennett, et al., 2006b). Raw counts of each index were scaled within each region and then averaged across the 5 regions. A composite score for overall burden of AD pathology was used in the analysis by averaging the scaled scores of the three indices. The measure was square root transformed in order to reduce skewness. Separately, Pathologic AD diagnosis followed the National Institute on Aging (NIA) Reagan criteria (Bennett, et al., 2006a). Macroscopic infarcts, microinfarcts and Lewy bodies were identified as described previously (Arvanitakis, et al., 2011a, Schneider, et al., 2012).
APOE genotyping
DNA was extracted from peripheral blood mononuclear cells, and in some cases DNA from frozen post-mortem brain tissue (cerebellum) was used. The APOE genotypes were determined by sequencing codon 112 (position 3937) and codon 158 (position 4075) in exon 4 (Boyle, et al., 2010). Cases with 1 or more copies of ε4 allele were considered ε4 carriers, and cases with 1 or more copies of ε2 allele were considered ε2 carriers.
Statistical analysis
Chi-square tests and Spearman correlations were used to examine the bivariate relationships of CAA with AD and other neuropathologic indices, as well as the APOE genotypes. Analysis of variance was used to examine the relationship of APOE with age at death. Ordinal logistic regression models tested the associations of the APOE genotypes with the odds of having more severe CAA. In the primary models, the semi-quantitative measure of CAA was the ordinal outcome, and the models included a term for APOE ε4 carriers and a separate term for ε2 carriers. The corresponding regression coefficients estimated the log odds ratios (ORs) of having more severe CAA among APOE ε4 (or ε2) carriers relative to the reference group (APOE ε3/3 carriers). We adjusted for age, sex and education in the initial analysis, and in subsequent models we further adjusted for a composite measure of AD pathology, as well as the presence of macroscopic infarcts, microinfarcts, and Lewy bodies. Secondary analysis included examination of the association of APOE with the presence of capillary CAA. We also investigated the dosage effect of the APOE genotypes. We then performed stratified analysis to examine whether the association of APOE with CAA differed in groups with and without capillary involvement.
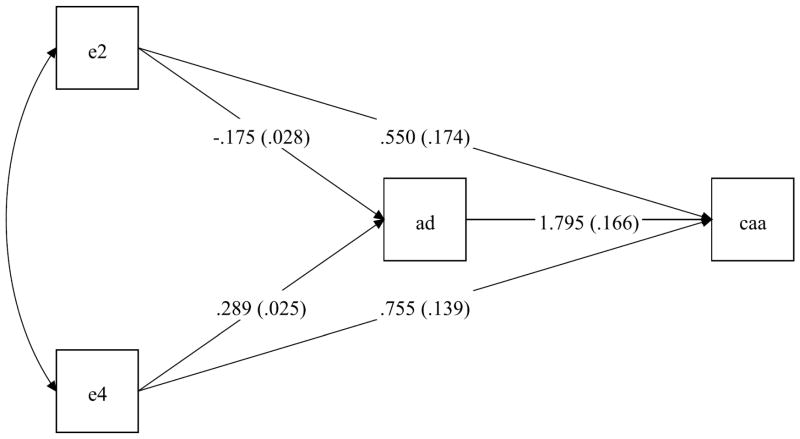
Considering that the APOE gene is implicated in AD pathology and CAA commonly coexisted with AD, we hypothesized that the relationship between the APOE genotypes and CAA may involve AD pathology. We tested this hypothesis by conducting path analysis. Path analysis is a special form of structural equation model, where we dissected the total effect of APOE genotypes on CAA into two contributing pathways, that is a direct effect and an indirect effect through AD pathology. The pathway diagram is presented in Figure 3.
Figure 3.
summarizes the path analysis of APOE genotypes and CAA where the model links the presence of APOE ε2 allele, and separately ε4 allele, with CAA, either directly or indirectly through an effect on AD pathology. The path coefficients (standard errors) estimate the associations of APOE genotypes with AD and subsequently CAA. All the coefficients are significant, and the result reveals that there is a significant direct effect of ε2 on CAA which is masked by the allele’s negative association with AD pathology.
The analyses were conducted using SAS/STAT software, version 9.3 (SAS Institute Inc. Cary, NC, USA) and Mplus 7.11 (Muthen & Muthen). A nominal level of α=0.05 was used to determine statistical significance.
Results
Amyloid angiopathy, AD and other common neuropathologies
The pathologic characteristics of the study group are presented in Table 1. Briefly, more than a third (N=374) had moderate to severe meningeal/parenchymal CAA, and 16% (N=170) had capillary CAA. As would be expected, the two measures were highly correlated such that the capillary involvement was associated with more severe meningeal/parenchymal CAA (p<0.001). About 18% of the subjects with capillary CAA (N=31) had mild CAA in meningeal/parenchymal vessels.
Table 1.
Descriptive of the study group (N=1,062)
| Age at death (mean, SD) | 88.7, 6.6 |
|
| |
| Female Sex (N, %) | 687, 64.7% |
|
| |
| Education (mean, SD) | 16.3, 3.6 |
|
| |
| Non-Hispanic Whites (N, %) | 1014, 95.6% |
|
| |
| APOE genotypes (N, %) | |
| ε2/2 | 7, 0.7% |
| ε2/3 | 134, 12.6% |
| ε2/4 | 21, 2.0% |
| ε3/3 | 644, 60.6% |
| ε3/4 | 240, 22.6% |
| ε4/4 | 16, 1.5% |
|
| |
| Composite measure of AD pathology (mean, SD)1 | 0.76, 0.40 |
|
| |
| Pathologic diagnosis of AD by NIA-Reagan criteria (N, %) | 672, 63.3% |
|
| |
| Macroscopic infarcts (N, %) | 368, 34.7% |
|
| |
| Microinfarcts (N, %) | 304, 28.6% |
|
| |
| Lewy Bodies (N, %) | 229, 21.6% |
|
| |
| Rating of CAA (N, %) | |
| None | 220, 20.7% |
| Mild | 468, 44.1% |
| Moderate | 245, 23.1% |
| Severe | 129, 12.1% |
|
| |
| Capillary CAA (N, %) | 170, 16.0% |
Square root transformed
A pathologic diagnosis of AD was made in 63% (N=672) of the subjects. The association between meningeal/parenchymal CAA and pathologic AD diagnosis was highly significant (p<0.001). 44.4% (N=298) of the subjects with pathologic AD diagnosis having moderate to severe CAA. By contrast, of the 390 subjects without pathologic AD, only 19.5% (N=76) had moderate to severe CAA. A similar association was observed between CAA and the composite measure of AD pathology (Spearman correlation coefficient=0.38, p<0.001). Notably, approximately 80% of all subjects with moderate to severe CAA had pathologic diagnosis of AD. Bivariate analyses did not show association of meningeal/parenchymal CAA with the presence of macroscopic infarcts (p=0.589), microinfarcts (p=0.143) or Lewy bodies (p=0.711).
The presence of capillary CAA was also strongly associated with pathologic AD (p<0.001). 20.7% (N=139) of subjects with pathologic diagnosis of AD had presence of capillary CAA, and the percentage was 8.0% (N=31) for those without pathologic AD diagnosis. Similar to meningeal/parenchymal CAA, a majority (> 80%) of the subjects with capillary CAA had pathologic AD. Bivariate analyses showed that capillary CAA was significantly associated with Lewy bodies (p=0.021), but not with macroscopic infarcts (p=0.587) or microinfarcts (p=0.056). Notably, after adjustment for AD pathology, the presence of Lewy bodies was no longer associated with capillary CAA (p=0.142).
Amyloid angiopathy and APOE genotypes
A majority (60.6%) of the subjects had APOE ε3/ε3 genotype, 15.3% were APOE ε2 carriers, and 26.1% were ε4 carriers. The mean age for the subjects with ε3/3 genotype was 88.9 years (SD=6.7). Comparing with ε3/3 carriers, ε4 carriers were on average 1 year younger at the time of death (p=0.017). We did not find significant age difference between ε3/3 carriers and ε2 carriers (p=0.196). In a series of logistic regression analyses, we examined associations of the APOE genotypes with pathologic indices of macroscopic infarcts, microinfarcts and Lewy bodies. Although we and others have previously reported associations of APOE ε4 with cerebral infarction and Lewy bodies (Schneider, et al., 2005, Tsuang, et al., 2013, Yu, et al., 2014), in this study we failed to replicate these associations after adjustment for demographics and AD pathology (data not shown).
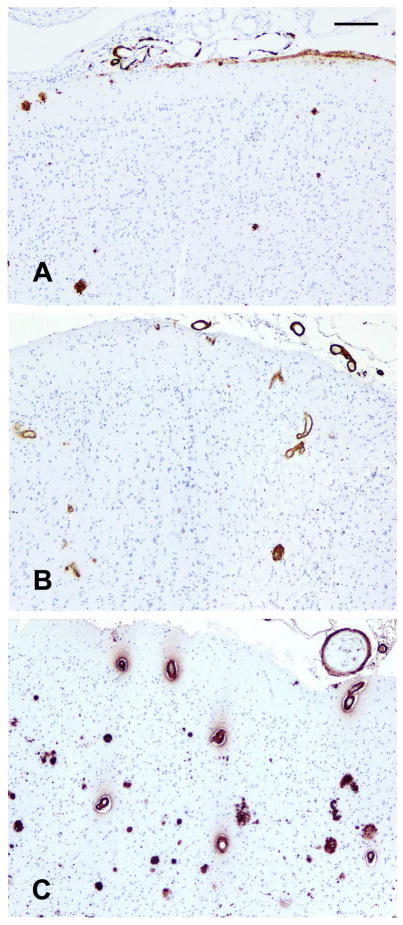
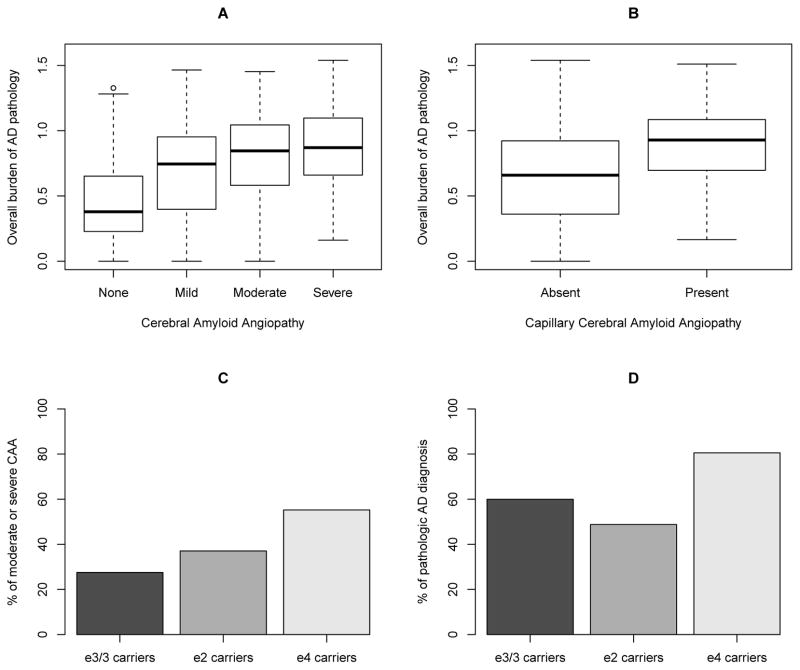
Next, we examined the association of APOE with meningeal/parenchymal CAA. Figure 1 shows representative CAA localization by the APOE genotypes. A subject with ε3/3 genotype shows sparse CAA which is confined to segmental deposition of β amyloid in only meningeal vessels, and the cortex shows few β amyloid plaques. Separately, a ε2 carrier shows CAA in meningeal and intracerebral cortical vessels but few β amyloid plaques. In contrast, a ε4 carrier shows marked CAA in meningeal and intracerebral cortical vessels as well as frequent β amyloid plaques in the cortex. 27.5% of the subjects with ε3/3 genotype had moderate to severe meningeal/parenchymal CAA, and the percentage increased to 37.0% and 55.2% respectively for the ε2 and ε4 carriers. This isoform-dependent pattern is illustrated in Figure 2C. As contrast, comparing with ε3/3 carriers, ε4 carriers had more AD pathology and ε2 carriers had less AD pathology. In particular, 59.9% of the ε3/3 carriers, 48.8% of the ε2 carriers, and 80.5% of the ε4 carriers had pathologic AD diagnosis (Figure 2D).
Figure 1.
illustrates representative CAA localization by the APOE genotypes. (A) shows a subject with ε3/3 genotype with sparse CAA which is confined to segmental deposition of β amyloid in only meningeal vessels, and the cortex has few β amyloid plaques. (B) shows a ε2 carrier with CAA present in meningeal and intracerebral cortical vessels, as well as few β amyloid plaques in the cortex. (C) shows a ε4 carrier with marked CAA in meningeal and intracerebral cortical vessels, as well as frequent β amyloid plaques in the cortex. Scale bar = 150 μm.
Figure 2.
illustrates relationship of AD pathology, CAA and APOE genotypes. (A) and (B) show the distribution of the composite measure of AD pathology by CAA. The association of CAA with more burden of AD pathology is evident. (C) shows the percentages of moderate to severe CAA by APOE genotypes. The figure illustrates isoform-dependent pattern of APOE (i.e. ε4>ε2>ε3) in relation to CAA. (D) shows the percentages of pathologic AD diagnosis by APOE genotypes. Here, we observe a different pattern in relation to AD (i.e. ε4>ε3>ε2), as compared with CAA (C).
In an ordinal logistic regression model adjusted for age, sex and education, the odds of having more severe meningeal/parenchymal CAA was tripled for APOE ε4 carriers (OR=3.554, 95% CI=2.728–4.630, p<0.001) relative to the ε3/3 reference group. In the same model, we did not find a significant association of APOE ε2 with CAA (OR=1.212, 95% CI=0.887–1.658, p=0.228). However, after we augmented the model by adding a term for the composite measure of AD pathology, both ε4 (OR =2.284, 95% CI =1.730–3.014, p<0.001) and ε2 (OR=1.707, 95% CI =1.236–2.358, p=0.001) were independently associated with more severe CAA. These associations persisted after further adjustment for cerebral infarcts and Lewy bodies (Table 2). We repeated the analyses to examine the associations of APOE with capillary CAA (Table 3). APOE ε4 remained strongly associated with the presence of capillary CAA (OR =4.149, 95% CI =2.835–6.073, p<0.001). The association of ε2 was not significant (OR=1.269, 95% CI =0.731–2.203, p=0.398).
Table 2.
Association of APOE with meningeal/parenchymal CAA
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|
| Age at death | 1.050 (1.032, 1.069)* | 1.033 (1.015, 1.052)* | 1.033 (1.014, 1.052)* |
| Male Sex | 1.038 (0.817, 1.320) | 1.175 (0.921, 1.500) | 1.184 (0.927, 1.511) |
| Education | 0.998 (0.967, 1.029) | 1.003 (0.971, 1.035) | 1.003 (0.972, 1.036) |
| AD1 | - | 5.537 (3.970, 7.721)* | 5.597 (4.009, 7.814)* |
| Macroscopic infarcts | - | - | 0.926 (0.724, 1.184) |
| Microinfarcts | - | - | 1.210 (0.937, 1.563) |
| Lewy Bodies | - | - | 0.954 (0.725, 1.257) |
| APOE ε2 | 1.212 (0.887, 1.658) | 1.707 (1.236, 2.358)* | 1.709 (1.237, 2.361)* |
| APOE ε4 | 3.554 (2.728, 4.630)* | 2.284 (1.730, 3.014)* | 2.296 (1.739, 3.032)* |
Composite measure of AD pathology;
p<0.05;
Model 1 was adjusted for demographics; Model 2 was adjusted for demographics and AD pathology; Model 3 was adjusted for demographics, AD pathology, as well as presence of infarcts and Lewy bodies.
Table 3.
Association of APOE with capillary CAA
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|
| Male Sex | 0.902 (0.616, 1.320) | 0.989 (0.670, 1.459) | 0.986 (0.667, 1.460) |
| Education | 1.045 (0.994, 1.098) | 1.055 (1.003, 1.110)* | 1.057 (1.004, 1.113)* |
| AD1 | - | 3.797 (2.247, 6.418)* | 3.816 (2.243, 6.494)* |
| Macroscopic infarcts | - | - | 0.936 (0.637, 1.375) |
| Microinfarcts | - | - | 1.534 (1.037, 2.270)* |
| Lewy Bodies | - | - | 1.374 (0.913, 2.067) |
| APOE ε2 | 0.966 (0.569, 1.640) | 1.250 (0.723, 2.161) | 1.269 (0.731, 2.203) |
| APOE ε4 | 5.747 (4.018, 8.221)* | 4.155 (2.845, 6.066)* | 4.149 (2.835, 6.073)* |
Composite measure of AD pathology;
p<0.05;
Model 1 is adjusted for demographics; Model 2 adjusted for demographics and AD pathology; Model 3 is adjusted for demographics, AD pathology, as well as presence of infarcts and Lewy bodies;
Next, we investigated the dosage effect of the APOE genotypes and the results were consistent. In the model adjusted for demographics, AD, infarcts and Lewy bodies pathologies, each additional copy of ε4 allele increased the odds of more severe meningeal/parenchymal CAA 2.5 fold (OR =2.507, 95% CI =1.939–3.243, p<0.001), and each additional copy of ε2 allele increased the odds of more severe CAA over 1.5 fold (OR =1.685, 95% CI =1.244–2.284, p<0.001).
Amyloid angiopathy and APOE genotypes by capillary involvement
A previous study (Thal, et al., 2002) suggests that the association of APOE genotypes with CAA may differ depending on the capillary involvement. Therefore, we examined the APOE association with CAA separately in groups with and without capillary involvement (Table 4). In the larger group of subjects without capillary CAA, relative to the ε3/3 reference group, both ε4 (OR=1.636, 95% CI =1.179–2.269, p=0.003) and ε2 carriers (OR=1.566, 95% CI =1.103–2.223, p=0.012) showed greater odds for more severe CAA. Similar associations were found in the smaller group of subjects with capillary involvement.
Table 4.
Association of APOE with CAA by capillary involvement
| Without capillaries OR (95% CI) |
With capillaries OR (95% CI) |
|
|---|---|---|
| Age at death | 1.028 (1.008, 1.049)* | 1.007 (0.951, 1.065) |
| Male Sex | 1.225 (0.936, 1.604) | 1.005 (0.523, 1.932) |
| Education | 0.995 (0.961, 1.031) | 0.967 (0.891, 1.049) |
| AD1 | 5.594 (3.881, 8.061)* | 1.494 (0.571, 3.910) |
| Macroscopic infarcts | 0.924 (0.703, 1.215) | 1.149 (0.611, 2.159) |
| Microinfarcts | 1.133 (0.851, 1.511) | 0.896 (0.476, 1.683) |
| Lewy Bodies | 0.843 (0.617, 1.153) | 0.947 (0.494, 1.815) |
| APOE ε2 | 1.566 (1.103, 2.223)* | 4.221 (1.487, 11.982)* |
| APOE ε4 | 1.636 (1.179, 2.269)* | 1.640 (0.863, 3.115) |
Composite measure of AD pathology;
p<0.05.
Both models were adjusted for demographics, AD pathology, as well as presence of infarcts and Lewy bodies;
APOE ε2 and amyloid angiopathy association is masked by AD
The logistic regression analysis suggests that the overall association of APOE ε2 with CAA may be masked by comorbid AD pathology. To examine this potential masking, we conducted a path analysis and dissected the total effect of APOE genotypes on CAA into direct and indirect effects through AD pathology. For the indirect pathway we placed AD upstream of CAA because recent data from animal models suggests that perivascular drainage of neuronal amyloid may be one mechanism for the development of CAA. The decision was also supported by our data that a predominant majority of subjects with CAA had pathologic AD (80%), while only less than half of those with pathologic AD had CAA (44%). The path coefficients and standard errors (SEs) for each pathway are illustrated in Figure 3. Similar to the logistic regression models, these path coefficients compare the log-odds of having more severe CAA between APOE genotypes.
In this analysis, the direct and indirect effects of both ε4 and ε2 genotypes were significant. We focused on APOE ε2. Consistent with the result from the logistic regression model, the direct link between ε2 and CAA had an OR of 1.733 (p=0.002), suggesting ε2 carriers had a higher risk for more severe CAA than ε3/3 carriers. Separately, we found that APOE ε2 was associated with less AD pathology, and AD pathology was associated with greater odds of more severe CAA. The indirect effect of ε2 on CAA through AD was in the opposite direction (OR =0.730, p<.0001). As the result, without disentangling the association with AD pathology, the total effect of ε2 on CAA was masked and not significant (p=0.196).
We repeated the path analysis by the presence of capillary CAA. The result for subjects without capillary CAA was similar and the masking was evident. The path coefficient that links ε2 to CAA was significant such that ε2 carriers had a higher risk for more severe CAA (OR=1.590, p=0.012), which was coupled with a negative association of ε2 with less AD pathology. We did not find the masking in subjects with capillary CAA, however the direct effect of ε2 on CAA remained significant (OR=4.380, p=0.007).
Finally, the percentages of moderate to severe CAA by APOE genotypes differed by the burden of AD pathology. In subjects with pathologic AD diagnosis, 34.5% of the ε3/3 carriers had moderate to severe CAA; this increased to 50.6% and 61.4% for the ε2 and ε 4 carriers, respectively. As contrast, in subjects without pathologic AD diagnosis, only 17.1% of the ε3/3 carrier had moderate to severe CAA, and the percentage increased to 24.1% and 29.6% for the ε2 and ε4 carriers, respectively. To test the hypothesis that the association of APOE genotypes with CAA may differ by AD pathology, we added ε2 by AD and ε4 by AD interactions to the ordinal logistic regression model. Indeed, both genotypes showed stronger associations with more severe CAA in the presence of more AD pathology. Adjusting for demographics, infarcts, and Lewy bodies, the odds ratio of having more severe CAA for the APOE ε2 by AD pathology interaction was 3.000 (95% CI 1.167–7.712, p=0.023), and the odds ratio for the ε4 by AD pathology interaction was 2.160 (95% CI 1.048–4.449, p=0.037).
Discussion
In this study, we show that both APOE ε2 and ε4 genotypes are associated with more severe CAA and the associations are independent of AD and other neuropathologies that are common in aging brain. We observe that APOE ε4, but not ε2, is associated with presence of capillary CAA. Accounting for capillary involvement did not alter the APOE associations with CAA.
The relation of APOE to CAA and CAA-related ICH has been previously reported. Earlier studies suggest that the ε2 and ε4 genotypes are related to ICH through different mechanisms, such that the ε4 allele promotes vascular amyloid accumulation while ε2 contributes to vessel rupture via other vasculopathic changes such as fibrinoid necrosis (Greenberg, et al., 1998, McCarron and Nicoll, 2000, McCarron, et al., 1999). Interestingly, there is new evidence that ε2 carriers have more severe CAA, suggesting a direct involvement of ε2 in accumulation of β-amyloid in cerebral vasculature. However, the association of APOE ε2 with CAA remains unclear, likely due to fewer cases with the ε2 genotype (Rannikmae, et al., 2014) or the data which reply on clinical cohorts. Using data from over 1,000 well characterized older persons, we confirm the direct association of ε2 with CAA.
The neuropathology of AD and CAA involve abnormal accumulation and clearance of β-amyloid; however, it is unclear whether the deposition of β-amyloid in the vessel wall has a neuronal origin. Three mechanistic hypotheses have been proposed with regards to cerebral amyloid deposition (Burgermeister, et al., 2000). First, the vessel wall theory hypothesizes that β-amyloid is produced and deposited locally by vascular smooth muscle cells. Second, the blood uptake theory suggests that circulating amyloid beta peptide binds to the receptor for advanced glycation end-products (RAGE) in endothelial cells and is transported through the blood-brain barrier into brain (Deane, et al., 2003). Third, the perivascular drainage theory suggests that amyloid originates from the central nervous system (CNS) and drains out of brain along perivascular spaces (Weller, et al., 1998, Weller, et al., 2008). Experiments using transgenic mice show that neuronal overexpression of mutant APP results in significant cerebral amyloid deposition (Calhoun, et al., 1999, Herzig, et al., 2004). In our data, 80% of the brains with moderate to severe CAA met pathologic AD criteria, and less than half of pathologic AD brain had moderate to severe CAA. Such asymmetric pattern is even more evident in the case of capillary CAA, where over 80% of the subjects with capillary CAA had pathologic diagnosis of AD, and only 21% of ADs had capillary CAA. These results are in support of the drainage pathway hypothesis.
The isoform dependent pattern of APOE in the clearance of brain amyloid makes it no surprise that the APOE ε4 allele was associated with more severe CAA (Alonzo, et al., 1998, Olichney, et al., 1996, Premkumar, et al., 1996). Yet the APOE ε2 results are interesting. Our data support that the ε2 allele was associated with less AD pathology; and after controlling for AD pathology, the allele was associated with more severe CAA. Separately, there is evidence that the association of APOE with CAA was stronger in persons with more AD pathology. These findings suggest that the role of APOE ε2 in the pathologic process of AD may be distinct from that of CAA. A biochemical study shows that the Aβ42: Aβ40 ratio differs between vascular and plaque amyloid such that amyloid in vessel walls is predominantly Aβ40 while Aβ42 is more abundant in cortical plaques (Van Dorpe, et al., 2000). A high Aβ40:Aβ42 ratio favors vascular amyloidosis and increase in the Aβ40:Aβ42 ratio may shift the amyloid deposition from parenchyma to vasculature (Herzig, et al., 2006). Immunotherapy studies also suggest that selective clearance of Aβ42 reduces the parenchymal amyloid deposition, but it induces vascular amyloid deposition (Herzig, et al., 2004). Further investigation on whether APOE ε2 contributes preferentially to Aβ42 clearance is warranted.
Multiple processes may be involved in amyloid deposition in CAA. One study on 41 postmortem brains with CAA shows that, depending on whether or not the disease involves cortical capillaries, there are two distinct morphological types of CAA; where cases with amyloid deposition in capillaries have higher ε4 allele frequency and those without capillaries deposition have higher ε2 allele frequency (Thal, et al., 2002). The study proposed that the amyloid deposition in capillaries shares a similar mechanism with amyloid plaques in cortices, for which the ε4 allele is a known risk factor. On the other hand, the ε2 allele promotes amyloid deposition that involves arterioles. We showed that APOE ε4 was also associated with the presence of capillary CAA but the association of APOE ε2 with capillary CAA didn’t reach statistical significance, which supports the previous study. On the other hand, we found that the ε2 and CAA association persisted regardless of capillary involvement.
The study has strengths and weaknesses. To our knowledge, this is by far the largest community based study that examines postmortem human brain for association of the APOE genotypes with CAA. Brains are collected from two longitudinal clinicopathologic cohort studies, which have very high follow-up and autopsy rates. Uniform clinical and pathologic evaluation protocols are applied in both studies by the same group of investigators, which makes it efficient to merge the data for combined analysis. One limitation is that lack of information on CAA related hemorrhages prevents us from further clarifying the relationship of APOE with CAA and the downstream effect on ICH. The quantification and classification of hemorrhages is currently not available in these cohorts. Separately, the study participants are older and have higher education comparing with the general population. Our findings await independent replications from other prospective studies.
Conclusions
We investigated the relationship of the APOE genotypes, CAA, and AD pathology utilizing genetic and neuropathologic data from over 1000 post-mortem human brains. We found that the APOE ε4 and ε2 alleles were both associated with more severe meningeal/parenchymal CAA, and that the association of ε2 with CAA was initially masked by comorbid AD pathology. APOE ε4, but not ε2, was associated with capillary CAA. Further, the associations of the APOE genotypes with CAA were significant with or without capillary involvement.
We examined the relationship of the APOE genotypes with cerebral amyloid angiopathy in the presence of variable amounts of Alzheimer’s pathology using data from over 1000 postmortem brains.
Both ε2 and ε4 alleles were associated with more severe cerebral amyloid angiopathy.
APOE ε4, but not ε2, was associated with the presence of capillary amyloid angiopathy.
The APOE associations with amyloid angiopathy persisted regardless of capillary involvement.
The relationship of ε2 with amyloid angiopathy was masked by ε2′s protective association with comorbid Alzheimer’s pathology.
Acknowledgments
This study is supported by the National Institutes of Aging grants: P30AG10161, R01AG17917, R01AG15819, R01AG043379, R01AG34374, R01NS084965, and the Illinois Department of Public Health. We are thankful for the participants of the Religious Orders Study and the Rush Memory and Aging Project. We also thank all the staff of the Rush Alzheimer’s Disease Center for their work.
Abbreviations
- CAA
cerebral amyloid angiopathy
- AD
Alzheimer’s disease
- ICH
intracerebral hemorrhage
- SD
standard deviation
- OR
odds ratio
- SE
standard error
- CI
confidence interval
- df
degrees of freedom
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM. Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. Journal of neuropathology and experimental neurology. 1998;57(4):353–9. doi: 10.1097/00005072-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Arold S, Sullivan P, Bilousova T, Teng E, Miller CA, Poon WW, Vinters HV, Cornwell LB, Saing T, Cole GM, Gylys KH. Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta neuropathologica. 2012;123(1):39–52. doi: 10.1007/s00401-011-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke; a journal of cerebral circulation. 2011a;42(3):722–7. doi: 10.1161/strokeaha.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Annals of neurology. 2011b;69(2):320–7. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Current atherosclerosis reports. 2012;14(4):343–50. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006a;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research. 2012a;9(6):628. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer research. 2012b;9(6):646. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. The Lancet Neurology. 2006b;5(5):406–12. doi: 10.1016/s1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, Jimenez-Conde J, Pires CR, Ayres AM, Schwab K, Cortellini L, Pera J, Urbanik A, Romero JM, Rost NS, Goldstein JN, Viswanathan A, Pichler A, Enzinger C, Rabionet R, Norrving B, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Broderick JP, Greenberg SM, Roquer J, Lindgren A, Slowik A, Schmidt R, Woo D, Rosand J. APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. The Lancet Neurology. 2011;10(8):702–9. doi: 10.1016/s1474-4422(11)70148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, Jimenez-Conde J, Hansen BM, Fernandez-Cadenas I, Cortellini L, Ayres A, Schwab K, Juchniewicz K, Urbanik A, Rost NS, Viswanathan A, Seifert-Held T, Stoegerer EM, Tomas M, Rabionet R, Estivill X, Brown DL, Silliman SL, Selim M, Worrall BB, Meschia JF, Montaner J, Lindgren A, Roquer J, Schmidt R, Greenberg SM, Slowik A, Broderick JP, Woo D, Rosand J. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Annals of neurology. 2010;68(6):934–43. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Kelly JF, Bennett DA. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology. 2010;34(1):43–9. doi: 10.1159/000256662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers HB, Biffi A, McNamara KA, Ayres AM, Valant V, Schwab K, Romero JM, Viswanathan A, Greenberg SM, Rosand J, Goldstein JN. Apolipoprotein E genotype is associated with CT angiography spot sign in lobar intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2012;43(8):2120–5. doi: 10.1161/strokeaha.112.659094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister P, Calhoun ME, Winkler DT, Jucker M. Mechanisms of cerebrovascular amyloid deposition. Lessons from mouse models. Annals of the New York Academy of Sciences. 2000;903:307–16. doi: 10.1111/j.1749-6632.2000.tb06381.x. [DOI] [PubMed] [Google Scholar]
- Cadavid D, Mena H, Koeller K, Frommelt RA. Cerebral beta amyloid angiopathy is a risk factor for cerebral ischemic infarction. A case control study in human brain biopsies. Journal of neuropathology and experimental neurology. 2000;59(9):768–73. doi: 10.1093/jnen/59.9.768. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):14088–93. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathology and applied neurobiology. 2008;34(2):131–44. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Science translational medicine. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9(7):907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, Kalaria RN. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78(14):1043–50. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan WJ, Falcone GJ, Anderson CD, Jagiella JM, Schmidt H, Hansen BM, Jimenez-Conde J, Giralt-Steinhauer E, Cuadrado-Godia E, Soriano C, Ayres AM, Schwab K, Kassis SB, Valant V, Pera J, Urbanik A, Viswanathan A, Rost NS, Goldstein JN, Freudenberger P, Stogerer EM, Norrving B, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Montaner J, Fernandez-Cadenas I, Delgado P, Greenberg SM, Roquer J, Lindgren A, Slowik A, Schmidt R, Woo D, Rosand J, Biffi A. Heritability estimates identify a substantial genetic contribution to risk and outcome of intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2013;44(6):1578–83. doi: 10.1161/strokeaha.111.000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Annals of neurology. 1995;38(2):254–9. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke; a journal of cerebral circulation. 1997;28(7):1418–22. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP, Segal AZ, Chiu RI, Clatworthy AE, Liao A, Hyman BT, Rebeck GW. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50(4):961–5. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral beta-amyloid angiopathy: murine and cellular models. Brain pathology (Zurich, Switzerland) 2006;16(1):40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nature neuroscience. 2004;7(9):954–60. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2892–7. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Chalmers K, Ince P, Esiri M, Attems J, Jellinger K, Yamada M, McCarron M, Minett T, Matthews F, Greenberg S, Mann D, Kehoe PG. Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. American journal of neurodegenerative disease. 2014;3(1):19–32. [PMC free article] [PubMed] [Google Scholar]
- Love S, Miners S, Palmer J, Chalmers K, Kehoe P. Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Frontiers in bioscience (Landmark edition) 2009;14:4778–92. doi: 10.2741/3567. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Annals of the New York Academy of Sciences. 2000;903:176–9. doi: 10.1111/j.1749-6632.2000.tb06366.x. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Nicoll JA, Stewart J, Ironside JW, Mann DM, Love S, Graham DI, Dewar D. The apolipoprotein E epsilon2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. Journal of neuropathology and experimental neurology. 1999;58(7):711–8. doi: 10.1097/00005072-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Pious NM, Jicha GA, Wilcock DM, Fardo DW, Estus S, Rebeck GW. APOE-epsilon2 and APOE-epsilon4 correlate with increased amyloid accumulation in cerebral vasculature. Journal of neuropathology and experimental neurology. 2013;72(7):708–15. doi: 10.1097/NEN.0b013e31829a25b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, Ferrari G, Coverlizza S, Merlini G. A novel AbetaPP mutation exclusively associated with cerebral amyloid angiopathy. Annals of neurology. 2005;58(4):639–44. doi: 10.1002/ana.20571. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology. 1996;47(1):190–6. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. The American journal of pathology. 1996;148(6):2083–95. [PMC free article] [PubMed] [Google Scholar]
- Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, Al-Shahi Salman R, Sudlow CL. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2014;85(3):300–5. doi: 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD, Saunders AM. APOE is a major susceptibility gene for Alzheimer’s disease. Current opinion in biotechnology. 1994;5(6):663–7. doi: 10.1016/0958-1669(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2012;83(3):275–81. doi: 10.1136/jnnp-2011-300371. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain : a journal of neurology. 2012;135(Pt 10):3005–14. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke; a journal of cerebral circulation. 2005;36(5):954–9. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT. APOEepsilon2 is associated with milder clinical and pathological Alzheimer’s disease. Annals of neurology. 2015 doi: 10.1002/ana.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. Journal of neuropathology and experimental neurology. 2002;61(3):282–93. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, Kramer P, Woltjer R, Trojanowski JQ, Weintraub D, Chen-Plotkin AS, Irwin DJ, Rick J, Schellenberg GD, Watson GS, Kukull W, Nelson PT, Jicha GA, Neltner JH, Galasko D, Masliah E, Quinn JF, Chung KA, Yearout D, Mata IF, Wan JY, Edwards KL, Montine TJ, Zabetian CP. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA neurology. 2013;70(2):223–8. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio C, Arima H, Harrap S, Anderson C, Godin O, Woodward M, Neal B, Bousser MG, Chalmers J, Cambien F, MacMahon S. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70(16):1322–8. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- Valant V, Keenan BT, Anderson CD, Shulman JM, Devan WJ, Ayres AM, Schwab K, Goldstein JN, Viswanathan A, Greenberg SM, Bennett DA, De Jager PL, Rosand J, Biffi A. TOMM40 in Cerebral Amyloid Angiopathy Related Intracerebral Hemorrhage: Comparative Genetic Analysis with Alzheimer’s Disease. Translational stroke research. 2012;3(Suppl 1):102–12. doi: 10.1007/s12975-012-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. The American journal of pathology. 2000;157(4):1283–98. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. The American journal of pathology. 1998;153(3):725–33. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain pathology (Zurich, Switzerland) 2008;18(2):253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA. Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiology of aging. 2014;35(4):819–26. doi: 10.1016/j.neurobiolaging.2013.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]