Abstract
Prospectively, 162 pleural fluid samples from patients with probable tuberculous pleural effusion were tested by the Xpert MTB/RIF assay and the Bactec MGIT-960 culture system. Of these, 43 (26.5%) were positive in the MGIT-960 culture, and 23 (14.2%), in the Xpert MTB/RIF assay. The sensitivity and specificity of the Xpert MTB/RIF compared with the MGIT-960 culture were 54.8% and 100%, respectively.
TEXT
Tuberculosis (TB) affects one third of the global population in developing countries, with annual estimates of 9.0 million new cases and 1.5 million deaths (1). While pulmonary tuberculosis (PTB) is the most common presentation, extrapulmonary tuberculosis (EPTB) is also an important clinical condition (2). Pleural TB occurs in up to 30% of patients concomitantly with pulmonary TB and contributes a major portion of extrapulmonary TB (3). The disease remains undiagnosed in the majority of cases due to its paucibacillary nature. The conventional methods of culture on solid and liquid media are gold standard for diagnosis, but the longer turnaround time (TAT) added with the relatively high cost for infrastructure development remain problems in resource-limited settings (4). Interferon gamma (IFN-γ) assay has also been reported as an alternative biological marker for pleural TB diagnosis by some workers (5).
The Xpert MTB/RIF assay (Cepheid, Inc., Sunnyvale, CA, USA) is a rapid, automated molecular test with good sensitivity for pulmonary TB. However, on its utility in pleural TB, only a few studies with small sample size have been carried out; most of these were from low-TB-burden countries (6–8), and none were from India.
This prospective observational study was conducted at the Government of India-approved culture & drug susceptibility testing (C&DST) laboratory, Division of Clinical Microbiology and Molecular Medicine, All India Institute of Medical Sciences, New Delhi. A total of 162 pleural fluid samples were obtained from patients having high suspicions of pleural TB. Eligibility for enrollment was based on standard clinical and radiological criteria, including a persistent cough of 2 weeks or more, unexplained fever for 2 weeks or more, unexplained weight loss with or without night sweats, chest pain, and radiological evidence of pleural effusion. Thus, no separate ethical clearance was required for this study. Patients who were receiving treatment at the time of enrollment were excluded from the study. All patients included were HIV negative.
One aliquot of the sample was used for the MGIT-960 culture, and the other, for the Xpert MTB/RIF assay. The Xpert MTB/RIF assay was performed directly on pleural fluid samples according to the manufacturer's instruction, using the newer version (G4) of cartridges and the newer software version 4.4a, as published earlier (9). Briefly, 1 ml of uncentrifuged pleural fluid sample was lysed with 3 ml of sample reagent (SR) buffer (3:1) and incubated for 15 min at room temperature; finally, 2 ml of mixture was loaded in cartridge. The instrument after DNA amplification detects the presence or absence of Mycobacterium tuberculosis along with recognition of rifampin (RIF) resistance. Semiquantitative estimation of Mycobacterium tuberculosis load is also determined as high, medium, low, or very low depending on cycle threshold (CT) value of the instrument.
For the MGIT-960 culture, pleural fluid samples were decontaminated using the N-acetyl-l-cysteine-sodium hydroxide (NALC-NaOH) method, as published elsewhere (9). Decontaminated samples were further used for Ziehl-Neelsen (ZN) staining, and 500 μl was inoculated in the MGIT-960 culture, as per the manufacturer's instructions (Becton Dickinson, Sparks, USA). Growth for determining time to positivity (TTP) values was continuously monitored by BD Epicentre (10). MGIT culture-positive samples were also subjected for drug susceptibility testing (DST) by using Sire test kits with the MGIT-960 method (9).
The Xpert MTB/RIF assay gives results on the same day, and the reports were dispatched on the same day rather than holding until culture results were obtained, because ours is a routine diagnostic service laboratory. However, during the data analysis and calculation of sensitivity, specificity, likelihood ratios, and P values for the Xpert MTB/RIF assay, the MGIT culture was considered standard, because it detected more cases.
Of 162 patients, 117 (72.2%) were males, and 45 (27.8%) females, with mean (± standard deviation [SD]) ages of 41.6 ± 19 years and 39.1 ± 18.6 years, respectively. The majority of cases (153 [94.4%]) were adults, and only 9 (5.6%) were children. Of the 162 cases, 145 (89.5%) had anorexia, 143 (88.3%) had dry cough, 142 (87.6%) had chest pain, and 140 (86.4%) had fever. Less common presentations were breathlessness in 98 (60.5%), night sweats in 72 (44.4%), and headache in 52 (32%). On chest x-ray, 42.7%, 39.7%, and 17.6% patients had left, right, and bilateral pleural effusion, respectively. Five of 162 (3.1%) samples (one sample each) were smear positive, and 43 (26.5%) flagged as MGIT-960 culture-positive. Forty-two (25.9%) samples grew Mycobacterium tuberculosis, but one smear and culture-positive sample grew Mycobacterium avium, as characterized by in-house multiplex PCR (11). Thus, 42 of 162 (25.9%) samples were detected as true positive for Mycobacterium tuberculosis.
The Xpert MTB/RIF assay overall detected only 23 (14.2%) of 162 samples as positive and 139 (85.8%) as negative. Error was detected in 3 (1.8%) samples, which were repeat tested again. However, considering the MGIT-960 culture as the reference, the diagnostic sensitivity of the Xpert MTB/RIF assay in Mycobacterium tuberculosis-positive samples was 54.8% (95% confidence interval [CI], 38.8% to 70.1.8%), while its specificity was 100% (95% CI, 96.8% to 100%) (Table 1 ).
TABLE 1.
Sensitivity and specificity of Xpert MTB/RIF assay (n = 23) in comparison with MGIT culture (n = 43d) on the pleural fluid samples (n = 162)
| Xpert MTB/RIF assay result | Sensitivity |
Specificity |
PPVa, % (95% CI) | NPVb, % (95% CI) | LRc positive | LR negative | P | ||
|---|---|---|---|---|---|---|---|---|---|
| No. positive/no. tested | % (95% CI) | No. negative/no. tested | % (95% CI) | ||||||
| Overall | 23/42d | 54.8 (38.8–70.1) | 119/119 | 100 (96.8–100) | 100 (85.2–100) | 86.2 (79.3–91.5) | 0.45 (0.32–0.63) | <0.01 | |
| Smear positive, culture positive | 4/4d | 100 (51.0–100) | 157/157 | 100 (97.7–100) | 100 (39.7–100) | 100 (97.1–100) | 0.00 | ||
| Smear negative, culture positive | 19/38 | 50.0 (54.8–65.1) | 124/124 | 100 (96.9–100) | 100 (83.2–100) | 86.2 (79.5–91.0) | 0.50 (0.36–69) | <0.01 | |
PPV, positive predictive value.
NPV, negative predictive value.
LR, likelihood ratio.
One culture grew M. avium and was excluded from the study.
We also observed that CT values of the tested samples were directly proportional to the semiquantitative mycobacterial (Mycobacterium tuberculosis) load (P < 0.01). The mean (± SD) CT values were 18.7 ± 1.4, 25.6 ± 2.0, and 34.4 ± 3.1 in samples showing mycobacterial load as medium, low, and very low, respectively. Of the total of 42 MGIT cultures positive for Mycobacterium tuberculosis, only 38 cultures were included for calculating TTP values, as 4 flagged-positive MGIT cultures became contaminated, and one was identified as M. avium. We found a positive correlation between TTP in the MGIT culture with CT values in the Xpert MTB/RIF assay (Fig. 1). As expected, the mean TTP value of samples detected negative by the Xpert MTB/RIF assay was significantly longer (24.6 ± 2.3 days) in MGIT cultures.
FIG 1.
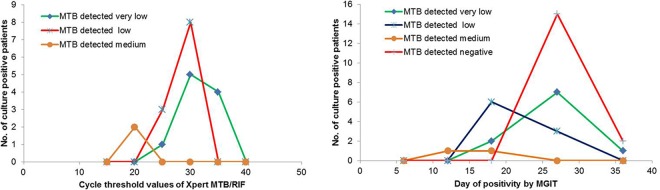
Correlation of mycobacterial load with Xpert MTB/RIF and MGIT culture positivity.
Of the 23 Xpert MTB/RIF assay-positive samples which were also MGIT culture-positive for Mycobacterium tuberculosis, 2 samples were rifampin resistant by MGIT-DST. Of these, 1 was rifampin resistant, and 1 was detected as indeterminate by the Xpert MTB/RIF assay. Seventeen samples were rifampin sensitive by both the Xpert MTB/RIF assay and the MGIT-DST. Two cultures were sensitive by culture DST and were detected as indeterminate by the Xpert MTB/RIF assay.
Efficacy of the Xpert MTB/RIF assay has widely been evaluated (12). However, for low-income, high-TB-burden countries there is need for more data, especially in paucibacillary specimens like pleural fluid (1, 12). Our study showed that the Xpert MTB/RIF assay test has very low diagnostic sensitivity, even in culture-proven cases. Previous studies have reported much lower sensitivities of between 15% and 48% (5, 13, 14). A few studies have assessed the diagnostic ability of the Xpert MTB/RIF assay by comparing centrifuged and uncentrifuged pleural fluid samples, but no significant increase in the TB detection rate was found (5, 14, 15). Pleural biopsy specimen has shown better diagnostic yield than pleural fluid samples. However, biopsy is not always feasible in most of the settings, due to the more invasive nature of the procedure which restricts its widespread use (5, 16). In our study, pleural biopsies were not attempted. However, had pleural biopsies been done and the specimens compared with the results of the Xpert MTB/RIF assay, the results would have been even more dismal (17).
The mean TTP values in the MGIT culture and the CT values in the Xpert MTB/RIF assay had direct correlations with mycobacterial load. The samples detected negative by the Xpert MTB/RIF assay had longer TTP, indicating that the bacterial load was too low to be detected by this molecular test (9). This observation is important and has a direct implication on the diagnostic ability of any test system. We conclude that the Xpert MTB/RIF assay has poor sensitivity in pleural fluid samples and should not be recommended for the detection of pleural TB, particularly in high-TB-burden settings.
ACKNOWLEDGMENTS
We thank Vinod Kumar, Virendra Kapil, and Brijesh Kumar Sharma for their technical help in this study.
REFERENCES
- 1.WHO. 2014. Global tuberculosis report. http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1.
- 2.Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, Borroni E, Mondo A, Piana F, Scarparo C, Coltella L, Lombardi G, Cirillo DM. 2012. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J 40:442–447. doi: 10.1183/09031936.00176311. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa K, Koga H, Hirakata Y, Tomono K, Tashiro T, Kohno S. 1997. Differential diagnosis of tuberculous pleurisy by measurement of cytokine concentrations in pleural effusion. Tuber Lung Dis 78:29–34. doi: 10.1016/S0962-8479(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 4.Maynard-Smith L, Larke N, Peters J, Lawn S. 2014. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing nonrespiratory samples: a systematic review. BMC Infect Dis 14:709. doi: 10.1186/s12879-014-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meldau R, Peter J, Theron G, Calligaro G, Allwood B, Symons G, Khalfey H, Ntombenhle G, Govender U, Binder A, Zyl-Smit R van, Dheda K. 2014. Comparison of same day diagnostic tools, including Gene Xpert and unstimulated IFN-γ for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulm Med 14:58. doi: 10.1186/1471-2466-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marlowe EM, Novak-Weekley SM, Cumpio J, Sharp SE, Momeny MA, Babst A, Carlson JS, Kawamura M, Pandori M. 2011. Evaluation of the Cepheid XpertMTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol 49:1621–1623. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, Munsamy V, Nicol M, Jones M, Persing DH, Hillemann D, Ruesch-Gerdes S, Leisegang F, Zamudio C, Rodrigues C, Boehme CC, Perkins MD, Alland D. 2011. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med 184:1076–1084. doi: 10.1164/rccm.201103-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, Bara W, Mungofa S, Pai M, Hoelscher M, Dowdy D, Pym A, Mwaba P, Mason P, Peter J, Dheda K, TB-NEAT team. 2014. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 9.Rufai SB, Kumar P, Singh A, Prajapati S, Balooni V, Singh S. 2014. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol 52:1846–1852. doi: 10.1128/JCM.03005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P, Balooni V, Sharma BK, Kapil V, Sachdeva KS, Singh S. 2014. High degree of multidrug resistance and hetero-resistance in pulmonary TB patients from Punjab state of India. Tuberculosis (Edinb) 94:73–80. doi: 10.1016/j.tube.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Gopinath K, Singh S. 2009. Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other mycobacterial species directly from clinical specimens. J Appl Microbiol 107:425–435. doi: 10.1111/j.1365-2672.2009.04218.x. [DOI] [PubMed] [Google Scholar]
- 12.Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. 2014. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. 2011. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol 49:4341–4342. doi: 10.1128/JCM.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christopher DJ, Schumacher SG, Michael JS, Luo R, Balamugesh T, Duraikannan P, Pollock NR, Pai M, Denkinger CM. 2013. Performance of Xpert MTB/RIF on pleural tissue for the diagnosis of pleural tuberculosis. Eur Respir J 42:1427–1429. doi: 10.1183/09031936.00103213. [DOI] [PubMed] [Google Scholar]
- 15.Lusiba JK, Nakiyingi L, Kirenga BJ, Kiragga A, Lukande R, Nsereko M, Ssengooba Katamba WA, Worodria W, Joloba ML, Mayanja-Kizza H. 2014. Evaluation of Cepheid's Xpert MTB/RIF test on pleural fluid in the diagnosis of pleural tuberculosis in a high prevalence HIV/TB setting. PLoS One 9:e102702. doi: 10.1371/journal.pone.0102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Hsu A, Lo C, Colt HG. 2007. Prospective evaluation of flex-rigid pleuroscopy for indeterminate pleural effusion: accuracy, safety and outcome. Respirology 12:881–886. doi: 10.1111/j.1440-1843.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- 17.Du J, Huang Z, Luo Q, Xiong G, Xu X, Li W, Liu X, Li J. 2015. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci 20:26–31. [PMC free article] [PubMed] [Google Scholar]


