Abstract
Our objectives were to study the prevalence, risk factors for carriage, and transmission dynamics of extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae (ESBLPE) in a national survey of cattle. This was a point prevalence study conducted from July to October 2013 in Israel. Stool samples were collected from 1,226 cows in 123 sections on 40 farms of all production types. ESBLPE were identified in 291 samples (23.7%): 287 contained Escherichia coli and 4 contained Klebsiella pneumoniae. The number of ESBLPE-positive cows was the highest in quarantine stations and on fattening farms and was the lowest on pasture farms (P = 0.03). The number of ESBLPE-positive cows was the lowest in sections containing adult cows (age, >25 months) and highest in sections containing calves (age, <4 months) (P < 0.001). Infrastructure variables that were significant risk factors for ESBLPE carriage included crowding, a lack of manure cleaning, and a lack of a cooling (P < 0.001 for each), all of which were more common in sections containing calves. Antimicrobial prophylaxis was given almost exclusively to calves and was associated with a high number of ESBLPE carriers (P < 0.001). The 287 E. coli isolates were typed into 106 repetitive extragenic palindromic (REP)-PCR types and mostly harbored blaCTX-M-1 or blaCTX-M-9 group genes. The isolates on the six farms with ≥15 isolates of ESBLPE were of 4 to 7 different REP-PCR types, with one dominant type being harbored by about half of the isolates. Fourteen types were identified on more than one farm, with only six of the farms being adjacent to each other. The prevalence of ESBLPE carriage is high in calves in cowsheds where the use of antimicrobial prophylaxis is common. ESBLPE disseminate within cowsheds mainly by clonal spread, with limited intercowshed transmission occurring.
INTRODUCTION
Since the advent of the first antimicrobials, antimicrobial-resistant bacteria (AMRB) have spread in conjunction with the use of the respective antimicrobial agents (1). Accordingly, the emergence of extended-spectrum β-lactamase (ESBL) enzymes as a global threat followed the introduction of third-generation cephalosporins (TGC), used mainly in health care settings (2). Although the ESBLs were first noted in the 1980s, a substantial increase in their prevalence in Escherichia coli was noted in the 2000s. This increase was mainly related to the emergence of a pandemic clone, designated sequence type (ST) 131. A particular worrisome feature of this clone was its predominance in community-onset cases of infection with ESBL-producing bacteria (3). This epidemiologic feature has attracted attention to the possibility of sources of acquisition other than health care settings, including the food and livestock industries. Indeed, many studies have documented the presence of ESBL-producing bacteria in a variety of meats and other livestock-origin food samples. Although the use of antimicrobials has often been suggested to be the main culprit for this phenomenon (4), there are in fact no studies that have looked into this question. Moreover, the data regarding other risk factors for carriage of ESBL-producing Enterobacteriaceae (ESBLPE) in livestock in general and cattle in particular are limited to those from the analysis of only a few factors (5, 6). Also, although molecular analysis of these isolates has been reported, such data have never been used for the analysis of the dissemination dynamics of ESBLPE in and between farms. In Israel, there is a large industry of cattle farms for the production of both dairy and meat products. Despite that, there are no data regarding the prevalence of AMRB in cattle in Israel. The objectives of this work were to study the prevalence of ESBLPE carriage among cattle in a nationwide survey in Israel, to analyze the dissemination dynamics of these strains using molecular studies, and to analyze the risk factors for carriage.
MATERIALS AND METHODS
Study design and data collection.
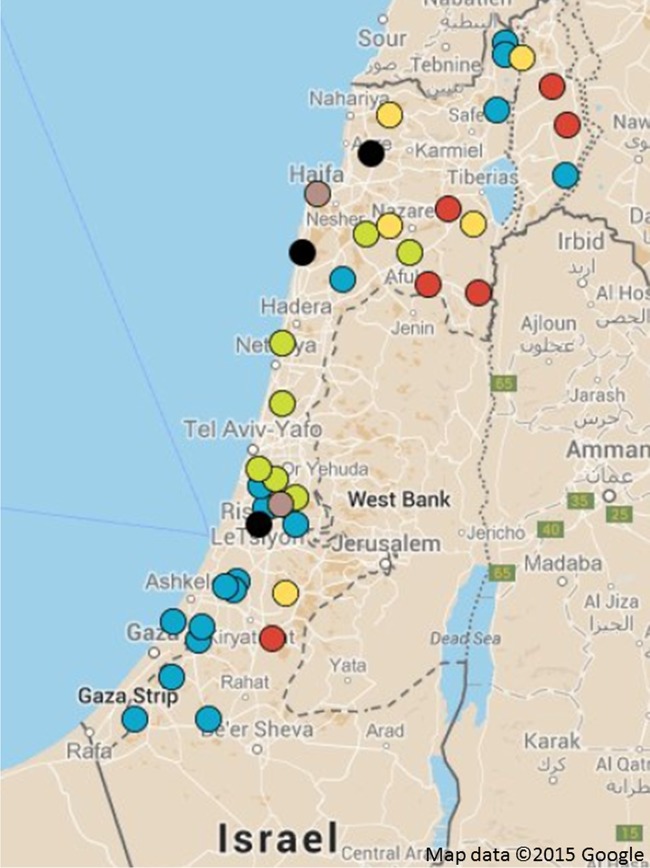
This was a point prevalence study conducted from July to October 2013 on cattle farms from the main farming locations in Israel (Fig. 1). The study included 1,226 cows placed in 123 sections on 40 farms of all types: dairy, fattening, pasture, and mixed (intensive dairy and fattening) farms. Dairy and fattening farms involve intensive farming without grazing. Farms are typically divided into separate sections according to age groups, and sampling was done accordingly. The study also included animals from two quarantine stations that hold imported calves prior to their transfer to fattening farms. The study included stool and data collection (see below). As cows are typically separated inside the farms according to age, sampling was done from approximately 10 heads of cattle in each section: for dairy farms, 4 sections; for fattening farms, 2 to 3 sections; and for pasture farms, sections with calves, adult females, and bulls. Sampling was done individually by rectal sampling (mainly in calves) or sampling from freshly excreted manure, and samples were delivered directly to the laboratory of the National Center for Infection Control.
FIG 1.
Geographical locations of farms according to farming type. Blue, dairy farms; green, fattening farms; red, pasture farms; yellow, mixed pasture and fattening farms; gray, quarantine stations; black, mixed dairy and fattening farms.
Data were collected by a single author (N.S.) by direct observation or by questioning of the farm's manager and included variables pertaining to the section or the entire farm. The section-related variables included age, crowdedness (number of heads per square meter), animal cleanliness (graded as the percentage of clean animals in the section), environmental cleanliness, infrastructure-related variables, and the use of antimicrobial prophylaxis. The farm-related variables included geographical location, farming type, the recent introduction of new calves, and veterinary care.
Microbiological and molecular methods.
A stool sample (∼1 g) was inoculated in brain heart infusion broth and incubated overnight at 36°C. A broth aliquot of 10 μl was subcultured onto CHROMagar ESBL agar plates (Hylabs, Rehovot, Israel) and incubated overnight. Suspicious colonies were identified according to the manufacturer's instructions. Identification was done using an Enterotest kit with a citrate test (Hylabs, Rehovot, Israel) and the Vitek 2 system (bioMérieux, Marcy l'Etoile, France) in equivocal cases. Testing for ESBLs was done by the combined disk method using ceftazidime and cefotaxime disks alone and with a clavulanic acid disk. Antimicrobial susceptibility testing (AST) was done by the disk diffusion method, and the results were interpreted according to CLSI criteria (7); susceptibility to colistin was determined by initial screening via the disk diffusion method followed by MIC testing via the gradient method (Etest; bioMérieux, Marcy l'Etoile, France) for isolates yielding a disk diffusion diameter of less than 10 mm.
Molecular typing was done by repetitive extragenic palindromic (REP)-PCR (8) or BOX-PCR (9) for ESBL-producing E. coli and Klebsiella pneumoniae isolates, respectively. PCR products were resolved using a capillary gel electrophoresis apparatus (QIAxcel; Qiagen, Hilden, Germany) and visually compared; isolates with an identical pattern were regarded as one strain. An example of this comparison is presented in Fig. S1 in the supplemental material. The blaESBL gene was determined by PCR for the group carrying the blaCTX-M allele (10) and by PCR and sequencing for the groups carrying the blaTEM and blaSHV alleles (11).
Statistical analysis.
With the exception of the microbiological data, all other data (nondependent variables) were collected per section and farm but not per individual cow. The values for the continuous parameters are presented as the means and standard deviations (SDs) of the variable and include the number of nonmissing values. The values for categorical variables are the presented as the numbers and percentages of sections in each category and the number (±SD) of positive ESBLPE carriers in each category.
The multiple imputation of missing data was conducted, but bivariate analyses with the imputed data for the relevant covariates did not converge; therefore all analyses were conducted with the available data only. Bivariate analyses were conducted with mixed Poisson regression models for each covariate separately, with a random effect of farms and an offset of the number of units within a section being used. The prevalence ratio (PR) and the P values are presented. A P value of less than 0.05 was considered statistically significant. Multivariate analyses including all covariates with P values (from the bivariate analysis) of ≤0.1, excluding those covariates with missing data, were conducted. Because of the pronounced differences in living conditions, analyses were done separately for nonpasture farms, in addition to the analysis for all farms. Statistical analyses were conducted with SAS software (version 9.2).
RESULTS
Farming type, location, and prevalence of ESBLPE on Israeli farms.
The study included 1,226 cows placed in 123 sections on 40 farms, the majority of which were dairy farms (Table 1). The number of cows sampled was 10 in 109 sections (including all pasture sections) and from 9 to 11 in an additional 115/123 sections (93.4%). Hence, the prevalence in the sections is represented by the number of ESBLPE carriers (Tables 1 and 2). Overall, ESBLPE were identified in 291 cows (23.7%), and the prevalence was the highest in the quarantine stations and on fattening farms and the lowest on pasture farms (Table 1). The farm type was not significant in multivariate analysis (PR = 0.053 for pasture farms, P = 0.079). The locations of the farms according to farming type are presented in Fig. 1. Dairy farms were located across the country and especially in the western Negev area (which has an arid to semiarid climate), whereas pasture farms were mainly in the Jezreel Valley and the Golan Heights (which have Mediterranean climates). Geographical location was not correlated with the prevalence of ESBLPE carriage (data not shown).
TABLE 1.
Farming types and prevalence of ESBLPE carriers on Israeli farms
| Farm type | No. of farms | No. of sections | Mean ± SD prevalence (%) of ESBLPE carriersa | PRb |
|---|---|---|---|---|
| Dairy | 17 | 66 | 2.6 ± 3.2 | 0.611 |
| Fattening | 7 | 16 | 2.5 ± 3.3 | 0.576 |
| Pasture | 6 | 13 | 0.4 ± 1.4 | 0.074 |
| Mixed pasture and fatteningc | 5 | 12 | 1.8 ± 2.6 | 0.436 |
| Quarantine station | 2 | 4 | 4.3 ± 3.3 | 1.000 |
| Mixed dairy and fatteningd | 3 | 12 | 3.0 ± 3.4 | 0.865 |
P = 0.03 for all farming types.
PR, prevalence ratio.
Six pastures and six feedlots.
Nine dairy sections and three feedlots.
TABLE 2.
Descriptive statistics and bivariate analysis of variables related to ESBLPE carriage in Israeli cattle
| Covariate | Mean ± SD prevalence (%) of carriers | No. (%) of sections in group | PRa | P value |
|---|---|---|---|---|
| Numeric covariates | ||||
| Cattle cleanliness (35.1% ± 34.5%)b | 2.4 ± 3.1 | 123 (100.0)c | 1.010 | <.001 |
| Trough (3.1 ± 2.4)b | 1.8 ± 2.5 | 111 (90.2)c | 0.927 | 0.053 |
| Categorical covariates | ||||
| Age (mo) | ||||
| <4 | 5.3 ± 3.6 | 32 (26.0) | 6.534 | <.001 |
| 5–10 | 1.7 ± 2.6 | 29 (23.6) | 2.136 | |
| 11–24 | 1.4 ± 1.9 | 36 (29.3) | 1.545 | |
| >25 | 0.9 ± 1.4 | 26 (21.1) | 1.000 | |
| Trough cleanliness | ||||
| Dirty | 2.6 ± 3.1 | 16 (13.0) | 0.242 | <.001 |
| Partially dirty | 1.7 ± 2.3 | 82 (66.7) | 0.226 | |
| Clean | 3.9 ± 4.1 | 21 (17.1) | 1.000 | |
| Information missing | 4 (3.3) | |||
| Cooling system | ||||
| Fan | 2.5 ± 3.2 | 39 (31.7) | 0.791 | <.001 |
| Fan and nebulizers | 1.2 ± 1.5 | 18 (14.6) | 0.261 | |
| None | 2.6 ± 3.2 | 66 (53.7) | 1.000 | |
| Crowdedness (no. of heads/m2) | ||||
| <5 | 5.9 ± 3.5 | 24 (19.5) | 8.656 | <.001 |
| 5–10 | 2.1 ± 2.9 | 23 (18.7) | 3.532 | |
| 11–20 | 1.6 ± 1.9 | 40 (32.5) | 1.988 | |
| 21–30 | 1.4 ± 2.3 | 20 (16.3) | 1.689 | |
| >30 | 0.6 ± 1.4 | 16 (13.0) | 1.000 | |
| Method for manure cleaning | ||||
| Tractor | 1.5 ± 2.1 | 38 (30.9) | 0.282 | <.001 |
| Automatic shovel | 1.7 ± 1.7 | 18 (14.6) | 0.187 | |
| Slatted floors | 0.0 ± 0.0 | 6 (4.9) | 0.000 | |
| None | 3.3 ± 3.6 | 46 (37.4) | 1.000 | |
| Information missing | 15 (12.2) | |||
| No. of heads/section | ||||
| <199 | 3.1 ± 3.3 | 18 (14.6) | 1.000 | <.001 |
| 200–499 | 2.8 ± 3.1 | 46 (37.4) | 0.710 | |
| 500–799 | 2.1 ± 3.1 | 43 (35.0) | 0.375 | |
| 800–999 | 1.0 ± 2.2 | 16 (13.0) | 0.340 | |
| No. of veterinarian visits/wk | ||||
| 0 | 1.2 ± 2.0 | 36 (29.3) | 0.176 | 0.032 |
| 1 | 2.4 ± 3.3 | 17 (13.8) | 0.392 | |
| 2 | 2.9 ± 3.2 | 60 (48.8) | 0.527 | |
| 3 | 3.4 ± 4.1 | 8 (6.5) | 0.690 | |
| 5 | 5.5 ± 4.9 | 2 (1.6) | 1.000 | |
| No. of new cattle on farm in preceding month | ||||
| None | 2.3 ± 3.0 | 91 (74.0) | 0.684 | 0.781 |
| From Israel | 2.3 ± 3.3 | 25 (20.3) | 0.779 | |
| From overseas | 3.1 ± 3.2 | 7 (5.7) | 1.000 | |
| Antimicrobial prophylaxis | ||||
| No | 1.3 ± 2.0 | 90 (73.2) | 0.231 | <.001 |
| Yes | 5.2 ± 3.7 | 33 (26.8) | 1.000 | |
| Vaccination other than mandatory | ||||
| Yes | 2.3 ± 2.9 | 23 (18.7) | 1.000 | 0.548 |
| No | 2.4 ± 3.1 | 100 (81.3) | 1.300 |
PR, prevalence ratio.
Data in parentheses represent the mean ± SD of the covariate.
The number of sections (in the continuous variable) where data were available.
Risk factors for ESBLPE carriage.
Descriptive statistics and bivariate analysis of the risk factors for ESBLPE carriage are presented in Table 2. The variables are presented in three groups: infrastructure and cleanliness related, veterinary treatment related, and farm related. Variables defined by distinct groups, including age and farm type, are further discussed below.
(i) Variables related to infrastructure and cleanliness, i.e., a lack of a cooling system, increased crowdedness, and a lack of manure cleaning, were all significantly associated with an increased risk for ESBLPE carriage (Table 2). Cooling with nebulizers (in addition to fans) and manure cleaning using slatted floors were associated with the lowest risk. In multivariate analysis, only the use of fans with nebulizers (PR = 0.2211, P = 0.036) was identified to be a significant factor protecting against ESBLPE carriage. Unexpectedly, the degree of cleanliness both of cows and of water troughs was related to ESBLPE carriage. A low number of cows per section was associated with an increased risk for ESBLPE carriage, and this is likely related to the specific risk groups (see below).
(ii) Variables related to veterinary treatment.
Antimicrobial prophylaxis was associated with an increased risk for ESBLPE carriage in the bivariate analysis (P < 0.001). It was administered in 33 sections overall, on all farm types, and most commonly (n = 26) in calves (age, <4 months). The most common agents were tetracycline (n = 26, 69%) as either chlortetracycline or doxycycline. Other agents included norfloxacin (n = 4), cephalosporin agents (cephalexin or ceftiofur, n = 4), anticoccidiosis agents (n = 3), sulfa agents (n = 3), gentamicin (n = 1), and monensin (n = 1). In eight sections, more than one agent was given, and the agent most commonly coadministered was a tetracycline. ESBLPE carriage increased with an increased frequency of veterinarian visits; vaccination with more than the mandatory vaccines was not associated with an increased risk.
(iii) Variables related to the farm.
The arrival of new cattle in the preceding month occurred in 26% of the sections. These cattle were mostly from other Israeli farms, and their arrival was not associated with an increased risk for ESBLPE carriage. Similarly, the geographical distribution of the farms (Fig. 1) was not associated with an increased risk (Table 2).
Farming groups associated with increased risk for ESBLPE carriage.
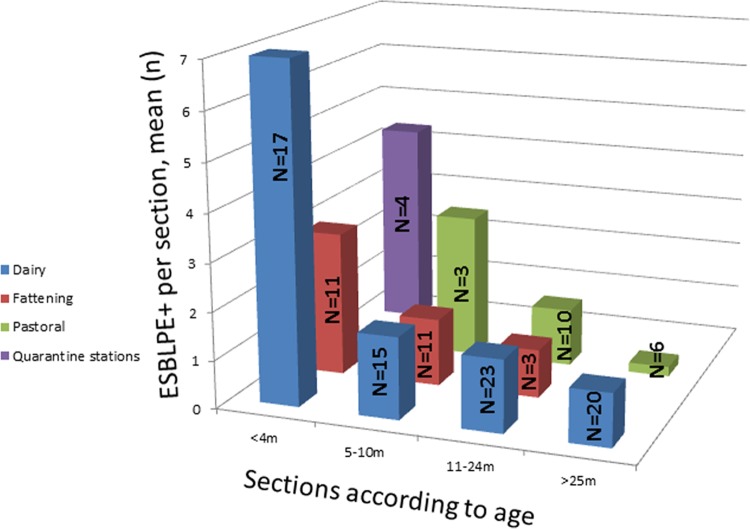
The mean prevalence of ESBLPE carriage was the highest in calves (PR = 5.3, SD = 3.6) and gradually declined with maturation in adult cows (PR = 0.9, SD = 1.4). This pattern was apparent on all farm types (Fig. 2) but was the most pronounced on dairy farms. This suggests that ESBLPE are mostly acquired by calves and that as the growing calves are transferred to the next age group carriage is gradually lost with maturation. Hence, we compared the most relevant risk factors between the age groups. Compared with the other age groups, calves had by far the highest rate of use of antimicrobial prophylaxis (78.6% versus 7.7%). Due to the vast difference in living conditions and physiology, a comparison of these factors in calves and older cows was problematic. For example, calves lived under the most crowded conditions (67.9% lived in areas of less than 5 m2 per head, whereas 5.5% of the animals in the other age groups lived in areas of this size). However, since calves on dairy farms are usually placed in individual pens, the relevance of these factors to ESBLPE carriage on these farms is less clear.
FIG 2.
Age-dependent carriage of ESBLPE according to farm type. The number of sections in each group is given inside the column. Sections on mixed farms were defined according to section.
As presented above (Table 1), the prevalence of ESBLPE carriage on different farm types varied significantly. However, although the distribution of age groups was similar overall, it differed widely on different farms (Fig. 3), thus affecting the overall prevalence of ESBLPE. For instance, quarantine stations included calves only and consequently had the highest prevalence of ESBLPE carriage. On the other hand, pastures did not include calves younger than 4 months of age and thus had the lowest prevalence. The latter animals had living conditions very different from those of the other animals in the study, and hence, comparisons of crowdedness, manure cleaning method, and cooling were irrelevant.
FIG 3.
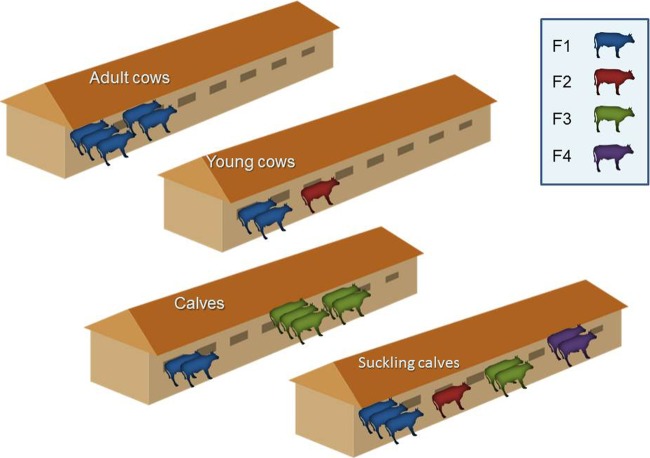
Distribution of ESBL-producing E. coli REP-PCR types in the different sections of farm 12. The REP-PCR types are designated F1 to F4.
The cross comparison of age and farm types identified the calves' (age, <4 months) sections on the dairy farms to be those with the highest prevalence of ESBLPE carriage (Fig. 3). We therefore compared these sections to the other sections on the dairy farms as well as to the calves' sections on the fattening farms (Table 3). Compared with both of these groups, calves' sections on dairy farms were more crowded (albeit the calves had separate pens) and had no manure cleaning, and the calves received antimicrobial prophylaxis almost universally. Use of cooling was more common in noncalves' sections on dairy farms.
TABLE 3.
Characteristics of calves'a sections on dairy farms versus other sections on dairy farms and calves' sections on fattening farms
| Covariate | No. (%) of sections |
||
|---|---|---|---|
| Dairy farms |
Calves' sections, fattening farms (n = 11) | ||
| Calves' sections (n = 17) | Other sections (n = 58) | ||
| Cooling system | |||
| Fan | 9 (52.9) | 17 (29.3) | 5 (45.4) |
| Fan and nebulizers | 0 | 18 (31) | 0 |
| None | 8 (47.1) | 23 (39.7) | 6 (54.6) |
| Crowdedness (no. of heads/m2) | |||
| <5 | 14 (82.3) | 4 (6.9) | 5 (45.4) |
| 5–10 | 2 (11.8) | 11 (19) | 4 (36.4) |
| 11–20 | 1 (5.9) | 25 (43.1) | 2 (18.2) |
| 21–30 | 0 | 16 (27.6) | 0 |
| >30 | 0 | 2 (3.4) | 0 |
| Method for manure cleaning | |||
| Tractor | 0 | 30 (52.6) | 4 (40) |
| Automatic shovel | 0 | 18 (31.6) | 0 |
| Slatted floors | 0 | 6 (10.5) | 0 |
| None | 14 (100) | 3 (5.3) | 6 (60) |
| Missing information | 3 | 1 | 1 |
| Antimicrobial prophylaxis | |||
| No | 1 (5.9) | 54 (93.1) | 5 (45.4) |
| Yes | 16 (94.1) | 4 (6.9) | 6 (54.6) |
Calves were animals <4 months of age.
Antimicrobial resistance patterns and molecular characteristics of ESBLPE isolates.
The majority of isolates were ESBL-producing E. coli (ESBLEC) isolates (n = 287, 98.6%), and the rest were K. pneumoniae isolates (n = 4). Nonsusceptibility to other antimicrobial agents was found as follows: tetracycline, 267 (91.7%) isolates; trimethoprim-sulfamethoxazole, 233 (80%) isolates; streptomycin, 123 (42.2%) isolates; chloramphenicol, 108 (37.1%) isolates; ciprofloxacin, 72 (24.7%) isolates; gentamicin, 58 (19.9%) isolates; and amoxicillin-clavulanate, 58 (19.9%) isolates. All isolates were susceptible to ertapenem, colistin, nitrofurantoin, and fosfomycin.
The most common blaESBL gene was of the blaCTXM-1 group (n = 233 isolates, 80%), followed by the blaCTXM-9 group (n = 28 isolates, 9.6%) and the blaSHV-12 group (n = 24 isolates, 8.2%). No blaESBL gene was identified in 6 isolates that tested positive by the ESBL combined disk test.
The 287 E. coli isolates were typed by REP-PCR and found to comprise 106 different types, of which 55 were singletons and 19 were common to two isolates. Monoclonal spread was not limited to a single farm: of the 26 types identified in ≥4 isolates, 16 types were identified on 1 farm only, 8 were identified on 2 farms, and 2 were identified on 3 farms. The three most frequent types were identified in 20 isolates (8 sections on 3 farms), 13 isolates (5 sections on 2 farms), and 11 isolates (3 sections on 3 farms), respectively. However, the route of dissemination was not apparent in most cases: of the 14 types that were found on more than one farm, geographical proximity was apparent for only 6 types, and all of them were on dairy farms in the western Negev area. Also, there were no types shared between calves in the quarantine station and on the fattening farms, with the latter being the usual destination of these calves.
The farm with the largest number of ESBLEC (farm 12, n = 23 isolates) showed a diverse clonal structure with one dominant type (n = 12 isolates) and three additional types. All four types were present in the ESBLPEC isolates retrieved from suckling calves, but only one or two types were identified in the other sections (Fig. 3). The other five farms with ≥15 isolates of ESBLEC per farm had six to seven different REP-PCR types identified at each farm, with one dominant type being present in up to half of the isolates. Similar to farm 12, a diverse population of types was represented in the isolates retrieved from suckling calves.
The four ESBL-producing K. pneumoniae isolates were divided into three BOX-PCR types that were present on three farms.
DISCUSSION
Surveillance of AMRBs in livestock in general and cattle in particular is typically done from an anthropocentric perspective, and thus, it is focused mainly on their implications for humans via either the food chain or the environment (12, 13). Thus, only a few studies have looked into the prevalence and risk factors for ESBLPE carriage in cattle (5, 6). A valid comparison of prevalence and risk factors for ESBLPE carriage between countries is very difficult even in human studies, due to the vast differences in the populations and the methodologies of the different studies (2). Such comparisons are probably even more problematic in veterinary studies that involve a wide variety of designs and methodologies, such as the sampling site and the type of cattle included in the survey. With that in mind, it is hardly surprising that the prevalence found in our study (23.7%) was very different from the prevalence found in Bavarian or Swiss studies (32.8% and 8.4%, respectively) (5, 6). A major difference relates to the sampling site, which was located on farms in both our study and the Bavarian study, whereas in the Swiss study the samples were collected at the slaughterhouse. This difference by itself has tremendous implications for the population selected in regard to both animal age and farming practices and may explain the lower prevalence found in the Swiss study. A wider comparison based on the routine surveillance programs undertaken in several European countries (14) is even more problematic. In these programs, E. coli isolates (one per epidemiological unit) are randomly picked at the slaughterhouse, and the burden of resistance is measured by the proportion of resistant isolates among those picked. Clearly, this methodology risks underestimating the actual prevalence, a likely explanation for the relatively low proportions of cefotaxime-resistant isolates (representing ESBL producers) found in these studies (e.g., 2.5% in Germany).
The highest prevalence of ESBLPE carriage was found in the youngest age groups, usually suckling calves, and with increasing age this gradually declined by as much as 6.5-fold for the prevalence in adult cows. This finding is similar to that of previous studies in cattle (5, 6). It is also reminiscent of the findings of other studies for surveillance of AMRB (e.g., methicillin-resistant Staphylococcus aureus) that were done in human infants in the first 2 years of life, which demonstrated a pattern of early acquisition followed by a gradual decline in prevalence (15). This proposed paradigm is supported by the findings of molecular typing analysis, which showed a diversity of strains in the youngest age group on a particular farm. Later, these strains were partially presented in the older age groups, with occasional spread within these groups (Fig. 3).
Methodologically, this observation (the early acquisition of ESBLPE followed by a gradual decline) suggests that a longitudinal design would have been more appropriate for risk factor analysis than the point prevalence design used in our study. Hence, we think that the bivariate and multivariate analyses that were done on the entire population might have been misleading in regard to some of the risk factors identified. For instance, the sampling of a higher proportion of calves on fattening farms than of calves on dairy farms explains the overall higher prevalence of ESBLPE on the former farms (Table 1), despite the higher prevalence of carriage by each of the age groups on the latter farms (Fig. 2). Therefore, we performed subgroup analyses (Table 3) that allowed partial compensation for the basic limitation of the study design.
From these analyses, it seems that the most conspicuous factor distinguishing the prevalence of ESBLPE in calves from that in the other groups is the use of antimicrobial prophylaxis. The most commonly used agents were tetracyclines (rather than cephalosporins), suggesting a connection between the use of these agents and ESBLPE carriage. Such a connection is not surprising, as numerous reports have correlated the use of agents other than cephalosporins (e.g., quinolones) with infections with or carriage of ESBLPE (2). This finding can be explained by the overall effect of broad-spectrum antimicrobials on the gut microbiota or by the presence of different resistance genes on a shared mobile element (16). Indeed, the rate of tetracycline resistance in these isolates was extremely high (91.7%). Although it was beyond the scope of this study to perform a risk-benefit analysis of the use of prophylaxis in this age group, this seems to be the most important factor that can be targeted for intervention. It is noteworthy that from an anthropocentric perspective, the decline in the prevalence of ESBLPE carriage with age suggests a decreased potential for transmission of ESBLPE to humans.
In addition to antimicrobial prophylaxis, these comparisons also highlighted the importance of several infrastructure factors, including crowdedness and a lack of cleanliness, associated with an increased prevalence of ESBLPE carriage. Unfortunately, we were unable to demonstrate these associations conclusively in the multivariate analysis due to the basic shortcoming of the design.
Although many studies have included molecular analysis of ESBLPE isolates from cattle, our study is unique in its use of typing data to provide an understanding of transmission dynamics in this population. In addition to its contribution to our understanding of intrafarm dissemination (discussed above), this analysis demonstrated the presence of identical isolates on different farms, most importantly, on neighboring farms. The blaESBL gene analysis showed a predominance of the blaCTX-M-1 group, similar to the findings of previous studies (5, 6). Along with the heterogeneity of the isolate population, the predominance of a single group suggests that horizontal gene transfer also plays an important role in the dissemination of these genes, as was also found to be the case with other resistance genes in E. coli (17).
In conclusion, despite the design-based limitations of this study, we were able to provide a combined epidemiological and molecular hypothesis on the cause of dissemination of ESBLPE on cattle farms and to identify modifiable risk factors for possible intervention. More detailed molecular data are required in order to explore the role of horizontal gene transfer in the dissemination of the blaESBL gene in this population and the risk of transfer to humans.
Supplementary Material
ACKNOWLEDGMENT
The work of the National Center for Infection Control, Ministry of Health, was performed in affiliation with the Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01915-15.
REFERENCES
- 1.Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ. 2013. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev 26:289–307. doi: 10.1128/CMR.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J Antimicrob Chemother 66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 4.Hammerum AM, Heuer OE. 2009. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin Infect Dis 48:916–921. doi: 10.1086/597292. [DOI] [PubMed] [Google Scholar]
- 5.Reist M, Geser N, Hächler H, Schärrer S, Stephan R. 2013. ESBL-producing Enterobacteriaceae: occurrence, risk factors for fecal carriage and strain traits in the Swiss slaughter cattle population younger than 2 years sampled at abattoir level. PLoS One 8:e71725. doi: 10.1371/journal.pone.0071725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid A, Hörmansdorfer S, Messelhäusser U, Käsbohrer A, Sauter-Louis C, Mansfeld R. 2013. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli on Bavarian dairy and beef cattle farms. Appl Environ Microbiol 79:3027–3032. doi: 10.1128/AEM.00204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2013. M100-S23. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, Pérez-Cano R, Pascual A. 2004. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol 42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao V, Lambert T, Nhu DQ, Loan HK, Hoang NK, Arlet G, Courvalin P. 2002. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob Agents Chemother 46:3739–3743. doi: 10.1128/AAC.46.12.3739-3743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother 57:154–155. [DOI] [PubMed] [Google Scholar]
- 11.Adler A, Solter E, Masarwa S, Miller-Roll T, Abu-Libdeh B, Khammash H, Najem K, Dekadek S, Stein-Zamir C, Nubani N, Kunbar A, Assous MV, Carmeli Y, Schwaber MJ. 2013. Epidemiological and microbiological characteristics of an outbreak caused by OXA-48-producing Enterobacteriaceae in a neonatal intensive care unit in Jerusalem, Israel. J Clin Microbiol 51:2926–2930. doi: 10.1128/JCM.01049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton RA, Randall LP, Snary EL, Cockrem H, Lotz S, Wearing H, Duncan D, Rabie A, McLaren I, Watson E, La Ragione RM, Coldham NG. 2011. Fecal carriage and shedding density of CTX-M extended-spectrum {beta}-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl Environ Microbiol 77:3715–3719. doi: 10.1128/AEM.02831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carattoli A. 2008. Animal reservoirs for extended spectrum beta-lactamase producers. Clin Microbiol Infect 14(Suppl 1):S117–S123. [DOI] [PubMed] [Google Scholar]
- 14.European Food Safety Authority. 2014. The European Union summary report on antimicrobial resistance in zoonotic antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in the European Union in 2012. EFSA J 12:3590. [Google Scholar]
- 15.Adler A, Givon-Lavi N, Moses AE, Block C, Dagan R. 2010. Carriage of community-associated methicillin-resistant Staphylococcus aureus in a cohort of infants in southern Israel: risk factors and molecular features. J Clin Microbiol 48:531–538. doi: 10.1128/JCM.02290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler A, Miller-Roll T, Assous MV, Geffen Y, Paikin S, Schwartz D, Weiner-Well Y, Hussein K, Cohen R, Carmeli Y. 2015. A multicenter study of the clonal structure and resistance mechanism of KPC-producing Escherichia coli isolates in Israel. Clin Microbiol Infect 21:230–235. doi: 10.1016/j.cmi.2014.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.