Abstract
Hydrophobic drug release from poly (lactic-co-glycolic acid) (PLGA) microspheres typically exhibits a tri-phasic profile with a burst release phase followed by a lag phase and a secondary release phase. High burst release can be associated with adverse effects and the efficacy of the formulation cannot be ensured during a long lag phase. Accordingly, the development of a long-acting microsphere product requires optimization of all drug release phases. The purpose of the current study was to investigate whether a blend of low and high molecular weight polymers can be used to reduce the burst release and eliminate/minimize the lag phase. A single emulsion solvent evaporation method was used to prepare microspheres using blends of two PLGA polymers (PLGA5050 (25KDa) and PLGA9010 (113KDa)). A central composite design approach was applied to investigate the effect of formulation composition on dexamethasone release from these microspheres. Mathematical models obtained from this design of experiments study were utilized to generate a design space with maximized microsphere drug loading and reduced burst release. Specifically, a drug loading close to 15% can be achieved and a burst release less than 10% when a composition of 80% PLGA9010 and 90 mg of dexamethasone is used. In order to better describe the lag phase, a heat map was generated based on dexamethasone release from the PLGA microsphere/PVA hydrogel composite coatings. Using the heat map an optimized formulation with minimum lag phase was selected. The microspheres were also characterized for particle size/size distribution, thermal properties and morphology. The particle size was demonstrated to be related to the polymer concentration and the ratio of the two polymers but not to the dexamethasone concentration.
Keywords: design of experiment, mathematical model, PLGA, polymer blends, release profile, microspheres
Graphical abstract
1. Introduction
Diabetes mellitus is a chronic metabolic disease affecting about 387 million people globally (2014 data) according to International Diabetes Federation (Cho, 2014). Fluctuations in blood glucose levels is a typical symptom of diabetic patients due to underproduction or underutilization of insulin. It is critical for diabetic patients to closely monitor their blood glucose levels in order to control disease progression and prevent severe complications (Fowler, 2008). At present, most diabetic patients rely on glucose strips along with hand held glucose meters to measure blood glucose levels via finger pricking (Samuelson and Gerber, 2009). Continuous glucose monitoring provides the advantage of accurately monitoring the blood glucose trend for precise calculation of the insulin dose, therefore eliminating the possibility of hypo/hyperglycemic conditions (Lodwig et al., 2014). Currently, commercially available continuous glucose monitoring devices can only function for up to 7 days with a glucose oxidase based transcutaneous amperometric sensor (Henning, 2009). These sensors lose functionality after one week due to the foreign body reaction (FBR) which is a series of sequential events that ultimately rejects the implanted biomaterials (Morais et al., 2010). The initial biofouling and sequential inflammatory cell attack can affect enzyme stability and reduce sensor sensitivity. Fibrous encapsulation, the final event of FBR, deprives the senor of adequate analyte supply leading to a loss in sensor signal. Inhibition of local FBR is one of the most promising strategies to extend sensor lifetime (Vaddiraju et al., 2010).
In order to achieve long-term continuous glucose monitoring, biocompatible coatings composed of poly (lactic-co-glycolic acid) (PLGA) microspheres embedded in a polyvinyl alcohol (PVA) hydrogel have been developed (Bhardwaj et al., 2007; Bhardwaj et al., 2010; Kastellorizios et al., 2015; Patil et al., 2007; Wang et al., 2013) for a totally implantable, miniaturized (0.5mm × 0.5 mm × 5 mm) glucose biosensor (being developed in our laboratory). The PVA hydrogel acts as a hydrophilic base to support the microspheres and to allow glucose to readily diffuse to the biosensor. PLGA microspheres serve as drug reservoirs to continuously release dexamethasone to inhibit local inflammation, therefore, preventing the foreign body reaction. However sustained release of dexamethasone is required over the entire sensor lifetime since a delayed tissue reaction can develop after exhaustion of the drug (Bhardwaj et al., 2007).
Dexamethasone loaded microspheres tend to have an initial burst phase, followed by a lag phase and then a secondary release phase (Zolnik and Burgess, 2008). A sufficient amount of dexamethasone should be released during the burst release phase to inhibit the acute inflammation that is caused by the trauma of implantation. However, too high an initial burst release may cause dose dumping, which can lead to severe side effects. High drug loading is desired as this can benefit both the daily dose and the duration of drug release. The lag phase, where the daily drug release is typically low, should be as short as possible in order to ensure efficacy during that period. It has always been a challenge to control the burst release and increase drug loading for PLGA based microspheres (Allison, 2008). The burst release of drug from microspheres can be adjusted through process controls such as stabilizing the primary emulsion and changing the solvent evaporation rate by modifying the agitation type/speed (Yeo and Park, 2004). The use of co-solvents is another effective strategy to reduce the initial burst release. For example, burst release of 10-hydroxycampetothecin from PLGA microspheres was reduced when dimethylformadide was used as a co-solvent with methylene chloride (Shenderova et al., 1997).
However, there has been a paucity of literature reports on how to control/eliminate the microsphere lag phase. Changing the composition of the formulation is the most straightforward and effective way to adjust microsphere release properties. Applying the concept of blending different polymers, it is possible to adjust the dexamethasone release profile by decreasing the burst release and shortening the lag phase to achieve sustained drug release for approximately 3 months (Wang et al., 2014). For ganciclovir microspheres prepared by blending PLGA7525 and Resomer RG 502H, drug encapsulation and release parameters were shown to be altered significantly (Duvvuri et al., 2006). Modified leuprolide release from PLGA microspheres was also reported by blending 8.6 KDa and 28.3 KDa polymers (Ravivarapu et al., 2000).
Based on the in vivo performance of previous formulations, further formulation optimization to increase the drug loading and control the burst release and lag phase is required to establish a long-term (6-month) effective microsphere formulation (Patil et al., 2004; Wang et al., 2013). Quality by design (QbD) and design of experiment (DoE) approaches have been widely used in the development of various pharmaceutical formulations (Gu et al., 2015; Kumar et al., 2014; Xu et al., 2012a). Mathematical models generated from DoE studies can also be used for response prediction and formulation optimization purposes (Xu et al., 2011, 2012b). In the current study, the drug loading and initial release phase of the dexamethasone microspheres were optimized using a DoE approach. In addition to burst release, the lag phase of the PLGA microspheres was also adjusted to achieve sufficient theoretical daily dose (0.17 μg/day from our previous study(Patil et al., 2004)) for in vivo efficacy. The release characteristics were correlated with the physico-chemical properties of the PLGA microspheres such as thermal properties, particle size as well as morphology.
2. Material and Methods
2.1. Materials
Dexamethasone was purchased from Cayman Chemical (Ann Arbor, MI), poly (vinyl alcohol) (PVA, Mw 30–70 KD), sodium chloride (NaCl, ACS grade), sodium azide (NaN3), sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), sodium phosphate monobasic (NaH2PO4) and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (St. Louis, MO). PVA (99% hydrolyzed, Mw 133 KD) was purchased from Polysciences, Inc. (Warrington, PA). PLGA Resomer® RG503H 5050 (RG503H, inherent viscosity 0.32–0.44 dl/g) was a gift from Boehringer-Ingelheim. PLGA 9010 DLG7E (DLG7E, inherent viscosity 0.6-0.8 dL/g) was purchased from Lakeshore Biomaterials (Birmingham, AL). RG503H has carboxylic acid end groups and DLG7E is end-capped with a lauryl ester group. Methylene chloride (DCM), acetonitrile (ACN, HPLC grade), and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). NanopureTM quality water (Barnstead, Dubuque, IA) was used for all studies.
2.2. Methods
2.2.1. Preparation of microspheres
Dexamethasone loaded microsphere formulations were prepared using an oil-in-water (o/w) emulsion solvent extraction/evaporation technique. The PLGA polymers (amounts and ratios as indicated in Table 1) were dissolved in 2 ml of methylene chloride and dexamethasone (amounts as indicated in Table 1) was dispersed in this solution and these dispersions were sonicated using a bath sonicator for 20 minutes. The dispersions were then further mixed using a T 25 digital ULTRA-TURRAX homogenizer (IKA Works, Inc., Wilmington, NC) at 10,000 rpm for 1 min. The organic phase was then slowly added to 10 ml of PVA solution (1% (w/v), average Mw 30–70 KDa) and homogenized at 10,000 rpm for 2.5 min. The emulsion was then transferred to 125 ml of an aqueous PVA solution (0.1% (w/v), Mw 30-70 KDa) and stirred at 600 rpm. A vacuum was applied to the aqueous phase for 2.5 hours to evaporate the methylene chloride and harden the microspheres. The hardened microspheres were transferred to 50 mL centrifuge tubes and collected via centrifugation at 1500 rpm for 2 minutes. The microspheres were then washed three times with deionized water (10 mL each time), collected using the same centrifugation procedure as before and dried using a freeze dryer. The prepared microspheres were stored at 4°C until further use.
Table 1. Variables and responses from the central composite design.
| Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Run # | Type of Design Point | Total Amount of PLGA (mg) | DLG7E Ratio (%) | Dexamethasone (mg) | Drug Loading (%) | Burst Release (%) | Encapsulatin Efficiency (%) |
| CCD-1 | Star | 500.00 | 20.00 | 75.00 | 9.71 | 37.21 | 74.44 |
| CCD-2 | Edge | 559.46 | 83.78 | 60.13 | 9.29 | 10.26 | 95.73 |
| CCD-3 | Star | 500.00 | 60.00 | 100.00 | 13.36 | 13.71 | 80.16 |
| CCD-4 | Edge | 559.46 | 83.78 | 89.87 | 13.18 | 10.33 | 95.23 |
| CCD-5 | Edge | 559.46 | 36.22 | 89.87 | 11.19 | 30.13 | 80.85 |
| CCD-6 | Star | 500.00 | 60.00 | 50.00 | 8.66 | 12.10 | 95.26 |
| CCD-7 | Center | 500.00 | 60.00 | 75.00 | 12.12 | 18.92 | 92.92 |
| CCD-8 | Star | 400.00 | 60.00 | 75.00 | 12.13 | 21.08 | 76.82 |
| CCD-9 | Center | 500.00 | 60.00 | 75.00 | 11.47 | 17.93 | 87.94 |
| CCD-10 | Edge | 440.54 | 83.78 | 60.13 | 11.25 | 9.75 | 93.67 |
| CCD-11 | Center | 500.00 | 60.00 | 75.00 | 11.55 | 17.87 | 88.55 |
| CCD-12 | Center | 500.00 | 60.00 | 75.00 | 11.21 | 15.17 | 85.94 |
| CCD-13 | Edge | 440.54 | 36.22 | 89.87 | 14.32 | 35.20 | 84.52 |
| CCD-14 | Edge | 559.46 | 36.22 | 60.13 | 8.77 | 15.20 | 90.37 |
| CCD-15 | Star | 500.00 | 100.00 | 75.00 | 12.84 | 5.18 | 98.44 |
| CCD-16 | Center | 500.00 | 60.00 | 75.00 | 11.32 | 14.28 | 86.79 |
| CCD-17 | Star | 600.00 | 60.00 | 75.00 | 9.81 | 14.27 | 88.29 |
| CCD-18 | Edge | 440.54 | 83.78 | 89.87 | 15.90 | 12.10 | 93.84 |
| CCD-19 | Edge | 440.54 | 36.22 | 60.13 | 11.23 | 20.47 | 93.51 |
| CCD-20 | Center | 500.00 | 60.00 | 75.00 | 12.41 | 15.38 | 95.14 |
2.2.2. Central composite design
A 3-factor 5-level central composite design was applied in order to optimize drug loading, burst release and lag phase by adjusting the total amount of PLGA, the percentage of DLG7E in the polymer blend and the amount of dexamethasone. The design involves preparation of 20 formulations including 6 center points (CCD-7, 9, 11, 12, 16, 20), 8 edge points (CCD-2, 4, 5, 10, 13, 14, 18, 19) and 6 star points (CCD-1, 3, 6, 8, 15, 17) as shown in Table 1.
2.2.3. Drug loading
Drug loading was determined by dissolving approximately 5 mg of dexamethasone-loaded PLGA microspheres in 10 ml THF. This solution was filtered (Millex® HV, PVDF 0.45 μm syringe filter) and the dexamethasone concentration was determined using a Perkin Elmer series 200 HPLC system (Shelton, CT) equipped with a UV absorbance detector (240 nm wave length). The mobile phase consisted of acetonitrile/water/phosphoric acid (35/70/0.5, v/v/v). A Zobax C18 (4.6 mm × 15 cm, Agilent, Santa Clara, CA) analytical column was used with a flow rate of 1 ml/min. The injection volume used for drug loading determination was 5 μl. The chromatographs were analyzed by PeakSimple™ Chromatography System (SRI instruments, Torrance, CA).
2.2.4. Burst release
Burst release was determined by incubating approximately 5 mg of microspheres in 100 mL of phosphate buffered saline (PBS, 10 mM, pH 7.4) solution at 37 °C. After 24 hours incubation, 10 mL of PBS was filtrated through a 0.45 μm syringe filter and the dexamethasone concentration was determined using the HPLC method described above except that a 20 μl sample was injected.
2.2.5. In vitro release testing
In vitro release testing was performed for the PLGA microsphere/PVA hydrogel (99% hydrolyzed, Mw 133 KD) composite formulations. The composites were prepared using a freeze-thaw method described previously (Wang et al., 2014). Briefly, an appropriate amount of PLGA microspheres was dispersed into the PVA hydrogel (5% w/v) solution, then this suspension was filled into a plastic tubing mold (5 mm inner diameter and 2 cm in length) and subjected to three freeze-thaw cycles consisting of 2 h freezing at −20°C followed by 1 h thawing at room temperature. The composites were air dried. Approximately 2 mg of each formulation was immersed in a 2 ml Eppendorf vial containing 1.8 ml of 10 mM PBS (pH 7.4) with 0.1% NaN3 and incubated at 37 °C under constant agitation. At pre-determined time points, all the release media was removed and replenished with fresh media. Sink conditions were maintained throughout. The samples were filtered through 0.45 μm syringe filters and the concentration of dexamethasone in each sample was determined using the HPLC method as described above. Normalized daily drug release from each central composite design formulation was calculated for up to 21 days in order to determine the lag phase of the formulations.
Cumulative dexamethasone release was plotted versus time in the release profile.
2.2.6. Particle size evaluation
An AccuSizer 780A autodiluter particle sizing system (Nicomp, Santa Barbara, CA) was used to determine the particle size of the prepared microspheres. Approximately 5 mg of microspheres were dispersed in 1 ml of 0.1% (w/v) PVA solution and 100 μl of the dispersion was injected into the system for particle size analysis. All measurements were conducted in triplicate and the results are reported as the volume based mean particle size ± SD. The standard deviation (indicating the distribution of the particle size) was also reported.
2.2.7. Thermal analysis
A TA Q1000 differential scanning calorimeter (DSC) (TA, New Castle, DE) was used to determine the glass transition temperature (Tg) of the prepared microspheres. Modulated DSC was performed with the cycle below: the samples were heated at a rate of 3 °C/min from 4°C to 80 °C at a modulating oscillatory frequency of 1°C/min. The thermograms were analyzed using Universal Analysis software (TA Instruments) to determine the glass transition temperatures.
2.2.8. Scanning electron microscopy (SEM)
A scanning electron microscope (FEI Nova™ NanoSEM 450) equipped with an ETD detector was used to evaluate the morphology of the prepared microspheres. Samples were mounted on carbon taped aluminum stubs and sputter coated with gold for 1.5 min at 6 mA. Images were taken with an accelerating voltage of 2.0 kV and a working distance of 4 mm.
2.2.9. Statistical analysis
Data collected for drug loading and burst release in each run were analyzed using Design Expert software (Version 9, StatEase, Minneapolis, MN, USA) and fitted into linear regression models. Analysis of variance (ANOVA) was used to determine the significance of the model and parameters. Model correctness was assessed by the lack of fit test. In the case of particle size and size distribution, paired Student's t-test was performed to determine whether there were statistically significant differences among the results.”
3. Results and discussion
3.1. Central composite design
The drug loading and burst release for all DoE formulations are shown in Table 1. CCD-18 has the highest drug loading (15.9%) among all the formulations. The encapsulation efficiency (EE, calculated by dividing the actual drug loading by the theoretical drug loading) is approximately 93.8%, indicating most of the dexamethasone added was loaded into the microspheres. CCD-6 has the lowest drug loading (8.66%) among all the formulations. This is due to the low theoretical drug loading of this formulation since the encapsulation efficiency is actually similar to that of CCD-18 (EE of CCD-6 is 95.3%). When comparing the encapsulation efficiency of all formulations, CCD-1 has the lowest EE around 75%. This formulation (CCD-1) has the highest ratio of low molecular weight PLGA which is likely to cause slower polymer precipitation and consequently loss of drug to the aqueous phase. It has been reported that low drug encapsulation is associated with slow PLGA precipitation (Makadia and Siegel, 2011). CCD-15, composed of only DLG7E (high molecular weight PLGA) and dexamethasone, was determined to have the lowest burst release of 5.18%. Thus, fast microsphere solidification is expected in this case which can lead to less surface associated or free dexamethasone present in the formulation (Zolnik and Burgess, 2008). The burst release of the CCD-1, 5 and 13 formulations are higher than 30%, which can be explained by lower DLG7E ratio and higher initial dexamethasone added into these formulations. A detailed statistical description for the DoE results regarding drug loading and burst release are discussed below.
3.2. Drug loading
A significant DoE model was obtained from the study according to the analysis of variance (ANOVA) table (Table. 2) with a p-value less than 0.0001 and Lack of Fit value of 0.2026 (larger than 0.05). Four terms were included to generate a mathematical model for drug loading prediction as shown below (also included in Figure 1-C).
Table 2.
ANOVA table of the DoE model used to predict dexamethasone loading.
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 57.58 | 4 | 14.40 | 35.49 | < 0.0001 | Significant |
| A-PLGA | 14.71 | 1 | 14.71 | 36.26 | < 0.0001 | |
| B-DLG7E% | 6.43 | 1 | 6.43 | 15.86 | 0.0012 | |
| C-Dexamethasone | 35.29 | 1 | 35.29 | 87.01 | < 0.0001 | |
| BC | 1.15 | 1 | 1.15 | 2.83 | 0.1133 | |
| Residual | 6.08 | 15 | 0.41 | |||
| Lack of Fit | 4.95 | 10 | 0.49 | 2.17 | 0.2026 | Not Significant |
| Pure Error | 1.14 | 5 | 0.23 | |||
| Correlation Total | 63.67 | 19 |
Figure 1.
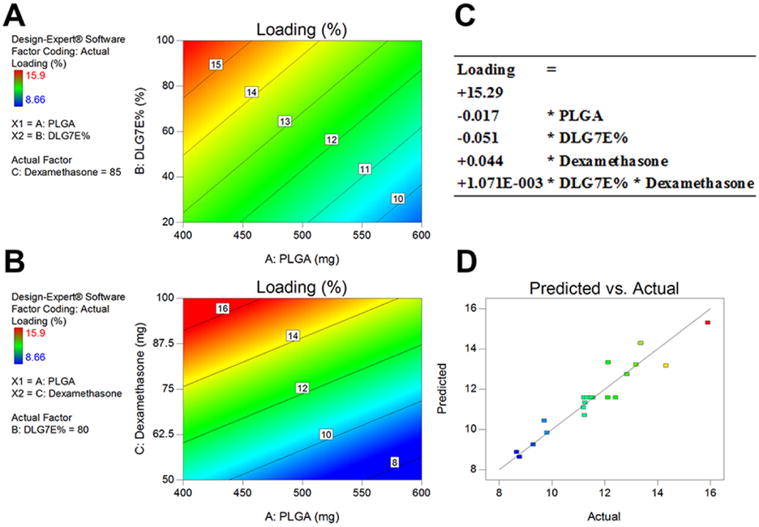
DoE model obtained for dexamethasone loading prediction of microspheres prepared using polymer blends. Contour plots (A and B) indicating correlation between drug loading and two independent variables. A: DLG7E ratio in the polymer blend and total amount of PLGA (the total amount of dexamethasone was set at 85 mg). B: Dexamethasone amount added and total amount of PLGA (DLG7E ratio was set at 80%). C: Mathematical equation used to predict dexamethasone loading. D: predicted versus actual experimental values based on the model, the line indicates a linear fit.
The p-values for the effect of total PLGA amount, ratio of DLG7E and total dexamethasone amount are much smaller than 0.05, indicating that all these factors significantly affect microsphere drug loading. The p-value for the interaction term (BC, DLG7E ratio and dexamethasone amount) is 0.1133, which is slightly higher than 0.05. A small interaction may exist between these two variables as increasing the amount of dexamethasone will leave excessive free drug to interact with the polymers, therefore affecting the drug loading of the formulation. The contour plots shown in Figures 1-A and 1-B indicate the effects of individual factors on the drug loading. Increase in the initial dexamethasone concentration or decrease in the total amount of PLGA increased the theoretical drug loading by adjusting the polymer/drug ratio and this is reflected in the actual drug loading. A higher ratio of the high molecular weight polymer (DLG7E) also leads to higher drug loading. This can be explained by fast precipitation of the high molecular weight polymer. When a higher ratio of DLG7E is used, fast solidification of the microspheres is expected which will lead to more dexamethasone encapsulation. The predicted versus actual drug loading values for all the CCD formulations are shown in Figure 1-D. A linear fit was obtained indicating that the model is valid.
3.3. Burst release
A significant DoE model (indicated by a p-value less than 0.0001 and Lack of Fit value higher than 0.05) for burst release was obtained The mathematical model for burst release prediction is shown below (also included in Figure 2-C):
Figure 2.
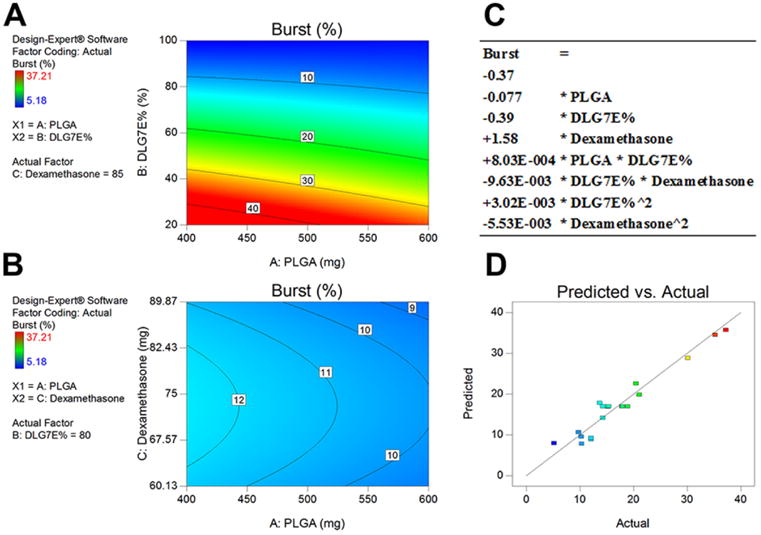
DoE model obtained for prediction of dexamethasone burst release from microspheres prepared using polymer blends. Contour plots (A and B) indicating correlation between burst release and two independent variables. A: DLG7E ratio in the polymer blend and total amount of PLGA (dexamethasone amount was set as 85 mg). B: Dexamethasone amount added and total amount of PLGA (DLG7E ratio was set as 80%)). C: Mathematical equation used to predict dexamethasone burst release. D: Predicted versus actual experimental values based on the model, the line indicates a linear fit.
More terms were included in this mathematical model indicating a more complex interaction for burst release compared to drug loading. The initial burst release is a result of free dexamethasone or microsphere surface associated dexamethasone which can be related to the microsphere composition as well as the process parameters. In order to rule out the effect of process parameters on burst release, all processes were carried out following exactly the same procedures including the solvent evaporation time, volume and time for microsphere washing. The process is highly reproducible as indicated by the reproducible center points (CCD-7, 9, 11, 12, 16 and 20) of the DoE. Since dexamethasone is only slightly soluble in methylene chloride, the majority of the drug exists as crystalline particles in the formulation. With change in the DLG7E ratio in the polymer composition, the burst release varies significantly as shown in the contour plot (Figure 2-A). When 80% of DLG7E was added into the polymer blends, the burst release remains around 11% although it varies between 9 and 12% according to the total amount of polymer and dexamethasone (Figure 2-B). The composition of the polymer blends is the most important factor affecting the burst release which is also indicated in the ANOVA table where the DLG7E ratio term has the lowest p-value. A significant interaction between the DLG7E ratio and the dexamethasone amount was observed (p-value equal to 0.0032). This may be explained by dexamethasone having different interactions with different polymers. The square of the DLG7E ratio (Bˆ2 in Table 3) is also significant. This is because the ratio of the two polymers affects many formulations aspects that may be related to burst release (such as polymer precipitation rate during microsphere formation as well as the diffusion rate of drug from the surface of the microspheres).
Table 3.
ANOVA table of the DoE model to predict dexamethasone burst release.
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1226.44 | 7 | 175.21 | 25.46 | < 0.0001 | Significant |
| A-PLGA | 38.91 | 1 | 38.91 | 5.65 | 0.0349 | |
| B-DLG7E% | 925.54 | 1 | 925.54 | 134.47 | < 0.0001 | |
| C-Dexamethasone | 88.61 | 1 | 88.61 | 12.87 | 0.0037 | |
| AB | 10.31 | 1 | 10.31 | 1.50 | 0.2446 | |
| BC | 92.75 | 1 | 92.75 | 13.48 | 0.0032 | |
| Bˆ2 | 42.53 | 1 | 42.53 | 6.18 | 0.0286 | |
| Cˆ2 | 21.72 | 1 | 21.72 | 3.16 | 0.1010 | |
| Residual | 82.60 | 12 | 6.88 | |||
| Lack of Fit | 64.92 | 7 | 9.27 | 2.62 | 0.1529 | Not Significant |
| Pure Error | 17.68 | 5 | 3.54 | |||
| Correlation Total | 1309.04 | 19 |
3.4. Formulation optimization and model validation
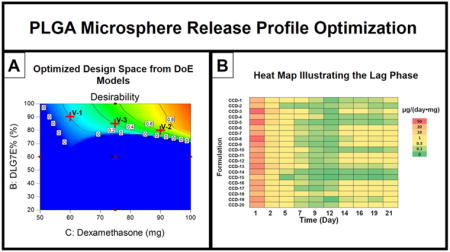
Following the establishment of the models for drug loading and burst release prediction, the design space for microsphere composition was optimized to maximize drug loading and minimize burst release (in a range of 8 to 12%) based on a batch size of 500 mg PLGA total. Figure 3 shows the optimized design space using a contour plot of desirability. Desired formulations require both a large amount of dexamethasone and a high percentage of DLG7E in the total polymer.
Figure 3.
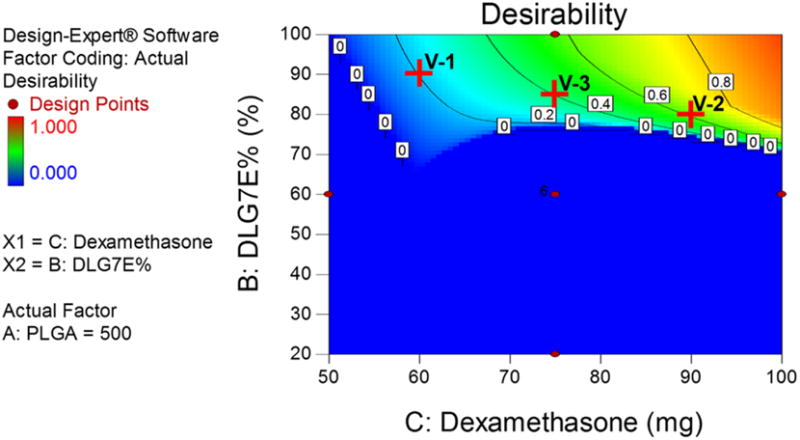
Optimized formulation composition to maximize drug loading and minimize burst release. The total amount of PLGA is set at 500 mg. Red crosses (V-1, V-2 and V-3) indicate conditions used for model validation.
Three points (marked as red crosses in Figure 3) in the design space were selected to verify the model. Three formulations (V-1, V-2 and V-3) were prepared according to the compositions listed in Table 4. Burst release and drug loading were analyzed for each formulation and compared to the predicted values for model validation. The burst release for all the formulations was close to 10% and the drug loading ranges from approximately 10% to 14%. The actual drug loading and burst release for all the formulations tested are consistent with the predicted values as shown in Table 3, indicating that the models are very predictive.
Table 4. Verification of CCD models using microspheres prepared under conditions within the optimized design space (n=3).
| Composition | Drug Loading (%, w/w) | Burst release (%, w/w) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| # | Total PLGA (mg) | DLG7E ratio (%, w/w) | Dexameth asone (mg) | Predicted | Actual | Predicted | Actual |
| V-1 | 500 | 90 | 60 | 10.62 | 10.2 ± 0.09 | 9.52 | 9.4 ± 0.3 |
| V-2 | 500 | 80 | 90 | 14.38 | 14.47 ± 0.18 | 9.45 | 9.4 ± 0.19 |
| V-3 | 500 | 85 | 75 | 12.58 | 12.31 ± 0.26 | 9.93 | 10.27 ± 0.52 |
3.5. Duration of lag phase
Bulk degradation is the typical release mechanism for PLGA based microsphere formulations. For dexamethasone release from microspheres, the release profile can usually be divided into three phases: a burst release, a lag phase with limited drug release and a linear release phase. Our previous data has indicated that dexamethasone release during the lag phase (approximately 10 days) from a 1-month formulation is sufficient to inhibit the foreign body reaction (Bhardwaj et al., 2010; Wang et al., 2013; Zolnik and Burgess, 2008). However, it is possible that this may not be the case for microsphere formulations with an elongated lag phase that are prepared with high molecular weight PLGA. For example, for a microsphere formulation prepared using 75 KDa PLGA, the lag phase was approximately 1 month (Bhardwaj et al., 2010). For higher Mw PLGA (such as 113 KDa used in the current study), the lag phase is expected to be even longer. Blending low and high molecular weight PLGA has been previously used in order to shorten the lag phase (Wang et al., 2014). The lag phase was reduced from 1 month to approximately 15 days by blending 25 KDa PLGA with 75 KDa PLGA.
PLGA microsphere/PVA hydrogel composites were prepared for all the microsphere formulations in the DoE study considering that the composite coatings are intended for application to biosensors for inhibition of the foreign body reaction. Figure 4-A shows the release profiles for the initial 21 days for the composite coatings. Release profiles plotted according to the different categories of design points (center, edge and star) is also available in supplementary data Figure S1. The lag phase for CCD-15 started from day 2 and lasted for the entire 21 days following a small burst release of less than 10 μg/mg. The low burst release for the CCD-15 composite coating is consistent with the low burst observed for the CCD-15 microspheres. CCD-2 and 6 showed slightly higher burst release (approximately 23 μg/mg) and reached a plateau at approximately day 2. The release profiles for the center points (CCD-7, 9, 11, 12, 16 and 20) are clustered in the middle of the graph with approximately 30 μg/mg burst at day 1 and the burst phase continues for these formulations till day 5 when the lag phase begins. The extended burst phase is probably due to a delayed effect from the PVA hydrogel. This is consistent with our previous observation that the release of dexamethasone from free drug embedded PVA hydrogels can last for up to 10 days (Galeska et al., 2005). CCD-3, 5, 8, and 13 (with high dexamethasone loading in the microspheres) have high burst release phases (more than 50 μg/mL) that also continue for approximately 5 days. The higher burst release exhibited by CCD-3, 5, 8, and 13 is considered to be due to the large amount of free/surface associated drug in the microspheres.
Figure 4.
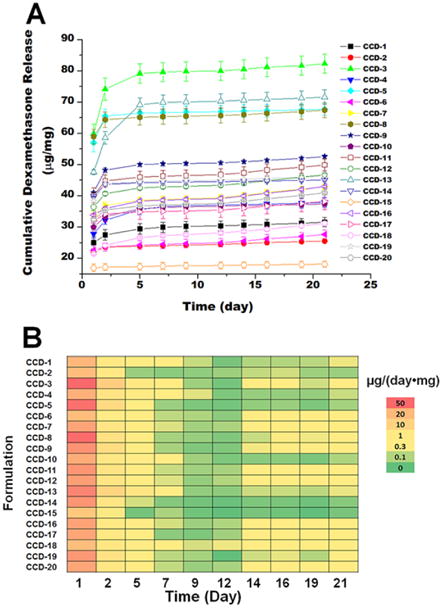
Release profile (A) and heat map (B) describing the initial drug release from the composite coatings prepared using the microspheres from the DoE study. A: Cumulative dexamethasone release was plotted versus time. B: The normalized daily dexamethasone release from the coatings was plotted in the heat map.
The burst and lag phases of all formulations were analyzed separately using various kinetics models. The models used for analyzing the burst release phase (till day 7) include: the first order release model; the Kosmeyer-Peppas model; and the Peppas-Sahlin model. The release kinetic parameters are shown in the supplementary data (Table-1). The first order release model did not provide a good approximations since the R square values were small for most of the formulations. The n-value obtained using the Kosmeyer-Peppas model is an indication of whether drug release is contributed by Fickian diffusion or polymer swelling (so called Case-II relaxation) (Siepmann and Peppas, 2001). However, the n-values obtained for these formulations were beyond the normal range of 0.43 to 0.85, indicating that a more complex mechanism is involved in the composite system. The microspheres are embedded in the PVA hydrogel which acts as an additional barrier for drug diffusion. Therefore, drug release was further analyzed using a more complex model, the Peppas-Sahlin model (Peppas and Sahlin, 1989), which returned better R square values (higher than 0.95 for most formulations). The Peppas-Sahlin model is able to simulate both the Fickian diffusion of dexamethasone from the microsphere surface and Case-II relaxation from the PVA hydrogel. With respect to the lag phase (from day 7 to 21), zero order release kinetics did fit the data of all formulations (linear release trends were observed with R square values higher than 0.95). Zero order drug release indicates concentration independent drug release. This can be explained since the release rate limiting factor during the lag phase is mainly the polymer matrix structure instead of the drug concentration.
The lag phases for most of the formulations shown in Figure 4-A started at approximately 5 days. During the lag phase small amounts of drug are released and it is difficult to differentiate the various formulations based on their release profiles. Thus, in order to further analyze the lag phase, the amount of drug released per day was plotted as a heat map (Figure 4-B). The heat map allowed better differentiation of the formulations. Analyzing the heat map, it became apparent that CCD-15 has the lowest amount of drug released during the 21 day study. This can be explained since CCD-15 is composed of only the high molecular weight PLGA. CCD-2 and CCD-14 also have relatively lower amounts of drug released during the lag phase compared to the other formulations. However, these two formulations have the lowest initial dexamethasone loading which will contribute to lower release during the lag phase. It is also apparent from the heat map that the daily drug release for CCD-18 is higher than that of the other formulations at most of the time points. The lowest daily dexamethasone release of CCD-18 is from day 7 to day 9 at approximately 0.15 μg/day, which is slightly less than the reported minimum effective daily dose (0.17 μg/day) to control the foreign body response in a rat model (Patil et al., 2004). However, a lag phase of 2 days can be tolerated (at least in this animal model) without onset of the inflammatory response (Bhardwaj et al., 2010; Wang et al., 2013; Zolnik and Burgess, 2008). Considering the high drug loading (15.9%, w/w) along with the burst release (12.1%, w/w) and short lag phase of CCD-18, this formulation is optimal among those investigated. The short lag phase observed for CCD-18 is probably due to: 1) the extended burst phase of this formulation; as well as 2) the reduced lag phase from blending low and high molecular weight polymers. The lag phase for the low molecular weight polymer (RG503H) is approximately 10 days. With the accumulation of lactic/glycolic acid monomers from hydrolysis of the low molecular weight PLGA, the degradation of the high molecular weight PLGA is accelerated due to decreased local pH. Accordingly, a continuous release pattern is expected for this formulation after the onset of drug release (Zolnik et al., 2006).
3.6. Particle size and size distribution
Microsphere particle size has been shown to be affected by the formulation composition (Meeus et al., 2015). The star points (6 formulations) from the CCD design were divided into three groups. Each group has two formulations that were prepared under the same conditions except that one of the independent variables is changed. The three groups are: 1) CCD-1 and 15 where the PLGA molecular weight ratio is varied; 2) CCD-8 and 17 where the total amount of PLGA is varied; and 3) CCD-3 and 6 where the amount of dexamethasone is varied. Therefore, by analyzing the star points in each group, the effect of each independent variable on the formulation characteristics was evaluated. The effect of each independent variable on the particle size and size distribution is shown in Figure 5. CCD-15 has significantly larger particle size compared to CCD-1. Larger particle size is also observed for CCD-17 compared to CCD-8. These observations indicate that increasing the amount of high molecular weight polymer as well as the initial polymer concentration can lead to larger particle size. This particle size increase can possibly be explained by increase in the polymer solution viscosity that occurs under these conditions. With high polymer solution viscosity, the energy input required to generate the primary emulsion is greater. Therefore larger particle size emulsion droplets were generated for formulations with high viscosity compared to those with low viscosity at the same homogenization speed (same energy input). It has also been reported that higher particle size is associated with higher drug encapsulation which is consistent with observations on these formulations (Siepmann et al., 2004). Particle size is also a major factor determining the diffusion rates of dexamethasone as the length of the diffusion pathways varies for different sized particles. However, no clear trend was observed when comparing the release rates of these formulations. This may be explained as the drug release is mainly controlled by the composition of these formulations. On comparing CCD-3 and CCD-6, no significant difference in particle size was observed since changing the amount of dexamethasone does not affect the viscosity. The standard deviation in the particle size for all formulations was approximately 15 μm indicating wide distributions with no significant difference between any of the groups. Wide particle size distribution is quite common for microspheres prepared via homogenization (Xu et al., 2009).
Figure 5.
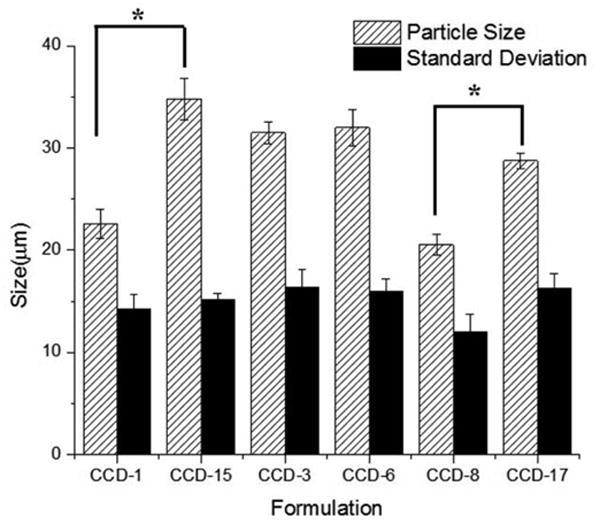
Particle size and size distribution (volume based) for the star points of the CCD microspheres (n=3). Center point parameters were used for these formulations except: 1) the DLG7E ratio was different for CCD-1 (20%) and CCD-15 (100%); 2) the dexamethasone amount was different for CCD-3 (50 mg) and CCD-6 (100 mg); and 3) the total polymer amount was different for CCD-8 (400 mg) and CCD-17 (600 mg). The paired student's t-test was performed to determine whether there were any statistically significant differences. P<0.05 was considered as a significant difference.
3.7. Modulated differential scanning calorimetry (mDSC)
Star points of the design were analyzed using mDSC to evaluate the thermal properties of the formulations as shown in Figure 6. Glass transition temperatures for all formulations except for CCD-15 were between 45-50 °C. CCD-15 has the highest glass transition temperature (Tg) of approximately 52 °C. The Tg of CCD-15 (composed solely of DLG7E polymer) is similar to the Tg of the polymer (52.97 °C, data not shown) indicating that there was no plasticization in the microspheres. Only one Tg was detected for all the formulations, suggesting that the polymers are miscible in nature and no phase separation occurs. When decreasing the ratio of DLG7E, the Tg decreases. Overall, the Tg(s) of these formulations are higher than body temperature. Based on this, these formulations are expected to be relatively stable compared to formulations with Tg values close to the body temperature following subcutaneous implantation as composite coatings for the biosensors. Those formulations with Tg values lower than body temperature may experience dose dumping once implanted.
Figure 6.
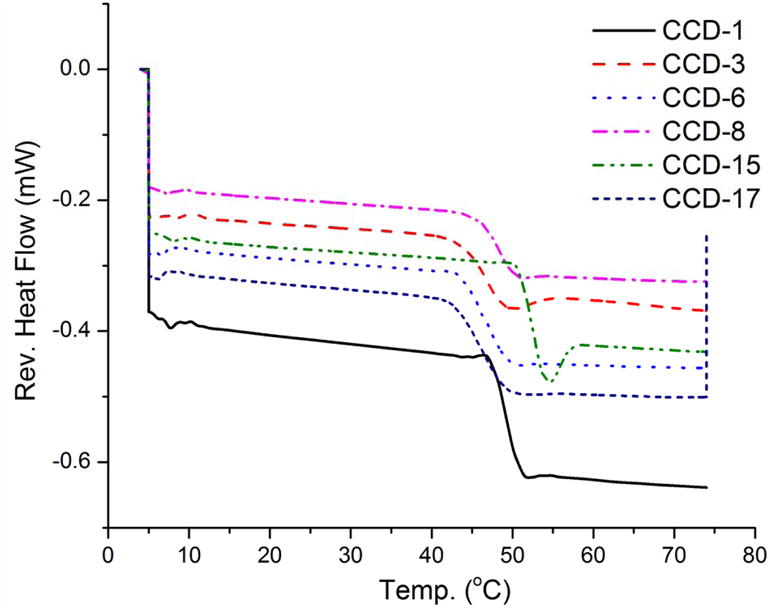
DSC thermograms of star points of the CCD microspheres. Center point parameters were used for these formulations except: 1) the DLG7E ratio was different for CCD-1 (20%) and CCD-15 (100%); 2) the dexamethasone amount was different for CCD-3 (50 mg) and CCD-6 (100 mg); and 3) the total polymer amount was different for CCD-8 (400 mg) and CCD-17 (600 mg).
3.8. Microsphere morphology
In order to investigate the morphology of the prepared microspheres, the star points were also evaluated using scanning electron microscopy. As shown from Figure 7, most of the formulations investigated are spherical. However, some irregular shaped or broken microspheres were observed for formulations other than CCD-15. CCD-15 is the only formulation composed of a single polymer. Some polymer segregation may occur within the polymer blend microspheres and this could cause breakage during the microsphere solidification step. In addition, heterogeneous solvent evaporation may occur when polymer blends were used which also can explain the morphology observed including the large pores and crevasses. Incomplete dexamethasone encapsulation was also observed for CCD-1 which may be a result of slow microsphere hardening due to the high ratio of small molecular weight polymer. Incomplete drug encapsulation can be correlated with the low encapsulation efficiency (EE=74.44%) for this formulation. On the other hand, high encapsulation efficiency (EE=98.44%) was observed for the CCD-15 microspheres which were spherical in shape with smooth surfaces. Some non-spherical particles were observed for CCD-3 (Figure 7-C) and CCD-8 (Figure 7-E), which appear to be free dexamethasone. This is in agreement with high burst release observed for these formulations. In addition, these formulations have a high drug/polymer ratio. On the other hand CCD-6 (Figure 7-D) and CCD-17 (Figure 7-F) which have low drug/polymer ratios, showed low burst release. The particle size for these formulations ranges from approximately 10 μm to 50 μm. A broad size distribution is observed for all the formulations. This is consistent with the particle size data shown in Figure 5.
Figure 7.
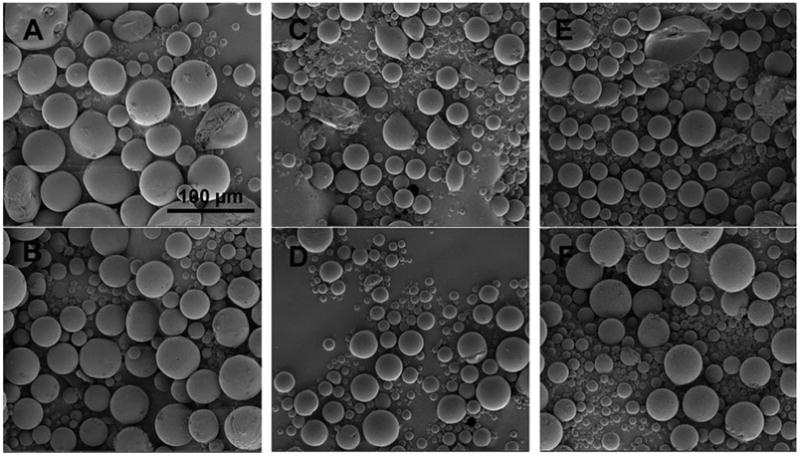
SEM images of star points of the CCD microspheres. Figures 7-A (top left panel), B (bottom left panel), C (top middle panel), D (bottom middle panel), E (top right panel), and F (bottom right panel) are corresponding to formulations CCD-1, 15, 3, 6, 8 and 17, respectively. Center point parameters were used for these formulations except: 1) the DLG7E ratio was different for CCD-1 (20%) and CCD-15 (100%); 2) the dexamethasone amount was different for CCD-3 (50 mg) and CCD-6 (100 mg); and 3) the total polymer amount was different for CCD-8 (400 mg) and CCD-17 (600 mg).
4. Conclusions
The strategy of blending different MW polymers was shown to be effective in reducing the microsphere burst release to less than 10% and shortening the release lag phase to less than one week. The optimized formulation could potentially be used to prevent the foreign body reaction associated with long-term fully implantable glucose sensors. The current study also demonstrated that the design of experiments (DoE) principles are beneficial for optimizing PLGA microsphere products to achieve the desired release properties. A design space with appropriate formulation outcomes (e.g. high drug loading and low burst release) was obtained based on the highly predictive DoE models. The optimized design space was shown to be valuable in predicting and controlling both drug loading and encapsulation efficiency. According to the design space, approximately 85 mg of dexamethasone and 85% of high molecular weight PLGA were required in order to achieve a formulation with 15% dexamethasone loading and 10% burst release. The design space can also serve as a blueprint to design PLGA blend based microsphere formulations with defined release attributes, such as drug loading and burst release. In addition, controlling drug release using polymer blending broadens the application of PLGA polymers in long-term drug delivery systems. A fundamental understanding of the effect of microsphere composition on the formulation performance was obtained by examining the formulation particle size, thermal properties as well as morphologies. Importantly, a novel heat map describing the daily drug release was developed to differentiate the various formulations during the lag phase where there is low/no drug release. Drug release properties are usually described using release profiles which may not be sufficiently detailed to describe the lag phase. In contrast, the drug release lag phase was illustrated clearly via the heat map and comparison between various formulations was easily made. Such heat maps are especially helpful for researchers who need to screen the release properties of a large number of formulations.
Supplementary Material
Acknowledgments
The authors thank US Army Medical Research (W81XWH0710688, W81XWH0910711), NIH (1R21HL09045801, R43EB011886, 9R01EB014586) and NSF/SBIR (1046902, 1230148) for funding. The authors also thank Dr. Xuanhao Sun and Dr. Marie Cantino from the Bioscience Electron Microscopy Laboratory for helping with the SEM imaging.
Abbreviations
- PLGA
poly (lactic-co-glycolic acid)
- FBR
foreign body reaction
- PVA
polyvinyl alcohol
- QbD
quality by design
- DoE
design of experiment
- DMSO
dimethyl sulfoxide
- DCM
methylene chloride
- ACN
acetonitrile
- THF
tetrahydrofuran
- DSC
differential scanning calorimeter
- Tg
glass transition temperature
- SEM
scanning electron microscopy
- EE
encapsulation efficiency
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5:615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling Acute Inflammation with Fast Releasing Dexamethasone-PLGA Microsphere/PVA Hydrogel Composites for Implantable Devices. Journal of Diabetes Science and Technology. 2007;1:8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. International Journal of Pharmaceutics. 2010;384:78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Cho NH. IDF Diabetes Atlas Sixth Edition Poster Update. International Diabetes Federation 2014 [Google Scholar]
- Duvvuri S, Gaurav Janoria K, Mitra A. Effect of Polymer Blending on the Release of Ganciclovir from PLGA Microspheres. Pharm Res. 2006;23:215–223. doi: 10.1007/s11095-005-9042-6. [DOI] [PubMed] [Google Scholar]
- Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clinical Diabetes. 2008;26:77–82. [Google Scholar]
- Galeska I, Kim TK, Patil S, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess D. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid- containing PVA hydrogels. AAPS J. 2005;7:E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Linehan B, Tseng YC. Optimization of the Büchi B-90 spray drying process using central composite design for preparation of solid dispersions. International Journal of Pharmaceutics. 2015;491:208–217. doi: 10.1016/j.ijpharm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Henning T. Commercially Available Continuous Glucose Monitoring Systems, In Vivo Glucose Sensing. John Wiley & Sons, Inc.; 2009. pp. 113–156. [Google Scholar]
- Kastellorizios M, Papadimitrakopoulos F, Burgess DJ. Prevention of foreign body reaction in a pre-clinical large animal model. J Control Release. 2015;202:101–107. doi: 10.1016/j.jconrel.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Kumar S, Xu X, Gokhale R, Burgess DJ. Formulation parameters of crystalline nanosuspensions on spray drying processing: A DoE approach. International Journal of Pharmaceutics. 2014;464:34–45. doi: 10.1016/j.ijpharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Lodwig V, Kulzer B, Schnell O, Heinemann L. Current Trends in Continuous Glucose Monitoring. Journal of Diabetes Science and Technology. 2014;8:390–396. doi: 10.1177/1932296814525826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeus J, Scurr DJ, Appeltans B, Amssoms K, Annaert P, Davies MC, Roberts CJ, Van den Mooter G. Influence of formulation composition and process on the characteristics and in vitro release from PLGA-based sustained release injectables. European Journal of Pharmaceutics and Biopharmaceutics. 2015;90:22–29. doi: 10.1016/j.ejpb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Morais J, Papadimitrakopoulos F, Burgess D. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. Journal of Controlled Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. International Journal of Pharmaceutics. 1989;57:169–172. [Google Scholar]
- Ravivarapu HB, Burton K, DeLuca PP. Polymer and microsphere blending to alter the release of a peptide from PLGA microspheres. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50:263–270. doi: 10.1016/s0939-6411(00)00099-0. [DOI] [PubMed] [Google Scholar]
- Samuelson LL, Gerber DA. Recent Developments in Less Invasive Technology to Monitor Blood Glucose Levels in Patients with Diabetes. Lab Medicine. 2009;40:607–610. [Google Scholar]
- Shenderova A, Burke TG, Schwendeman SP. Stabilization of 10-hydroxycamptothecin in poly(lactide-co-glycolide) microsphere delivery vehicles. Pharm Res. 1997;14:1406–1414. doi: 10.1023/a:1012172722246. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Faisant N, Akiki J, Richard J, Benoit JP. Effect of the size of biodegradable microparticles on drug release: experiment and theory. Journal of Controlled Release. 2004;96:123–134. doi: 10.1016/j.jconrel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Advanced Drug Delivery Reviews. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. Journal of Diabetes Science and Technology. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu B, Burgess D. Microspheres Prepared with PLGA Blends for Delivery of Dexamethasone for Implantable Medical Devices. Pharm Res. 2014;31:373–381. doi: 10.1007/s11095-013-1166-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. Journal of Controlled Release. 2013;169:341–347. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow- focusing Device for Controlled Drug Delivery. Small (Weinheim an der Bergstrasse, Germany) 2009;5:1575–1581. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Costa AP, Khan MA, Burgess DJ. Application of quality by design to formulation and processing of protein liposomes. Int J Pharm. 2012a;434:349–359. doi: 10.1016/j.ijpharm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. International Journal of Pharmaceutics. 2011;419:52–59. doi: 10.1016/j.ijpharm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. International Journal of Pharmaceutics. 2012b;423:543–553. doi: 10.1016/j.ijpharm.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27:1–12. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Burgess DJ. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. Journal of Controlled Release. 2008;127:137–145. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. Journal of Controlled Release. 2006;112:293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


