Abstract
Dysregulation of microRNA-21 (miR-21) is independently associated with human immunodeficiency virus (HIV) infection, pulmonary arterial hypertension (PAH), and hepatitis C virus (HCV) infection. To assess expression of miR-21 in these overlapping comorbidities, we measured plasma miR-21 in HIV with and without PAH and then stratified by concomitant HCV infection. miR-21 was increased in HIV and HIV-PAH versus uninfected subjects, but did not differ between these groups. HIV/HCV co-infection correlated with even higher miR-21 levels within the HIV-infected population. These data reveal specific regulation of plasma miR-21 in HIV, HIV/HCV co-infection, and PAH and suggest that miR-21 may integrate complex disease-specific signaling in the setting of HIV infection.
Keywords: MicroRNA, HIV/HCV co-infection, HIV-PAH
Introduction
Persons infected with the human immunodeficiency virus (HIV) suffer from numerous co-morbid conditions, causing complex disease progression. For example, pulmonary arterial hypertension (PAH) is a deadly disease with increased prevalence in the HIV-infected population 1,2. Additionally, co-infection with hepatitis C virus (HCV) is thought to accelerate morbidity in the HIV-infected population and may contribute to the pathogenesis of PAH, either related to portopulmonary hypertension or from a direct viral toxicity to the pulmonary vasculature 3,4.
MicroRNAs (miRNAs) are non-coding RNA molecules that are implicated as regulators of pathogenesis in both HIV and PAH 5–10. In addition, HCV infection dysregulates multiple host cell miRNAs, and some reports suggest that disease acceleration in HIV/HCV co-infection may be modulated by miRNAs 4,11,12. Recently, it was demonstrated that miRNAs are stably secreted into the bloodstream at rest 13 and in provocative conditions. Alterations in such expression often reflect the fundamental actions of each miRNA 14–16. Thus, coordinate regulation of plasma miRNAs by comorbid diseases may reveal specific miRNAs as molecular lynchpins of complex pathology.
miR-21 is a ubiquitous and pleiotropic miRNA, and its relative circulating expression in patients with HIV, HIV/HCV co-infection, and HIV-PAH may inform our understanding of the integrated pathogenesis of these diseases. Intracellular miR-21 is controlled by signaling pathways important to HIV and modulates PAH pathogenesis 17–22. Interestingly, plasma miR-21 has been identified as a marker of acute lentiviral infection in simian immunodeficiency virus 23,24 and is increased in the B-cells and serum of HIV patients prior to developing AIDS-associated non-Hodgkin’s lymphomas 25,26. Plasma miR-21 levels are also elevated during chronic HCV infection 27.
Given its actions related to HIV, HCV, and PAH, miR-21 may integrate signaling between these concomitant diseases, and its release into the circulating plasma may reflect this overlapping biology. As a result, we sought to determine whether plasma miR-21 is additively regulated by HIV infection and HIV-PAH and whether such alterations are further modulated by HCV co-infection.
Methods
Subjects
Three groups of human subjects (HIV alone (without PAH), HIV-PAH, and uninfected subjects) were recruited from 1) a cohort of HIV patients with and without PAH at San Francisco General Hospital (SFGH), 2) a cohort of HIV-PAH patients at University of California, San Francisco (UCSF), and 3) a cohort of uninfected patients without PAH at the Massachusetts General Hospital (MGH) and SFGH. Patients were confirmed HIV-infected by letter of diagnosis and/or standard ELISA testing. All controls were tested for HIV infection and confirmed as HIV-uninfected prior to study enrollment. All subjects gave written consent before participation. These methods were approved by institutional review boards at SFGH and UCSF (the Committee on Human Research), as well as MGH (Partners Human Research Committee).
Clinical data
Data on past medical history, NYHA status, medications and laboratory data were collected both from patient questionnaires and chart review within 1 month of the date of plasma collection.
Distance walked on six-minute walk test, right heart catheterization, and echocardiography were performed for patients after appropriate consent was obtained as previously described 28. Catheterization was performed by cardiologists at UCSF and SFGH hospitals using standard equipment and technique.
PAH was defined as a mean pulmonary arterial pressure (mPAP) greater than 25 mmHg with a pulmonary capillary wedge pressure less than 15 mmHg by right heart catheterization 29.
HCV co-infection was defined as a positive HCV antibody at any time prior to plasma collection. Eleven patients did not have HCV antibody titer available.
Measurement of plasma miRNA
Venous blood was collected in standard anticoagulant (EDTA)-treated vacutainer tubes. Cellular elements were pelleted in each blood sample following blood draw via centrifugation at 2000 × g for 10 min. The supernatant plasma was then aliquoted and immediately frozen at −80°C. A miRNA with poor endogenous expression in human plasma (hsa-miR-422b mimic, ThermoScientific) was added to plasma samples (4 fmol per 150 μl plasma) for quantitative normalization of plasma miRNA levels, as has been standard operating procedure in previous work 30. Such a spiked-in control facilitates technical rather than biological normalization of expression. Notably, at this time, there is no universal consensus for an endogenous biological normalizer for small RNAs in plasma. As previously described 31, total RNA extraction was performed using a MicroRNA Extraction Kit (Benevbio). Reverse transcription was performed to generate complementary DNA (cDNA) representing levels of mature miRNA molecules (MicroRNA Assay Kit, ThermoScientific). An Applied Biosystems 7900HT Fast Real Time PCR device was used to amplify cDNA using fluorescently labeled Taqman probe and primer sets. Fold change of RNA species was calculated using the formula 2(-ΔΔCt) relative to the exogenous miRNA-422b mimic. Taqman primer/probes for quantification were used as follows (ThermoScientific): hsa-miR-21 [AB 4427975 (000397)], hsa-miR-145-5p [AB 4427975 (002278)] and hsa-miR-422b [AB 4427975 (000575)].
Statistical Analyses
Using Stata®, logistic and linear regression, ANOVA and student’s T-test were employed. For missing data, subjects were excluded from analysis for that variable rather than perform an imputation-based estimate.
Results
A total of 57 HIV-infected and 16 uninfected controls were studied (Table 1). The HIV-infected patients included 39 HIV-PAH patients [median age 46 years, 79% male, average mean pulmonary artery pressure (mPAP) 43 mmHg], 18 HIV-infected patients without PAH [median age 51 years, 72% male, 66.7% on antiretroviral therapy (ART)], and 16 uninfected individuals [median age 49 years, 62% male]. Among all HIV patients, the median CD4 count was 484 (322–640) cells/μL, 73% were on ART and 48% of individuals had an undetectable HIV RNA level. In the subset of HIV patients without PAH, the median CD4 count was 500 (366–654) cells/μL, and 44% had an undetectable viral load. In the subset of HIV-PAH patients, the median CD4 count was 425.5 cells/μL (IQR 277–632 cells/μL) and 50% of individuals had an undetectable viral load. The proportion of individuals on ART, with an undetectable HIV RNA level, and current CD4 count were similar between HIV patients with and without PAH. Traditional cardiovascular risk factors were common in the HIV-infected population (including those with and without PAH) and included 40% with hypertension, 11% with history of myocardial infarction or stroke, 17% with hyperlipidemia, and 75% current smokers. Additionally, among the HIV-positive patients, 18% reported methamphetamine use and 24% were HCV positive.
Table 1.
Population characteristics
| Un-infected (N=16) | All HIV-infected Patients (N=57) | HIV (Without PAH) (N=18) | HIV-PAH (N=39) | p-value | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 10 (62%) | 45 (79%) | 13 (72%) | 32 (82%) | 0.33 |
| Female | 6 | 12 | 5 | 7 | |
|
| |||||
| Age (median (IQR)) | 49 years (42–55) | 47 years (41–52) | 51 years (42–56) | 46 years (41–50) | 0.34 |
|
| |||||
| Current ART | |||||
| Yes | N/A | 30 (73%) | 12 (67%) | 18 (78%) | 0.41 |
| No | 11 | 6 | 5 | ||
|
| |||||
| CD4+ T-cell count (median (IQR)) | N/A | 484 (322–640) cells/ μL | 500 (366–654) cells/ μL | 425.5 (277–632) cells/μL | 0.67 |
|
| |||||
| HIV viral load (%undetectable) | N/A | 48% undetectable | 44% undetectable | 50% undetectable | 0.72 |
|
| |||||
| Mean Pulmonary Artery Pressure (mean(SD)) | 18 (16–23) mmHg | 45 (32–51) mmHg | <0.0001 | ||
|
| |||||
| PASP by echocardiography (median (IQR)) | 29 (25–35) mmHg | 70 (54–80) mmHg | <0.0001 | ||
|
| |||||
| 6 minute walk distance (median (IQR)) | 497 (438–575) feet | 424 (306–454) feet | 0.005 | ||
|
| |||||
| History of MI/CVA | |||||
| Yes | 0 (0%) | 5 (11.4%) | 0 (0%) | 5 (23.8%) | 0.06 |
| No | 9 | 39 | 18 | 21 | |
|
| |||||
| Hypertension | |||||
| Yes | 2 (22%) | 19 (40.4%) | 5 (27.8%) | 14 (48%) | 0.23 |
| No | 7 | 28 | 13 | 15 | |
|
| |||||
| Hyperlipidemia treatment | |||||
| Yes | 3 (33%) | 8 (17.4%) | 3 (17.6%) | 5 (17.2%) | 0.56 |
| No | 6 | 38 | 14 | 24 | |
|
| |||||
| Current tobacco | |||||
| Yes | 5 (55%) | 35 (75%) | 12 (66.7%) | 23 (79%) | 0.34 |
| No | 4 | 12 | 6 | 6 | |
|
| |||||
| Prior methamphetamine | |||||
| Yes | 7 (18.4%) | 2 (11%) | 5 (20%) | 0.90 | |
| No | 16 | 7 | 9 | ||
|
| |||||
| HCV antibody positive | |||||
| Yes | 0 (0%) | 11 (24.4%) | 6 (33.3%) | 5 (17.8%) | 0.04 |
| No | 16 | 35 | 12 | 23 | |
N/A= not applicable. Empty cell indicates data not available. ART= Antiretriviral therapy. MI/CVA= myocardial infarction or stroke. HCV= hepatitis C virus.
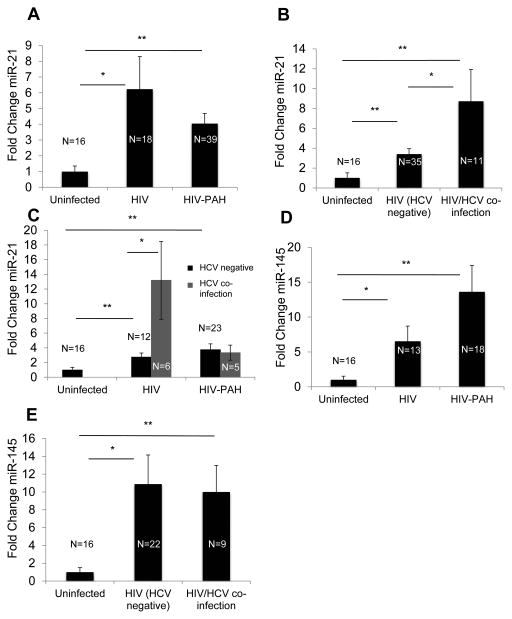
Plasma miR-21 was significantly up-regulated in HIV-infected and HIV-PAH individuals compared with uninfected controls, although no difference was identified between HIV and HIV-PAH (Fig. 1A). Linear and logistic regression demonstrated a correlation of circulating miR-21 expression only with HCV co-infection. No other hemodynamic or clinical disease characteristics correlated with plasma miR-21 expression (Supplemental Digital Content 1). To assess alterations of plasma miR-21 in HIV/HCV co-infected individuals, a subset of the original HIV-positive cohort was analyzed, as 11 of the 57 original HIV-infected cohort members were missing data for HCV infection and were excluded from this analysis (Table 1). Importantly, among the remaining HIV-infected subjects (including those with and without PAH), circulating miR-21 was increased in individuals with HCV as compared with persons without HCV (Fig. 1B). Additional analyses demonstrated that this finding was preserved among HIV patients without PAH but was not present among HIV-PAH patients (Fig. 1C). Circulating miR-21 was significantly increased in the HIV and HIV-PAH populations without HCV as compared to uninfected subjects, indicating that the increased prevalence of HCV in the HIV-only population was not the sole explanation for elevated circulating miR-21 in HIV-infected individuals (Fig. 1C, Table 1).
Figure 1. HIV and its comorbid conditions, HIV-PAH and HIV/HCV co-infection, are associated with distinct and overlapping effects on plasma miR-21 expression.
A. Plasma miR-21 was increased in HIV and HIV-PAH compared with controls. [Uninfected, 1±0.35 fold change (mean±SD); HIV, 6.23±2.07 fold change (p=0.02 vs uninfected); HIV-PAH, 4.04±0.65 fold change (p=0.006 vs uninfected and p=0.20 vs HIV)]. Plasma miRNA expression in the uninfected population was normalized to a fold change of 1, to which all other conditions were compared. B. Plasma miR-21 was increased in HIV/HCV co-infection. [Uninfected, 1±0.35 fold change; HCV-negative HIV, 3.4±0.56 fold change (p=0.007 vs uninfected); HIV/HCV co-infection, 8.72±3.2 fold change (p=0.008 vs uninfected and p=0.01 vs HCV-negative HIV)]. C. HCV co-infection increased plasma miR-21 expression in HIV infection alone but not HIV-PAH. [Uninfected, 1±0.35 fold change; HCV-negative HIV alone, 2.8±0.55 fold change; HCV-co-infected HIV alone 13.2±5.3 fold change; HCV-negative HIV-PAH, 3.8±0.8 fold change; HCV-co-infected HIV-PAH, 3.3±1.0 fold change]. P-values were calculated as follows: HCV–co-infected HIV vs uninfected: p=0.001; HCV-negative HIV vs uninfected: p=0.009; HCV-co-infected HIV vs HCV-negative HIV: p=0.01. D. Plasma miR-145 was increased in HIV infection and displayed a trend toward even higher levels in HIV-PAH compared with HIV. [Uninfected, 1.0±0.52 fold change; HIV, 16.0±5.2 fold change (p=0.01 vs uninfected); HIV-PAH, 33.5±9.1 fold change (p=0.004 vs uninfected and p=0.16 vs HIV)]. E. Plasma miR-145 was unchanged in HCV-co-infected HIV infection compared with HIV infection alone (including patients with and without PAH). [Uninfected, 1±0.52 fold change; HCV-negative HIV, 10.88±3.28 fold change (p=0.02 vs uninfected); HIV/HCV co-infection 10.01±3.00 fold change (p=0.001 vs uninfected and p=0.87 vs HCV-negative HIV)].
To determine the specificity of these changes in plasma miR-21, plasma miR-145 was tested in a smaller sub-cohort selected for extremes of mPAP, given its known association with PAH pathogenesis 32. Notably, circulating miR-145 expression was increased in HIV-infected as compared with uninfected controls and displayed a trend toward further increase in HIV-PAH compared with HIV infection alone (Fig. 1D). However, unlike miR-21, plasma miR-145 was not affected by HCV co-infection (Fig. 1E). Thus, the intricate regulation of circulating miR-21 by HCV, HIV, and PAH was specific and displayed a pattern distinct from miRNAs not previously associated with HIV or HCV.
Discussion
Here, we report the increased expression of plasma miR-21 specifically associated with HIV, HIV/HCV co-infection, and HIV-associated PAH. We found an increase in plasma miR-21 expression in both HIV-infected subjects and HIV-PAH subjects compared with uninfected subjects, as well as additive increases in miR-21 with HCV co-infection. Such findings have potential implications for our understanding of the complicated and likely overlapping pathogenesis of these comorbid diseases.
The specific alterations of plasma miR-21 demonstrated in our study may reflect the convergence of complex pathogenic mechanisms of chronic HIV/HCV co-infection. Certainly, multiple host miRNAs have been implicated in HIV and HCV 12, but our findings may provide mechanistic insight into the additive pathologic effects observed in HIV/HCV co-infection 33,34. Possible mediators of this phenomenon could include known targets of miR-21 such as programmed cell death 4 (PDCD4) or phosphotensin homologue (PTEN), which are involved in liver fibrosis 35 and HCV infection 36,37 as well as T-cell survival and death in HIV infection 38,39. Furthermore, the unique and dynamic alterations of plasma miR-21 suggest its potential utility as a biomarker reflecting disease progression during chronic HIV/HCV co-infection, though prospective studies will be required to investigate this.
Interestingly, PAH did not appear to increase plasma miR-21 above levels observed in HIV infection alone, and, within the HIV-PAH group, plasma miR-21 was not statistically associated with clinical correlates of PAH (Supplemental Digital Content 1). These findings are consistent with recent reports of a lack of substantial association of plasma miR-21 with clinical markers such as pulmonary artery pressure in non-HIV associated PAH 40. Certainly, miR-21 expression is associated with a variety of pathologies and this may detract from its utility as a biomarker of single diseases 41. It should be noted that, though plasma miRNA expression in some cases has been demonstrated to mimic intracellular patterns, the regulation of circulating or plasma miRNAs remains poorly understood. Thus, a lack of correlation with clinical markers and the lack of specificity among HIV, HCV and HIV-PAH comorbidities does not preclude the pathologic importance of this intracellular molecule. These findings reflect regulatory overlap between diseases for which miR-21 may represent an important molecular intersection.
Analyses of HIV-infected and HIV-PAH subjects suggested that plasma miR-21 may be modulated by PAH in HIV/HCV co-infection (Fig. 1C). Certain studies in animal models have demonstrated a protective role for miR-21 in PAH, while others reported that miR-21 antagonism improves PAH pathology, suggesting complex, context-specific disease regulation by this molecule 18–22,42. The complexity of such context-dependent expression is reflected in our findings. Based on our observations, it appears that PAH may counteract the effect of HCV on overall plasma miR-21 expression. Unfortunately, our small sample size of HCV-positive patients limits our ability to fully interpret this finding. Further analyses are warranted to examine disease-specific miRNA modulation in the setting of HIV/HCV co-infection. Importantly, increased miR-21 in HIV/HCV co-infection was unique as compared to miR-145, which we also found to be increased in HIV and HIV-PAH as compared to uninfected subjects. Interestingly, this is the first report of increased circulating miR-145 in HIV infection or HIV-PAH. Although there is no known mechanistic link between HIV and miR-145 expression, there is a report of BMP-receptor down-regulation in HIV-positive intravenous drug users, which could theoretically lead to miR-145 up-regulation and PAH 32,43
Certainly, limitations exist in this cross-sectional study – namely, the lack of prospective data. Additionally, although our findings are robust with respect to the HIV/HCV co-infection group, the relatively small sample size in this pilot study precluded testing clinically relevant variables within this group, including markers of HIV disease severity, HCV viral load, and additional HIV comorbidities. Additionally, although data in matched patients afflicted only with HCV were not available, circulating miR-21 has been reported in this group previously 27. Although subjects were not actively matched for all potential comorbidities affecting HIV-infected patients, common cardiovascular risk factors did not differ between groups, indicating a relatively homogeneous population as least with respect to this relatively prevalent HIV comorbidity (Table 1). Moreover, while the addition of a cohort of patients from a second clinical site (MGH) in the uninfected control population was necessary to improve statistical power, such inclusion could have introduced unanticipated confounding variables to the analysis. To allay some of those concerns, we found that miR-21 expression did not differ between the MGH and SFGH uninfected populations (p=0.37). Lastly, the inclusion of HIV-negative PH and HIV-negative HCV comparison groups would provide further information regarding the additive effects of these diseases on circulating miR-21 expression and should be investigated in the future.
Nonetheless, taken together, these data provide clear evidence of dysregulation of plasma miR-21 during HIV infection, HIV-PAH, and HIV/HCV co-infection. Such modulation is distinct for miR-21 and reflects the integration of several comorbid disease processes. Future study of plasma miR-21 and other miRNAs is warranted to determine their prognostic capabilities and potential biological actions in HIV, a disease whose natural history is driven in large part by the overlap and synergy of its comorbidities.
Supplementary Material
Acknowledgments
Source of Funding: NIH (grants K08HL096834; R01HL124021 (SYC) and K24AI112393; R01HL095130 (PH)); the McArthur-Radovsky, Lerner, Harris, and Watkins Funds; the American Heart Association (14GRNT19600012) (SYC); and the Pulmonary Hypertension Association (SYC). PH has received honoraria from Gilead. TD is a consultant for Gilead and Actelion and has research grants from United Therapeutics.
We thank Stephanie Tribuna for expert administrative assistance.
Footnotes
Conflicts of Interest: For the remaining authors, none were declared.
Citations
- 1.Sitbon O. HIV-related pulmonary arterial hypertension: clinical presentation and management. AIDS. 2008;22 (Suppl 3):S55–62. doi: 10.1097/01.aids.0000327517.62665.ec. [DOI] [PubMed] [Google Scholar]
- 2.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 3.Cool CD, Voelkel NF, Bull T. Viral infection and pulmonary hypertension: is there an association? Expert Rev Respir Med. 2011;5(2):207–216. doi: 10.1586/ers.11.17. [DOI] [PubMed] [Google Scholar]
- 4.Moorman J, Saad M, Kosseifi S, Krishnaswamy G. Hepatitis C virus and the lung: implications for therapy. Chest. 2005;128(4):2882–2892. doi: 10.1378/chest.128.4.2882. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan S, Suzuki K, Seddiki N, et al. Differential regulation of the Let-7 family of microRNAs in CD4+ T cells alters IL-10 expression. J Immunol. 188(12):6238–6246. doi: 10.4049/jimmunol.1101196. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 7.Triboulet R, Mari B, Lin YL, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315(5818):1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- 8.Sun G, Li H, Wu X, et al. Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res. 40(5):2181–2196. doi: 10.1093/nar/gkr961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant JS, White K, MacLean MR, Baker AH. MicroRNAs in pulmonary arterial remodeling. Cell Mol Life Sci. 2013;70(23):4479–4494. doi: 10.1007/s00018-013-1382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swaminathan S, Murray DD, Kelleher AD. miRNAs and HIV: unforeseen determinants of host-pathogen interaction. Immunol Rev. 254(1):265–280. doi: 10.1111/imr.12077. [DOI] [PubMed] [Google Scholar]
- 11.Conrad KD, Niepmann M. The role of microRNAs in hepatitis C virus RNA replication. Arch Virol. 2014;159(5):849–862. doi: 10.1007/s00705-013-1883-4. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Swaminathan G, Martin-Garcia J, Navas-Martin S. MicroRNAs, hepatitis C virus, and HCV/HIV-1 co-infection: new insights in pathogenesis and therapy. Viruses. 2012;4(11):2485–2513. doi: 10.3390/v4112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichtlscherer S, De Rosa S, Fox H, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 15.Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10(5):543–550. doi: 10.1016/j.coph.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55(11):1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 17.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bockmeyer CL, Maegel L, Janciauskiene S, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012;31(7):764–772. doi: 10.1016/j.healun.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Drake KM, Zygmunt D, Mavrakis L, et al. Altered MicroRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am J Respir Crit Care Med. 2011;184(12):1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White K, Dempsie Y, Caruso P, et al. Endothelial Apoptosis in Pulmonary Hypertension Is Controlled by a microRNA/Programmed Cell Death 4/Caspase-3 Axis. Hypertension. 2014;64(1):185–194. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Banerjee S, Freitas A, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L521–529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh VN, Jin RC, Rabello S, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witwer KW, Sarbanes SL, Liu J, Clements JE. A plasma microRNA signature of acute lentiviral infection: biomarkers of central nervous system disease. AIDS. 2011;25(17):2057–2067. doi: 10.1097/QAD.0b013e32834b95bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thapa DR, Bhatia K, Bream JH, et al. B-cell activation induced microRNA-21 is elevated in circulating B cells preceding the diagnosis of AIDS-related non-Hodgkin lymphomas. AIDS. 2012;26(9):1177–1180. doi: 10.1097/QAD.0b013e3283543e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thapa DR, Hussain SK, Tran WC, et al. Serum microRNAs in HIV-infected individuals as pre-diagnosis biomarkers for AIDS-NHL. J Acquir Immune Defic Syndr. 2014;66(2):229–237. doi: 10.1097/QAI.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bihrer V, Waidmann O, Friedrich-Rust M, et al. Serum microRNA-21 as marker for necroinflammation in hepatitis C patients with and without hepatocellular carcinoma. PLoS One. 2011;6(10):e26971. doi: 10.1371/journal.pone.0026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26(15):1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saggar R, Sitbon O. Hemodynamics in pulmonary arterial hypertension: current and future perspectives. Am J Cardiol. 2012;110(6 Suppl):9S–15S. doi: 10.1016/j.amjcard.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Baggish AL, Hale A, Weiner RB, et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol. 2011;589(Pt 16):3983–3994. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caruso P, Dempsie Y, Stevens HC, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111(3):290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 33.Feuth T, Arends JE, Fransen JH, et al. Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One. 2013;8(3):e59302. doi: 10.1371/journal.pone.0059302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W, Weinberg EM, Chung RT. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J Infect Dis. 2013;207 (Suppl 1):S13–18. doi: 10.1093/infdis/jis926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Zha Y, Hu W, et al. The auto-regulatory feedback loop of microRNA-21/Programmed cell death protein 4/Activation Protein-1 (miR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288(52):37082–93. doi: 10.1074/jbc.M113.517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clement S, Peyrou M, Sanchez-Pareja A, et al. Down-regulation of phosphatase and tensin homolog by hepatitis C virus core 3a in hepatocytes triggers the formation of large lipid droplets. Hepatology. 2013;54(1):38–49. doi: 10.1002/hep.24340. [DOI] [PubMed] [Google Scholar]
- 37.Yan-Nan B, Zhao-Yan Y, Li-Xi L, Jiang Y, Qing-Jie X, Yong Z. MicroRNA-21 accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN. Biochem Biophys Res Commun. 2014;443(3):802–807. doi: 10.1016/j.bbrc.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 38.Dabrowska A, Kim N, Aldovini A. Tat-induced FOXO3a is a key mediator of apoptosis in HIV-1-infected human CD4+ T lymphocytes. J Immunol. 2008;181(12):8460–8477. doi: 10.4049/jimmunol.181.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim N, Kukkonen S, Gupta S, Aldovini A. Association of Tat with promoters of PTEN and PP2A subunits is key to transcriptional activation of apoptotic pathways in HIV-infected CD4+ T cells. PLoS Pathog. 6(9):e1001103. doi: 10.1371/journal.ppat.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei C, Henderson H, Spradley C, et al. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8(5):e64396. doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider BA, Baras AS, McCall MN, Hertel JA, Cornish TC, Halushka MK. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLoS One. 2014;9(2):e89565. doi: 10.1371/journal.pone.0089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannone L, Zhao L, Dubois O, et al. MiRNA-21/DDAH1 pathway regulates pulmonary vascular responses to hypoxia. Biochem J. 2014;462(1):103–12. doi: 10.1042/BJ20140486. [DOI] [PubMed] [Google Scholar]
- 43.Dalvi P, O’Brien-Ladner A, Dhillon NK. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: implications for HIV-related pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2013;33(11):2585–2595. doi: 10.1161/ATVBAHA.113.302054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.