Abstract
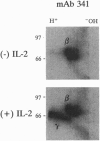
The interleukin 2 receptor (IL-2R) consists of at least two subunits, alpha and beta, both of which can bind interleukin 2 (IL-2). Recent studies have demonstrated the existence of a third subunit, a 64-kDa molecule termed IL-2R gamma chain, and have suggested that gamma chain functions to regulate the rate of IL-2 dissociation from the receptor. In the present report we have addressed whether the gamma chain modulates IL-2R affinity by contributing contact sites for IL-2 binding. Using reagents that allow the IL-2R complex to be immunoprecipitated through the IL-2 molecule itself, we demonstrate the existence of a stable IL-2-IL-2R gamma-chain complex. These studies thus establish that the IL-2R gamma chain directly contributes to the IL-2-binding site, consistent with the hypothesis that gamma chain influences IL-2R affinity through its direct interaction with IL-2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima N., Kamio M., Okuma M., Ju G., Uchiyama T. The IL-2 receptor alpha-chain alters the binding of IL-2 to the beta-chain. J Immunol. 1991 Nov 15;147(10):3396–3401. [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Unraveling the structure of IL-2. Science. 1992 Jul 17;257(5068):410–413. doi: 10.1126/science.1631562. [DOI] [PubMed] [Google Scholar]
- Brandhuber B. J., Boone T., Kenney W. C., McKay D. B. Three-dimensional structure of interleukin-2. Science. 1987 Dec 18;238(4834):1707–1709. doi: 10.1126/science.3500515. [DOI] [PubMed] [Google Scholar]
- Collins L., Tsien W. H., Seals C., Hakimi J., Weber D., Bailon P., Hoskings J., Greene W. C., Toome V., Ju G. Identification of specific residues of human interleukin 2 that affect binding to the 70-kDa subunit (p70) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7709–7713. doi: 10.1073/pnas.85.20.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Garcia G. G., Evans G. A., Michiel D. F., Farrar W. L. Characterization of a tyrosine kinase activity associated with the high-affinity interleukin 2 receptor complex. Biochem J. 1992 Aug 1;285(Pt 3):851–856. doi: 10.1042/bj2850851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. J., Roessler E., Ju G., Tsudo M., Sugamura K., Waldmann T. A. The interleukin 2 receptor (IL-2R): the IL-2R alpha subunit alters the function of the IL-2R beta subunit to enhance IL-2 binding and signaling by mechanisms that do not require binding of IL-2 to IL-2R alpha subunit. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2165–2169. doi: 10.1073/pnas.89.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi J., Seals C., Anderson L. E., Podlaski F. J., Lin P., Danho W., Jenson J. C., Perkins A., Donadio P. E., Familletti P. C. Biochemical and functional analysis of soluble human interleukin-2 receptor produced in rodent cells. Solid-phase reconstitution of a receptor-ligand binding reaction. J Biol Chem. 1987 Dec 25;262(36):17336–17341. [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Imler J. L., Zurawski G. Receptor binding and internalization of mouse interleukin-2 derivatives that are partial agonists. J Biol Chem. 1992 Jul 5;267(19):13185–13190. [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Ju G., Collins L., Kaffka K. L., Tsien W. H., Chizzonite R., Crowl R., Bhatt R., Kilian P. L. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987 Apr 25;262(12):5723–5731. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Kuo L. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. I. Localization of a receptor binding site on IL 2. J Immunol. 1986 Sep 1;137(5):1538–1543. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Lin Y., Robb R. J., Gray J. E., Simon P. The construction and characterization of a biologically active recombinant IL-2 containing a lysine-rich C-terminal extension. J Immunol. 1988 Dec 1;141(11):3847–3851. [PubMed] [Google Scholar]
- McKay D. B. Response. Science. 1992 Jul 17;257(5068):412–413. doi: 10.1126/science.257.5068.412. [DOI] [PubMed] [Google Scholar]
- Michiel D. F., Garcia G. G., Evans G. A., Farrar W. L. Regulation of the interleukin 2 receptor complex tyrosine kinase activity in vitro. Cytokine. 1991 Sep;3(5):428–438. doi: 10.1016/1043-4666(91)90047-h. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ringheim G. E., Freimark B. D., Robb R. J. Quantitative characterization of the intrinsic ligand-binding affinity of the interleukin 2 receptor beta chain and its modulation by the alpha chain and a second affinity-modulating element. Lymphokine Cytokine Res. 1991 Jun;10(3):219–224. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Neeper M. P. Structure-function relationships for the interleukin 2 receptor: location of ligand and antibody binding sites on the Tac receptor chain by mutational analysis. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5654–5658. doi: 10.1073/pnas.85.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvé K., Nachman M., Spence C., Bailon P., Campbell E., Tsien W. H., Kondas J. A., Hakimi J., Ju G. Localization in human interleukin 2 of the binding site to the alpha chain (p55) of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4636–4640. doi: 10.1073/pnas.88.11.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Sharon M., Siegel J. P., Tosato G., Yodoi J., Gerrard T. L., Leonard W. J. The human interleukin 2 receptor beta chain (p70). Direct identification, partial purification, and patterns of expression on peripheral blood mononuclear cells. J Exp Med. 1988 Mar 1;167(3):1265–1270. doi: 10.1084/jem.167.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Suzuki J., Takeshita T., Ohbo K., Asao H., Tada K., Sugamura K. IL-2 receptor subunit, p75: direct demonstration of its IL-2 binding ability by using a novel monoclonal antibody. Int Immunol. 1989;1(4):373–377. doi: 10.1093/intimm/1.4.373. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Suzuki J., Sugamura K. An associated molecule, p64, with high-affinity interleukin 2 receptor. Int Immunol. 1990;2(5):477–480. doi: 10.1093/intimm/2.5.477. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Ohtani K., Asao H., Kumaki S., Nakamura M., Sugamura K. An associated molecule, p64, with IL-2 receptor beta chain. Its possible involvement in the formation of the functional intermediate-affinity IL-2 receptor complex. J Immunol. 1992 Apr 1;148(7):2154–2158. [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Karasuyama H., Kitamura F., Tanaka T., Kubo S., Yamamura Y., Tamatani T., Hatakeyama M., Taniguchi T., Miyasaka M. The IL-2 receptor beta-chain (p70). Ligand binding ability of the cDNA-encoding membrane and secreted forms. J Immunol. 1990 Jul 15;145(2):599–606. [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Robb R. J., Weil-Hillman G., Hank J. A., Sugamura K., Tsudo M., Sondel P. M. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp Med. 1990 Oct 1;172(4):1101–1114. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Sondel P. M., Robb R. J. Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R gamma chain with the IL-2R beta chain in functional intermediate-affinity IL-2R. J Exp Med. 1992 Aug 1;176(2):531–541. doi: 10.1084/jem.176.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]
- Zurawski S. M., Imler J. L., Zurawski G. Partial agonist/antagonist mouse interleukin-2 proteins indicate that a third component of the receptor complex functions in signal transduction. EMBO J. 1990 Dec;9(12):3899–3905. doi: 10.1002/j.1460-2075.1990.tb07610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]