Abstract
Objective
To characterize immune modulation as expressed by cytokine assays at three time-points in human pregnancy.
Study Design
This is a prospective, longitudinal study of a broad panel of cytokine expression during singleton pregnancies resulting in an uncomplicated, full-term, live births. Peripheral blood was obtained at 8–14, 18–22, and 28–32 weeks gestation. Six cytokines—IFN-γ, IL-4, TNF-α, IL-1β, IL-6, and IL-10—were measured in supernatants obtained from whole blood stimulations with PHA or LPS and were compared to unstimulated controls. Samples were processed by Luminex-100 MAP®. We used Generalized Linear Models (GLM) to evaluate cytokine trajectories.
Results
Complete data were obtained for forty-five uncomplicated pregnancies. Overall, peripheral blood WBC’s demonstrated dampened cytokine responses. However, over the course of pregnancy, we found enhanced counter-regulatory cytokine expression (e.g., shown by increased IL-10).
Conclusion
The overall decrease in pro-inflammatory cytokines and increase in counter-regulatory cytokines as uncomplicated pregnancy progresses supports the evolving concepts of immunoregulation for the maintenance of a viable pregnancy.
Keywords: cytokines, immune modulation, tolerance, pregnancy
1. INTRODUCTION
1.1 Role of the Immune System in Pregnancy
The immune system plays an important role in pregnancy, both in normal and pathologic states. The maternal immune response must be modulated to allow establishment and maintenance of a viable pregnancy, comprising allogeneic tissues, without rejection [1]. Infectious complications and dysregulated immune responses have been associated with reproductive pathology and increased risks of abortion [2]. TNF-α, IL-1β, and IL-6 are just a few of the cytokines shown to play a key role in implantation and first trimester miscarriage. Later in pregnancy, elevated TNF-α levels have been associated with pre-eclampsia while decreased IL-10 levels have been associated with pre-term birth [3].
Given the association between immune parameters and obstetric outcomes, early identification of alterations in the immune system may help predict and/or impact pregnancy outcomes. The longitudinal immunologic profile over the course of human pregnancy, however, has yet to be well defined, thus, complicating efforts to characterize the normal modulation of the immune system and to identify systemic immune parameters associated with specific reproductive pathologies and outcomes.
1.2 Cytokines and the Functional Immune Response
Cytokines are a diverse family of soluble small proteins, expressed by various cells and tissue types that act as immune mediators. Their expression profile has been used to categorize immune responses and the functional status of the immune system. Although cytokines are secreted by a number of immune cell types, T cells have often been characterized as playing a key role in determining the nature of an immune response [4]. If there is a predominance of T helper 1 (Th1) cells, the immune system will generate a cell-mediated or cytotoxic response, targeting intracellular pathogens or cancerogenic cells [5]. In contrast, a predominance of T helper 2 (Th2) cells would favor an antibody-mediated or humoral response to target extracellular pathogens like bacteria [6]. Th1 and Th2 responses were among the first classes of immune responses to be characterized, generally function in opposition to one another, and have been extensively studied [7]. The number of human cytokines described continues to increase with at least 33 interleukins (ILs) identified to date [8]. In humans, Th1 associated cytokines include interferon gamma (IFN-γ) and IL-12 while Th2-associated cytokines include IL-4,-5, and -13 [1]. Another cytokine category, which includes TNF-α, IL-1β, and IL-6, is considered to exhibit pro-inflammatory function [4].
1.3 T reg cells and Immune Suppression
More recently, regulatory T cells (T reg) or T helper type 3 (Th3) responses have been identified [9, 10] and have been found to play an immune suppressive role including in the setting of pregnancy [11]. Although the exact mechanism by which regulatory T cells function has yet to be firmly established, the cytokine IL-10 appears to play a critical role [12,13]. IL-10 is a particularly intriguing cytokine. In humans, IL-10 is a pleiotropic cytokine with both immune stimulatory and counter-regulatory (immune suppressive) functions that place it outside of the Th1–Th2 paradigm, although it was originally described as a Th2-associated cytokine in rodents [14, 15].
1.4 Innate versus Adaptive Arms of the Immune System
Cytokines can be secreted predominantly by cellular elements of the innate arm of the immune system such as monocytes (monokines), predominantly by components of the adaptive arm of the immune system such as lymphocytes (lymphokines), or by both [6]. For instance, monocytes are the predominant source of IL-1β while lymphocytes are the predominant source of IL-4 and IFN–γ [7]. Monocytes and lymphocytes express TNF-α, IL-6, and IL-10 [4]. Monocytes and lymphocytes respond to different stimuli, and thus the expressed cytokines along with the limitations inherent in the collection of human biological specimens, necessitate critical attention to the conditions under which specific samples are examined. For example, selection of a stimuli targeting one cell type (e.g., LPS stimulation of monocytes) will not provide information regarding primary stimulation of lymphocytes, thus limiting the capacity to evaluate their contribution to the physiologic or pathologic process in question [6,7].
1.5 Current Paradigms and Study Objective
Various paradigms have been proposed for the modulation of the immune system that maintains a viable pregnancy [3, 16]. These paradigms have focused on phenomena such as “missing self” [17], “Th2 immunologic bias” [18] and more recently “immunosubversion” [19]. Current data support a significant role for placental mechanisms that may dampen immune responses rather than a shift in the immune system during pregnancy from a Th1–Th2 balance to a Th2 bias [20]. Many of the studies supporting these various paradigms were conducted in murine models with problems inherent in generalizing rodent systems to humans. Existing human data are mostly limited to individuals with a history of reproductive pathology or is limited by cross-sectional study design [21–26].
Thus, we conducted a prospective longitudinal epidemiologic study of recently diagnosed pregnant women over the course of their pregnancy in which we used three incubation conditions for whole blood samples to obtain measures of the longitudinal systemic cytokine expression profile associated with pregnancy. Ex vivo analysis offers the clinically important advantages of 1) retaining all blood components, including serum, 2) maintaining the target cell types at their in vivo ratios with other cell types and non-cellular components, 3) ease of handling (does not involve cell purification), and 4) economy in processing costs [27].
2. MATERIALS AND METHODS
2.1 Study Design
This study is part of the Philadelphia Collaborative Preterm Prevention Project sponsored by The Children’s Hospital of Philadelphia (CHOP) and Drexel University. IRB approval has been obtained from both universities (CHOP IRB 09-007191, date of approval 12/28/2009 and Drexel IRB 40568, date of approval 9/10/01, renewal 9/10/09). We initiated a prospective, longitudinal investigation of immune and neuroendocrine parameters associated with stress, infection, and preterm birth.
2.2 Recruitment
For this study, all women attending their first prenatal visit at a hospital-based clinic in Philadelphia, PA between March 2001 and November 2002 were screened for eligibility. Eligible women were enrolled after providing written informed consent. Women were considered eligible for participation if they, 1) were less than fifteen weeks gestation, 2) were seventeen years of age or older, 3) had a singleton intrauterine pregnancy, and 4) were English or Spanish speaking. Additionally, women were excluded from participating if they had taken antibiotics in the fifteen days prior to enrollment, had taken systemic corticosteroids six months prior to enrollment, or if they had ever been diagnosed with HIV/AIDS, sarcoidosis, rheumatoid arthritis or any other immunologic disorder.
Over the twenty month recruitment period, 681 women were screened for this study with 267 women being found to be eligible. Among the 267 eligible women, 234 (87.6%) consented to participate and subsequently completed the enrollment survey and provided a baseline phlebotomy sample. At the 2nd trimester visit, 201 women remained eligible for the study, and, of these, 174 (86.6%) completed their study questions and phlebotomy. At the 3rd trimester visit, 192 study participants remained eligible, and, of these, 164 (85.4%) completed their survey questions and phlebotomy.
Women became ineligible at the second or third trimester visits because of the following reasons: (1) they were no longer pregnant because they had an abortion (N=12), a stillbirth (N=2), or a miscarriage (N=21), (2) they developed a medical condition that excluded them from participation (N=1), or (3) they moved out-of-state and could no longer take part in the study (N=2).
For these analyses, we included all participants with complete immune data for their first three study visits and available medical record data providing documentation of a live birth. Cytokine analysis was discontinued during the course of the study due to cost of the assays, resulting in a total study group of pregnancies that includes forty-five eligible participants with complete data to establish cytokine profiles over the three trimesters of a “normal pregnancy” defined as singleton pregnancies that concluded in a live birth at ≥ 37 weeks gestation in the absence of adverse outcome (e.g., pre-eclampsia, preterm delivery, growth restriction, low birth weight, infection, diabetes).
2.3 Data Collection
Baseline medical history, demographic, behavioral, and psychosocial data were collected from study participants at the time of enrollment by trained female interviewers. Baseline phlebotomy was collected during the first trimester (8–14 weeks gestation). Study participants were followed at their regularly scheduled prenatal appointments with updated psychosocial status and medical history data along with phlebotomy specimens obtained at two additional intervals—once during their second trimester (between 18 and 22 weeks’ gestation) and once during their third trimester (between 28 and 32 weeks’ gestation).
Medical records were reviewed to obtain and verify antepartum, intrapartum, and post-partum information about the study participant’s medical history and medical conditions as well as documentation of a live birth from available medical records. To ensure accuracy of the medical record information, medical records were abstracted by trained personnel with all controversial aspects of the chart flagged and reviewed by three consulting physicians for eligibility purposes.
2.4 Immunologic Assays
At each of three time points, phlebotomy samples were collected by standard antecubital venipuncture with vacutainer collection in sodium heparin anticoagulant, for in vitro cytokine release assays. For the cytokine release assay, the sample of anti-coagulated maternal blood was collected and transported on ice within thirty minutes to the on-site laboratory for processing as follows: the cytokine release analysis was performed on incubated supernatants from stimulated whole blood. For the cytokine release assay, twenty-four well plates were prepared in advance with 1.5 ml of media consisting of RPMI 1640 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and penicillin/streptomycin (Invitrogen, Carlsbad, CA) at 1:100 dilution added to individual wells. Plates were stored at −20°C, thawed and brought to room temperature immediately before use. Heparin anti-coagulated blood samples were distributed, 0.5 ml of whole blood, into four individual wells of the previously prepared twenty-four well plate containing one of the three conditions: 1) supplemented with phytohemagglutinin (PHA) (Sigma, St. Louis, MO) at a final concentration of 5 μg/ml, 2) supplemented with lipopolysaccharide (LPS) −2 (Sigma, St. Louis, MO) at a final concentration of 1 μg/ml, or 3) unsupplemented as the control. All plates were incubated for twenty-four hours at 37° C in 5% humidified CO2. At the conclusion of this period, plates were placed on ice and the contents of the four wells for each condition were transferred to 15 ml conical tubes. Samples were centrifuged at 4°C for ten minutes at 300 x g followed by distribution of the supernatant into 500 μl aliquots that were immediately frozen at −80° C and maintained frozen until analysis in triplicate by Luminex-100 MAP® methods using commercially available kits (Linco Research Inc. St. Charles, MO) according to manufacturer’s instructions and as previously reported [27].
We selected the following cytokines for analysis: IFN γ, the prototypical Th1-associated cytokine; IL-4, a Th2-associated cytokine; the less specific pro-inflammatory cytokines TNF-α, IL-1β, and IL-6; and IL-10, a predominant counter-regulatory cytokine associated with suppression of T cell maturation. We employed two primary stimuli: 1) LPS that activates CD14 & TLR-4 expressing cells, i.e. monocytes, and 2) PHA that activates predominantly T lymphocytes. Together, these stimuli provide measures of induced cytokine expression for monocytes, components of the innate arm of the immune system, and for lymphocytes, integral elements of the adaptive arm of the immune system. Notably, the utilization of a mitogen has some limitations, as it does not precisely replicate an antigen-specific response (i.e., in vivo interactions between monocytes/dendritic cells and T cells). However, mitogen use has been validated and is a widely accepted approach for the study of general, polyclonal immune responses [18, 27]. Cytokine expression was evaluated at twenty-four hours to limit the effect of secondary induction of cytokine byproducts derived from the primary stimulus.
2.5 Statistical Analysis
Replicate cytokine determinations were evaluated for potential outliers within the triplicate values for each sample using the Grubbs formula at the 5% critical Z-value [28]. Mean determinations for replicates from each sample were used for subsequent analysis.
To assess immune modulation during pregnancy, we fit a regression model to assess the trajectory of each cytokine throughout pregnancy. Specifically for all regression analyses, we used Generalized Linear Models with the Gamma family of distributions and a log link [28]. Such models accounted for the distribution of the data. Since the cytokine values were measured repeatedly over time, and thus correlated for each subject, we calculated robust standard errors using the Huber-White sandwich estimator [28]. Statistical significance was defined at p<0.05.
3. RESULTS
We conducted a longitudinal study of eligible pregnant women in an attempt to describe cytokine profiles across uncomplicated pregnancy. Forty-five subjects had complete medical record data and assayed phlebotomies at all three time points. Physician review verified that the pregnancies resulted in singleton livebirths at ≥ 37 weeks gestation and that they were uncomplicated; i.e., none of the subjects included in the analysis had pre-eclampsia, diabetes, low birthweight/intrauterine growth restriction, or diagnosis of antepartum/intrapartum infection.
3.1 Population Demographics
Demographic characteristics of the study sample are presented in Table 1. All participants enrolled during the first trimester of pregnancy. These women were mostly young, black, born in the U.S., multiparous, and had limited income and education.
Table 1.
Sociodemographic Characteristics of Sample (N=45).
| Age--mean years (sd) | 23.1 (5.1) |
| Race/Ethnicity--no. (%) | |
| Non-hispanic White | 3 (6.7) |
| Hispanic/Latina | 6 (13.3) |
| Non-hispanic Black | 34 (75.6) |
| Other | 2 (4.4) |
| US Born--no. (%) | 37 (82.2) |
| Education--no. (%) | |
| Did not finish High School | 14 (31.1) |
| High School or GED | 22 (48.9) |
| Continued Post High School | 9 (20.0) |
| Marital Status, single—no. (%) | 32 (71.1) |
| Annual Income--median dollars (range) | 10838 (0,36120) |
| Nulliparous--no. (%) | 17 (37.8) |
| Gestational age at recruitment--mean weeks (sd) | 10.3 (2.4) |
3.2 Th1 Response
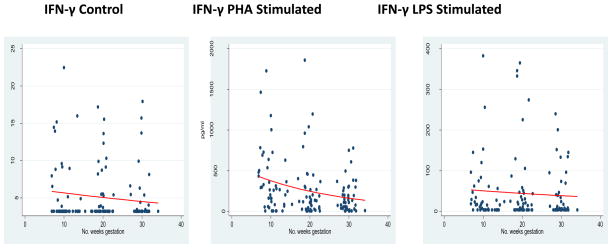
Median values and interquartile ranges for each measured cytokine are summarized in Table 2. Regression coefficients and percent change in cytokine measurements for gestational age weeks along with respective confidence intervals are presented in Table 3. To evaluate modulation in Th1 response, we measured IFN-γ. In both control and PHA-stimulated conditions, significant decreases occurred in maternal cytokine responses—IFN-γ control β= −0.011 (95% CI −0.022 to −0.001; p=0.03), and IFN-γ PHA-stimulated β = −0.042 (95% CI −0.055 to −0.029; p<0.01). No significant decrease occurred in that of the LPS-stimulated condition, INF-γ LPS stimulation β = −0.014 (95% CI −0.043 to 0.016; p=0.3). In effect, the observed trend was toward a dampened maternal Th1 response as pregnancy progressed, Figure 1, Table 3.
Table 2.
Median and Inter-quartile Range IQR* Culture Supernatant Cytokine Concentrations (pg/ml) By Trimester
| Sample | 1st Trimester** | 2nd Trimester** | 3rd Trimester** | |||
|---|---|---|---|---|---|---|
| median | IQR | median | IQR | median | IQR | |
| IFNγ--Control | 3.2 | 3.2, 5.2 | 3.2 | 3.2, 5.5 | 3.2 | 3.2, 3.4 |
| IFNγ--PHA | 264.2 | 84.1, 502.0 | 118.7 | 24.9, 305.6 | 105.1 | 34.1, 202.3 |
| IFNγ--LPS | 15.8 | 3.2, 55.9 | 14.1 | 3.2, 44.7 | 5.5 | 3.2, 39.1 |
| IL4--Control | 3.2*** | 3.2, 3.3 | 3.2 | 3.2, 3.2 | 3.2 | 3.2, 3.2 |
| IL4--PHA | 168.3 | 42.1, 281.8 | 93.2 | 20.8, 252.0 | 70.3 | 27.7, 181.1 |
| IL4--LPS | 3.2 | 3.2, 3.2 | 3.2 | 3.2, 3.2 | 3.2 | 3.2, 3.2 |
| TNFα--Control | 79.8 | 24.9, 189.0 | 66.9 | 30.9, 120.2 | 47.0 | 25.0, 139.1 |
| TNFα--PHA | 344.5 | 265.8, 464.4 | 281.9 | 162.5, 437.9 | 268.3 | 185.9, 416.3 |
| TNFα--LPS | 591.4 | 400.8, 790.5 | 626.2 | 362.7, 906.6 | 580.8 | 356.3, 959.2 |
| IL1β--Control | 39.6 | 4.8, 77.0 | 18.3 | 4.9, 36.9 | 6.3 | 3.2, 20.9 |
| IL1β--PHA | 86.3 | 48.3, 115.9 | 71.2 | 42.4, 105.3 | 48.6 | 35.7, 83.0 |
| IL1β--LPS | 345.6 | 272.7, 613.6 | 364.2 | 206.1, 654.3 | 345.3 | 170.2, 581.0 |
| IL6--Control | 4110.7 | 865.6, 9285.0 | 3240.4 | 1393.6, 8808.8 | 2019.7 | 617.4, 7760.8 |
| IL6--PHA | 9542.1 | 8596.3, 10796.4 | 9987.4 | 6802.7, 10854.6 | 9610.4 | 7168.1, 10963.6 |
| IL6--LPS | 11070.9 | 10246.6, 11778.9 | 11270.4 | 10111.8, 11845.2 | 11136.8 | 10072.4, 12273.2 |
| IL10--Control | 195.9 | 28.9, 332.1 | 79.2 | 32.2, 331.6 | 62.8 | 22.5, 176.7 |
| IL10--PHA | 1515.1 | 1095.2, 2002.7 | 1456.6 | 785.7, 1928.2 | 1570.1 | 897.9, 2126.8 |
| IL10--LPS | 1584.4 | 1191.2, 2093.4 | 2006.8 | 1233.0, 2619.8 | 2208.6 | 1467.1, 2902.4 |
IQR represents the 25th and 75th percentile values for each cytokine within trimester.
N=45 subjects for T1, T2, and T3.
3.2 pg/ml was the lower limit of detection established by the reagent kit manufacturer and the lowest standard on the standard curve.
Table 3.
Regression Coefficients, Percent Change, and 95% Confidence Intervals for Longitudinal Cytokine Trajectories.
| Dependent Variable | β coefficient for gestational age weeks | 95% CI | %Change for gestational age weeks | 95% CI for % change |
|---|---|---|---|---|
| IFNγ--Control | −0.011* | −0.022, −0.001 | −1.1* | (−2.2, −0.09) |
| IFNγ--PHA | −0.042* | −0.055, −0.029 | −4.1* | (−5.4, −2.9) |
| IFNγ--LPS | −0.014 | −0.043, 0.016 | −1.4 | (−4.2, 1.6) |
| IL4--Control | 0.002 | −0.004, 0.008 | −0.20 | (−0.4, 0.80) |
| IL4--PHA | −0.021* | −0.035, −0.006 | −2.1* | (−3.4, −0.6) |
| IL4--LPS | −0.002 | −0.015, 0.011 | −0.19 | (−1.5, 1.1) |
| TNFα--Control | −0.015* | −0.029, −0.015 | −1.5* | (−2.9, −1.5) |
| TNFα--PHA | −0.014* | −0.023, −0.005 | −1.4* | (−2.3, −0.5) |
| TNFα--LPS | 0.006 | −0.004, 0.015 | 0.6 | (−0.4, 1.5) |
| IL1β--Control | −0.069* | −0.086, −0.051 | −6.6* | (−8.2, −5.0) |
| IL1β--PHA | −0.028* | −0.040, −0.016 | −2.8* | (−3.9, −1.6) |
| IL1β--LPS | −0.010 | −0.028, 0.009 | −0.99 | (−2.7, 0.90) |
| IL6--Control | −0.019* | −0.033, −0.005 | −1.9* | (−3.2, −0.5) |
| IL6--PHA | −0.003* | −0.006, −0.001 | −0.3* | (−0.6, −0.1) |
| IL6--LPS | 0.002 | −0.003, 0.006 | 0.2 | (−0.3, 0.6) |
| IL10--Control | −0.016 | −0.040, 0.008 | −1.6 | (−3.9, 0.8) |
| IL10--PHA | −0.004 | −0.012, 0.004 | −0.4 | (−1.2, 0.4) |
| IL10--LPS | 0.011* | 0.001, 0.021 | 1.1* | (0.1, 2.1) |
Data derived from the entire study population (N=45) was analyzed for longitudinal linear trends for each cytokine and culture condition. Significant trends are denoted by an asterisk to identify p-values <0.05.
Figure 1.
Th1 Response Over Gestation
*GLM models for IFN-γ in each incubation condition.
3.3 Th2 Response
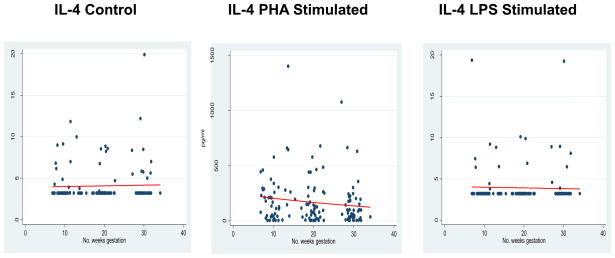
IL-4 was used as the surrogate measure for the evaluation of Th2 response. No significance longitudinal change was observed in the absence of antigenic stimulation; e.g., IL-4 Control β = 0.002 (95% CI −0.004 to 0.008; p=0.50). Likewise, no significant modulation occurred in the presence of LPS-stimulation; β = −0.002 (95% CI −0.015 to 0.011; p=0.74). Oddly, a significant decrease in response to the lymphocyte mitogen, PHA, was demonstrated; β =−0.021 (95% CI −0.035 to −0.006; p<0.01) as depicted in Figure 2 and Table 3.
Figure 2.
Th2 Response
*GLM models for IL-4 expression in each incubation condition.
3.4 Pleiotrophic Pro-inflammatory Response
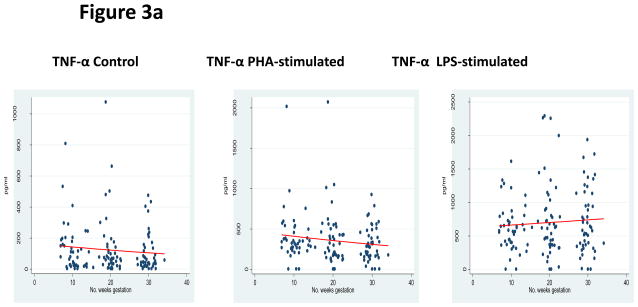
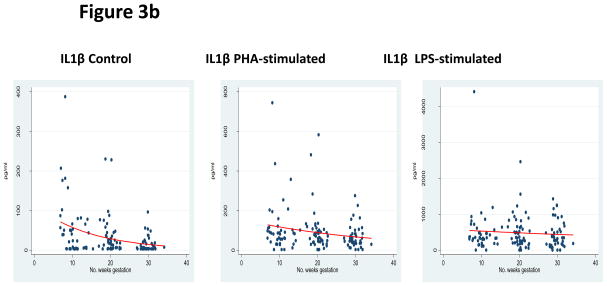
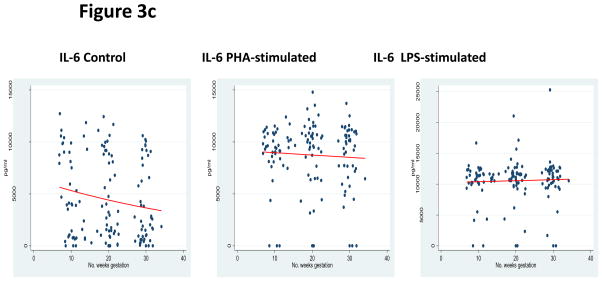
Assessment of the pleiotrophic pro-inflammatory cytokines was performed by measuring TNF-α, IL-1β, and IL-6. In both control and PHA-stimulated incubations, significantly decreased responses were observed as pregnancy progressed—TNF-α control β −0.015 (95% CI −0.029 to −0.015; p=0.03), TNF-α PHA stimulation β= −0.014 (95% CI −0.023 to −0.005; p<0.01), IL-1β control β = −0.069 (95% CI −0.086 to −0.051; p<0.01), IL-1β PHA-stimulated β = −0.028 (95% CI −0.040 to −0.016), IL-6 control β = −0.019 (95% CI −0.033 to −0.005; p<0.01), and IL-6 PHA stimulation β = −0.003 (95% CI −0.006 to −0.001; p<0.01). LPS-stimulation did not result in any significant changes: TNF-α LPS stimulation β = 0.006 (95% CI −0.004 to 0.015; p=0.29), IL-1β LPS stimulation β= −0.010 (95% CI −0.028 to 0.009; p=0.32), and IL-6 LPS stimulation β = 0.002 (95% CI −0.003 to 0.006; p=0.50). Overall, there was a diminished pleiotrophic pro-inflammatory response over the course of pregnancy, as shown in Figures 3a, 3b, and 3c and Table 3.
Figure 3.
Figure 3a: Pleiotrophic Pro-inflammatory Response
*GLM models for TNF-α expression in each incubation condition.
Figure 3b: Pleiotrophic Pro-inflammatory Response
*GLM models for IL1β expression in each incubation condition.
Figure 3c: Pleiotrophic Pro-inflammatory Response
*GLM models for IL-6 expression in each incubation condition.
3.5 Counter-regulatory Response
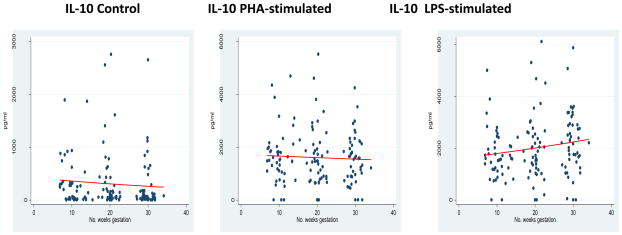
The longitudinal measurement of the counter-regulatory cytokine IL-10 demonstrated some interesting patterns. No significant change of IL-10 expression occured in either the absence of antigenic stimulation (control) or in response to PHA-stimulation. However, in response to the chosen monocyte stimulant, LPS, IL-10 expression increased as pregnancy progressed, β = 0.011 (95% CI 0.001 to 0.021). See Figure 4 and Tables 2 and 3.
Figure 4.
Counter-Regulatory Response
*GLM models for IL-10 expression in each incubation condition.
Recall that we evaluated replicate cytokine determinations for potential outliers in each condition and that we utilized mean determinations for replicates from each sample in the analysis to produce the above results. Notably, we performed our analysis with and without the outliers. No changes in statistical significance were noted when removing outliers from the analysis, indicating that our analysis and data sets were robust.
4. DISCUSSION
Evaluation of our study population revealed a general trend over pregnancy toward enhanced counter-regulatory cytokine expression, and a dampening of Th1-associated and pleiotrophic cytokine expression. Longitudinal modulation of Th2-associated cytokine expression was less clearly demonstrated but did demonstrate a muted response during lymphocyte stimulation.
Studies published to date have been limited by relatively small sample sizes and absence of longitudinal sampling for individual subjects. In effect, the complete immune profile in normal pregnancy has yet to be fully described. Because immune modulation is thought to be important in the maintenance of a viable pregnancy as well as the development of normal or pathologic outcomes, we sought to characterize immune system changes in pregnancy. Our study was designed to prospectively follow whole blood cytokine profiles in singleton pregnancies throughout pregnancy.
In our study population, PHA-stimulated expression of most of the measured cytokines was generally observed to decrease over pregnancy, including a significant decrease in IFN-γ, representative of the Th1 class of immune response. Recall that this response is directed towards intracellular pathogens, such as viruses, and is responsible for hyperacute allograft rejection, suggesting that the immune system is modulating itself for tolerance of the fetal allograft. Likewise, significant decreases in response were observed in the pleiotropic pro-inflammatory cytokines (TNFα, IL-1β, and IL-6) that were measured, further supporting immune modulation’s role in pregnancy. One could speculate that this decreased inflammation not only permits tolerance of the fetal allograft but also highlights the importance of decreased inflammation so as to promote carrying a pregnancy to term. These findings are consistent with the evolving paradigm of immunologic modulation associated with pregnancy.
Zenclussen and colleagues found T regulatory cell activity to play a critical role in murine models of pregnancy [11]. Our observation—the trajectory of increasing IL-10 expression was seen with LPS stimulation but not that of PHA stimulation—at first glance, may be confusing. However, dendritic cells (DCs) at the maternal-fetal interface possess the capacity to bridge between the innate and adaptive arms of the immune system. IL-10 appears to be critical for the induction of tolerogenic dendritic cells, which are likewise critical in the induction of T suppressor cells and other regulatory cells. In effect, our observation of a longitudinal increase in stimulated IL-10 expression is placed squarely within the evolving reproductive immunology paradigm [1]. Our understanding of T regulatory cell biology and mechanisms of tolerance in humans is evolving and future studies may implicate other cytokines with counter-regulatory or immune suppressive activities, such as TGF beta. Nevertheless, we speculate that this increased expression in IL-10 is a driving force in suppressing the generation of a Th1 and pro-inflammatory cytokine class responses to achieve an overall suppression in maturation of T cells during pregnancy.
The importance of this suppression could be tested by comparing uncomplicated pregnancy to complicated pregnancy (e.g., spontaneous preterm deliveries, preeclampsia, and intra-uterine growth restriction). If cytokine trajectories vary by outcome, we could identify patients at risk for adverse outcomes by immunologic profiling during the course of pregnancy. Furthermore, learning which controllable factors modulate cytokine trajectories may help us guide patient care in terms of prevention, risk reduction for adverse outcome, and development of potential interventions.
Our data indicate an overall decrease in pro-inflammatory cytokine trajectories in the innate and adaptive arms of the immune system and an increase in counter-regulatory cytokines as pregnancy progresses; these changes support the role of immune modulation to permit maintenance of a viable pregnancy. Future studies in this area may prove useful in the care of the obstetrical patient to both identify predictive deviations from this normal longitudinal pattern and to potentially identify interventions to modify the outcomes for at risk populations.
Acknowledgments
Patient care and data collection for this study are supported by the Pennsylvania Department of Health and The March of Dimes. Cytokine assays were performed at the UC Irvine Development, Health and Disease Research Program Laboratory by Edward Nelson, MD and Pathik D. Wadhwa, MD, PhD., and were supported, in part, by US PHS (NIH) grants RO1 HD-041696 and PO1 HD-047609 to PDW.
Footnotes
Author’s disclosure: All of the authors state that they have no financial interests that would be viewed as a potential conflict of interest. Moreover, we report that the data contained in this study has not been presented in any other published manuscript. These data were presented as an oral session on February 2, 2008 at the Annual Pregnancy Meeting for the Society of Maternal-Fetal Medicine in Dallas, Texas and published in abstract format in the December 2007 edition of The American Journal of Obstetrics & Gynecology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaouat G, et al. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004;92(3):207–14. doi: 10.1016/j.imlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Kwak-Kim JY, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chem Immunol Allergy. 2005;88:64–79. doi: 10.1159/000087821. [DOI] [PubMed] [Google Scholar]
- 3.Raghupathy R. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin Immunol. 2001;13(4):219–27. doi: 10.1006/smim.2001.0316. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald K, editor. The Factsbook Series. 2. Academic Press; San Diego, CA: 2001. The Cytokine Factsbook; p. 515. [Google Scholar]
- 5.Wilczynski JR. Cancer and pregnancy share similar mechanisms of immunological escape. Chemother. 2006;52(3):107–10. doi: 10.1159/000092537. [DOI] [PubMed] [Google Scholar]
- 6.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223–46. [PubMed] [Google Scholar]
- 7.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10(12):542–50. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Carroll HP, Gadina M. The newest interleukins: recent additions to the ever-growing cytokine family. Vitam Horm. 2006;74:207–28. doi: 10.1016/S0083-6729(06)74008-0. [DOI] [PubMed] [Google Scholar]
- 9.Curotto de Lafaille MA, et al. Do regulatory T cells play a role in the control of homeostatic proliferation? Int Rev Immunol. 2005;24(3–4):269–84. doi: 10.1080/08830180590935001. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 11.Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65(2):101–10. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Taylor A, et al. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunol. 2006;117(4):433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunol. 2001;103(2):131–6. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti P, et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86(2):123–9. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 15.Wakkach A, Cottrez F, Groux H. Can interleukin-10 be used as a true immunoregulatory cytokine? Eur Cytokine Netw. 2000;11(2):153–60. [PubMed] [Google Scholar]
- 16.Raghupathy R, et al. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod. 2000;15(3):713–8. doi: 10.1093/humrep/15.3.713. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JS, et al. HLA-G and immune tolerance in pregnancy. Faseb J. 2005;19(7):681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 18.Makhseed M, et al. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol. 1999;42(5):273–81. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 19.Block MS, Markovic S. The Tumor/Immune Interface: Clinical Evidence of Cancer Immunosurveillance, Immunoediting and Immunosubversion. Am J Immunol. 2009;5(1):29–49. [Google Scholar]
- 20.Wegmann TG, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 21.Marzi M, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. Am J Reprod Immunol. 2005;54(1):30–7. doi: 10.1111/j.1600-0897.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 23.El-Shazly S, et al. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol. 2004;52(1):45–52. doi: 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 24.Makhseed M, et al. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49(5):308–18. doi: 10.1034/j.1600-0897.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 25.Raghupathy R, et al. Cytokine patterns in maternal blood after premature rupture of membranes. Obstet Gynecol. 2001;98(1):122–6. doi: 10.1016/s0029-7844(01)01408-9. [DOI] [PubMed] [Google Scholar]
- 26.Steinborn A, et al. Anti-fetal immune response mechanisms may be involved in the pathogenesis of placental abruption. Clin Immunol. 2004;110(1):45–54. doi: 10.1016/j.clim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.dupont NC, et al. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66(2):175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheskin D. In: Handbook of Parametric and Nonparametric Statistical Procedures. Hall C, editor. Boca Raton, FL: CRC Press; 2004. [Google Scholar]