Abstract
Influenza is a vaccine-preventable contagious respiratory illness caused by influenza (flu) viruses which can lead to hospitalization and sometimes even death. Current flu vaccines delivered intramuscularly (IM) or intradermally (ID) are less effective at eliciting protective mucosal immune responses and vaccines delivered intranasally (IN) possess potential safety concerns. Sublingual (SL) vaccination is a promising alternative route for vaccine delivery which has been indicated as safe and effective at inducing protective immune responses in both systemic and mucosal compartments. We evaluated the efficacy of methylglycol chitosan (MGC) and a synthetic toll-like receptor 4 agonist (CRX-601), alone or in combination, for improving systemic and mucosal immune responses to a monovalent detergent-split flu virus vaccine delivered SL. SL vaccination of mice with split-flu vaccine formulated with either MGC or CRX-601 resulted in specific serum IgG and mucosal IgA titers that were significantly greater than titers from non-adjuvanted vaccination and equivalent to or greater than titers in mice vaccinated IM. Our results demonstrate that SL vaccination utilizing MGC or CRX-601 as adjuvants is a viable alternative route of vaccination for flu which can elicit systemic immune responses equivalent to or greater than IM vaccination with the added benefit of stimulating a robust specific mucosal immune response.
Keywords: sublingual, TLR-4, chitosan, influenza, mucosal vaccination, CRX-601
Introduction
Influenza is a common contagious respiratory tract infection responsible for notable morbidity and mortality during recurrent epidemics and occasional pandemic outbreaks. Annual vaccination is currently the most effective strategy for preventing or containing flu infections [1, 2]. Current licensed vaccines against flu viruses are principally live attenuated, whole inactivated, split virion or subunit vaccines. Available vaccines are administered either intramuscularly (IM), intradermally (ID) or intranasally (IN) and promote a humoral immune response against the viral surface glycoprotein hemagglutinin (HA) and neuraminidase (NA) [2, 3]. Vaccines administered parenterally are generally effective at stimulating systemic antibody mediated immune responses but are less effective at inducing mucosal immunity [4–6]. Since flu viruses enter the host via the respiratory tract mucosa, prospective improved vaccination strategies should not only elicit an effective systemic immune response but also neutralizing mucosal antibodies, particularly IgA, at the initial site of infection.
Mucosal vaccination has been explored as an alternative strategy to parenteral administration to more efficiently elicit mucosal and systemic immune responses [7–10]. Mucosal vaccination is generally associated with oral or IN routes. Vaccines delivered orally are potentially degraded by gut microflora or stomach pH during the passage through the gut and require specialized formulations to prevent degradation while antigens or adjuvants delivered IN can potentially be redirected to the central nervous system through the olfactory nerve epithelium, thereby causing adverse side effects and reducing vaccine safety [11–16]. Sublingual (SL) administration is a promising alternative mucosal vaccination method which bypasses the potential degradation in the gut or olfactory bulb redirection and has been demonstrated as safe and effective for both bacteria and virus vaccines, including influenza [17–27].
Although delivery through the oral mucosa avoids alteration by gastric fluids and enzymes present in the gastrointestinal tract, various factors exist which act as barriers and hinder absorption of certain vaccine components, especially larger molecules. These barriers include the permeability of the mucosa to the vaccine components, saliva, mucus, membrane coating granules, basement membrane etc., all of which can limit the absorption through the mucosa, depending on the physiochemical characteristics of the vaccine components [8]. In order to address the challenge of delivering vaccine components via the SL route and eliciting an adequate immune response, a number of enhancement strategies have been explored, including the use of adjuvants and/or improved vaccine formulations [8, 9]. SL vaccination with flu vaccine in particular has been shown to require high doses of antigen and/or use of an adjuvant to elicit a robust immune response [6].
CRX-601 is an aminoalkyl glucosaminide 4-phosphate (AGP), a new class of synthetic lipid A mimetics engineered to effectively trigger toll-like receptor 4 (TLR4), which has recently been shown to enhance the immune response against flu vaccination following IN administration [28]. In addition, chitosan and chitosan derivatives have shown promise as safe and effective adjuvants and delivery systems for enhancing immunogenicity of mucosally administered vaccines [29–41]. Chitosan is the generic term for a family of linear polysaccharides which exist as copolymers of β-(1–4)-linked glucosamine and N-acetylglucosamine and is produced from the exoskeletons of crustacean or the cell walls of fungi. Chitosan possess the favorable biological properties for formulation with mucosal vaccines such as biocompatibility, biodegradability, mucoadhesive properties and permeation-enhancing ability; however, chitosan has limited solubility at physiological pH. Therefore, various chitosan derivatives with improved solubility profiles more suited for inclusion in vaccine formulations have been explored [30, 42–44].
In this study, we evaluated chitosan derivatives formulated with a monovalent detergent-split flu vaccine to be delivered mucosally via the SL route. We also evaluated the compatibility of lead chitosan derivatives for co-formulation with the synthetic mucosal adjuvant CRX-601. The immunogenicity of SL flu vaccines containing either methylglycol chitosan (MGC) or CRX-601 was evaluated in a mouse model and compared to flu vaccine administered IM. In addition, to determine if combinations of MGC and CRX-601 confer any added immunological benefits, vaccines containing MGC co-formulated with CRX-601 (MGC-CRX-601) were tested for immunogenicity following SL administration in mice.
Materials and Methods
Chitosan derivatives and CRX-601
Synthesis, purification and analysis of the AGP CRX-601 have been described previously [28, 45]. Aqueous buffered dispersions of CRX-601 were prepared by suspending CRX-601 (0.05 –2 mg/mL) in 10 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid buffered saline (HEPES-saline, pH 7.0) in a borosilicate glass vial (2 – 5 mL batch) and sonication on a water bath sonicator (Elma-Lab line, Singen, Germany). The bath temperature was maintained below 45 °C, and the dispersion was sonicated (1 – 2 h) until a clear to slightly hazy dispersion with an average particle size (as measured by dynamic light scattering, DLS) of 60 – 100 nm was obtained. The aqueous dispersion was further sterile filtered using a 0.22 μm Millex-GV filter (Millipore, Bedford, MA). The concentration of CRX-601 in formulations was determined by ion-pair reverse phase high-performance liquid chromatography (RP-HPLC, Waters Alliance 2690/2695, Milford, MA) on a C8 column (Ace 3, 3μm, 50mm × 3.0mm; Mac-Mod Analytical, Chadds Ford, PA) and UV detection at 210 nm (Waters model 2487 or 996 PDA detector). Elution consisted of a linear gradient at 0.8 mL/min from 50% to 100% B over 10 min and 100% B for 5 min. Solvent A consisted of 8% ACN, 2% buffer and 90% water. Solvent B consisted of 2% buffer in ACN. Buffer was prepared from 62.5 mL of 0.4 M tetrabutylammonium hydroxide in water with pH adjustment to 6.0 with 15 M phosphoric acid and a final volume of 100 mL. Samples were diluted in tetrahydrofuran (THF) (1:1 v/v) and analyzed against a set of CRX-601 standards (0.25, 0.5, 1, 1.5, and 2 mg/mL in THF) with system suitability injections at the start and the end of the run. The chitosan derivatives methylglycol chitosan (MGC), chitosan oligosaccharide lactate (CO) and glycol chitosan (GC, Sigma-Aldrich, St Louis, MO), were prepared in either 10 mM HEPES (pH 7.0) or 10 mM HEPES-saline (0.9% saline, pH 7.0) and sterile filtered using a 0.22 μm filter. For preparation of chitosan-CRX-601 complexes, the solution of a chitosan derivative was admixed with aqueous CRX-601 dispersion and vortexed for 30 sec. Zeta-potential (ZP), particle size, and polydispersity indices (PDI) of formulations were determined by DLS using a Malvern Zetasizer Nano ZS (Malvern Instruments, Westborough, MA). Samples (8 μL) were diluted with 800 μL ultrapure water before measurement.
Vaccination of mice
Stock solutions of MGC were prepared at either 1.0 or 5.0 mg/mL in 10 mM HEPES-saline (0.9% saline, pH 7.0). CRX-601 was prepared in HEPES-saline at 1 mg/mL, with or without MGC at either 1.0 mg/mL or 5.0 mg/mL. For the mouse study involving MGC formulated with a suboptimal dose of CRX-601, complexes were prepared at a higher concentration of MGC (12.5 mg/mL) and lower concentrations of CRX-601 (0.005 and 0.05 mg/mL). These preparations did not exhibit any precipitation and were used as such without further characterization. For mouse dosing, MGC and/or CRX-601 were diluted in HEPES-saline and admixed with H3N2 monovalent detergent-split flu (A/Victoria/210/2009) and mixed by vortexing immediately prior to each study to obtain final vaccine formulations which were visually inspected for flocculation or precipitation (none observed for any formulations used). Final formulations were administered to mice within two hours of antigen addition. Female BALB/c mice (6 to 8 weeks of age) were obtained from Charles River Laboratories, Wilmington, MA. For vaccinations, mice anesthetized by intraperitoneal administration of ketamine (80–150 mg/kg) and xylazine (8–15 mg/kg) were given vaccine by either SL administration (6 μL/mouse deposited under the tongue toward the floor of the mouth) or IM administration (50 μL/mouse in the quadricep muscle) at 21 day intervals. Mice receiving vaccine by SL administration were vaccinated at total of either two or three times on days 0, 21 and 42, respectively. Mice receiving vaccine by IM administration were vaccinated two times on either days 21 and 42 or days 0 and 21. For each vaccination, mice (except for the naïve group) received the indicated dose of flu antigen (0.3 or 3.0 μg) alone or in combination with the indicated dose of either MGC, CRX-601, or CRX-601 co-formulated with MGC. All animals were treated in accordance with guidelines established by the U.S. Department of Health and Human Services Office of Laboratory Animal Welfare and the Institutional Animal Care and Use Committee at GSK Vaccines, Hamilton, Montana.
Serum and mucosal wash collections
On day 35, (14 days post-secondary [14dp2°] SL vaccination and either 14dp2° or 14dp1° IM vaccination, blood samples were obtained from the superficial temporal vein of mice and sera collected using Microtainer Serum Separator Tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and stored at −70 °C prior to analysis for antigen specific antibody responses. In addition, 14 days after the final vaccination, terminal serum, vaginal wash and tracheal wash samples were obtained upon completion of each study (day 35 for studies with only two SL vaccinations or day 56 for studies with three SL vaccinations) as previously described [28] and stored at −70 °C prior to analysis.
Specific antibody responses
Anti-flu IgG, IgG1 and IgG2a titers in mouse serum samples and IgA levels in both mucosal vaginal and tracheal wash samples were analyzed by ELISA as previously described [28], with the following modifications. 96-well nunc MaxiSorp plates (Thermo Fisher Scientific, Rochester, NY) were coated overnight with 2.0 μg/mL of monovalent detergent-split flu antigen in Dulbecco’s phosphate buffered saline (DPBS, Sigma, St. Louis, MO). For standard curves, wells were coated with goat anti-mouse IgG or IgA (1 μg/mL or 4 μg/mL, respectively). Plates were washed and blocked with Super Blok (ScyTek, Logan, UT) for 1 h at 37 °C. Serum, mucosal wash samples were added in diluting buffer (DPBS, 1% bovine serum albumin, 0.1%Tween 20, 5% heat inactivated fetal bovine serum; 1/100 sample dilution) and titrated in two-fold serial dilutions down the assay plate. For standard curves, murine IgG, IgG1, IgG2a (Sigma), or IgA (Thermo Fisher Scientific) were added in dilution buffer (at 200 ng/mL, 200 ng/mL, 125 ng/mL or 125 ng/mL, respectively) and similarly titrated in two-fold serial dilutions down the assay plate. Following 1 h of incubation at 37 °C, bound antibody was detected with peroxidase goat anti-mouse IgG, IgG1, IgG2a or IgA (Southern Biotech, Birmingham, AL, 1 h at 37 °C) and followed by 3,3′,5,5′-tetramethylbenzidine substrate and the reaction was stopped with sulfuric acid. The optical density was read at 450 nm and antibody titers were extrapolated from the corresponding standard curve. IgG1 titers were divided by IgG2a titers for each mouse to obtain IgG1 to IgG2a ratios.
Influenza hemagglutination inhibition assay
Quantitative analysis of the functional antibody response in mice vaccinated with flu vaccines was performed using a standard Hemagglutination Inhibition Assay (HAI). Briefly, freshly collected chicken red blood cells (RBCs) were washed once with Alsever’s solution (Sigma) and two times with DPBS and concentration was adjusted by colorimetry. Serum samples from vaccinated mice were treated with a 0.4% solution of receptor destroying enzyme (RDE, Sigma), to eliminate non-specific inhibitors of hemagglutination (18 h at 37 °C), prior to addition of sodium citrate solution (Sigma) at 0.75% final concentration for 30 min at 56 °C. RDE-treated serum samples were incubated with a 2.5% solution of washed-RBCs for 60 min at 4 °C, to eliminate non-specific hemagglutination by endogenous serum constituents (supernatants were collected and background hemagglutination was evaluated by incubation with 0.5% RBC suspension for 45 min at room temperature [RT]). Flu virus (H3N2, A/Victoria/210/2009) was back-titrated to ensure 8 HA units/50 μL immediately prior to and after performing the HAI assay. RDE/RBC-treated serum samples (1/20 dilution) were added in duplicate to wells of a 96-well microtiter plate and serially diluted by two-fold dilutions down the plate. Diluted flu virus (8 HA units/50 μL) was added to serum samples and incubated for 45 min at RT. 0.5% RBC suspension was added to each well and incubated for 45 min at RT. Plates were immediately evaluated for hemagglutination (tear-drop pattern). The functional antibody (HAI) titer represents the reciprocal of the last sample dilution that does not produce a complete or partial agglutination of RBCs.
Statistics
Data were analyzed by one-way ANOVA with Dunnett post-test using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego, CA) or by Student t-test (two-tails, equal variance) with Microsoft® Excel 2007 (Microsoft Corporation, Redmond, WA).
Results
Evaluation of deacetylated chitosans and chitosan derivatives for formulation with flu antigen
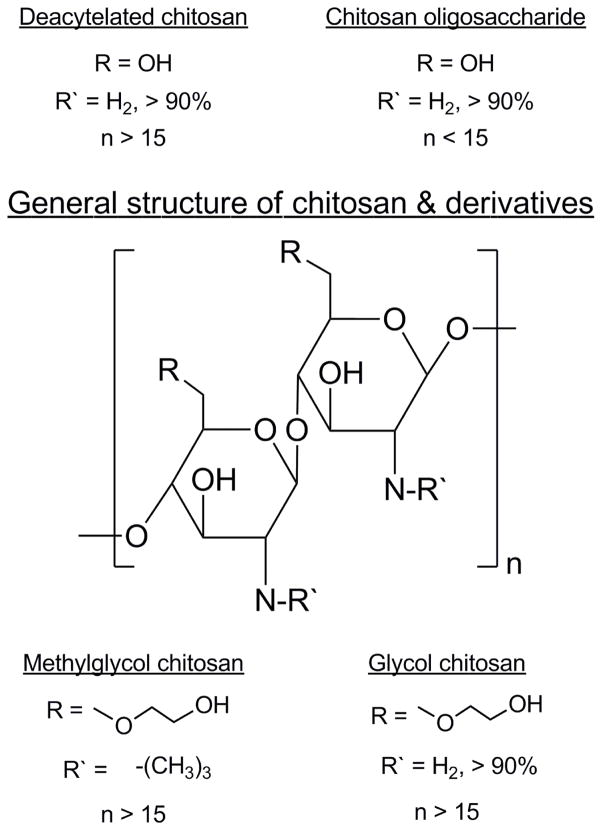
Deacetylated chitosans and other chitosan derivatives identified as promising candidates for inclusion in a vaccine for SL administration were evaluated for formulation with flu antigen. Deacetylated chitosans with average molecular weights of 120 kDa, 280 kDa and 342 kDa were sparingly soluble (solubility < 1 mg/mL in 10 mM HEPES-saline, pH 7.0) and a solution with particle sizes below 200 nm could not be achieved despite sonication in a water bath for 4 – 6 h. In contrast, the chitosan derivatives GC, MGC and CO (Figure 1) had superior solubility profiles (soluble at >10 mg/mL in HEPES-saline, pH 7.0). Due to their poor solubility, the deacetylated chitosans were removed from consideration and the chitosan derivatives were selected for additional compatibility testing in formulation with the adjuvant CRX-601.
Figure 1. Chemical structures of deacetylated chitosan and chitosan derivatives evaluated in this study.
The chitosans and derivatives used had > 90% deacetylation as indicated by the manufacturer. Chitosan oligosaccharide lactate is a low molecular weight oligosaccharide available as the lactic acid salt. Glycol chitosan and methylglycol chitosan have the glycol modification with MGC additionally being N-trimethylated.
Identification of MGC as the lead chitosan-derivative for formulation with CRX-601
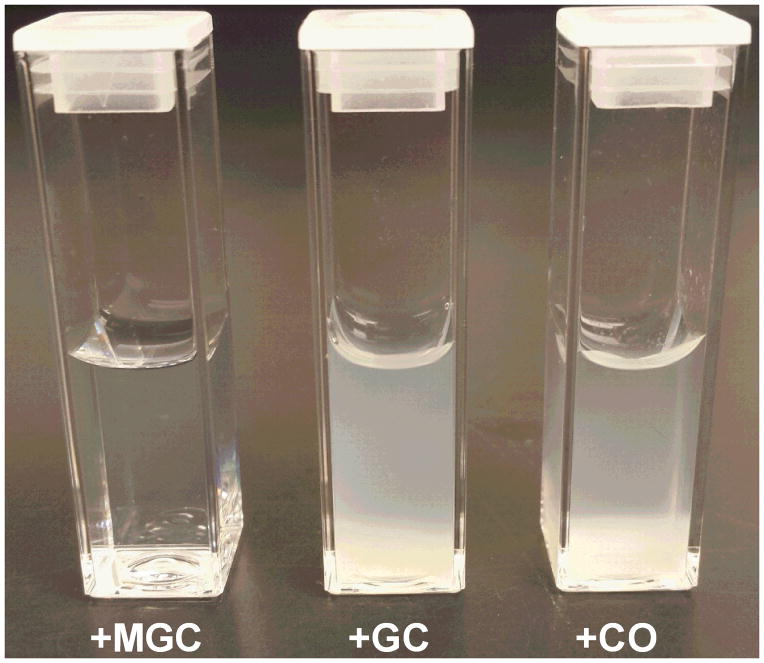
CRX-601 vaccine formulations have been previously described for delivery via the intranasal route of inoculation [28]. CRX-601 has also been found to be suitable for formulation in either 10 mM HEPES or HEPES-saline (data not shown). To evaluate whether lead chitosan derivatives are compatible for formulation with CRX-601, CRX-601 at 1 mg/mL was formulated with each compound of interest and the zeta-potential (ZP), particle size and distribution and polydispersity indices (PDI) were evaluated. Due to its amphipathic nature, CRX-601 forms anionic nanoparticles in the size range of 60 – 120 nm in solution (Table 1). Chitosan derivatives are polycations and plausibly form polyelectrolyte complexes with CRX-601 particles. GC and CO in formulation with CRX-601 resulted in significant aggregation and precipitation at all concentrations tested (0.2 – 5 mg/mL GC or CO) in 10 mM HEPES (Figure 2). Conversely, formulations of CRX-601 with MGC at MGC:CRX-601 ratios below 0.5 (corresponding to MGC concentrations of 0.1–0.4 mg/mL) and above 0.6 (corresponding to MGC concentrations ≥0.7 mg/mL) were found to be colloidally stable (Table 1). A reversal in ZP was observed at MGC:CRX-601 ratios exceeding 0.6, which is consistent with surface coating of CRX-601 particles with MGC. An increase in particle size from ~80 nm without MGC to >200 nm at MGC:CRX-601 ratios exceeding 0.7 indicates formation of multi-particle complexes with MGC. These formulations, however had a multimodal size distribution and PDI of 0.6 – 0.8. The MGC:CRX-601 combinations in the range of 0.5 – 0.7 resulted in formulations with near neutral ZP and formation of visible precipitates. This data indicates that CRX-601 particles in solution are charge stabilized, neutralization of which drives the particles to aggregate. Reversal of charge exceeding +10 mV again provides the necessary charge-charge repulsion for colloidal stabilization. Therefore, because of the lack of precipitation with CRX-601 at specified concentrations, MGC was identified as the lead chitosan derivative for continued formulation optimization.
Table 1.
Compatibility of MGC co-formulated with CRX-601 in 10 mM HEPES
| MGC concentration (mg/ml)a | Parameters for MGC-CRX-601 complexes
|
||
|---|---|---|---|
| Diameter (nm)b | PDIc | ζ-potential (mV) | |
| 0 | 88 ± 27 | 0.31 ± 0.04 | −26 ± 7 |
| 0.1 | 141 ± 28 | 0.35 ± 0.07 | −28 ± 2 |
| 0.2 | 175 ± 38 | 0.29 ± 0.07 | −36 ± 10 |
| 0.3 | 231 ± 66 | 0.43 ± 0.17 | −38 ± 5 |
| 0.4 | 221 ± 58 | 0.33 ± 0.08 | −39 ± 10 |
| 0.5 | Precipitatesd | −8 ± 26 | |
| 0.6 | Precipitatesd | 9 ± 11 | |
| 0.7 | 503 ± 241 | 0.79 ± 0.19 | 32 ± 5 |
| 0.8 | 331 ± 99 | 0.66 ± 0.13 | 23 ± 6 |
| 0.9 | 245 ± 101 | 0.60 ± 0.12 | 25 ± 12 |
| 1 | 228 ± 74 | 0.60 ± 0.10 | 33 ± 14 |
CRX-601 concentration is 1 mg/mL in all cases
Is the mean ± standard deviation (n = 3) of z-average particle sizes obtained by dynamic light scattering (DLS)
PDI: Polydispersity index, mean ± standard deviation (n = 3)
precipitates were visible to the naked eye
Figure 2. Colloidal stability of CRX-601 with chitosan derivatives.
Photograph of aqueous solution of CRX-601 (1 mg/mL) incubated with the indicated chitosan derivative (5 mg/mL) in 10 mM HEPES for 24h. The picture shows MGC-CRX-601 complexes are colloidally stable whereas visible precipitation is observed with GC and CO. MGC: methylglycol chitosan; GC: glycol chitosan; CO: chitosan oligosaccharide lactate.
Optimization of vaccine formulations containing MGC and CRX-601
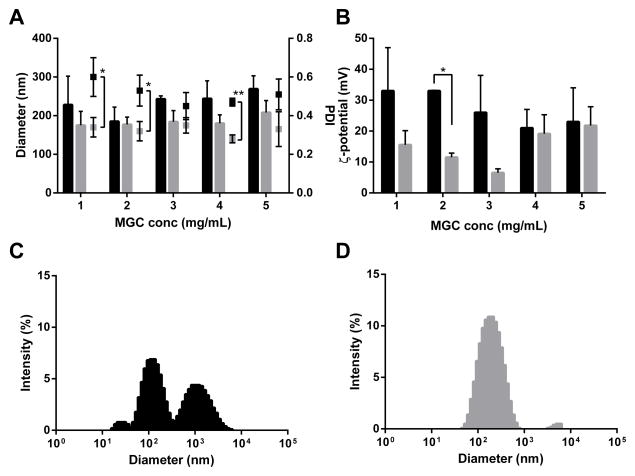
Ionic strength is known to influence the aggregation behavior of polyelectrolyte complexes [46]. With the intention of improving formulation polydispersity, size distribution and colloidal stability at MGC:CRX-601 ratios ≥1 (targeted in vivo formulations sufficiently coated with MGC), MGC-CRX-601 complexes prepared in 10 mM HEPES were compared to those prepared in HEPES-saline. While MGC-CRX-601 formulations in either buffer yielded comparable particle sizes (Figure 3A), formulations in HEPES-saline, in general were less polydisperse. For example, at a MGC:CRX-601 ratio of 2 (2 mg/mL MGC), the distribution was largely unimodal (Figure 3D) with a PDI of ~0.3 (Figure 3A) in 10 mM HEPES-Saline versus being multimodal (Figure 3C) with a PDI >0.5 (Figure 3A) for the preparation in 10 mM HEPES. Interestingly, for complexes prepared in HEPES-saline the neutralization and subsequent reversal of zeta-potential occurred at a lower MGC:CRX-601 ratio of 0.2 (data not shown). The zeta potential values were in general lower for complexes prepared in HEPES-saline (Figure 3B). The difference in zeta potential was found to be significant (p < 0.05) at MGC concentration of 2 mg/mL for complexes prepared in HEPES-saline versus those prepared in HEPES. Furthermore, the zeta potential distribution (data not shown) was more uniform (single peak, low zeta deviation) for complexes in HEPES-saline compared to those in HEPES alone. Direct correlations between zeta potential, PDI and size distribution were not obvious and further work is necessary to fully understand the solution dynamics of these formulations. Overall, due to the improved formulation characteristics (largely unimodal size distribution, lower PDI and uniform zeta potential distribution) at the target vaccine concentration range, HEPES-saline was identified as the optimal vehicle for development of a SL flu vaccine containing MGC-CRX-601 and all vaccine formulations were prepared using sterile starting materials using aseptic technique to eliminate the need for sterile filtration.
Figure 3. MGC-CRX-601 formulations prepared in 10 mM HEPES or HEPES-Saline.
Particle size (diameter)/polydispersity (PDI) (A) and zeta-potential (ζ-potential) (B) values as a function of increasing MGC concentration for formulations prepared in 10 mM HEPES (■) vs. 10 mM HEPES-Saline (■) indicating lower polydispersity for formulations prepared in HEPES-Saline. All formulations contained CRX-601 at 1 mg/mL. Particle size is graphically represented by bars while PDI is shown as dot plot. Representative size distribution profiles obtained by dynamic light scattering (DLS) for MGC-CRX-601 complexes at 2 mg/mL MGC prepared in 10 mM HEPES (C) indicating multimodal size distribution vs. those obtained in 10 mM HEPES-Saline (D) indicating a largely unimodal distribution. The data in (A) and (B) represents mean ± standard deviation of three independent experiments. Data were analyzed by t-test (two-tails, equal variance). *, p <0.05, **, p <0.005
SL vaccination with split-flu antigen and MGC or CRX-601 increases anti-flu IgG titers
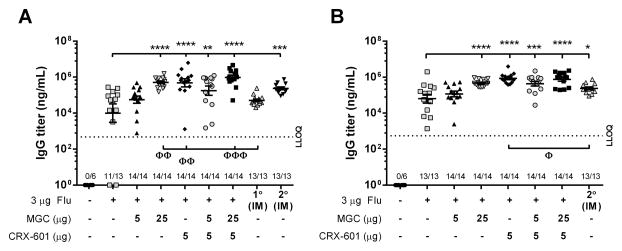
The effectiveness of lead SL flu antigen vaccine formulations comprised of MGC, CRX-601 or MGC-CRX-601 were evaluated in a murine model. Mice were vaccinated SL with either flu antigen alone or flu antigen admixed with MGC, CRX-601 or MGC-CRX-601. As a benchmark control, one group of mice was vaccinated IM on days 21 and 42 with an equivalent dose of flu antigen alone. Preliminary studies evaluating the immune response of mice vaccinated SL with flu antigen (3 μg) admixed with various doses of MGC (1 to 50 μg) or CRX-601 (0.01 to 10 μg) indicated that maximal serum anti-flu antibodies were achieved with an MGC dose between 5 to 25 μg and a CRX-601 dose of 1 to 5 μg, with no added benefit observed with higher doses (data not shown). In a head to head comparison study, serum anti-flu IgG titers in mice vaccinated SL with flu alone were detectable but variable 14 days post-secondary (14dp2°) vaccination (with a mean titer ± standard error of the mean [SEM] of 8.1 × 104 ± 2.5 × 104 pg/mL). Titers from mice vaccinated twice SL with flu antigen alone were comparable to the IM benchmark samples at 14dp1° vaccination (6.4 × 104 ± 1.6 × 104 pg/mL, p>0.05) but were significantly less than the IM benchmark 14dp2° vaccination (2.9 × 105 ± 5.6 × 104 pg/mL, p<0.0005) (Figure 4A). Mice receiving flu antigen admixed with either MGC (25 μg), CRX-601 (5 μg) or MGC (5 or 25 μg) co-formulated with 5 μg CRX-601 had serum IgG titers significantly (p<0.005) greater than mice vaccinated SL with flu antigen alone 14dp2° SL vaccination. Mice receiving flu antigen formulated with 25 μg MGC, 5 μg CRX-601 or 25 μg MGC co-formulated with 5 μg CRX-601 also had serum IgG titers 14dp2° vaccination that were significantly (p<0.05) greater than the IM control 14dp1° vaccination, and equivalent to or greater than the IM group 14dp2° vaccination (Figure 4A).
Figure 4. MGC and CRX-601 enhance anti-flu IgG titers in mice following SL vaccination.
Mice were vaccinated SL with the indicated dose of either flu antigen alone or flu antigen in combination with MGC, CRX-601 or MGC-CRX-601. Serum anti-flu IgG titers (A) 14 days post-secondary SL vaccination (day 35) and (B) 14 days post-tertiary SL vaccination (day 56). Titers obtained from mice vaccinated IM one time (1° IM, collected on day 35) or two times (2° IM, collected on day 56) are included for comparison. The horizontal dashed line represents the lower limit of quantification (LLOQ) for the assay (473.4 ng/mL and 553.1 ng/mL, respectively). Values equal to or less than LLOQ at 1:100 sample dilutions are represented as a value of 1. The number of mice per group that had titers greater than LLOQ, out of the total mice per group, is shown on the X-axis. The mean and standard error are indicated. Data were analyzed by one-way ANOVA with Dunnett post-test. *, p <0.05, **, p <0.005, ***, p <0.0005, ****, p <0.0001 compared to the SL flu antigen only group. Φ, p <0.01, ΦΦ, p <0.05, ΦΦΦ, p <0.005 compared to the indicated IM group.
Mice receiving a third SL vaccination on average had higher serum IgG titers 14dp3° vaccination (Figure 4B), compared to 14dp2° vaccination (Figure 4A). Serum IgG titers from mice vaccinated SL with either 25 μg MGC or 5 μg CRX-601 with either 5 or 25 μg MGC were significantly (p<0.0005) higher than the corresponding SL flu antigen alone group 14dp3° vaccination and were comparable to or greater than titers of the IM benchmark 14dp2° vaccination (Figure 4B). In addition, mice vaccinated SL with flu antigen plus 5 μg CRX-601 had serum IgG titers 14dp3° vaccination significantly (p<0.01) higher than the IM benchmark at 14dp2° vaccination (Figure 4B). There were no significant differences in IgG titers 14dp3° vaccination between mice receiving 25 μg MGC, 5 μg CRX-601 or 5 μg CRX-601 co-formulated with 5 and 25 μg MGC (Figure 4B). On average, mice vaccinated SL with flu antigen alone had a higher ratio of IgG1 to IgG2a antibodies compared to mice vaccinated IM with flu antigen alone (Supplementary Figure 1). Addition of MGC or CRX-601 to the flu antigen increased IgG2a titers in mice vaccinated three times SL without notably increasing IgG1 titers, resulting in decreased IgG1 to IgG2a ratios (Supplementary Figure 1). Together, our data indicate that MGC and CRX-601 enhance anti-flu IgG titers in mice vaccinated SL and stimulate higher IgG2a titers, resulting in a more balanced IgG1 to IgG2a ratio that is more comparable to the immune response seen following IM vaccination than SL vaccination with flu antigen alone.
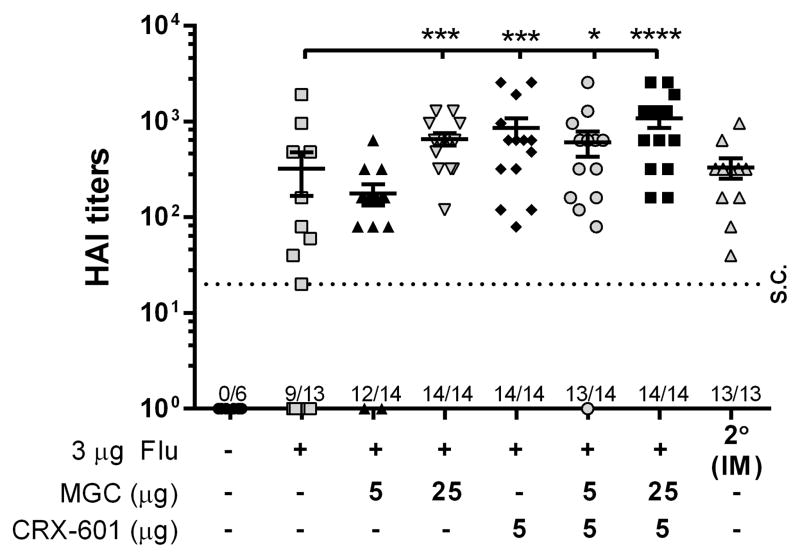
Functional antibody titers are greater following SL vaccination with formulations containing CRX-601 or MGC
Serum samples from mice vaccinated either SL (three times) or IM (two times) with flu antigen alone or in combination with MGC, CRX-601 or MGC-CRX-601 were evaluated for anti-HA antibody titers (functional antibody) by hemagglutination inhibition assay (HAI). In agreement with serum IgG titers, HAI titers from mice receiving flu antigen alone SL were detectable but variable, with 9 out of the 13 mice seroconverting (Figure 5). Vaccination SL with flu antigen and 25 μg MGC or 5 μg CRX-601 reduced the variability between vaccinated mice, compared to flu antigen alone, and significantly increased HAI titers to levels equivalent to the IM vaccinated group (Figure 5). Vaccination of mice SL with 5 μg CRX-601 co-formulated with either 5 or 25 μg MGC resulted in HAI titers comparable to vaccination with either MGC or CRX-601 alone (Figure 5). This data suggest that IgG titers correlate with HAI titers and further indicate that MGC and CRX-601 enhance the immune response to flu antigen following SL vaccination of mice.
Figure 5. Functional antibody titers are greater following SL vaccination with formulations containing CRX-601 or MGC.
Mice were vaccinated SL with the indicated dose of either flu antigen alone or flu antigen in combination with MGC, CRX-601 or MGC-CRX-601. IM positive control mice were vaccinated two times and are included for comparison. Serum collected on day 56 was analyzed by HAI assay for functional antibody titers. The horizontal dashed line represents the titer necessary for seroconversion (S.C.). Serum samples that did not demonstrate hemagglutination at the lowest dilution (1/20) are represented as a value of 1. The number of mice per group that seroconverted (samples were not diluted), out of the total mice per group, is shown on the X-axis. The mean and standard error are indicated. Data were analyzed by one-way ANOVA with Dunnett post-test. *, p <0.05, **, p <0.005, ***, p <0.0005, ****, p <0.0001 compared to the flu antigen only group.
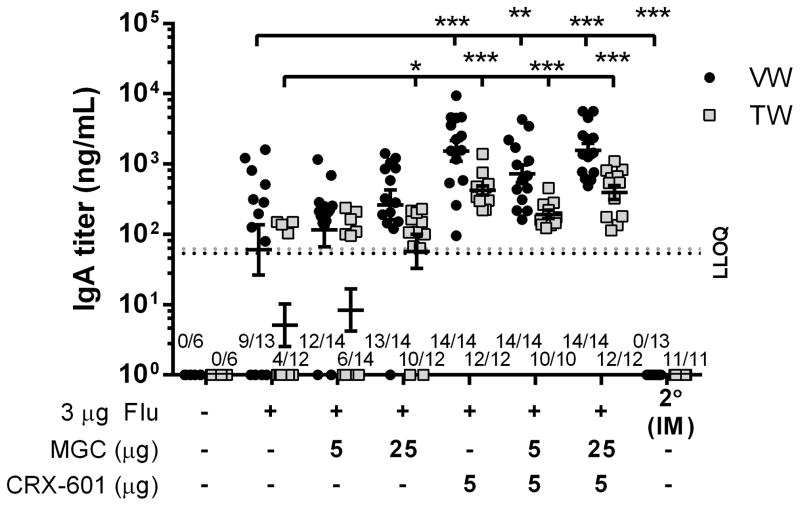
SL vaccination elicits a local and distal mucosal IgA response which is enhanced by the addition of CRX-601 or MGC
Anti-flu IgA titers in mucosal vaginal (VW) and tracheal (TW) wash samples were also determined following SL vaccination of mice three times with flu antigen alone or in combination with MGC, CRX-601 or MGC-CRX-601. Mice vaccinated SL with any of the flu antigen formulations had detectable IgA titers in both VW and TW samples (Figure 6). In comparison, none of the mice vaccinated IM had detectable IgA titers in either TW or VW samples (Figure 6). Mice vaccinated SL with flu antigen formulated with 25 μg MGC had more consistent and higher average IgA titers than mice receiving flu antigen alone, with significantly higher titers observed in TW samples (Figure 6). All of the mice vaccinated SL with formulations containing 5 μg CRX-601 had detectable anti-flu IgA titers which, in both VW and TW samples, were on average higher than those of any other formulations tested, with significantly higher titers in both VW and TW samples compared to samples from mice vaccinated with flu antigen alone (Figure 6). There were no notable differences in IgA titers between groups receiving 5 μg CRX-601 alone or 5 μg CRX-601 co-formulated with 5 or 25 μg MGC (Figure 6). Our data confirm that SL vaccination with the flu antigen elicits a notable mucosal IgA response that was lacking in mice vaccinated IM and demonstrates that formulations of MGC and CRX-601 can enhance mucosal IgA titers following SL vaccination in mice.
Figure 6. IgA titers following SL vaccination are enhanced by the addition of CRX-601 or MGC.
Mice were vaccinated SL with the indicated dose of either flu antigen alone or flu antigen in combination with MGC, CRX-601 or MGC-CRX-601. Anti-flu IgA titers in vaginal wash samples (VW, black circles) and tracheal wash samples (TW, grey squares) collected on day 56 (14dp3 for SL and 14dp2 for IM) are shown. The horizontal dashed line represents the LLOQ for the VW (black line, ~54.02 ng/mL) and TW (grey line, ~62.18 ng/mL) ELISA assays. Values equal to or less than the LLOQ at 1:40 sample dilution are represented as a value of 1. The number of mice per group that had titers greater than LLOQ, out of the total mice per group, is shown on the X-axis (some TW samples were not assayed due to contamination with blood). The mean and standard error are indicated. Data were analyzed by one-way ANOVA with Dunnett post-test. *, p <0.005, **, p≤0.001, ***, p <0.0001 compared to the flu antigen only group.
Improved immune response following SL vaccination of mice with MGC formulated with a suboptimal dose of CRX-601 and flu antigen
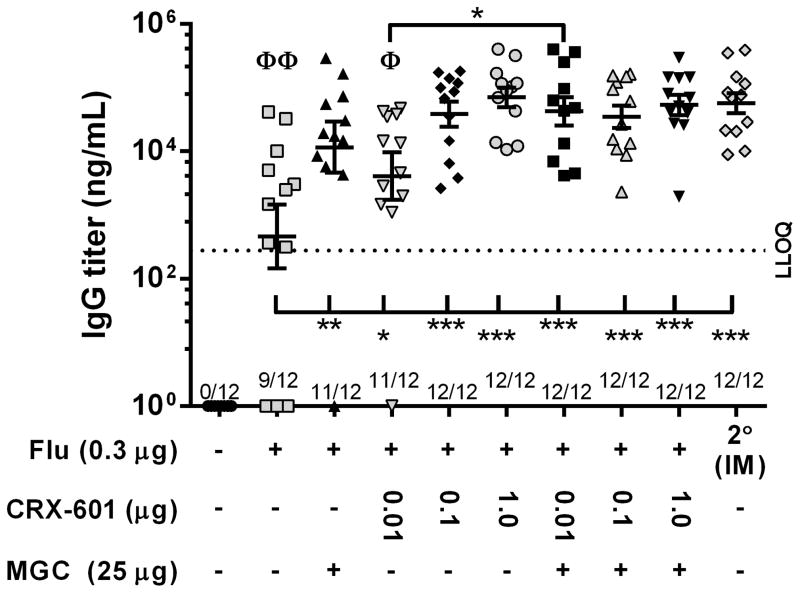
To further evaluate whether there is a benefit for co-formulation of MGC with CRX-601 in SL flu vaccine formulations, mice were vaccinated SL two times with a suboptimal dose of flu antigen (0.3 μg) alone or admixed with CRX-601 (0.01, 0.1 or 1.0 μg), either with or without MGC (25 μg). As a benchmark control, one group of mice was vaccinated IM two times with an equivalent dose of flu antigen alone. Mice receiving 0.3 μg of flu antigen alone SL had an average serum IgG titer of 8.1 × 103 ± 4.0 × 103 ng/mL, which was significantly (p<0.05) lower than average IgG titers in groups of mice receiving vaccines containing either MGC or CRX-601, with IgG titers increasing with increased CRX-601 dose (Figure 7). Mice vaccinated with MGC co-formulated with 0.01 μg CRX-601 had significantly (p<0.05) higher IgG titers than mice vaccinated with 0.01 μg CRX-601 alone (1.7 × 104 ± 5.3 × 103 ng/mL compared to 1.2 × 105 ± 4.4 × 104 ng/mL, respectively); however, although average IgG titers from any group of mice vaccinated with MGC-CRX-601 (0.01, 0.1, or 1.0 μg)were generally higher than IgG titers from mice vaccinated with MGC alone, there were no significant differences (Figure 7). In addition, all groups vaccinated SL with MGC, CRX-601 or MGC-CRX-601 had anti-flu IgG titers equivalent to the IM benchmark, except for 0.01 μg CRX-601 alone (Figure 7). This data further demonstrates that vaccination of mice SL with either MGC or CRX-601 increases serum IgG titers to levels comparable to IgG titers in mice vaccinated IM and that an added benefit from co-formulation of MGC with CRX-601 could be achieved by further optimization of adjuvant doses in combination with antigen dose-sparing.
Figure 7. Improved IgG titers when MGC is co-formulated with a suboptimal concentration of CRX-601 and flu antigen.
Mice were vaccinated SL with the indicated dose of either flu antigen alone or flu antigen in combination with MGC, CRX-601 or MGC-CRX-601. Serum anti-flu IgG titers 14 days post-secondary SL and IM vaccination (day 35) are shown. The horizontal dashed line represents the LLOQ for the ELISA assays (~278.7 ng/mL). Values equal to or less than the LLOQ at 1:100 sample dilutions are represented as a value of 1. The number of mice per group that had titers greater than LLOQ, out of the total mice per group, is shown on the X-axis. The mean and standard error are indicated. Data were analyzed by one-way ANOVA with Dunnett post-test. *, p <0.05, **, p≤0.005, ***, p <0.0001 compared to the indicated group. Φ, p <0.05, ΦΦ, p <0.0001 compared to the IM group. Except where indicated, there were no significant differences between the 2° IM group and groups vaccinated with either MGC or CRX-601.
Discussion
In this study, we evaluated immunogenicity of SL vaccine formulations comprised of monovalent detergent-split flu antigen alone or admixed with the chitosan derivative MGC, CRX-601 (a demonstrated mucosal adjuvant [28]), or MGC-CRX-601. Our data show that SL administration of a split-flu antigen can elicit a mucosal immune response and further demonstrate that inclusion of MGC and/or CRX-601 in SL vaccine formulations can enhance serum antibody and HAI titers to levels comparable to or greater than those achieved with un-adjuvanted IM vaccination.
Adjuvants are increasingly used to enhance and broaden immune responses induced by inactivated or subunit vaccines. In general, adjuvants can modulate the immune response, improve the immunogenicity of the vaccine, and allow decreased antigen doses [3]. Chitosan and chitosan derivatives have previously been shown to act as adjuvants in vaccines and possess desirable mucoadhesive and permeation-enhancing properties [29–41]. Chitosan is soluble in a variety of acids, but is sparingly soluble at neutral and alkaline pH values. This is because the primary amine of chitosan has a pKa value of about 6.5 [47]. Therefore, we focused our attention on chitosan derivatives with increased solubility, especially at neutral pH values. GC, MGC and CO, due to quaternization of primary amines and reduced molecular weight, were identified to be highly soluble at neutral pH and compatible for formulation with the split-flu antigen in a liquid SL vaccine.
Aside from evaluating chitosan derivatives alone, we also sought to compare chitosan derivatives in formulation with the previously identified mucosal adjuvant, CRX-601 [28]. In particular, we wanted to determine if there was an added benefit in co-formulating MGC with CRX-601. Therefore, lead chitosan derivatives were further evaluated for formulation compatibility with CRX-601. Upon evaluation of the lead chitosan derivatives, MGC was identified as the most compatible for co-formulation with CRX-601, resulting in the most uniform and stable formulations at concentrations desired for SL vaccination. Therefore, of the chitosan derivatives tested, MGC was identified as the lead SL candidate chitosan derivative.
MGC differs from other derivatives in that a fraction of the amines are quaternized providing a permanent positive charge [48]. Whereas the other chitosan derivatives tested caused notable aggregation or precipitation when co-formulated with CRX-601, MGC-CRX-601 complexes were colloidally stable. It is likely that only MGC had a sufficient charge density to achieve complete neutralization and reversal of charge on CRX-601 complexes. This reversal of charge by MGC is necessary to achieve charge-charge repulsion for colloidal stabilization, which other derivatives lacked. In addition, in the process of optimizing MGC-CRX-601 formulations, HEPES-saline was identified as a more effective vehicle, compared to 10 mM HEPES. The influence of ionic strength on the aggregation behavior of polyelectrolyte complexes has been described previously [46]. It is possible that MGC conformation in saline shifts from a more stiff to a flexible coiled structure due to the shielding of polycation Coulombic repulsions in saline. Higher chain flexibility and a more coiled structure might have permitted easier conformational adaptation of MGC chains, leading to a lower level of aggregation.
Our data demonstrate that both MGC and CRX-601 are effective adjuvants for split flu antigen delivered SL, which is in agreement with previous studies showing increased immunogenicity of vaccines containing chitosan or chitosan derivatives [29–41] or CRX-601 [28]. Our data show that inclusion of MGC or CRX-601 in SL vaccine formulations significantly increased serum IgG and functional HAI titers as well as mucosal IgA titers. HA-specific antibodies are recognized as a major correlate of protection against influenza infection and disease [49, 50] and both serum IgG and mucosal IgA are efficient at inhibiting flu virus attachment and entry [51]. When present at sufficiently high titers, protective immunity to flu virus has been demonstrated to be mediated by IgG and IgA in the respiratory tract [52–57]. SL vaccination with flu antigen alone was able to elicit notable serum IgG and functional HAI titers as well as IgA titers in mice; however, titers were highly variable between mice and on average significantly less than titers from mice vaccinated IM. Inclusion of MGC or CRX-601 significantly increased anti-flu antibody titers in a dose dependent manner. We also evaluated if there were any notable improvements in vaccine immunogenicity if MGC was co-formulated with CRX-601. Although anti-flu antibody titers generally increase in mice vaccinated with co-formulations of MGC-CRX-601, no significant improvements were noted compared to vaccination with an equivalent dose of MGC or CRX-601 alone (Figure 7 and data not shown). Additional testing utilizing greater numbers of mice and/or further optimization of both MGC and CRX-601 doses may demonstrate a significant benefit of co-formulating MGC and CRX-601 for SL vaccines and may substantiate the use of lower adjuvant and/or antigen doses. Nonetheless, MGC and/or CRX-601, when given alone at optimal doses, elicited antibody titers equivalent to or greater than those seen in the IM control.
Analysis of IgG subtypes, IgG1 and IgG2a, indicated that SL vaccination with flu alone elicits a predominately IgG1 response, which is indicative of a T helper (Th) 2 skewed response in mice [58]. In comparison, SL vaccination with formulations containing either MGC or CRX-601 increased IgG2a levels, indicative of Th1 predominance [58], resulting in a decreased IgG1 to IgG2a ratio that was more comparable to that seen following IM vaccination with flu alone (Supplementary Figure 1). Influenza infection is known to induce a Th1 skewed response while influenza subunit vaccines are associated with a Th2 response [59]. Several other adjuvants have also been shown to induce Th2 responses when administered mucosally, including immunostimulating complexes (ISCOMs) [60], cholera toxin (CT) [61] and Escherichia coli heat labile enterotoxin (LT) [62, 63]. Protection against influenza A is suggested to benefit from a predominately Th1 or a balanced Th response [59]. Thus, our data indicating that SL vaccination with split-flu antigen adjuvanted with either MGC or CRX-601 resulted in increased IgG2a (indicative of Th1 cell activation), compared to SL vaccination with flu antigen alone, and a more balanced IgG2a to IgG1 ratio, comparable to that seen following IM vaccination, would be beneficial for protection against influenza. Of note, Maroof et. at. recently demonstrated a detrimental role for Th17/IL-17A following influenza challenge of mice previously vaccinated intranasaly with CRX-601 plus split-flu antigen [28]. Intracellular cytokine staining of lung or splenic T-lymphocytes using the method described by Maroof et. al. were below the limit of detection in all SL vaccinated mice suggesting T-cell responses were lower in SL vaccinated mice as compared to previously reported intranasal vaccine experiments with CRX-601 and split-flu (data not shown) [28]. Further studies are required in both naïve and primed vaccination models further explore the role of T-cells in mucosal immunity to influenza virus.
Being that anti-HA antibody titer determined by HAI is generally considered to be a useful marker of protective immunity against infection with influenza, our results also suggest that comparable (or greater) HAI titers following SL vaccination with MGC or CRX-601 adjuvanted formulations would also likely correspond to at least an equivalent immunity to challenge with the flu virus as traditional IM vaccination. In addition, whereas IM vaccination failed to elicit any detectable mucosal IgA, SL vaccination consistently resulted in detectable IgA titers. IgA in the respiratory tract has been indicated as more effective than IgG at neutralization of the flu virus before an infection is established [64–66]. Therefore, increased specific IgA titers following SL vaccination, in addition to comparable systemic IgG titers with MGC or CRX-601 adjuvanted formulations, may confer enhanced protection to flu infection over traditional IM vaccination strategies.
In conclusion, our results demonstrate for the first time that MGC and/or CRX-601 adjuvanted split-flu vaccines administered SL elicit an improved mucosal and at least an equivalent systemic immune response to flu vaccine delivered IM. SL vaccination requires a relatively simple, painless and needle-free vaccine administration and does not have the reported safety risks associated with IN or the complications of oral vaccination. The ability to stimulate equivalent or greater immune responses following SL vaccination using novel safe and effective adjuvants such as MGC and/or CRX-601 presents an attractive SL vaccination strategy for future vaccine development.
Supplementary Material
Acknowledgments
We thank Kendal Ryter and George Ettenger from GSK chemistry for providing CRX-601. We thank Audrey Morasse, Jon Ward, Robert Child, Margaret Whitacre, Connie Mullen and Cindy Nilles for their technical support. This work was supported by National Institutes of Health Contract HHSN272200900008C.
Abbreviations
- Flu
influenza
- MGC
methylglycol chitosan
- IM
intramuscular
- ID
intradermal
- IN
intranasal
- SL
sublingual
- Ig
immunoglobulin
- HA
hemagglutinin
- NA
neuraminidase
- AGP
aminoalkyl glucosaminide 4-phosphate
- TLR4
toll-like receptor 4
- MGC-CRX-601
MGC co-formulated with CRX-601
- HPLC
high-performance liquid chromatography
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- ZP
zeta-potential
- PDI
polydispersity indices
- DLS
dynamic light scattering
- 14dp2°
14 days post-secondary
- 14dp3°
14 days post-tertiary
- ELISA
enzyme-linked immunosorbent assay
- HAI
hemagglutination inhibition assay
- DPBS
Dulbecco’s phosphate buffered saline
- RBC
red blood cell
- RDE
receptor destroying enzyme
- RT
room temperature
- LLOQ
lower limit of quantification
- VW
vaginal wash
- TW
tracheal wash
- Th
T helper
- NT
not tested
- Uni
unimodal
- Multi
multimodal
- Bi
bimodal
- S.C
seroconversion
- THF
tetrahydrofuran
Footnotes
Authorship
J.S., H.O., Y.Y., D.B., D.P., M.P. and J.E were involved in the conception of the study and critical analysis of data. All authors contributed to the design and performance of experiments. J.S. and H.O. wrote the manuscript.
Conflict of Interest Disclosure
All authors were employees of GSK-Vaccines during performance of the work reported herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couch RB, Kasel JA, Glezen WP, Cate TR, Six HR, Taber LH, et al. Influenza: its control in persons and populations. J Infect Dis. 1986;153:431–40. doi: 10.1093/infdis/153.3.431. [DOI] [PubMed] [Google Scholar]
- 2.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YT, Kim KH, Ko EJ, Lee YN, Kim MC, Kwon YM, et al. New vaccines against influenza virus. Clin Exp Vaccine Res. 2014;3:12–28. doi: 10.7774/cevr.2014.3.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–15. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 5.Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. doi: 10.1002/rmv.398. [DOI] [PubMed] [Google Scholar]
- 6.Shim BS, Choi Y, Cheon IS, Song MK. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013;13:81–5. doi: 10.4110/in.2013.13.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 8.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–92. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 10.Poles J, Alvarez Y, Hioe CE. Induction of intestinal immunity by mucosal vaccines as a means of controlling HIV infection. AIDS Res Hum Retroviruses. 2014;30:1027–40. doi: 10.1089/aid.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong ME, Lavelle EC, Loscher CE, Lynch MA, Mills KH. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. J Infect Dis. 2005;192:1628–33. doi: 10.1086/491739. [DOI] [PubMed] [Google Scholar]
- 12.Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, et al. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77:10078–87. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20:2431–8. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 14.van Ginkel FW, Nguyen HH, McGhee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000;6:123–32. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 17.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–9. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011;18:150–60. doi: 10.1128/CVI.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Picking WL, Tzipori S. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect. 2014;16:796–803. doi: 10.1016/j.micinf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Amuguni JH, Lee S, Kerstein KO, Brown DW, Belitsky BR, Herrmann JE, et al. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine. 2011;29:4778–84. doi: 10.1016/j.vaccine.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 21.Amuguni H, Lee S, Kerstein K, Brown D, Belitsky B, Herrmann J, et al. Sublingual immunization with an engineered Bacillus subtilis strain expressing tetanus toxin fragment C induces systemic and mucosal immune responses in piglets. Microbes Infect. 2012;14:447–56. doi: 10.1016/j.micinf.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Gallorini S, Taccone M, Bonci A, Nardelli F, Casini D, Bonificio A, et al. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine. 2014;32:2382–8. doi: 10.1016/j.vaccine.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 23.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Park HJ, Ferko B, Byun YH, Song JH, Han GY, Roethl E, et al. Sublingual immunization with a live attenuated influenza a virus lacking the nonstructural protein 1 induces broad protective immunity in mice. PLoS One. 2012;7:e39921. doi: 10.1371/journal.pone.0039921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murugappan S, Patil HP, Frijlink HW, Huckriede A, Hinrichs WL. Simplifying influenza vaccination during pandemics: sublingual priming and intramuscular boosting of immune responses with heterologous whole inactivated influenza vaccine. AAPS J. 2014;16:342–9. doi: 10.1208/s12248-014-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim BS, Choi YK, Yun CH, Lee EG, Jeon YS, Park SM, et al. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS One. 2011;6:e27953. doi: 10.1371/journal.pone.0027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim BS, Choi JA, Song HH, Park SM, Cheon IS, Jang JE, et al. Sublingual administration of bacteria-expressed influenza virus hemagglutinin 1 (HA1) induces protection against infection with 2009 pandemic H1N1 influenza virus. J Microbiol. 2013;51:130–5. doi: 10.1007/s12275-013-2399-z. [DOI] [PubMed] [Google Scholar]
- 28.Maroof A, Yorgensen YM, Li Y, Evans JT. Intranasal vaccination promotes detrimental Th17-mediated immunity against influenza infection. PLoS Pathog. 2014;10:e1003875. doi: 10.1371/journal.ppat.1003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann AJ, Noulin N, Catchpole A, Stittelaar KJ, de Waal L, Veldhuis Kroeze EJ, et al. Intranasal H5N1 vaccines, adjuvanted with chitosan derivatives, protect ferrets against highly pathogenic influenza intranasal and intratracheal challenge. PLoS One. 2014;9:e93761. doi: 10.1371/journal.pone.0093761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10:797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkova MA, Irza AV, Chvala IA, Frolov SF, Drygin VV, Kapczynski DR. Adjuvant effects of chitosan and calcium phosphate particles in an inactivated Newcastle disease vaccine. Avian Dis. 2014;58:46–52. doi: 10.1637/10510-020413-Reg.1. [DOI] [PubMed] [Google Scholar]
- 32.Svindland SC, Pedersen GK, Pathirana RD, Bredholt G, Nostbakken JK, Jul-Larsen A, et al. A study of Chitosan and c-di-GMP as mucosal adjuvants for intranasal influenza H5N1 vaccine. Influenza Other Respir Viruses. 2013;7:1181–93. doi: 10.1111/irv.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawar D, Jaganathan KS. Mucoadhesive glycol chitosan nanoparticles for intranasal delivery of hepatitis B vaccine: enhancement of mucosal and systemic immune response. Drug Deliv. 2014:1–11. doi: 10.3109/10717544.2014.908427. [DOI] [PubMed] [Google Scholar]
- 34.Dehghan S, Tafaghodi M, Bolourieh T, Mazaheri V, Torabi A, Abnous K, et al. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int J Pharm. 2014;475:1–8. doi: 10.1016/j.ijpharm.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Fukushima K, Sannan T, Saito N, Takiguchi Y, Sato Y, et al. Evaluation of the effectiveness and safety of chitosan derivatives as adjuvants for intranasal vaccines. Viral Immunol. 2013;26:133–42. doi: 10.1089/vim.2012.0057. [DOI] [PubMed] [Google Scholar]
- 36.Jabbal-Gill I, Watts P, Smith A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv. 2012;9:1051–67. doi: 10.1517/17425247.2012.697455. [DOI] [PubMed] [Google Scholar]
- 37.Sawaengsak C, Mori Y, Yamanishi K, Srimanote P, Chaicumpa W, Mitrevej A, et al. Intranasal chitosan-DNA vaccines that protect across influenza virus subtypes. Int J Pharm. 2014;473:113–25. doi: 10.1016/j.ijpharm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Sawaengsak C, Mori Y, Yamanishi K, Mitrevej A, Sinchaipanid N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014;15:317–25. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buffa V, Klein K, Fischetti L, Shattock RJ. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PLoS One. 2012;7:e50529. doi: 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein K, Mann JF, Rogers P, Shattock RJ. Polymeric penetration enhancers promote humoral immune responses to mucosal vaccines. J Control Release. 2014;183:43–50. doi: 10.1016/j.jconrel.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Mannila J, Jarvinen K, Holappa J, Matilainen L, Auriola S, Jarho P. Cyclodextrins and chitosan derivatives in sublingual delivery of low solubility peptides: A study using cyclosporin A, alpha-cyclodextrin and quaternary chitosan N-betainate. Int J Pharm. 2009;381:19–24. doi: 10.1016/j.ijpharm.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Chopra S, Mahdi S, Kaur J, Iqbal Z, Talegaonkar S, Ahmad FJ. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J Pharm Pharmacol. 2006;58:1021–32. doi: 10.1211/jpp.58.8.0002. [DOI] [PubMed] [Google Scholar]
- 43.Artursson P, Lindmark T, Davis SS, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm Res. 1994;11:1358–61. doi: 10.1023/a:1018967116988. [DOI] [PubMed] [Google Scholar]
- 44.Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24:979–93. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 45.Bazin HG, Murray TJ, Bowen WS, Mozaffarian A, Fling SP, Bess LS, et al. The ‘Ethereal’ nature of TLR4 agonism and antagonism in the AGP class of lipid A mimetics. Bioorg Med Chem Lett. 2008;18:5350–4. doi: 10.1016/j.bmcl.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 46.Dautzenberg H. Polyelectrolyte complex formation in highly aggregating systems. Macromolecules. 1997;30:7810–5. [Google Scholar]
- 47.Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, et al. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26:2705–11. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2009;20:1057–79. doi: 10.1007/s10856-008-3659-z. [DOI] [PubMed] [Google Scholar]
- 49.Rimmelzwaan GF, McElhaney JE. Correlates of protection: novel generations of influenza vaccines. Vaccine. 2008;26 (Suppl 4):D41–4. doi: 10.1016/j.vaccine.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 50.Li CK, Rappuoli R, Xu XN. Correlates of protection against influenza infection in humans--on the path to a universal vaccine? Curr Opin Immunol. 2013;25:470–6. doi: 10.1016/j.coi.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Reperant LA, Rimmelzwaan GF, Osterhaus AD. Advances in influenza vaccination. F1000Prime Rep. 2014;6:47. doi: 10.12703/P6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ainai A, Tamura S, Suzuki T, Ito R, Asanuma H, Tanimoto T, et al. Characterization of neutralizing antibodies in adults after intranasal vaccination with an inactivated influenza vaccine. J Med Virol. 2012;84:336–44. doi: 10.1002/jmv.22273. [DOI] [PubMed] [Google Scholar]
- 53.Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, Suzuki Y, Tamura S, Kurata T, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74:328–35. doi: 10.1002/jmv.20173. [DOI] [PubMed] [Google Scholar]
- 54.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 55.Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol. 1989;146:107–16. doi: 10.1007/978-3-642-74529-4_12. [DOI] [PubMed] [Google Scholar]
- 56.Brown TA, Murphy BR, Radl J, Haaijman JJ, Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985;22:259–64. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandtzaeg P, Berstad AE, Farstad IN, Haraldsen G, Helgeland L, Jahnsen FL, et al. Mucosal immunity--a major adaptive defence mechanism. Behring Inst Mitt. 1997:1–23. [PubMed] [Google Scholar]
- 58.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012;8:689–93. doi: 10.4161/hv.19568. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen G, Major D, Roseby S, Wood J, Madhun AS, Cox RJ. Matrix-M adjuvanted virosomal H5N1 vaccine confers protection against lethal viral challenge in a murine model. Influenza Other Respir Viruses. 2011;5:426–37. doi: 10.1111/j.1750-2659.2011.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lycke N. Is the choice of vaccine adjuvant critical for long-term memory development? Expert Rev Vaccines. 2010;9:1357–61. doi: 10.1586/erv.10.136. [DOI] [PubMed] [Google Scholar]
- 63.Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3:556–66. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- 64.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–86. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 65.Isaka M, Zhao Y, Nobusawa E, Nakajima S, Nakajima K, Yasuda Y, et al. Protective effect of nasal immunization of influenza virus hemagglutinin with recombinant cholera toxin B subunit as a mucosal adjuvant in mice. Microbiol Immunol. 2008;52:55–63. doi: 10.1111/j.1348-0421.2008.00010.x. [DOI] [PubMed] [Google Scholar]
- 66.Asanuma H, Fujihashi K, Miyakoshi T, Yoshikawa T, Fujita-Yamaguchi Y, Kojima N, et al. Long- and short-time immunological memory in different strains of mice given nasally an adjuvant-combined nasal influenza vaccine. Vaccine. 2007;25:6975–80. doi: 10.1016/j.vaccine.2007.06.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.